Abstract
Objective(s):
Phage therapy is a potential alternative treatment for infections caused by Acinetobacter baumannii, a significant nosocomial pathogen, which has evolved resistance to almost all conventional antimicrobial drugs in poor hygiene and conflicts areas such as Iraq.
Materials and Methods:
Bacteriophages were isolated to highly resistant isolates of A. baumannii to form therapeutic phage cocktail, and to extract and evaluate native endolysin activity. Bacterial samples were collected in Al-Imamein Al-kadhimein Medical City Hospital. Phages were isolated from different regions in Baghdad city including (soil, sewage, irrigation channels). Phage endolysin was extracted from highly lytic phages that produced halo-like appearance around inhibition zone.
Results:
Up to 23 isolates of extensive- and pan- drug resistant (XDR, PDR) A. baumannii were isolated from patients with various infections, and 136 lytic phages specific to A. baumannii were isolated. Each bacterial isolate was sensitive to at least one lytic phage. Accordingly, a phage cocktail was formulated which remarkably minimized bacterial resistance to lysis by phages when compared to individual lytic phages. And, the phage cocktail succeeded in treating and saving life of all bacteremic mice with A. baumannii versus the non-treated group. In addition, the endolysin native activity to A. baumannii was evaluated in this study; endolysin revealed a potent antibacterial activity (> 1 log) reduction of bacterial density in just one hour of endolysin treatment.
Conclusion:
The phage therapy assessed in this study showed an ability to efficiently solve the problems of “superbug” bacteria by lysing effectively most XDR, PDR bacteria in vitro and in vivo. And, phage cocktail was shown to be superior over single-phage preparations in treating A. baumannii with much less resistance rate to therapeutic phages. Furthermore, intrinsic activity of native endolysin revealed promising results to tackling superbug pathogens.
Key Words: Acinetobacter baumannii, Bacteriophages, Drug resistance, Endolysin, Phage therapy
Introduction
Antibiotic resistance is an emerging global health disaster, resulting from the constant use and misuse of antibiotics in healthcare (1, 2). Acenitobacter baumannii is a Gram-negative, capsulated, opportunistic pathogen that is effortlessly spread in hospital intensive care units (ICU) (3). Most of A. baumannii clinical isolates are multi-drug resistant (MDR), extensively drug-resistant (XDR), and pan-drug resistant (PDR) bacteria, which greatly restricts the available treatment choices (4). To prevent returning to the dark “post antibiotics” era, there is an urgent need for new therapeutic agents against the MDR, XDR, PDR pathogens. To fight these bacteria, the scientists suggest a number of new therapeutics alternatives or complements to antibiotics against the “superbug” pathogens, of which A. baumannii. Interestingly, bacteriophage, or phage, therapy has been placed at the top of table presenting a possible alternative mean to tackle refractory bacterial infections (5). Phage therapy refers to the utilization of phages to treat bacterial diseases (6). Phages are very abundant in nature (7) and every bacterium is likely to have their own specific viruses that could be utilized as antibacterial agents (8-10). The host range of a given phage is often very specific to the sub-species level, which may confer an advantage over antibiotics by targeting pathogens without damaging commensal members of the host microbial community. However, this could be a drawback as it is not easy to find a phage for every pathogenic strain (11). Moreover, bacteria develop resistance to phages 10-fold easier than to chemical antibiotics (12). Therefore, using a mixture of lytic and specific phages to certain pathogenic bacteria would address the problems of narrow host range and anti-phage resistance (13). Hence, the formation of phage cocktail could save lives of uncountable patients suffering from serious and devastating A. baumannii infections resistant to the conventional antibiotics. This highlights the importance of using phage cocktails especially in a country like Iraq where A. baumannii flourishes in poor hygiene and areas of conflicts (14).Similar to phage cocktails, phage endolysins are lytic enzymes produced by bacteriophages during the last step of their replicative cycle. The enzymes degrade the cell wall of bacterial hosts and lead to cell lysis and phage progeny release (15). Endolysins are classified as a new class of antimicrobials for the treatment of drug-resistant bacterial infection because of their rapid action, low evidence of resistance development and low cytotoxicity against mammalian cells (15). In the case of Gram-negative bacteria, applications of specific endolysins are limited because the outer cell membrane (OM) prevents exogenously applied endolysins from attracting the peptidoglycan layer (15), Thus, many studies have focused on the enhancement of OM permeability using chelators such as EDTA (16), or by fusion of polycationic peptides to the Gram-negative endolysin facilitates outer membrane penetration allowing these new so-called Artilysin®s access to the Gram-negative peptidoglycan (17).
Accordingly, the current study aims at testing the efficacy and biosafety of phage therapy, via using a single phage, and a phage cocktail to treat infections with MDR A. baumannii bacteria in vitro and in vivo and to extract anti-A. baumannii phage endolysins and determine their intrinsic lytic activity.
Materials and Methods
Specimen collection and identification
Samples of bacteria were collected in Al-Imamein Al-kadhimein Medical City Hospital in Alkadymiya, Baghdad. Bacterial sampling was carried out during the period from September 2016 to November 2016. A total of twenty three, 23, different A. baumannii isolates (11 XDR, 12 PDR), belonging to hospitalized patients with various infections including septicemia, skin infection, severe UTI, pneumonia, and meningitis, were obtained from the central laboratory of the hospital. At the same day, samples were transferred to the laboratory of the Medical Microbiology Department in the College of Medicine, Al-Nahrain University to sub-culture bacteria on nutrient, MacConkey and blood agar then incubated at 37 ⁰C for 18-24 hr. Next day, all bacterial isolates were subjected to a full set of diagnosis including Gram staining, culture, and bio-chemical tests (18). Furthermore, the results of the identification of A. baumannii were confirmed by API 20E system.
Antibiotic susceptibility test
Antibiotic susceptibility test was carried out on A. baumannii isolates using Kirby-Bauer method (19). In this assay, 17 types of antibiotic disks were used as following imipenem (10 μg), ciprofloxacin (5 μg), colistin (10 μg), tigacyclin (15 μg ), gentamicin (10 μg), cefotaxime (30 μg), ceftazidime (30 μg), ceftriaxone (30 μg), trimethoprim/ sulphamethaxazole (1.25/23.75 μg), cefepime (30 μg), levofloxacin (10 μg), piperacillin(100 μg), tobramycin(10 μg), amikacin (30 μg), meropenem (10 μg), aztreonam (30 μg) and amoxicillin-clavulanicacid (20/10 μg). A 0.5 McFarland standards of bacteria was used and inoculated and spread by a sterile swab on Muller-Hinton agar Medium. Antibiotic discs were then placed on inoculated agar plates by forceps. The plates were left in incubator upside down at 37 ⁰C for 18-24 hr.
Bacteriophage sampling, isolation
Different crude samples for phage isolation were obtained from different regions in Baghdad city including sewage, farm soil, feces of sheep, chicken litter, and swab from surgical lounge of several hospitals in Baghdad. Overnight bacterial broth (100 μl) was mixed with 2-3 ml of crude samplesand incubated overnight at 37 ⁰C until obtain specific lytic phage (21).
Optimization and charachtarization of isolated phages
Plaque characteristics were determined using top layer plaque assay and according to the following parameters: a) Diameter (mm) of the plaque. b) Shape of the plaque. c) Depth of the plaque. d) Margin cut. e) Clarity or turbidity of the plaque. Accordingly, the clearest and largest plaques were selected; moreover, small or turbid plaques were subjected to optimization by conducting serial passage in top layer plaque assays; at each run, the best of the best plaques, in terms of the above mentioned parameters, were selected in order to acquire better virulence characteristics of the isolated lytic phages.
Testing of bacterial resistance rate of A. baumannii to infecting bacteriophages
The resistance rate of bacteria to infecting phages was measured. A piece from the bacterial lawn of the target bacteria equal in diameter to the phage lysis spot was cut by a sterile loop and put in 1.5 ml sterile Eppendorf tube containing one ml of normal saline to obtain the same number of bacteria that was present in the phage spot lysis zone.Then, the bacterial resistance rate was calculated as the following:
Resistance rate=Number of resistant colonies per phage lysis spot/ number of bacterial colonies formed from the same size cut of bacterial lawn.
Determination of the coverage rate of bacteriophage cocktails to A. baumannii
In this approach, after mixing numerous phages in one suspension, randomly sampled 10 A. baumannii isolates were collected from patients in Al-Imamein Al-kadhimein Medical City Hospital. Ten (10) μl of the prepared bacteriophage cocktail suspension were spotted on to the surface of the overnight bacterial lawn and were allowed to dry before incubating at 37 ⁰C for 24 hr. On the next day, if a zone of lysis was developed at the spot where the phages suspension was applied, a susceptible bacterial isolate to phage cocktail was found. Then, the coverage rate of the formed bacteriophage cocktails was measured using this formula:
Coverage rate=(number of bacterial isolates lysed by the phage cocktail / total number of bacterial isolates treated with the phage cocktail) X 100%.
The assessment of the native activity of phage endolysin on A. baumannii bacteria extraction of endolysin
About 100 ml of A. baumannii broth were incubated for 18-24 hr at 37 ⁰C. Next day, 250 ml of broth medium were added to the bacterial growth and incubated for another 3 hr at titer 1×109 CFU/ml. Up to 10 ml of phage, titer 1×1011 PFU/ml (1:100 MOI), were mixed with bacteria for 20 min and were then put directly in ice and centrifuged at 10,000×g for 20 min and the sediment was collected. The sediment was suspended in 10 ml of 0.05 M phosphate buffer+5 mg deoxyribonuclease and incubated for 60 min at 37 ⁰C. EDTA(0.005 M) was added and centrifugation at 10,000×g for 1 hr and then the supernatant was taken. Disodium tetrathionate (0.3 M) was added and mixed for 1 hr at 4 ⁰C. Ammonium sulfate was added to 85% saturation and incubated for 18-24 hr at 4 ⁰C. Next day, centrifugation at 10,000g for 1 h and was resuspended in 5 ml of 0.05 M phosphate buffer saline (pH 7.5). Dialysis against 200 ml of the buffer at 4 ⁰C was conducted. The resultant solution was added to column chromatography sephadex G.100 in 0.1 M phosphate buffer saline pH 7.5, in 18×0.5 cm column. Each one ml of the resultant filtrate was collected in Eppendorf tube. From each Eppendorf tube, 10 μl of the filtrate were dropped by automatic pippete onto A. baumannii bacterial lawns of the specific bacteria to see which Eppendorf tube contains the lytic and native activity of endolysin.
Measurement of the native activity of endolysin on A. baumannii bacteria
The endolysin activity was first checked by lysis on bacterial lawn and by decreasing the optical density of the bacterial broth when measured by a spectrophotometer. A. baumannii broth, composed of bacterial cells at mid-log phase OD(600=0.6), was centrifuged (4000×g, 30 min, 4 ⁰C) and then re-suspended in a phosphate-buffered saline (PBS) at pH 7.5. After assigning the tube that showed lysis in the bacterial lawn assay, 30 μl of this supposed-to-be endolysin-containing elute were added to 270 μl of the prepared bacterial broth at room temperature. Then, the optical density was measured spectrophotometrically every ten min for 1 hr at 600 nm (22).
In vivo experiments of phage therapy on mice infected with highly resistant A. baumannii
In these experiments, white mice males of mean body weight 17±1.5 g and age 4-5 weeks were used. Mice were grouped into: mice injected with 0.3 ml of 108 PFU/ml of a therapeutic phage alone (the phage group), 0.3 ml of 106 CFU/ml of bacteria alone (the bacteremic group), 0.3 ml of 106 CFU/ml of bacteria then, after 2 hr, with 0.3 ml of 108 PFU/ml of a therapeutic phage (the test group) intreperitoneally (IP) in order to evaluate the phage side effects, bacterial virulence, and the efficacy of isolated and optimized phages to treat infected mice in vivo, respectively. In addition, a fourth group of mice that did not receive any kind of injections, the negative control group, was included. Besides, a mice group received 0.3 ml of 108 PFU/ml of phage then after 2 weeks was treated as a test group in order to evaluate the in vivo phage therapy on immunized mice. For all these experiments, the physical activity of mice was monitored every day and the survival of mice was monitored for 9 days, or until the time of death. The health status of mice in each group was measured on a scale from 5 (normal) to 0 (death), based on the progressive status of bacteremia that is reflected by several clinical signs and physical activities (alertness, response to light or voice, feeding frequencies)(23).
Results
Characteristics of the isolates of Acinetobacter baumannii
The isolates of A. baumannii came from patients infected with serious and life-threatening diseases including urinary tract infection, septicemia, wound infection, pneumonia, and meningitis. A total of 23 A. baumanii isolates were collected. The specimens were as follows: blood 7/23 (30.4%), urine 2/23 (9%), wound swab 7/23 (30.4%), diabetic foot 3/23 (13%), sputum 2/23 (9%), and C.S.F 2/23 (9%). The diseases, from which A. baumanii bacteria were isolated, were wound infection 10/23 (43.4%), urinary tract infection 2/23 (9%), septicemia 9/23 (39.1%), pneumonia 2/23 (9%), and meningitis 2/23 (9%). Hence, the most prevalent prevalent disease related to A. baumanii was wound infection followed by septicemia.
The age of patients ranged from 1 day to 70 years and male to female ratio was 2.3.
Antibiotic susceptibility test
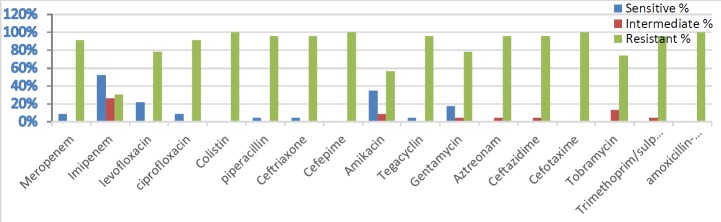
The results showed that different A. baumannii isolates had different antibiotic sensitivity profiles; of 23 isolates included in the current study, 11 were XDR and 12 were PDR as shown in Figure 1.
Figure 1.
The rate of antibiotic sensitivity/resistance of 23 Acinetobacter baumannii isolates to a panel of 17 antibiotic disks commonly used in Iraq
The characteristic features of the isolated and optimized phages
The characteristics of the plaque assay of the isolated phages showed that plaques clarity ranged from clear, semi-clear, turbid, to semi-turbid, plaques size ranged from 0.5 to 6.5 mm, margin cut ranged from regular to irregular, and plaques shape from oval to circular. One hundred and thirty six (136) phages specific for 23 A. baumannii bacteria were isolated. Most of the isolated phages were highly lytic and produced obvious inhibition zone on target A. baumannii bacteria where plaque size was higher than 3 mm with full clarity of plaques; therefore, further optimization was not needed except for 25 phages which required further optimization in order to increase their lytic characteristics, (Table 1).
Table 1.
Morphological features of the isolated phages to Acinetobacter baumannii bacteria before and after optimization via top layer plaque assay
|
Phage symbol
|
Plaque size (mm)
|
Plaque clarity
|
Plaque shape
|
Margin cut
|
||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| AB1P1 | 0.5 | 1.5 | Turbid | Semi-clear | Round | Round | Un-obvious | Regular |
| AB1P2 | 0.3 | 1 | Semi-turbid | Clear | Round | Round | Irregular | Irregular |
| AB2P1 | 2 | 2.5 | Semi-clear | Semi-clear | Round | Round | Regular | Regular |
| AB3P1 | 0.8 | 1.5 | Semi-turbid | Semi-clear | Oval | Oval | Irregular | Irregular |
| AB3P2 | 0.5 | 0.5 | Semi-clear | Clear | Oval | Oval | Regular | Regular |
| AB3P3 | 2.5 | 4 | Semi-Clear | Clear | Round | Round | Irregular | Irregular |
| AB3P4 | 3.5 | 3.5 | Semi-Clear | Clear | Oval | Oval | Irregular | Irregular |
| AB4P1 | 3.5 | 7 | Clear | Clear | Round | Round | Irregular | Irregular |
| AB5P1 | 1 | 1.5 | Turbid | Clear | Oval | Oval | Un-obvious | Irregular |
| AB6P1 | 2 | 2 | Semi-turbid | Semi-clear | Semi-round | Round | Irregular | Regular |
| AB6P2 | 2 | 3.5 | Semi-clear | Semi-clear | Oval | Oval | Irregular | Irregular |
| AB9P1 | 0.5 | 0.5 | Semi-Clear | Clear | Round | Round | Regular | Regular |
| AB10P1 | 1.2 | 2.5 | Clear | Clear | Round | Round | Regular | Regular |
| AB10P2 | 1.9 | 5.5 | Semi-turbid | Clear | Round | Round | Regular | Regular |
| AB12P1 | 1 | 1 | Semi-turbid | Semi-turbid | Oval | Oval | Irregular | Irregular |
| AB15P1 | 3.5 | 5.5 | Turbid | Clear | Round | Round | Un-obvious | Regular |
| AB15P2 | 0.8 | 1 | Semi-turbid | Clear | Round | Round | Regular | Regular |
| AB17P1 | 1.7 | 3.5 | Semi-Clear | Clear | Oval | Oval | Irregular | Irregular |
| AB19P1 | 0.5 | 3 | Semi-turbid | Semi-turbid | Round | Round | Un-obvious | Irregular |
| AB19P2 | 0.8 | 1.5 | Turbid | Clear | Round | Round | Un-obvious | Regular |
| AB20P1 | 0.5 | 2 | Semi-Clear | Clear | Oval | Oval | Regular | Regular |
| AB21P1 | 1.5 | 6.5 | Turbid | Semi-clear | Oval | Oval | Regular | Regular |
| AB21P2 | 1 | 2 | Semi-turbid | Semi-clear | Round | Round | Irregular | Irregular |
| AB22P1 | 0.5 | 1.5 | Turbid | Turbid | Oval | Oval | Un-obvious | Regular |
| AB22P2 | 2.3 | 4.5 | Semi-turbid | Semi-turbid | Oval | Oval | Irregular | Irregular |
The titer of the lytic phages isolated and optimized to A. baumanii were amplified and measured by using top layer plaque assay. Most phages reached high titers ranging between 108-1011 PFU/ml using top layer plaque assay.
The optimized specific and lytic phages were shown to be able to completely lyse the bacterial host in whatever manner of application of phages as demonstrated in Figures 2-3.
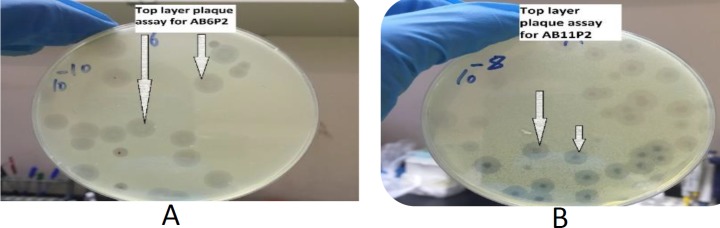
Figure 2.
(A) Plaques produced by phage AB6P2 via top-layer plaque assay (B) plaques produced by phage AB11P2 via top-layer plaque assay
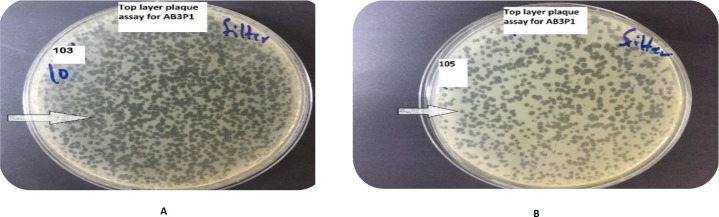
Figure 3.
Top layer plaque assay for phage AB3P1 with different concentrations (A) AB3P1 with 103 PFU/ml (B) AB3P1 with 105 PFU/ml
Table (5).
Health score of AB3 non-immunized and AB3 immunized mice groups treated with AB3-specific phage
| Hour |
Health scores of
immunized mice treated with phage |
Median of health
scores of immunized mice treated with phage |
Health scores of non-immunized mice treated with phage | Median of health scores of non-immunized mice treated with phage |
P-value (Mann Whitney test) |
|---|---|---|---|---|---|
| 0.5 | 4,4,5,5,5 | 5 | 4,4,4,5,5 | 4 | 0.84 |
| 1 | 4,3,4,4,4 | 4 | 4,4,4,4,5 | 4 | 0.64 |
| 1.5 | 3,3,3,4,4 | 3 | 3,3,4,3,4 | 3 | 0.76 |
| 2 | 3,3,3,3,3 | 3 | 3,3,3,4,3 | 3 | 0.8 |
| 2.5 | 4,4,4,4,4 | 4 | 4,3,4,4,4 | 4 | 1 |
| 3 | 4,4,5,4,5 | 4 | 5,4,5,5,5 | 5 | 0.52 |
| 3.5 | 5,5,5,4,5 | 5 | 5,5,5,5,5 | 5 | 1 |
| 4 | 5,5,5,4,5 | 5 | 5,5,5,5,5 | 5 | 1 |
The characteristics of the isolated and optimized phages in terms of biokinetic assay
In the current study, 10 bacteriophages to differentbacterial isolates were randomly selected to give representative values of biokinetic characteristics. The results in this study showed that the average burst time (BT) was 73.5 min ranging between 30 to 45 min. The maximum burst size (BS) was 245 progeny, while the minimum BS was 130 progeny and the average BS was 187.5 progeny. The average infective percentage (IP %) was 85.45% ranging between 74.4% and 94.5%, as shown in Table 2.
Table 2.
The biokinetic parameters of optimized phages: Infective percentage (IP %), Burst time (BT) in min, and Burst size (BS) in number of progenies of the randomly selected bacteriophages to Acinetobacter baumannii bacteria
| IP % | BT | BS | |
|---|---|---|---|
| AB1P2 | 91.3 | 40 | 170 |
| AB3P4 | 94.5 | 45 | 245 |
| AB5P1 | 76.4 | 35 | 220 |
| AB7P3 | 85.2 | 30 | 160 |
| AB9P1 | 88 | 45 | 210 |
| AB10P2 | 86.6 | 45 | 145 |
| AB14P1 | 77.5 | 40 | 190 |
| AB15P3 | 82 | 30 | 200 |
| AB17P2 | 80 | 40 | 130 |
| AB20P1 | 79 | 35 | 185 |
Formation of phage cocktail to A. baumannii
A phage cocktail was formed by mixing 64 phages specific for 23 A. baumannii isolates (AB1-AB23). All bacterial isolates, except AB2 and AB8, were targeted by more than one phage; the most targeted isolate was AB3 where 6 different phages shared the same specificity towards this isolate.
Bacterial resistance to a single phage versus phage cocktail
Up to 18/23 (78.3%) of A. baumannii bacteria were completely sensitive to the applied lytic phages with zero resistant bacterial colonies. So, only 5 out of 23 isolates (21.7%) of A. baumannii were shown to develop some level of resistant colonies in the inhibition zone at the spot of lytic phage application. On the other hand, the formed phage cocktail was shown to remarkably minimize the number of the resistant bacterial colonies appeared to individual phages. The results revealed that once A. baumannii isolate develops resistance to a one
member of the phage cocktail, this bacterial isolate was still sensitive to other phage members in the same cocktail as shown in Figure 4.
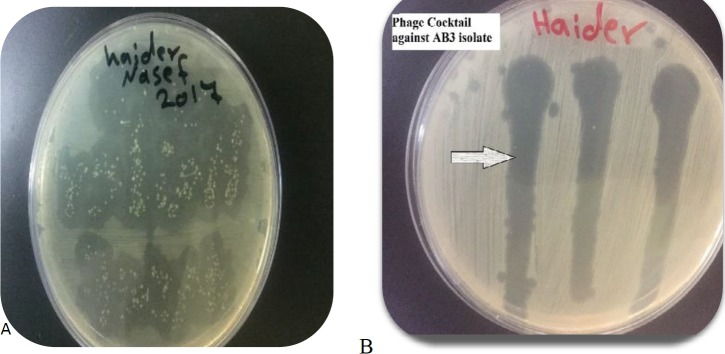
Figure 4.
(A) Resistant Acinetobacter baumannii bacteria (AB3) to a single specific phage (AB3P1) (B) the phage cocktail completely lysed A. baumannii (AB3) without development of any resistant colonies
The coverage rate of the formed phage cocktail to A. baumannii bacteria
Ten (10) A. baumannii isolates were collected randomly after the formation of the phage cocktail. The collected specimens were not biased towards particular disease, site of infection, or patients’ age or sex. The formed phage cocktail was able to form a clear inhibition zone on the most tested bacterial lawns. The coverage rate of the formed phage cocktail was calculated. The phage cocktail was shown to be able to lyse 7/10 (70%) of A. baumannii and thus the coverage rate was 70%.
Determination of the native activity of phage endolysin on A. baumanii
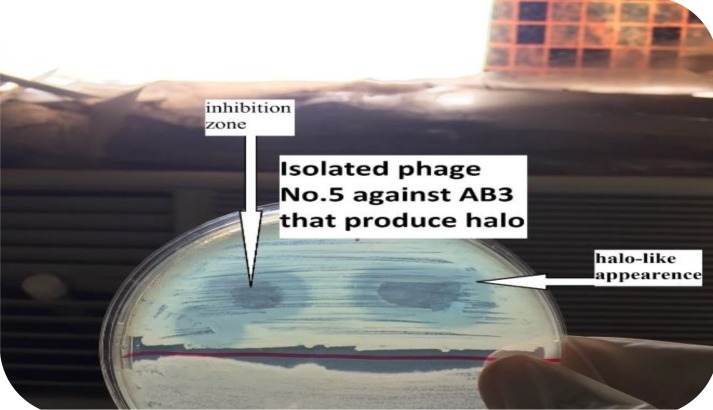
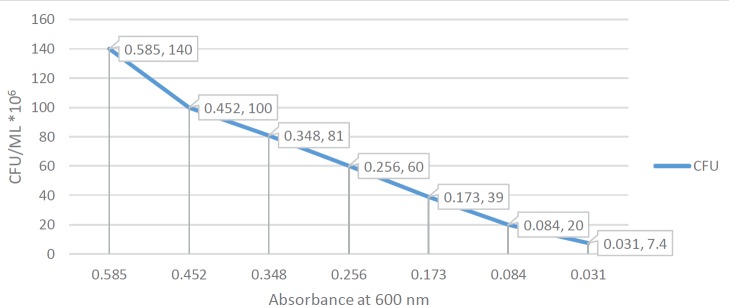
Some phages were found to produce a halo-like appearance around the inhibition zone produced by some lytic phages as shown in Figure 5. This halo-like appearance suggested a native endolysin production from phage. A specific phage endolysin to A. baumannii was extracted successfully by using sephadex G100 column chromatography. The Eppendorf tube number two showed positive results for phage endolysin. The optical density of A. baumannii broth was measured initially at zero time, just before the addition of the corresponding endolysin, then it was measured every 10 min up to 1 hr and it showed obvious decline in optical density of bacterial broth with time. According to t-distribution test, there was a significant difference between the test groups, bacteria treated with endolysin and control group, bacteria alone with PBS, (P=0.00134). Moreover, the overall enzymatic activity of extracted native endolysin was quantified by turbidometric reduction analysis, 270 μl of exponentially growing A. baumannii (AB3) cultures (1.4 x 108 CFU/ml) were challenged to 30 μl of extracted native endolysin at room temperature. A. baumannii optical density and viability counts were reduced from 0.585 to 0.031 after one hour of treatment, compared with the untreated control group that continued to grow (from 577 to 624 after one hour). It was shown to be 0.0092 ΔOD/min. By using standard curve measurements to interpolate OD values to bacterial count, it was shown that the endolysin native activity surpassed 1.4 log reduction threshold after one hour of treatment as shown in Figure 6.
Figure 5.
An obvious halo-like appearance around the inhibition zone produced by the lytic phage AB3P5 on the lawn of Acinetobacter baumannii isolate AB3
Figure 6.
Interpolation of bacterial count in colony forming unit/ml with the optical density (600nm) of Acinetobacter baumannii broth treated with AB3 phage endolysin
In vivo experiments of phage therapy
The results in the current study showed that the test group, which was injected IP with AB3 isolate and in 2 hr was treated IP with AB3-specific phages, stayed alive for more than 6 weeks, with full physical activity. By using Mann Whitney test, there was no significant difference in the median of health score between the bacteremic (without phage therapy) and test (with phage therapy) groups (P=0.64) during the 2 hr period after the bacterial injection and before the phage injection, Table 3. On the other hand, 2.5 to 4 hr after phage administration, the median of health score of the test group was far higher than the bacteremic group (P< 0.01), Table 3. Therefore, 4 hr of bacteremia with AB3 A. baumannii were enough to kill the bacteremic mice while mice treated with phage 2 hr after the start of bacteremia were 100% saved and survived in healthy condition as shown in Table (3). Referring to the phage group, that was injected IP with AB3-specific phage at concentration 108 PFU/ml alone, mice stayed alive, without any change in their health score (P-value =1) as shown in Table (4). More to the in vivo experiment, there was no significant difference in the median of the health score between AB3-specific phage immunized versus non-immunized groups of mice (P>0.05).
Table (3).
The health score of the test group of mice injected IP with AB3 bacteria and AB3P phage in comparison with the bacteremic group injected only with AB3 bacteria
| Hour |
Health scores
Bacteremic group |
Median of health scores of acteremic group |
Health scores
of test group |
Median of health
scores of test group |
P-value (Mann Whitney test) |
|---|---|---|---|---|---|
| 0.5 | 4,4,5,5,5 | 5 | 4,4,4,5,5 | 4 | 0.84 |
| 1 | 4,4,4,4,3 | 4 | 4,4,4,4,5 | 4 | 0.64 |
| 1.5 | 3,3,3,4,4 | 3 | 3,3,4,3,4 | 3 | 0.76 |
| 2 | 3,2,3,3,3 | 3 | 3,3,3,4,3 | 3 | 0.64 |
| 2.5 | 3,2,2,2,2 | 2 | 4,3,4,4,4 | 4 | 0.04 |
| 3 | 2,1,2,1,1 | 1 | 5,4,5,5,5 | 5 | >0.01 |
| 3.5 | 1,0,1,0,0 | 0 | 5,5,5,5,5 | 5 | >0.01 |
| 4 | 0,0,0,0,0 | 0 | 5,5,5,5,5 | 5 | >0.01 |
Table (4).
Health score of the phage group injected IP with AB3-specific phage alone in comparison with the negative control group of mice
| weeks |
Health scores
of phage group |
Median of health
scores of phage group |
Health scores of
negative control group |
Median of health
scores of negative control group |
P-value (Mann Whitney test) |
|---|---|---|---|---|---|
| 1 | 5,5,5,4,5 | 5 | 5,5,5,5,5 | 5 | 1 |
| 2 | 5,5,5,5,5 | 5 | 5,5,5,5,5 | 5 | 1 |
| 3 | 5,5,5,5,5 | 5 | 5,5,5,5,5 | 5 | 1 |
| 4 | 5,4,5,5,5 | 5 | 5,5,5,5,5 | 5 | 1 |
| 5 | 5,5,5,5,4 | 5 | 5,5,5,5,5 | 5 | 1 |
Discussion
All of the isolates of A. baumannii were shown to be completely resistant to several antibiotics as follows:
Cefepime, Cefotaxime, colistin, and amoxicillin-clavulanic acid. However, the resistance rate to other antibiotics was less than 100% and ranged from 95.65% to 30.43%. The multiple drug resistant status reported in current study is in agreement with the findings of other recent studies carried in Iraq (24, 25)but this study disagrees with a study carried out in USA which reported that approximately 50% of patients are with colistin-resistant A. baumannii (26). The variation in the results may be due to differences in the time when the studies were conducted, or simply differences in the geographic areas. The complete resistance of A. baumannii isolates collected in this study to colistin might be attributed to the major mechanism of colistin resistance in A. baumannii, namely modification of lipopolysaccharide (LPS) outer membrane via adding phosphor ethanol amine to the hepta -acylated lipid A structure (27-30). In this regard, phage therapy could offer one of the best applicable solutions to overwhelm the problem of antibiotics resistance of bacteria in Iraq, the Middle East, and in the world (31). One of the striking merits of using phages over antibiotics in a country like Iraq is that phages are self-amplifying in the site of infection; so, phages can be given to patients in a single dose. Hence, incompliance of patients will not affect the success of the course of therapy. In this study, the lytic and specific phages to A. baumannii were isolated from various environmental sources; the main source was sewage; this finding is in line with other studies (32, 33). Another main source of phages in this study was waste water (34). The current study revealed that sewage was the best source to isolate highly lytic and specific phages to A. baumannii (35).
The current study showed successful in vitro use of both single phage and phage cocktail to lyse A. baumannii XDR or PDR isolates. Nevertheless, this study revealed a superiority of the phage cocktail over the single phage in lysing A. baumannii bacteria without development of resistant colonies to phage therapy. Consequently, such phage cocktails are good candidates to prevent the emergence of phage-resistant mutants (21, 36). The results of the current study revealed that using phage cocktails provides several advantages. Firstly, phage cocktails broaden the strain-specific range of infective phages. This permits effective therapy of a broader spectrum of bacteria within the same A. baumannii species (37, 13). Secondly, phage cocktails solve the serious obstacle of the development of A. baumannii resistance to attacking phages. It was stated that using phage cocktails is the finest choice for effective phage therapy without suspicions of rapid emergence of bacterial resistance (38). Therefore, the phage cocktail used in this study ensured these two important goals.
Each A. baumannii isolate have more than one receptor and each receptor is recognized by a different phage to attach and invade (13). This explains why each bacterial isolate was invaded by more than one different phage. Therefore, when a bacterial isolate develops resistance to one phage in the phage cocktail, it is still sensitive to other phages in the same phage cocktail. From the findings of the top layer plaque assay, each member of the phage cocktail was unique, and from the findings of the bacterial resistance rate to single phage versus phage cocktail, the phages used in this study seem to target different receptors on the cell wall of A. baumannii. This provides evidence on the superiority of using phage cocktails.
The coverage rate of the formed phage cocktail in this study was shown to be very high, up to 70%. Such high coverage paves the road to successful and ready-to-use therapy of serious and life-threatening infections of A. baumannii. Nevertheless, in this study, it was proven that in few months, a phage cocktail of 64 anti-A. baumannii specific phages was formed. The formed phage cocktail could save lives of uncountable patients suffering from serious and devastating A. baumannii infections resistant to the conventional antibiotics. This highlights the importance of using phage cocktails especially in a country like Iraq where A. baumannii flourishes in poor hygiene and areas of conflicts (39).
The native activity of endolysin shown in the current study is in harmony with few studies examined the native activity of endolysin from phages infect Gram-negative bacteria such as A. baumannii, Pseudomonas aeruginosa and Escherichia coli (40-45). The current study highlights the intrinsic antimicrobial activity of endolysin produced from phages against Gram-negative bacterial pathogens. Native endolysin activity is a good candidate for the therapeutic/disinfectant endeavor to control nosocomial infections caused by MDR bacteria, particularly MDR A. baumannii (40). The intrinsic antibacterial activity of endolysin against Gram-negative needs the ability of endolysin to get through the outer membrane of these bacteria. This might explain why endolysins from phages infecting Gram-negative hosts are mostly small single-domain globular proteins (molecular mass between 15 and 20 kDa), and usually without a specific CBD module (41). These lysins likely better fulfill the catalytic role of classical enzymes (aiding multiple catalytic reactions during cell lysis), as opposed to their Gram-positive counterparts, which are proposed to bind to one site and have a very low off-rate (46-49).
Referring to the in vivo results, the current study highlighted several points to discuss. First, we selected one of the most pathogenic bacteria to mice, which have the capability to kill mice within only 3.5-4 hr. Second, the therapeutic phage used in this study, AB3-specific phage, stopped and reversed the worsening health score of mice and succeeded in eradicating the morbidly effects of the septicemic state of A. baumannii AB3. Even better, the therapeutic phage succeeded in rescuing life of the bacteremic mice group. This was a definitive proof on the efficacy and eligibility of using specific, optimized, highly lytic phages in treating deadly A. baumannii infections. These findings are in harmony with findings of several previous reports (50, 51). Third, the findings of this study revealed solid proof on the safety of therapeutic phages. The experimental mice group that was injected IP with 108 PFU/ml of therapeutic phage alone showed no change in health score and physical activity when compared to the negative control group. Moreover, mice were clear from any side effect for more than 4 weeks. This outcome is in line with other studies conducted to evaluate the safety of therapeutic phages in vivo (52, 53). The safety of bacteriophages is attributed to the fact that phages attach and react to receptors only found on prokaryotic cells. So, phages are incapable to invade eukaryotic cells and cause infections because no receptors specific for phage are on the eukaryotic cells (15).
Referring to in vivo phage therapy to immunized and non-immunized mice groups, the group of mice previously exposed to therapeutic phage (immunized) was unable to mount an effective immune response to the same phage at the second encounter. This might be explained by several points of view. First, bacteriophages are hyper-available in our environment, and plentifully found in what we eat and drink (54), so, our bodies in general and our immune system in particular get used to the existence of phages and no violent and aggressive immune reaction occur against phages (55). Second, if immune response was mounted, it is evident that it was not enough to hinder therapeutic phage from reaching and lysing A. baumannii. Third, host bacteria act as a shelter to attacking phages from the hostile environment of immune system; therefore, the higher the extent of bacterial infection, the more shelter provided by host bacteria to the attacking phages and vice versa. Fourth, the phage kinetics, as shown in the current study with burst size up to 150 and infection percentage up to 94%, are far more efficient than that of bacteria leading to an effective eradication of bacterial pathogen rapidly before the immune response become capable of wiping out the attacking phages. Therefore, the immune response produced by mice did not hinder the efficacy of phage therapy, these results are in line with previous reports (55, 56).
Conclusion
Taken together, the findings of this study indicate that A. baumannii in Iraq are mostly XDR and PDR bacteria; such abnormally high rate of multiple drug resistance necessities novel methods to tackle this impeding health risk on community. Therefore, phage therapy assessed in this study was shown to be able to solve the problem of superbug bacteria by lysing effectively most XDR and PDR bacteria in vitro. And, phage cocktails were shown to be superior over single-phage preparations in treating A. baumannii with much less rate of resistance to therapeutic phages. In addition, the native activity of endolysin from phages specific to A. baumannii revealed a potent antibacterial activity, >1 log reduction of bacterial density in just 1 hr of endolysin treatment; this provided evidence to tackle Gram negative bacteria by using low molecular weight endolysins which are of high level of native antibacterial activity. The results from in vivo experiments provide a solid proof that the formed phage cocktail as well as the single phage preparations are highly effective in treating life-threatening bacteremic cases and can quickly restore health condition back to normal; moreover, phage therapy was shown to be safe when given systemically without any side effects.
Acknowledgment
The results described in this paper were part of student thesis. The funding source for this research is from the author’s team of this study. Regards are directed to the staff members of Department of Medical Microbiology as well as the staff of the laboratory animal house at the College of Medicine, Al-Nahrain University for their support and assistance.
Conflicts of Interest
The authors declared no conflicts of interest.
References
- 1.Cantas L, Shah S, Cavaco L, Manaia C, Walsh F, Popowska M, et al. A brief multi-disciplinary review on antimicrobial resistance in medicine and its linkage to the global environmental microbiota. Front Microbiol. 2013;4:96–110. doi: 10.3389/fmicb.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Antimicrobial resistance: global report on surveillance. World Health Organization ; 2014. [Google Scholar]
- 3.Murray CK, Yun HC, Griffith ME, Thompson B, Crouch HK, Monson LS, et al. Recovery of multidrug-resistant bacteria from combat personnel evacuated from Iraq and Afghanistan at a single military treatment facility. Mil Med. 2009;174:598–604. doi: 10.7205/milmed-d-03-8008. [DOI] [PubMed] [Google Scholar]
- 4.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:510–526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 5.O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. HM Government and Wellcome trust 2016: There is no corresponding record for this reference; 2016. [Google Scholar]
- 6.Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM. Phage treatment of human infections. Bacteriophage. 2011;1:66–85. doi: 10.4161/bact.1.2.15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendrix RW, Smith MC, Burns RN, Ford ME, Hatfull GF. Evolutionary relationships among diverse bacteriophages and prophages: all the world’sa phage. Proc Natl Acad Sci U S A. 1999;96:2192–2197. doi: 10.1073/pnas.96.5.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clokie MR, Millard AD, Letarov AV, Heaphy S. Phages in nature. Bacteriophage. 2011;1:31–45. doi: 10.4161/bact.1.1.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores CO, Meyer JR, Valverde S, Farr L, Weitz JS. Statistical structure of host–phage interactions. Proc Natl Acad Sci U S A. 2011;108:E288–E297. doi: 10.1073/pnas.1101595108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Örmälä AM, Jalasvuori M. Phage therapy: should bacterial resistance to phages be a concern, even in the long run? Bacteriophage. 2013;3:24219–24222. doi: 10.4161/bact.24219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattila S, Ruotsalainen P, Jalasvuori M. On-demand isolation of bacteriophages against drug-resistant bacteria for personalized phage therapy. Front Microbiol. 2015;6:1271–1278. doi: 10.3389/fmicb.2015.01271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulakvelidze A, Alavidze Z, Morris JG. Bacteriophage therapy. Antimicrob Agents Chemother. 2001;45:649–659. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan BK, Abedon ST. Phage therapy pharmacology: phage cocktails. Adv Appl Microbiol. 2012;78:1–23. doi: 10.1016/B978-0-12-394805-2.00001-4. [DOI] [PubMed] [Google Scholar]
- 14.Falagas ME, Vardakas KZ, Kapaskelis A, Triarides NA, Roussos NS. Tetracyclines for multidrug-resistant Acinetobacter baumannii infections. Int J Antimicrob Agents. 2015;45:455–460. doi: 10.1016/j.ijantimicag.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 15.Schmelcher M, Donovan DM, Loessner MJ. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012;7:1147–1171. doi: 10.2217/fmb.12.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briers Y, Walmagh M, Lavigne R. Use of bacteriophage endolysin EL188 and outer membrane permeabilizers against Pseudomonas aeruginosa. J Appl Microbiol. 2011;110:778–785. doi: 10.1111/j.1365-2672.2010.04931.x. [DOI] [PubMed] [Google Scholar]
- 17.Briers Y, Walmagh M, Van Puyenbroeck V, Cornelissen A, Cenens W, Aertsen A, et al. Engineered endolysin-based “Artilysins” to combat multidrug-resistant Gram-negative pathogens. MBio. 2014;5:e01379–01314. doi: 10.1128/mBio.01379-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merril CR, Scholl D, Adhya SL. The prospect for bacteriophage therapy in Western medicine. Nat Rev Drug Discov. 2003;2:489–497. doi: 10.1038/nrd1111. [DOI] [PubMed] [Google Scholar]
- 19.WHO (World Health Organization) Basic laboratory procedures in clinical bacteriology . 2nd ed. Geneva, Switzerland: 2003. pp. 103–121. [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: Twenty-fourth informational supplement, M100-S24. Clinical and Laboratory Standards Institute (CLSI) ; 2014. p. 34. [Google Scholar]
- 21.Jassim SA, Abdualamir AS, Abu baker F. Methods for bacteriophage design in "international application published under the patent cooperation (PCT)" (W.I.P. organization, ed.) 2010. Vol.WO2010064044 A1. [Google Scholar]
- 22.Biswas B, Adhya S, Washart P, Paul B, Trostel AN, Powell B, et al. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect immun. 2002;70:204–210. doi: 10.1128/IAI.70.1.204-210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmons M, Donovan DM, Siragusa GR, Seal BS. Recombinant expression of two bacteriophage proteins that lyse Clostridium perfringens and share identical sequences in the C-terminal cell wall binding domain of the molecules but are dissimilar in their N-terminal active domains. J Agric Food Chem. 2010;58:10330–10337. doi: 10.1021/jf101387v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mshachal MA, Abdulrahman TR, Khudair MS, Hassan JS. Molecular detection of multidrug resistance Acinetobacter baumannii from different clinical samples. Iraqi J Med Sci. 2017;15:314–323. [Google Scholar]
- 25.Al Marjani M, Al-Ammar M, Kadhem E. Occurrence of ESBL and MBL genes in Pseudomonas aeruginosa and Acinetobacter baumannii isolated from Baghdad, Iraq. Int J Cur Res. 2013;5:2482–2486. [Google Scholar]
- 26.Qureshi ZA, Hittle LE, O'hara JA, Rivera JI, Syed A, Shields RK, et al. Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clin Infect Dis. 2015;60:1295–1303. doi: 10.1093/cid/civ048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelletier MR, Casella LG, Jones JW, Adams MD, Zurawski DV, Hazlett KR, et al. Unique structural modifications are present in the LPS from colistin-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother. 2013;51:4831–4840. doi: 10.1128/AAC.00865-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arroyo LA, Herrera CM, Fernandez L, Hankins JV, Trent MS, Hancock RE. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob Agents Chemother. 2011;55:3743–3751. doi: 10.1128/AAC.00256-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain R, Danziger LH. Multidrug-resistant Acinetobacter infections: an emerging challenge to clinicians. Ann Pharmacother. 2004;38:1449–1459. doi: 10.1345/aph.1D592. [DOI] [PubMed] [Google Scholar]
- 30.Rodríguez-Martínez JM, Nordmann P, Ronco E, Poirel L. Extended-spectrum cephalosporinase in Acinetobacter baumannii. Antimicrob Agents Chemother. 2010;54:3484–3488. doi: 10.1128/AAC.00050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bragg R, van der Westhuizen W, Lee JY, Coetsee E, Boucher C. Bacteriophages as potential treatment option for antibiotic resistant bacteria. Adv Exp Med Biol. 2014;807:97–110. doi: 10.1007/978-81-322-1777-0_7. [DOI] [PubMed] [Google Scholar]
- 32.Kusradze I, Karumidze N, Rigvava S, Dvalidze T, Katsitadze M, Amiranashvili I, et al. Characterization and testing the efficiency of Acinetobacter baumannii phage vB-GEC_Ab-M-G7 as an antibacterial agent. Front Microbiol. 2016;7:1590–1597. doi: 10.3389/fmicb.2016.01590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regeimbal JM, Jacobs AC, Corey BW, Henry MS, Thompson MG, Pavlicek RL, et al. Personalized therapeutic cocktail of wild environmental phages rescues mice from Acinetobacter baumannii wound infections. Antimicrob Agents Chemother. 2016;60:5806–5816. doi: 10.1128/AAC.02877-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merabishvili M, Vandenheuvel D, Kropinski AM, Mast J, De Vos D, Verbeken G, et al. Characterization of newly isolated lytic bacteriophages active against Acinetobacter baumannii. PLoS ONE. 2014;9:104853–104864. doi: 10.1371/journal.pone.0104853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Łobocka M, Hejnowicz MS, Gagała U, Weber-Dabrowska B, Wegrzyn G, Dadlez M. The first step to bacteriophage therapy—How to choose the correct phage. In: Borysowski, J, Miedzybrodzki, R, Górski, A, editors. Phage Therapy: Current Research and Applications. 2014. pp. 23–69. [Google Scholar]
- 36.Jassim S, Abdulamir A, Abu Bakar F. Phage-based limulus amoebocyte lysate assay for rapid detection of bacteria. WO2011/098820A1. 2011 [Google Scholar]
- 37.Kelly D, McAuliffe O, O’Mahony J, Coffey A. Development of a broad-host-range phage cocktail for biocontrol. Bioeng bugs. 2011;2:31–37. doi: 10.4161/bbug.2.1.13657. [DOI] [PubMed] [Google Scholar]
- 38.Benjamin K Chan, Stephen T Abedon. Catherine Loc-Carrillo Future. Microbiol. 2013;8:769–783. doi: 10.2217/fmb.13.47. [DOI] [PubMed] [Google Scholar]
- 39.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai MJ, Lin NT, Hu A, Soo PC, Chen LK, Chen LH, et al. Antibacterial activity of Acinetobacter baumannii phage ϕAB2 endolysin (LysAB2) against both Gram-positive and Gram-negative bacteria. Appl Microbiol Biotechnol. 2011;90:529–539. doi: 10.1007/s00253-011-3104-y. [DOI] [PubMed] [Google Scholar]
- 41.Briers Y, Volckaert G, Cornelissen A, Lagaert S, Michiels CW, Hertveldt K, et al. Muralytic activity and modular structure of the endolysins of Pseudomonas aeruginosa bacteriophages φKZ and EL. Mol Microbiol. 2007;65:1334–1344. doi: 10.1111/j.1365-2958.2007.05870.x. [DOI] [PubMed] [Google Scholar]
- 42.Miroshnikov K, Faizullina N, Sykilinda N, Mesyanzhinov V. Properties of the endolytic transglycosylase encoded by gene 144 of Pseudomonas aeruginosa bacteriophage phiKZ. Biochem (Mosc) 2006;71:300–305. doi: 10.1134/s0006297906030102. [DOI] [PubMed] [Google Scholar]
- 43.Paradis-Bleau C, Cloutier I, Lemieux L, Sanschagrin F, Laroche J, Auger M, et al. Peptidoglycan lytic activity of the Pseudomonas aeruginosa phage φKZ gp144 lytic transglycosylase. FEMS Microbiol Lett. 2007;266:201–209. doi: 10.1111/j.1574-6968.2006.00523.x. [DOI] [PubMed] [Google Scholar]
- 44.Junn HJ, Youn J, Suh KH, Lee SS. Cloning and expression of Klebsiella phage K11 lysozyme gene. Protein Expr Purif. 2005;42:78–84. doi: 10.1016/j.pep.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 45.Mikoulinskaia GV, Odinokova IV, Zimin AA, Lysanskaya VY, Feofanov SA, Stepnaya OA. Identification and characterization of the metal ion-dependent l-alanoyl-d-glutamate peptidase encoded by bacteriophage T5. FEBS J. 2009;276:7329–7342. doi: 10.1111/j.1742-4658.2009.07443.x. [DOI] [PubMed] [Google Scholar]
- 46.Schmelcher M, Shabarova T, Eugster MR, Eichenseher F, Tchang VS, Banz M, et al. Rapid multiplex detection and differentiation of Listeria cells by use of fluorescent phage endolysin cell wall binding domains. Appl Environ Microbiol. 2010;76:5745–5756. doi: 10.1128/AEM.00801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loessner MJ, Kramer K, Ebel F, Scherer S. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol Microbiol. 2002;44:335–349. doi: 10.1046/j.1365-2958.2002.02889.x. [DOI] [PubMed] [Google Scholar]
- 48.Lood R, Winer BY, Pelzek AJ, Diez-Martinez R, Thandar M, Euler CW, et al. Novel phage lysin capable of killing the multidrug-resistant Gram negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrob Agents Chemother. 2015;59:1983–1991. doi: 10.1128/AAC.04641-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hibbing ME, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol. 2010;8:15–25. doi: 10.1038/nrmicro2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 51.Debarbieux L, Leduc D, Maura D, Morello E, Criscuolo A, Grossi O, et al. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis. 2010;201:1096–1104. doi: 10.1086/651135. [DOI] [PubMed] [Google Scholar]
- 52.Pires DP, Vilas Boas D, Sillankorva S, Azeredo J. Phage therapy: a step forward in the treatment of Pseudomonas aeruginosa infections. J Virol. 2015;89:7449–7456. doi: 10.1128/JVI.00385-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCallin S, Alam Sarker S, Barretto C, Sultana S, Berger B, Huq S, et al. Safety analysis of a Russian phage cocktail: from metagenomics analysis to oral application in healthy human subjects. Virology. 2013;443:187–196. doi: 10.1016/j.virol.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 54.Reyes A, Semenkovich NP, Whiteson K, Rohwer F, Gordon JI. Going viral: next-generation sequencing applied to phage populations in the human gut. Nat Rev Microbiol. 2012;10:607–617. doi: 10.1038/nrmicro2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skurnik M, Pajunen M, Kiljunen S. Biotechnological challenges of phage therapy. Biotechnol Lett. 2007;29:995–1003. doi: 10.1007/s10529-007-9346-1. [DOI] [PubMed] [Google Scholar]
- 56.Merril CR, Scholl D, Adhya S. Phage Therapy. In: Calendar R, editor. The Bacteriophages. New York: Oxford University Press; 2006. pp. 725–741. [Google Scholar]