Abstract
Background:
Since the 1860’s hypercoagulability and malignancy have been linked. However, the impact of different neoplasms on multiple components of the coagulation system remains poorly understood. Thrombelastography (TEG) enables measurement of coagulation incorporating clotting through fibrinolysis. We hypothesize that specific TEG indices are associated with hypercoagulability can be appreciated in patients with adenocarcinoma undergoing pancreatic resection.
Study Design:
Blood samples were obtained in patients undergoing pancreatic resection before surgical incision and assayed with TEG. The four indices of coagulation measure by TEG included in the analysis were R time, Angle, MA (maximum amplitude) and LY30. Patient tumor type, nodal disease, and mass resectability were contrasted to TEG indices.
Results:
One-hundred patients were enrolled over 18-months. The majority of patients had adenocarcinoma (ACA) (63%). Patients with ACA had increased angle compared to other lesions (49° [37-59] vs 43° [32-49] p=0.011). When excluding patients that underwent neoadjuvant therapy, patients with ACA had shorter R-times (13 min [9-16] vs 14 [12-18] p=0.051), steeper angles (49° [40-59] vs 43° [32-49] p=0.010), and higher MA (67 mm [61-69] vs 62 mm [57-67] p=0.017). Nodal disease was associated with a significantly increased angle (49° [42-59] vs 40° [32-50] p=0.002) and MA (64 mm [61-69] vs 62 mm [56-67] p=0.017). Patients who underwent successful mass resection had longer R times (14 min [11-17] vs 10 min [9-15] p=0.033) and shorter angles (44 [35-55] vs 58 [45-66] p=0.025).
Conclusions:
Patients with adenocarcinoma undergoing pancreatic resection have multiple TEG abnormalities consistent with hypercoagulability. These TEG outputs are associated with tumor type, nodal disease, and probability of a successful resection. The use of preoperative TEG has the potential to aid surgeon and patient discussions on anticipated disease burden and prognosis prior to resection.
Precis
Adenocarcinoma of the pancreas is associated with specific coagulation changes. These coagulation changes are detectable with thrombelastography. Specific thrombelastography measurements in patients with pancreatic masses correlates to disease burden and resectability, in addition to risk of postoperative pulmonary embolism.
Introduction
Since Trousseau’s observation of thrombosis associated with malignancy, there has been an interest in the identifying the mechanistic links between cancer and coagulation. Patients presenting with new onset bilateral deep venous thrombosis have been reported to have an undiagnosed malignancy in as many as 40% of patients(1). These thrombotic complications can be lethal, as it is appreciated that patients with known pancreatic cancer have a high rate of pulmonary embolism(2). Furthermore, patients undergoing pancreatic resection also have an increased rate of thrombotic complications in the perioperative period, which have been reported to exceed 25%(3).
There are several speculated mechanisms on what drives the derangement in coagulation including increased activity of platelets(4), release of tissue factor(5), anti-fibrinolytic protein(6), and inflammation(4, 7). Beyond changing coagulation, growing evidence suggests that platelets(8) endothelium(9) and neutrophils(9) are modified by tumor related factors and play a role in cancer metastasis. This is supported by recent data that oncologic patients’ risk of thrombotic complications correlates with their stage of disease(10). Therefore, classification of a patient’s coagulation status prior to surgery may not only predict thrombotic complications, but could serve as a prognostic tool to predict the severity of disease and resectability.
One of the largest dilemmas in identifying patients at risk of thrombosis with malignancy is a lack of tools to quantify hypercoagulability. Measurement of the hypercoagulable state is limited with conventional plasma based assays, but are detectable with viscoelastic assays such as thrombelastography (TEG) employed in trauma (11). This has also been appreciated in surgical oncology patients, in which TEG detected hypercoagulability is appreciated in colorectal and breast cancer patients despite having normal fibrinogen and platelet levels(12). As advance pancreatic cancer patients are known to have high rates of thrombotic complications(13) our study targeted a cohort of patients undergoing pancreatic resection to identify if pre-operative coagulation evaluation with TEG could predict nodal disease, and resectability of the patient’s pancreatic mass as well as thrombotic complications. We hypothesize that specific TEG indices associated with hypercoagulability are more common in patients with adenocarcinoma of the pancreas compared to other lesions.
Methods
Patient Population and Sample Collection
Patients undergoing pancreatic surgery for resection of a mass (or cyst) were prospectively enrolled over an 18-month period under protocols approved by the Colorado Institutional Review Board from January 2016- June 2017. Patients were included in the study if they had a pancreatic mass deemed resectable by their treating surgeon. The decision to proceed to resection was often based on the consensus of the University of Colorado Multidisciplinary Pancreatic clinic, which includes additional surgical oncologist, radiologist, oncologists, radiation oncologists. Patient selection was limited to patients undergoing pancreatic resection as it would provide the definitive pathologic diagnosis, in addition to the presence of nodal disease. This study was a pragmatic convenient sample based on the patient’s willingness to consent for research and availability of the research assistant to collect and process samples. Blood samples were obtained in 3.5 ml citrated tubes before surgical incision and after general anesthesia induction through a radial placed arterial line. Healthy volunteer native TEG data on 96 individuals was also included to serve as a control for expected normal ranges. These results and patient demographics have previously been reported(14).
Thrombelastography
Citrated blood samples were all run by a single technician (PL) with experience conducting over 1,000 TEG assays. Samples were all run within 2 hours of blood draw in accordance with manufacturers recommendations. TEGs were run as native non activated assays, rather than kaolin or rapid TEG, based on previous work demonstrating a native TEG having a higher sensitivity for detecting hypercoagulability(14). The four indices of coagulation measure by TEG included in the analysis were R time (minutes ~ coagulation factors) angle (degrees ~ fibrinogen function) MA (mm ~ platelets function) and LY30 (%~ fibrinolysis).
Demographics and Outcomes
Patient demographics and outcomes were prospectively recorded through a central data collection registry. Demographics of interest included the patients age, sex, location of tumor, pre-operative tumor markers, neoadjuvant chemotherapy/radiation, and planned operative intervention. Outcomes of interest included tumor type, nodal disease, metastatic disease, tumor grade, neuronal and lympho-vascular invasion, mass resectability, and deep vein thrombosis (DVT) and pulmonary embolism (PE). These thrombotic complications (DVT, PE) we identified if the patient had symptoms with subsequent follow up confirmatory testing with ultrasound or computed tomography, and not identified by any screening tests. The patient’s disease burden was determined after final pathology assessment. Patients with a preoperative diagnosis of IPMN or other cystic neoplasm that had evidence of malignancy were categorized as having malignancy, including patients with carcinoma in situ. Patients were classified as having hypercoagulability if there TEG indices were outside of the interquartile range of healthy volunteers (R time < 13 minutes, Angle >51%, MA> 60mm and LY30 <0.9%)(14). All patients had routine standardized post-operative deep vein thrombosis prophylaxis with three times a day subcutaneous heparin and sequential compression devices on lower extremities.
Statistical Analysis
SPSS version 23 (IBM, Armonk, NY, USA) was used for statistical analysis. TEG measurements are described as median and interquartile values. TEG indices were contrasted between tumor types [adenocarcinoma (ACA) vs non] with a Mann Whitney U test. Additional analyses were performed to contrast TEG values between patients with nodal disease vs local disease, resectable vs non resectable masses, and patients that had thrombotic complications vs non thrombotic events. Patients were then stratified based on if they recieved neoadjuvant therapy versus direct to surgery and the same analyses were repeated. The prevalence of hypercoagulability for each of the TEG indices was described per tumor type, and significance was assessed using a goodness of fitness test based on the expected distribution of hypercoagulability (25%) based on healthy volunteer data. Burden of disease (local, nodal, metastatic) was correlated with Spearman’s Rho Test. Receiver operating characteristic (ROC) curves were used to contrast TEG indices performance to predict resectability compared to CA19-9. While CA19-9 is not clinically used at our institution to proceed with surgery, it is has been proposed a potential biomarker to determine if patients are resectable regardless of imaging(15). Overall model significance of CA19-9 vs TEG indices was determined by area under the curve of a receiver operator characteristic curve (ROCAUC).
Results
Patient Population
During the 18-month study period 186 patients underwent surgery for pancreatic resection, of which, 100 patients were successfully enrolled. The median age of patients was 66 years ranging from 21 to 82. The most common operation was a pancreaticoduodenectomy (75%) followed by distal pancreatectomy (20%) and central pancreatectomy (5%). The majority of patients had adenocarcinoma (63%) with the remaining masses having a roughly equal distribution of neuroendocrine tumors (15%), intraductal papillary mucinous neoplasm (IPMN) and 11% inflammatory/cystic mass. Tumors were predominantly located in the pancreas (89%) but were also found in the common bile duct (9%) and duodenum (2%). Neoadjuvant chemo radiation was used in 17% of patients. An overview of the patient demographics in patients with adenocarcinoma versus other malignancy are summarized in Table 1.
Table 1.
Patient Demographics and Tumor Characteristics
| Variable | Non-ACA | ACA |
|---|---|---|
| Age, y (25th-75th percentile) | 60 (53-69) | 67 (61-73) |
| Female, % | 48 | 49 |
| Tumor location, % | ||
| Pancreas | 95 | 86 |
| Common bile duct | 5 | 11 |
| Duodenum | 0 | 3 |
| Pancreatic mass location, % | ||
| Ampulla | 11 | 13 |
| Head | 46 | 65 |
| Body | 14 | 6 |
| Tail | 29 | 17 |
| Neoadjuvant therapy, % | 0 | 27 |
| Median tumor size, cm (25th-75th percentile) | 2.4 (1.6-4.7) | 2.5 (1.6-3.5) |
| Perineural invasion, % | 14 | 67 |
| Lymphovascular invasion, % | 22 | 51 |
| Node positive disease, % | 19 | 67 |
| Metastatic disease, % | 3 | 17 |
| Resectable, % | 100 | 83 |
| Deep vein thrombosis, % | 12 | 10 |
| Pulmonary embolism, % | 3 | 3 |
ACA, adenocarcinoma.
Thrombelastography Characteristics of Patients with Adenocarcinoma
Descriptive changes between different histopathologic diagnoses and TEG indices are listed in Table 2. There was no significant difference in patients with malignant (ACA or PNET) vs nonmalignant masses (R time p=0.868, angle p=.380, MA p=0.396, p=0.543). The prevalence of hypercoagulability in the overall patient population for the different TEG measurements were; R time 48% (p<0.001 compared to healthy), Angle 36% (p=0.038), MA 65% (p<0.001), LY30 73% (P<0.001). Patients with adenocarcinoma of the pancreas had a significantly increased angle compared to other lesions [49° (37-59) vs 43° (32-49) p=0.011]. When excluding patients that underwent neoadjuvant therapy, patients with ACA had shorter R-times [13 min (9-16) vs 14 (12-18) P=0.051], steeper angles [49° (40-59) vs 43° (32-49) p=0.010], and higher MA [67mm (61-69) vs 62 (57-67) p=0.017] compared to other lesions (Figure 1). Hypercoagulability in this patient population was significantly more common compared to healthy volunteers; R time 57% (p<0.001), Angle 44% (p<0.001), MA 80% (p<0.001), LY30 74% (p<0.001). However, within the patients undergoing pancreatic resection hypercoagulability was only significantly more prevalent in patient with ACA compared to other lesions in regards to Angle (44% vs 16% P=0.009) and MA (80% vs 60% p=0.036), while no statistical difference was appreciated with R time (57% vs 41% p=0.148) and LY30 (74% vs 81% p=0.440). Within the cohort of patient with ACA, patients that underwent neoadjuvant chemotherapy had a significantly reduced MA [58mm (55-65) vs 67mm (61-69) p=0.024] but no differences between other TEG indices with blood draws the day of surgery before incision.
Table 2.
Thrombelastography Variables Stratified by Tumor Histology
| Variables | ACA | IPMN | PNET | INFL/Cystic/other |
|---|---|---|---|---|
| R Time, min | 13 (10-16) | 12 (11-16) | 15 (14-20) | 13 (11-17) |
| Angle, degree | 49 (37-59) | 37 (33-49) | 37 (28-49) | 49 (43-54) |
| MA, mm | 63 (58-69) | 61 (57-64) | 61 (54-69) | 65 (56-68) |
| LY30, % | 0.4 (0-1.0) | 0.2 (0-0.7) | 0.4 (0-0.9) | 0.4 (0.1-0.6) |
Data in parentheses are 25th to 75th percentile.
ACA, adenocarcinoma; IPMN, intraductal papillary mucinous neoplasm; LY30, lysis at 30 min; MA, maximum amplitude; PNET, pancreatic neuroendocrine tumor; INFL/Cystic/other, inflammatory, cystic mass, or other mass type not mentioned.
Figure 1.
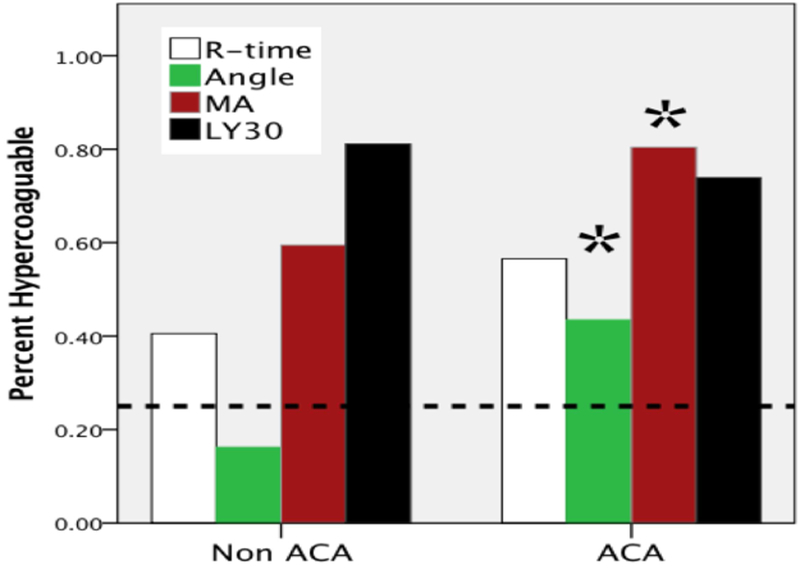
Percent of patients determined to have hypercoagulability stratified by adenocarcinoma compared to other lesions. Dotted line, expected percentage of patients to have thrombelastography indices suggestive of hypercoagulability based on the healthy volunteer population (25%). While hypercoagulability determined by thrombelastography measurements was significant for all four variables in the overall population, only angle and MA hypercoagulability were significantly increased in patients with adenocarcinoma compared to other pancreatic lesions. *p<0.05 between ACA and non ACA. ACA, adenocarcinoma; MA, maximum amplitude; LY30, lysis at 30 minutes.
Coagulation Measurements are Association with Regional and Metastatic Disease
Node positive disease was identified in 51% of patients. In the overall patient cohort nodal disease was associated with a significantly increased angle [49° (42-59) vs 40° (32-50)p=0.002] and MA [64mm (61-69) vs 62mm (56-67) p=0.017]. This association only existed within the cohort of patients with ACA (angle p=0.003 MA p=0.038 Figure 2), which was consistent with tumors that had lymphvascular invasion having higher angles [53 (44-61) vs 42 (35-58) p=0.042]. There was no association identified with TEG indices and perineural invasion, histologic grade, or tumor size. While underpowered (n=17) to show a significant difference, patient’s who underwent neoadjuvant chemotherapy show a similar relationship with nodal disease had steeper angles [55° (34-59) vs 41° (25-56) p=0.315] and higher MA [61mm (54-68) vs 57mm (55-58) p=0.475] compared to patients with node free disease.
Figure 2.
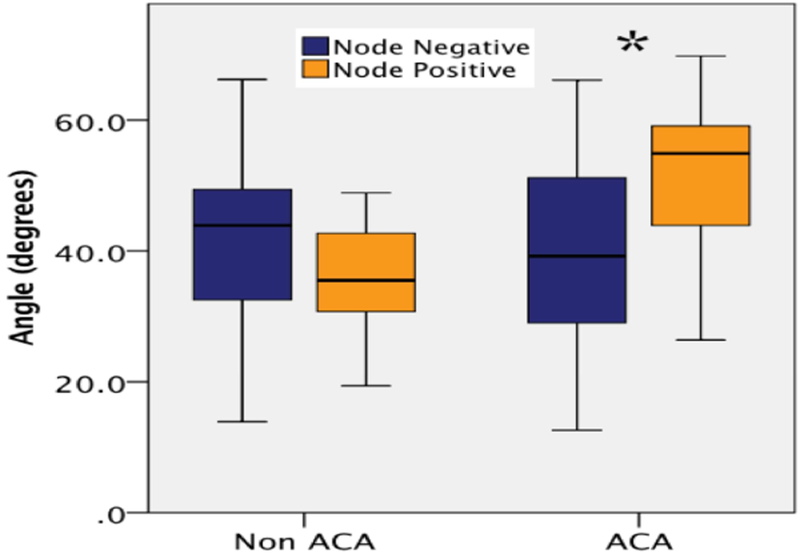
Differences in angle between patients with adenocarcinoma vs non-adenocarcinoma lesions stratified by node-positive disease. *p<0.05. ACA, adenocarcinoma.
Coagulation Measurements Predict Successful Mass Resection
Masses were successfully resected in 89% of patients, with the remaining 11 patients having aborted resection due to evidence of occult metastatic disease after laparotomy or invasion into vascular structures. Patients whom underwent successful mass resection had longer R times [14 min (11-17) vs 10 min (9-15) p=0.033] and shorter angles [44 (35-55) vs 58 (45-66) p=0.025]. When comparing TEG indices to CA19-9 (collected on 81 patients preoperative) ROC curve analysis identified both R time (ROCAUC 0.708 p=0.034) and Angle (ROCAUC 0.712 p=0.030) as better predictors for successful resection compared to CA19-9 (ROCAUC 0.581 p=0.409 Figure 3).
Figure 3.
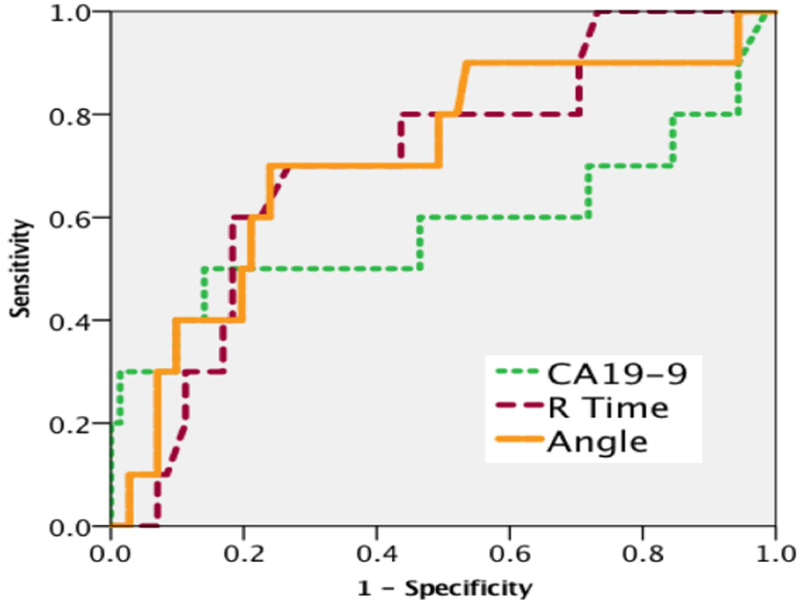
Differences in receiver operating characteristic curve between CA19-9 vs thrombelastography measurements of R time and angle to predict resectability.
Thrombotic Outcomes
Thrombotic complications occurred in 11% of patients (10 DVT and 3 PE). The majority of the DVTs occurred in patients with ACA (n=6) followed by inflammatory/cystic (n=3) and neuroendocrine (n=1). PE occurred in ACA (n=2) and inflammatory/cystic (n=1). Both patients with ACA and PE also had a DVT, but the single patient with an inflammatory mass had an isolated PE with no evidence of DVT. There were no specific TEG variables associated with DVT in the overall cohort or in patients with the diagnosis of ACA. However, multiple TEG variables were associated with PE including a short R time [8 min (8-9) vs 14 (11-17) p=0.020], increased angle [57 (44-58) vs 45 (36-56) p=0.044], and low LY30 [0 (0-0) vs 0.4 (0-0.9) p=0.022]. All of the patients who developed a PE had all four TEG measurements consistent with hypercoagulability.
Discussion
Preoperative TEG in patients undergoing pancreatic resection identified patients with adenocarcinoma of the pancreas who have unique coagulation patterns that can aid in risk stratification for the presence of nodal disease and resectability. TEG also identified surgical patients undergoing a pancreatic resection at risk of pulmonary embolism. While all TEG indices in this cohort had a significantly higher rate of hypercoagulability comparted to a healthy volunteer population, only specific indices were associated with oncologic burden of disease. Angle, was found to be the most predictive variable in differentiating patients with ACA versus other pathology, having node positive disease, and had better performance than CA19-9 to predict which patients could likely undergo complete surgical resection.
Our study is in agreement with previous findings that preoperative hypercoagulability measured by thrombelastography is associated with solid tumor malignancy(12). Francis et al(12) reported that roughly half of patients with colorectal cancer (n=8/17) had shortened R time, increased angle, and MA compared to healthy controls. Similar observations of preoperative hypercoagulability in patients with solid tumor malignancies have also been appreciated with ROTEM, a different type of viscoelastic assay(16, 17). However, coagulation differences in these older patients may not be causes by malignancy, and could be related to the patient age and comorbidities. Individuals who donated blood for our previous healthy volunteer study had a median age of 31years (twice the age of the median age in this study) and did not have diabetes, or known cardiovascular disease(14). Advanced age(18) and diabetes(19) have been associated with hypercoagulability and thrombotic risks. This is of clinical significance as the patient’s comorbidities alone could be a risk factor for thrombosis prior to a significant abdominal surgery, regardless of underlying pathology. Even more concerning, evidence suggests that patients undergoing hepatobiliary surgery have a tendency to have increased hypercoagulability on post-operative day 1(20). This may explain why preoperative TEG measurements did not identify a risk for DVT in this study. The overall patient population was had a higher prevalence of hypercoagulability compared to healthy 30 year olds, which could be related to their advanced age and comorbidities. A larger patient population would be required to control for these confounders to identify if adenocarcinoma and a specific TEG measurements are independent predictor of DVTs.
A recent retrospective study of nearly 150,000 oncologic patients identified an association between a patient’s stage of malignancy at initial diagnosis and risk of subsequent thrombotic event(10). While the mechanistic link is lacking, pre-existing data on TEG abnormality in patients with new onset DVT, identified that angle was the most commonly elevated TEG variable in these patients (21). Angle in viscoelastic testing is most commonly ascribed to the fibrinogen function of whole blood(22). Previous reports have identified increased fibrinogen levels in patients with nodal and metastatic disease in esophageal cancer(23, 24). Our study is consistent with these previous descriptive studies in which an elevated angle was associated with patients having nodal disease, and pulmonary embolism.
Our data suggests a benefit of a pre-operative role of viscoelastic assessment in patients undergoing pancreatic resection. The current gold standard biomarker CA19-9 for diagnosis of pancreatic cancer and prediction of tumor resectability(25) has been reported to perform well in predicting successful mass resection(26). However current consensus guidelines for boarderline resectable pancreatic masses do not advocate using CA19-9 to predict resectability, rather it should be used to decide if surgical resection will benefit the patient(27). The TEG indices R time and angle both outperformed CA19-9 to predict resection in our patients (Figure 3). Continued monitoring of the patient coagulation status after surgery may also be important for the acute and long-term care of the patient. Pancreatic resection is associated with an acquired hypercoagulability by post-operative day one(20). This acquired hypercoagulability also been reported in an older study, in which impairment of fibrinolysis was associated with thrombotic complications(28). This would set up a dangerous scenario in which a patient has increased fibrinogen function prior to surgery, post operatively has an impaired ability to break down fibrin clot. During trauma the development of an acquired fibrinolysis resistance can occur within 2 hours of injury(29). Patients who developed post-operative pulmonary embolisms in our study has shortened clotting times, increased angles, and lower LY30s, suggesting that they had a multifactorial process driving their clotting abnormalities. Heparin alone is unlikely to be an effective prophylaxis for these patients prothrombotic state, as it only targets thrombin generation and not hyperfibrinogenemia. The role of multi anticoagulant/platelet prophylaxis is becoming common practice in other high-risk patient populations(30) and a personalized patient specific targeted anticoagulation regimen would be appealing to reduce the risk of thrombotic versus bleeding.
After discharge, continued viscoelastic testing may also be warranted to monitor for disease recurrence indicated by failure to reverse hypercoagulability. It has been previously demonstrated that failure to correct CA19-9 is associated with early recurrence and shorter disease-free survival(31). The same effect could be true with TEG measurements. Early data from our study suggests the patients who have had successful neoadjuvant chemotherapy with node free disease have lower angles and MA, compared to those with nodal disease. Others investigators have proposed that CA19-9 in combination with coagulation measurements (fibrinogen and D-Dimer) can aid in monitoring patient for risk of recurrence(32).
Our study is limited to measuring one time point in a cancer patient’s long treatment trajectory, and lacks long-term follow up data for cancer recurrence and survival. In addition, the cut points for pathologic hypercoagulability in older patients undergoing major abdominal operations is not clearly defined, and multiple definitions exist and lack adjustment for multiple confounders which could be attributable to thrombotic risk(17, 20, 33–35). Based on our healthy volunteer data, patients undergoing pancreatic resection have dissimilar coagulation profiles compared to younger adults lacking medical comorbidities. We used a 25th percentile cut point to crudely describe coagulation differenced and should not necessarily be used as a cut point to define risk of thrombosis. Future studies with large patient populations are needed to define the specific pathologic cut points for hypercoagulability with viscoelastic assays and risk for thrombotic complications in patients undergoing pancreatic surgery after adjusting for confounders. The rates of thrombotic complications in our study was not sufficient to enable a multivariate regression analysis to determine if TEG indices are independent predictors of thrombotic complications, limiting our results to an observation which require subsequent validation. Finally, while our convenient patient sample population suggests that fibrinogen is likely the contributory cause of hypercoagulability in patients with adenocarcinoma of the pancreas, ongoing investigation is needed to validate this finding. Alternative explanations for hypercoagulability and pancreatic cancer have been from tumor derived microparticles driving thrombotic complications(36), while older data suggest that mucin from tumors is an etiology of thrombosis(37). However, neither of these hypotheses would explain why angle is increased in patient with advanced pancreatic cancer. The increase in angle could also represent an adaptive response to the tumor. It has been appreciated that the innate immunity evolved from the coagulation system(38). Other pathologic conditions with chronic inflammatory states have also been associated with hypercoagulability including primary biliary sclerosis(35), inflammatory bowel disease(34) and obesity(33). Ongoing work is needed to determine the underlying cause of increased angle and hypercoagulability in patients with adenocarcinoma of the pancreas, to differentiate if these measurements are biomarkers or intrinsic to changes for the underlying disease. We also acknowledge that our study population is limited to a convenient sample and is at risk of selection bias to patients deemed resectable by the surgeon, and results cannot be generalized to all patients presenting to the hospital for evaluation of the removal of a pancreatic mass.
In conclusion, a pre-operative TEG in patients undergoing pancreatic resection has potential utility in risk stratifying patients for burden of disease for patient with adenocarcinoma, in addition to identifying patients at risk of pulmonary embolism. Ongoing investigation is needed to validate these findings in a large patient population to adjust for confounders, in addition to identifying the mechanisms of what drive these coagulation changes.
Acknowledgments
Support: This study was supported in part by National Institute of General Medical Sciences (NIGMS) grants: T32-GM008315 and department of surgery at the University of Colorado academic enrichment fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: Drs H Moore and E Moore have shared intellectual property with Haemonetics and University of Colorado. Both Dr Moore’s are co-founders of Thrombo Therapeutics Incorporated and Dr H Moore is a board member; Dr H Moore received a payment for a lecture to Instrument Laboratories.
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS or the National Institutes of Health.
Presented at the Western Surgical Association 125th Scientific Session, Scottsdale, AZ, November 2017.
References:
- 1.Stein GY, Schwartz A, Neuman H, Rozenblat Y, Zeidman A. [Bilateral deep vein thrombosis as presenting symptom of malignancy]. Harefuah 2007;146:800–802, 812. [PubMed] [Google Scholar]
- 2.Shinagare AB, Guo M, Hatabu H, et al. Incidence of pulmonary embolism in oncologic outpatients at a tertiary cancer center. Cancer 2011;117:3860–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smoot RL, Christein JD, Farnell MB. Durability of portal venous reconstruction following resection during pancreaticoduodenectomy. J Gastrointest Surg 2006;10:1371–1375. [DOI] [PubMed] [Google Scholar]
- 4.Shao B, Wahrenbrock MG, Yao L, et al. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood 2011;118:4015–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khorana AA, Ahrendt SA, Ryan CK, et al. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res 2007;13:2870–2875. [DOI] [PubMed] [Google Scholar]
- 6.Andren-Sandberg A, Lecander I, Martinsson G, Astedt B. Peaks in plasma plasminogen activator inhibitor-1 concentration may explain thrombotic events in cases of pancreatic carcinoma. Cancer 1992;69:2884–2887. [DOI] [PubMed] [Google Scholar]
- 7.Li YH, Kuo CH, Shi GY, Wu HL. The role of thrombomodulin lectin-like domain in inflammation. J Biomed Sci 2012;19:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Nat Academy Sciences 2014;111:E3053–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov 2012;2:1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gade IL, Braekkan SK, Naess IA, et al. The impact of initial cancer stage on the incidence of venous thromboembolism: the Scandinavian Thrombosis and Cancer (STAC) Cohort. J Thrombosis Haemostasis 2017;15:1567–1575. [DOI] [PubMed] [Google Scholar]
- 11.Park MS, Martini WZ, Dubick MA, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma 2009;67:266–275; discussion 275–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis JL, Francis DA, Gunathilagan GJ. Assessment of hypercoagulability in patients with cancer using the Sonoclot Analyzer and thromboelastography. Thrombosis Res 1994;74:335–346. [DOI] [PubMed] [Google Scholar]
- 13.Tun NM, Guevara E, Oo TH. Benefit and risk of primary thromboprophylaxis in ambulatory patients with advanced pancreatic cancer receiving chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Blood Coagulation Fibrinolysis 2016;27:270–274. [DOI] [PubMed] [Google Scholar]
- 14.Moore HB, Moore EE, Chapman MP, et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. J Thrombosis Haemostasis 2015;13:1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilic M, Gocmen E, Tez M, et al. Value of preoperative serum CA 19–9 levels in predicting resectability for pancreatic cancer. Can J Surg 2006;49:241–244. [PMC free article] [PubMed] [Google Scholar]
- 16.Papa ML, Capasso F, Pudore L, et al. Thromboelastographic profiles as a tool for thrombotic risk in digestive tract cancer. Exp Oncol 2007;29:111–115. [PubMed] [Google Scholar]
- 17.Akay OM, Ustuner Z, Canturk Z, et al. Laboratory investigation of hypercoagulability in cancer patients using rotation thrombelastography. Med Oncol 2009;26:358–364. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Guan Z, Xu Q, Zhao L, Song Y, Wang H. Relation of thromboelastography parameters to conventional coagulation tests used to evaluate the hypercoagulable state of aged fracture patients. Medicine (Baltimore) 2016;95:e3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim HS, MacFadyen RJ, Bakris G, Lip GY. The role of hyperglycaemia and the hypercoagulable state in the pathogenesis of cardiovascular events in diabetes mellitus: implications for hypertension management. Curr Pharm Des 2006;12:1567–1579. [DOI] [PubMed] [Google Scholar]
- 20.De Pietri L, Montalti R, Begliomini B, et al. Thromboelastographic changes in liver and pancreatic cancer surgery: hypercoagulability, hypocoagulability or normocoagulability? Eur J Anaesthesiol 2010;27:608–616. [DOI] [PubMed] [Google Scholar]
- 21.Spiezia L, Marchioro P, Radu C, et al. Whole blood coagulation assessment using rotation thrombelastogram thromboelastometry in patients with acute deep vein thrombosis. Blood Coagulation Fibrinolysis 2008;19:355–360. [DOI] [PubMed] [Google Scholar]
- 22.Schlimp CJ, Solomon C, Ranucci M, et al. The effectiveness of different functional fibrinogen polymerization assays in eliminating platelet contribution to clot strength in thromboelastometry. Anesthesia Analgesia 2014;118:269–276. [DOI] [PubMed] [Google Scholar]
- 23.Wayman J, O’Hanlon D, Hayes N, Shaw I, Griffin SM. Fibrinogen levels correlate with stage of disease in patients with oesophageal cancer. Br J Surg 1997;84:185–188. [PubMed] [Google Scholar]
- 24.Takeuchi H, Ikeuchi S, Kitagawa Y, et al. Pretreatment plasma fibrinogen level correlates with tumor progression and metastasis in patients with squamous cell carcinoma of the esophagus. J Gastroenterol Hepatol 2007;22:2222–2227. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal S Potential prognostic biomarkers in pancreatic juice of resectable pancreatic ductal adenocarcinoma. World J Clin Oncol 2017;8:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartwig W, Strobel O, Hinz U, et al. CA19–9 in potentially resectable pancreatic cancer: perspective to adjust surgical and perioperative therapy. Ann Surg Oncol 2013;20:2188–2196. [DOI] [PubMed] [Google Scholar]
- 27.Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977–988. [DOI] [PubMed] [Google Scholar]
- 28.Mellbring G, Dahlgren S, Wiman B, Sunnegardh O. Relationship between preoperative status of the fibrinolytic system and occurrence of deep vein thrombosis after major abdominal surgery. Thrombosis Res 1985;39:157–163. [DOI] [PubMed] [Google Scholar]
- 29.Moore HB ME, Gonzalez E, Huebner BJ, et al. Reperfusion shutdown: delayed onset of fibrinolysis resistance after resuscitation from hemorrhagic shock is associated with increased circulating levels of plasminogen activator inhibitor-1 and postinjury complications. Blood 2016;128:206. [Google Scholar]
- 30.Gordon RJ, Lombard FW. Perioperative venous thromboembolism: a review. Anesthesia Analgesia 2017;125:403–412. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Misih SR, Hatzaras I, Schmidt C, et al. Failure of normalization of CA19–9 following resection for pancreatic cancer is tantamount to metastatic disease. Ann Surg Oncol 2011;18:1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao J, Fu Z, Gao L, et al. Evaluation of serum D-dimer, fibrinogen, and CA19–9 for postoperative monitoring and survival prediction in resectable pancreatic carcinoma. World J Surg Oncol 2017;15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kupcinskiene K, Trepenaitis D, Petereit R, et al. Monitoring of hypercoagulability by thromboelastography in bariatric surgery. Med Sci Monit 2017;23:1819–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senchenkova E, Seifert H, Granger DN. Hypercoagulability and platelet abnormalities in inflammatory bowel disease. Sem Thrombosis Hemostasis 2015;41:582–589. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Ari Z, Panagou M, Patch D, et al. Hypercoagulability in patients with primary biliary cirrhosis and primary sclerosing cholangitis evaluated by thrombelastography. J Hepatol 1997;26:554–559. [DOI] [PubMed] [Google Scholar]
- 36.Tesselaar ME, Romijn FP, Van Der Linden IK, et al. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thrombosis Haemostasis 2007;5:520–527. [DOI] [PubMed] [Google Scholar]
- 37.Pratt-Thomas HR, Smith RM, McMaster KR, Earlywine GR. Intravascular mucinosis with mucin emboli and thrombosis accompanying adenocarcinomas. Pathol Res Pract 1980;170:328–337. [DOI] [PubMed] [Google Scholar]
- 38.Muta T, Iwanaga S. The role of hemolymph coagulation in innate immunity. Curr Opin Immunol 1996;8:41–47. [DOI] [PubMed] [Google Scholar]


