Abstract
We have fabricated a temperature-sensitive hydrogel through copolymerization of N-isopropylacrylamide (NIPAAm) and Acrylamide (AAm) inside a macroporous silicon structure and demonstrated fast thermal response compared to its bulk structure. The presented method allows physical arrangement of micro-sized hydrogels within a predefined arrayed structure. Static and dynamic temperature responses of the fabricated structure are successfully demonstrated through optical transmission measurement. The measured temporal response reveals that presented structure can allow fast response time of the implemented hydrogels. Furthermore, spatial thermo-distribution pattern can be observed through pixel-like, arrayed macropores, which indicates a potential for addressing individual or single-channel hydrogel sensors or actuators through temperature stimulation.
Index Terms: Hydrogel-based sensors and actuators, Macroporous Silicon, Optical sensor, Temperature Sensor
I. INTRODUCTION
Stimuli-responsive hydrogels have been of great interest because of their abilities to respond physically or chemically to external changes. Different stimuli methods including temperature, pH, light, ions, electric and magnetic field [1] have been demonstrated in many fields, such as controlled drug release [2], [3], sensors [4], actuators [5], [6], and tissue engineering [7]. Among different hydrogels, temperature-responsive poly(N-isopropylacrylamide) (pNIPAAm) is the most widely studied. The response of pNIPAAm is typically characterized by a lower critical solution temperature (LCST). At this temperature, the pNIPAAm undergoes significant physical changes in its polymer network.
In many sensor or actuator applications, stimuli-response time can be an important factor which significantly contributes towards device performance. Typical demonstrations using hydrogels showed very long response time, ranging from several minutes to hours. Many efforts, especially in material synthesis, have been made to improve the response time of bulk hydrogels. These include decreasing the polymerization reaction temperature [8], creating macroporous network structure [9]–[11], and incorporating inorganic nano-composite material [12] or nanospheres [13]. However, it is still not suitable to use hydrogels in demanding applications requiring rapid response. Adding to the new material properties, stimuli-response time is also expected to be affected by the size of the hydrogel. For example, sub-mm sized hydrogels prepared from emulsion method show a few minutes of response time [14].
In this letter, we incorporate thermo-responsive hydrogels inside a macroporous silicon membrane structure and demonstrate their thermal response through optical transmission measurement. The macroporous silicon structure creates physical arrangement of hydrogels within its pores. This allows the control of hydrogel volume to be determined by the pore dimension. At the same time, physical locations of hydrogels are confined within defined pore arrays. Compared to other demonstrations, the presented structure shows much faster, reaching sub-second response time.
II. MATERIALS AND METHODS
A. Structure schematic and operation
In this work, we fabricated a temperature-sensitive hydrogel through copolymerization of N-isopropylacrylamide (NIPAAm) and Acrylamide (AAm) inside a macroporous silicon structure and demonstrated fast thermal response compared to its bulk structure. Depending on its composition ratio, copolymerization of NIPAAm and AAm affects its mechanical strength and response temperature [15]–[18]. Generally, higher concentration of AAm increases mechanical strength and LCST, while sacrificing its responsive volume change.
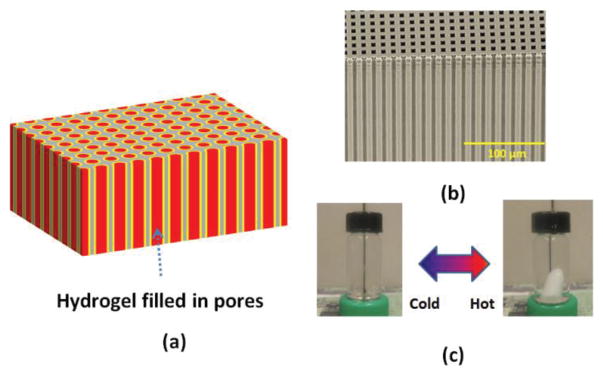
Fig. 1(a) shows a schematic of the proposed structure. The structure presents highly ordered macroporous silicon membrane geometries which define physical locations of the hydrogel. Each pore incorporates the hydrogel. Potentially, its response can be independently addressed by the implemented hydrogel within the pore. Fig. 1(b) depicts a sample optical image of our macroporous silicon structure used for the device fabrication. The structure has periodically ordered holes in silicon. Fig. 1(c) shows the temperature response of a bulk NIPAAm/AAm-based hydrogel stored in water. When the temperature is less than its LCST, it is transparent. On the contrary, when the temperature is more than the LCST, the hydrogel turns opaque. Typical response time of a bulk hydrogel as depicted in Fig. 1(c) depends on temperature differences and its volume. For example, a transparent bulk hydrogel (volume of 0.32 cm3) turns opaque in around 22 sec and 55 sec for final water temperatures of 55 °C (from 19 °C to 55 °C) and 43 °C (from 19 °C to 43 °C) respectively. The hydrogel undergoes a reversible response depending on its temperature. The presented structure combines these unique material properties to present pixel-like temperature sensing capability through its optical response. Since the implemented hydrogel volume can be confined within the pore size, the response time from our proposed device can be much faster than other conventional bulk structures.
Fig. 1.
(a) Schematic of device structure. (b) Scanning electron microscope image of a macroporous silicon sample used in the device fabrication. (c) Temperature response of a bulk hydrogel block in water.
B. Fabrication of Hydrogels embedded in a macroporous silicon structure
AAm was obtained from Alfa Aesar. NIPAAm was obtained from TGI America. N, N′-methylend-bis-acrylamide (MBAAm), N, N, N′, N′ –tetramethylethylenediamine (TEMED), and ammonium persulphate (AP) were obtained from VWR. They were used as received without further purification.
Thermo-responsive hydrogels were prepared with copolymerization of NIPAAm and AAm monomers. 3g NIPAAm (monomer), 0.3g AAm (comonomer) and 0.06g MBAAm (crosslinker) were dissolved in 30 ml deionized water and degassed under nitrogen gas for 30 min. Separately, 5% AP solution in water was also prepared. TEMED was added right before the embedding process. In the device fabrication process, 1 ml solution of monomers and crosslinker was mixed with 20 μl of 5% AP solution and 1 μl of TEMED. The mixed solution was applied for device fabrication.
Periodically arranged macroporous silicon, which has 5 μm pore diameters with 12 μm pitch and 400 μm pore length was obtained from Smart Membranes, Germany. To reveal the pores from the macroporous silicon, the back-side of a sample was lapped using a lapping machine (Buehler Minimet). First, a macroporous silicon sample was mounted using a wax on a lapping mount and lapped using a slurry solution containing 1 μm alumina powders until pores were visible from the back of the sample. Additional back-side polishing was performed using slurry with 0.25 μm alumina powders to obtain a smooth surface. Pore diameter affects light transmission characteristics. Therefore, in our study we enlarged the pore diameter through repeated thermal-oxidation and hydrofluoric acid etching procedures. At the final stage, the membrane sample was thermally oxidized resulting in a 500 nm thick silicon dioxide along the pores. The final pore diameter was around 8 μm.
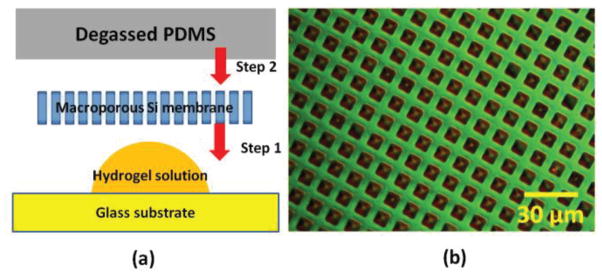
Inspired from degassing-assisted patterning method [19], which allows liquid solution to be aspirated through channels or cavities, we employed micro-aspiration induced with a degassed polydimethylsiloxane (PDMS) to fill the pores of the membrane structure with hydrogels. To prepare the PDMS block, Dow Corning Sylgard 182 PDMS elastomer and curing agent were mixed with a weight ratio of 10:1, respectively. The PDMS mixture was degassed for 30 min until air bubbles were removed from the PDMS mixture. Next, the PDMS mixture was poured on top of the silicon substrate, which had an aluminum mold. The mixture was baked at 100°C for 30 minutes to fully cure the PDMS layer and allowed to cool down to room temperature. The cured PDMS was cut into a proper sized block. The PDMS block was first treated with trimethylchlorosilane (TMCS) vapor for 5 min and degassed for 30 min in vacuum. Separately, a glass substrate was also TMCS–treated to reduce the adhesion between hydrogels and the glass substrate. A macroporous silicon membrane sample was treated through vapor deposition of hexamethyldisilazane (HMDS) surface adhesion layer. Fig. 2(a) shows a schematic of the procedure for the hydrogel embedding process. A drop of the fresh hydrogel solution mixed with AP and TEMED was applied on a TMCS-treated glass substrate. A HMDS treated membrane was placed on top of the hydrogel droplet. Finally, the degassed PDMS block was placed on the membrane sample. Within several minutes, the pore cavities were filled with the hydrogel solution due to the PDMS degassing-induced aspiration. The whole structure was cured in a nitrogen environment for 12 hours. Once the curing process was completed, the membrane sample was separated from the glass and PDMS block, rinsed with DI water, and soaked in DI water overnight. Fig. 2(b) shows a microscope image of a membrane sample with embedded hydrogels within the pores. The overall dimension of the fabricated membrane device is 8 mm × 8 mm with a thickness of 400 μm.
Fig. 2.
(a) Fabrication procedure of hydrogels embedded inside pores of a macroporous membrane sample. (b) Nomarski contrast image of a fabricated structure.
C. Measurement Setup
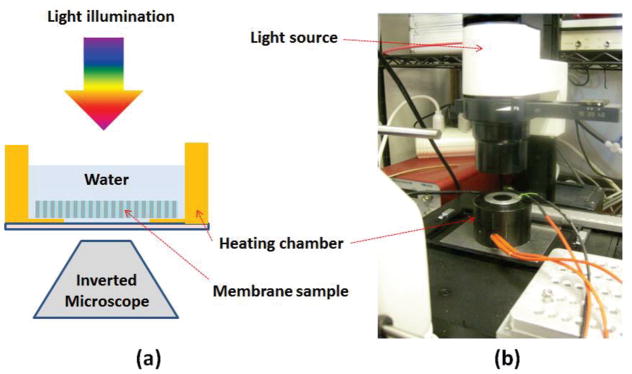
Fig. 3(a) shows a schematic of measurement setup used to characterize the fabricated device. Leica inverted microscope (DMIL) was used to measure light transmission response of the fabricated sample. A custom-built temperature-regulated chamber shown in Fig. 3(b) was used to control water temperature within the chamber. A thin glass-window on the bottom of the chamber allowed the visualization of the membrane surface using a digital CCD camera on the inverted microscope.
Fig. 3.
(a) Schematic arrangement of the measurement setup. (b) An image of the setup used to characterize the device.
Static thermal response of the fabricated hydrogel embedded membrane was measured by increasing and decreasing water temperature in the chamber. Light transmission through the sample was recorded as a series of images for different temperatures.
Dynamic thermal response of the membrane was also studied. For this study, the membrane sample was soaked in a 3 inch Petri dish containing cold water at 25 °C and hot water droplets (~60 °C) using a plastic pipette was applied on top of the membrane. During this experiment, the dynamic optical transmission was recorded in a video format through the microscope camera.
III. RESULTS
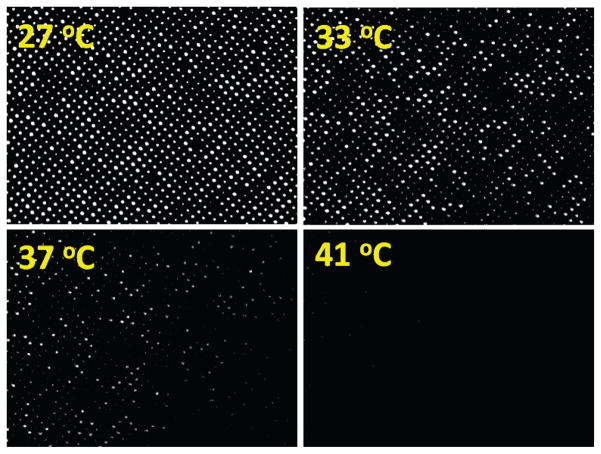
Fig. 4 shows a series of light transmission images from the fabricated membrane sample. When the temperature was lower than the LCST of hydrogels, bright light transmission through the pores was observed. Over 90% of pores were successfully opened. A few dead pores were observed due to imperfection of pores and processing contaminations. As the temperature was increased, the intensities of the transmitted light through the pores were decreased. At around 40 °C, the transmitted light was substantially reduced. This clearly indicates that the presented embedding process of hydrogel within pores is successful.
Fig. 4.
A sequence of images of the sample response at different temperatures.
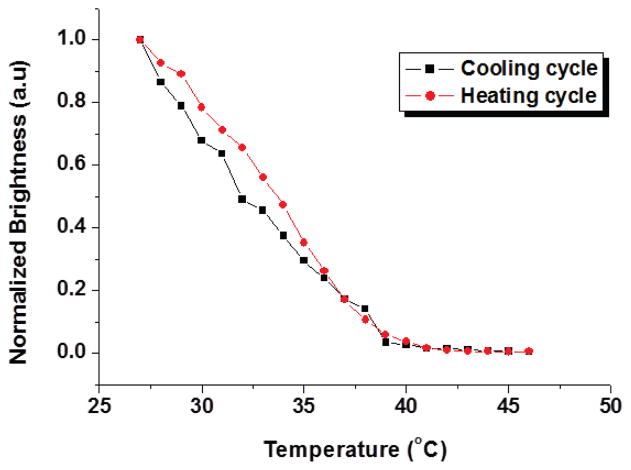
Fig. 5 shows normalized brightness changes as a function of water temperature in the heating chamber. The sum of the brightness values of the pixels in each temperature image was calculated. In the heating cycle, the water temperature was increased from 27 °C to 46 °C at the rate of 1 °C per 5 sec. In the cooling cycle, the water temperature was decreased naturally to room temperature. Light transmission images were taken and processed to obtain average intensities for different temperatures. It was observed that the temperature response was fairly repeatable during the temperature cycle.
Fig. 5.
Normalized brightness changes for cooling cycle and heating cycle.
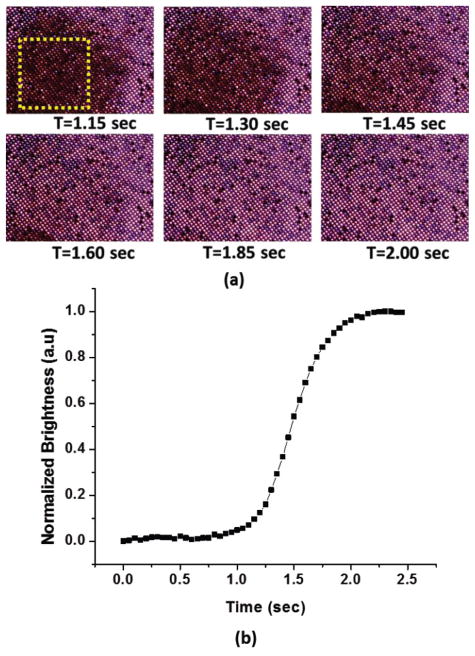
Fig. 6(a) shows frame images from a video while a few drops of hot water were applied on the surface of the membrane sample. As expected, the light transmission in the high temperature region due to the hot water droplet was weakened as indicated by the dark area. As the temperature on the dark area was stabilized to the background water temperature, light transmission is visible through the pores and the area changed back from dark to bright. Fig. 6(b) shows the normalized brightness change as a function of time. The square area (in yellow) indicated in Fig. 6(a) was used to obtain changes in brightness as a function of time. The extracted temporal response (90% change) from the hydrogels embedded within the macropores was less than 700 msec. The temporal response of this work is among the fastest NIPAAM-based hydrogel structures reported till date. Furthermore, spatial temperature pattern can be observed through pixel-like, arrayed macropores, which indicates a potential for individually addressable hydrogel sensors or actuators through temperature stimulation. Better thermal isolation between pores can be achieved through thicker thermal oxide layers on the macroporous silicon substrate.
Fig. 6.
Dynamic thermal response from the sample.
IV. CONCLUSION
We have demonstrated a fast thermo-responsive optical membrane using hydrogels embedded in a macroporous silicon structure. The unique structural configuration offers fast response of the hydrogel under stimulated temperature changes. Optical transmission through the pore was utilized to characterize the implemented structure. Inspired from volume change of pNIPAAm by thermal stimulation, it is also possible to utilize this concept in stimulation-induced drug delivery applications.
Acknowledgments
The authors thank Ritesh Ray Chaudhuri and Youngsik Song for helpful discussions. This work was supported in part by the National Institutes of Health (Grant No.: R21EY024644) and PSC-CUNY grant.
References
- 1.Koetting MC, et al. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Materials Science and Engineering: R: Reports. 2015;93:1–49. doi: 10.1016/j.mser.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49(8):1993–2007. [Google Scholar]
- 3.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53(3):321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 4.Richter A, et al. Review on hydrogel-based pH sensors and microsensors. Sensors. 2008;8(1):561–581. doi: 10.3390/s8010561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eddington DT, Beebe DJ. Flow control with hydrogels. Adv Drug Deliv Rev. 2004;56(2):199–210. doi: 10.1016/j.addr.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 6.D’eramo L, et al. Microfluidic actuators based on temperature-responsive hydrogels. Microsystems & Nanoengineering. 2018;4:17069. [Google Scholar]
- 7.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Zhuo R. Preparation of fast responsive, temperature-sensitive poly (N-isopropylacrylamide) hydrogel. Macromolecular Chemistry and Physics. 1999;200(12):2602–2605. [Google Scholar]
- 9.Zhang X, Zhuo R. Synthesis and properties of thermosensitive poly (N-isopropylacrylamide-co-methyl methacrylate) hydrogel with rapid response. Mater Lett. 2002;52(1–2):5–9. [Google Scholar]
- 10.Zhang X, Wang F, Chu C. Thermoresponsive hydrogel with rapid response dynamics. J Mater Sci Mater Med. 2003;14(5):451–455. doi: 10.1023/a:1023219019500. [DOI] [PubMed] [Google Scholar]
- 11.Xue W, et al. Rapid swelling and deswelling in cryogels of crosslinked poly (N-isopropylacrylamide-co-acrylic acid) European Polymer Journal. 2004;40(3):467–476. [Google Scholar]
- 12.Xiang Y, Peng Z, Chen D. A new polymer/clay nano-composite hydrogel with improved response rate and tensile mechanical properties. European Polymer Journal. 2006;42(9):2125–2132. [Google Scholar]
- 13.Tan Y, et al. High mechanical strength and rapid response rate of poly (N-isopropyl acrylamide) hydrogel crosslinked by starch-based nanospheres. Soft Matter. 2010;6(7):1467–1471. [Google Scholar]
- 14.Mou C, et al. Monodisperse and fast-responsive poly (N-isopropylacrylamide) microgels with open-celled porous structure. Langmuir. 2014;30(5):1455–1464. doi: 10.1021/la4046379. [DOI] [PubMed] [Google Scholar]
- 15.Janovák L, et al. Investigation of the structure and swelling of poly (N-isopropyl-acrylamide-acrylamide) and poly (N-isopropyl-acrylamide-acrylic acid) based copolymer and composite hydrogels. Colloid Polym Sci. 2008;286(14–15):1575–1585. [Google Scholar]
- 16.Chen J, et al. Synthesis and properties of poly (n-isopropylacrylamide-co-acrylamide) hydrogels. Macromolecular Symposia. 2005 [Google Scholar]
- 17.Sousa RG, Freitas RF, Magalhães WF. Structural characterization of poly (N-isopropylacrylamide) gels and some of their copolymers with acrylamide through positron annihilation lifetime spectroscopy. Polymer. 1998;39(16):3815–3819. [Google Scholar]
- 18.Caykara T, Kiper S, Demirel G. Thermosensitive poly (N-isopropylacrylamide-co-acrylamide) hydrogels: synthesis, swelling and interaction with ionic surfactants. European Polymer Journal. 2006;42(2):348–355. [Google Scholar]
- 19.Luo C, et al. Degassing-assisted patterning of cell culture surfaces. Biotechnol Bioeng. 2010;105(4):854–859. doi: 10.1002/bit.22586. [DOI] [PubMed] [Google Scholar]