Abstract
Objective
To identify associations between circulating endocannabinoids and craving during the luteal phase of the menstrual cycle. This report is a secondary analysis of a trial registered in clinicaltrials.gov as NCT01407692.
Methods
Seventeen premenopausal women were studied during the follicular and luteal phases of their menstrual cycle. Previously we had reported fasting plasma estradiol, progesterone, leptin associations with luteal phase cravings for carbohydrate, fat, sweet-rich foods, and eating behavior. Here, we measured fasting plasma endocannabinoids (ECs) endocannabinoid-like substances (ECLs), and postprandial metabolic responses to a mixed meal challenge. Structural equation modeling was used to evaluate relationships between measured variables and cravings.
Results
Oleoylethanolamide (OEA) and postprandial lipids were inversely associated with craving sweet-rich foods, while progesterone was positively associated (RMSEA = 0.041, χ2 p: 0.416 i.e. hypothetical and physiological models not different). OEA, progesterone and disinhibition were positively associated with craving carbohydrates (RMSEA: <0.001, χ2 p: 0.919). ECs and ECLs combined were stronger predictors of craving than clinical metabolic parameters, ECs only, satiety hormones or gonadocorticoids.
Conclusions
Our theoretical model suggests that ECs and ECLs influence craving. Since these metabolites can be modulated via dietary fat intake, they could be potential targets to alter menstrual cycle cravings.
Keywords: Menstrual cycle craving, endocannabinoids, progesterone, oleoylethanolamide
1. Introduction
Food intake regulation is a crucial component of body weight control. Satiety and appetite-modulating endocrine and central nervous system circuits determine food intake. Endocannabinoids (ECs) are lipid mediators that include amides, esters and ethers of polyunsaturated fatty acids (PUFAs) that are endogenous ligands of the cannabinoid receptors (1), with myriad functions including the regulation of eating and craving behaviors (2–4). Moreover, the satiety hormone leptin has been shown to suppress hypothalamic EC levels following acute administration in mice (5). The most well studied ECs are the arachidonate-derived ananadamide or N-arachidonoylethanolamide (AEA) and 2-arachidonoylglycerol (2-AG) (6). In addition, several structurally similar N-acylethanolamide- (NAE) and monoacylglycerol- (MAG) derivatives of PUFAs (collectively referred to as endocannabinoid-like substances or ECLs) exist (7).
Signaling in the endocannabinoid system is complex. ECs and ECLs bind to several receptors with different degrees of affinity, inducing multifaceted physiological effects (8, 9). AEA and 2-AG, along with several NAEs (e.g. docosahexenoylethanolamide, DHEA and eicosapentenoylethanolamide, EPEA), bind to cannabinoid receptors 1 and 2 (CB1/2), both in the brain and peripheral tissues (7). 2-oleoylglycerol (2-OG) and oleoylethanolamide (OEA) are poor CB1/2 ligands, but induce their effects by binding to PPARα, or other G-protein coupled receptors (GPCRs) (7). For instance, OEA, linoleoylethanolamide (LEA) and OGs are potent agonists of the orphaned GPR119 (10), activation of which stimulates incretin hormone release in response to meals (11).
The luteal phase of the menstrual cycle is associated with strong food cravings, especially for sweet-, carbohydrate- and fat-rich foods, contributing to higher energy intake and weight gain over time (12, 13). In a previous study, referred to henceforth as ‘our previous report’, we investigated the metabolic underpinnings of cravings focused on whether the primary ovarian hormones, estradiol and progesterone, as well as leptin were associated with craving for sweet, fat and carbohydrate rich foods (14). This is a follow-up ancillary study which has added additional measures (endocannabinoid system, clinical responses to a standard mixed meal challenge test) and a novel approach (feature selection followed by covariance based structural equation modeling) to evaluate factors that predict craving behaviors using the same research cohort (14). In particular, we evaluate eating behaviors that include cognitive restraint, i.e. a “tendency to consciously restrict or control food intake” and disinhibition, i.e. a “tendency to overeat in the presence of palatable foods or other stimuli such as stress” which have been strongly associated with weight gain and higher BMI in women (15).
The primary objective of the current study was to identify factors strongly/causally related to craving behaviors, while accounting for their inter-relational covariance, amidst a milieu of metabolic, anthropometric, endocrine and cognitive components. Here, we use structural equation modeling (SEM), to explore the relationships between ECs, ECLs, eating behaviors and clinical measures as predictors of craving in the luteal phase of the menstrual cycle. The SEM is used as a hypothesis generating tool, creating a theoretical framework of associations between the endocannabinoid, gonadal and satiety hormone systems and their impacts on cravings that can be tested in future studies.
2. Methods
2.1. Study design:
This is an observational study of the follicular and luteal phases of the menstrual cycle details of which can be found in our previous report, including descriptive statistics about our participants (14). Briefly, healthy women between the ages of 18–45 y, with regular menstrual cycles, not on hormonal contraceptives women were studied twice during their menstrual cycle, once during the late follicular phase, and once in the mid-late luteal phase. Further details about this questionnaire, and its scoring are discussed in detail in our previous report (14). The study was approved by the Institutional Review Board of the University of California Davis, and all participants signed an informed consent form at enrolment. This study is registered on ClinicalTrails.gov as NCT01407692
2.2. Standard meal challenge:
On the scheduled test day, participants arrived at the test center following an overnight fast, to consume a standardized breakfast (08:00) and lunch (11:30). Blood was collected at three time points – upon arrival (fasting), 1h after lunch (1hPP), and 2h after lunch (2hPP). The postprandial blood draws were done following lunch to avoid the second meal effect from their self-selected meals the previous night (Supplementary Table 1).
2.3. Clinical markers:
Plasma insulin, glucose, triglycerides, total cholesterol, LDL-c and HDL-c were measured at the fasting and two postprandial time points. Plasma glucose and lipids were measured using chemiluminescence technology on a Clinical Chemistry Analyser (COBAS Integra 400+, Roche Diagnostics Corp., Indianapolis, IN). Fasting and postprandial plasma insulin and fasting plasma leptin, serum estradiol and progesterone were measured using electro-chemiluminescence on a Sector Imager 2400 (Model 1250, Meso-Scale Discovery, Gaithersburg, MD). The fasting serum was used to measure DHEAS (dehydroepiandrosterone-sulphate), which was also measured using chemiluminescence on the COBAS Integra 400+ (Roche). Sex hormone binding globulin (SHBG) was measured using a Quantikine (R) ELISA kit (Minneapolis, MN). The fasting values were previously reported in our primary manuscript as outcomes (14).
2.4. Questionnaires:
The food craving inventory, created and validated by White et al, (14) was used to obtain responses from participants during the luteal phase, about cravings for fat-rich, sweet-tasting and carbohydrate rich foods. Briefly, the questionnaire includes lists of foods that belong to 5 different categories – sweets, high fat, starches/carbohydrate, fast foods and sweet beverages. Participants scored their craving for each of them during the luteal phase testing on a likert scale ranging from never to daily. Scores were totaled per category and used as a measure of craving each food type. The Eating Inventory was administered at screening to all women enrolled in the study. This instrument is the commercially available version of the Three Factor Eating questionnaire (16), and has been established and validated by Stunkard AJ and Messick S. The questionnaire asked participants about their eating behaviors, and provided scores for three dimensions of eating behaviors – cognitive restraint, disinhibition and hunger.
2.5. Measurement of Endocannabinoids and endocannabinoid-like substances measurement:
ECs and ECLs were isolated from fasting serum collected on both test days using modifications of a previously published protocol (17, 18). Briefly, 100 μL aliquots of serum were enriched with 10 μL of 1 μM deuterated analytical surrogates (18, 19), after which 5 μL of an anti-oxidant solution (0.2 mg/mL butylated hydroxytoluene/EDTA in 1:1 methanol:water) and 300 μL acetonitrile with 1% formic acid was added to each sample. Samples were extracted by elution through an Ostro Sample Preparation Plate (Waters Corp., Milford, MA), and the eluent was dried by vacuum evaporation and reconstituted in 100 μL of an internal standard solution containing 100 nM each of 1-cyclohexyl-3-ureido dodecanoic acid (Sigma Aldrich, St. Louis, MO) and 1-phenyl,3-ureido hexanoic acid (gift from B. D. Hammock, University of California-Davis) in 1:1 (v/v) methanol/acetonitrile.
Quantification of extracted ECs and ECLs using targeted ultra-performance liquid chromatography coupled to tandem mass spectrometry was conducted using a previously published method (18, 19). Briefly, analytes were separated on a 2.1 × 150 mm, 1.7 μm BEH C18 column (Waters) and detected by positive mode electrospray ionization tandem mass spectrometry on an API 4000 QTRAP (Sciex, Framingham, MA). Analytes were quantified by internal standard methodology using five to seven point calibration curves (r ≥ 0.997) and data were processed in MultiQuant v3.0.2 (Sciex). All calibration standards and analytical surrogates were purchased from Cayman Chemical (Ann Arbor, MI) or Avanti Polar Lipids Inc. (Alabaster, AL).
Measured analytes included monoacylglycerol derivatives of arachidonate, linoleate and oleate (1- and 2-AG, LG and OG, respectively), and the acylethanolamide derivatives of arachidonate, oleate, docosatetraenoate, docosahexaenoate, alpha-linolenate and dihomo-gamma-linoleate (AEA, OEA, DEA, DHEA, aLEA and DGLEA, respectively).
2.6. Statistical analysis:
Data analysis was done in R (R statistical software, Vienna Austria (20)) using packages nlme, lavaan, and semPlot, and JMP Pro 13.1 (SAS Institute, Cary, NC). Preliminary differences between the luteal and follicular phase fasting variables have been presented in our previous report (14). The newly measured meal challenge response variables, endocannabinoids, and previously reported TFEQ and FCI scores were tested for normality using Shapiro-Wilk test. Grubbs test was used to detect outliers and none were found. Since data were not normally distributed, non-parametric tests were used unless specified otherwise. Wilcoxon’s rank tests were used to identify differences between phases in endocannabinoids and eating behavior factors. Lipids, glucose and insulin responses to the standard meal challenge responses were log transformed, and phase differences were evaluated using linear mixed model analysis, with participant as the random effect, and time of blood draw as the repeated factor. Spearman’s correlations were used to identify associations between ovarian hormones, endocannabinoids and leptin, irrespective of phase.
2.7. Feature selection and structural equation modeling:
Data were range scaled prior to analysis (21), since SEM is vulnerable to differences in magnitude and scale across multiple variables (22). Since the degrees of freedom for an analysis where n = 17 is small, the number of variables that can be entered as independent or latent variables in the SEM is limited. Hence, we performed feature selection prior to building SEMs. Feature selection was accomplished using cluster analysis (eigenvectors used to derive Euclidean distance), which identified groups of correlated and co-linear variables. This was used for both clinical data (fasting and postprandial responses to the standard meal challenge test), as well as endocannabinoids (combined ECs and ECLs). Cluster analysis grouped variables together that were most correlated with each other in each phase (based on R2). A single representative variable from each cluster was chosen for use in the model because it displayed the highest correlation with the other variables in that cluster explained the highest proportion of variation within that cluster, and was also the least correlated with the neighboring cluster. Cluster analysis was not used to identify representative variables from eating behaviors or ovarian hormones because it was possible to include all of them without needing a larger sample size. Rather, variable inclusion was decided based on statistical fitness of the final model. Hence, to build the SEM models, the following variables were used: representative variables chosen by cluster analysis of clinical data and ECLs; eating behaviors (hunger score, cognitive restraint and disinhibition); and ovarian/satiety hormones (estradiol, progesterone, SHBG and leptin) [R code is available in Supplemental Material]. Models were built backwards by elimination until model convergence, to identify the best fit, retaining ideal variable(s) possible while also improving chi square p-value. The FCI was only administered during the luteal phase testing to capture craving tendencies for that phase. However, we used both follicular and luteal phase clinical and endocrine milieu to predict craving during the luteal phase. It is possible that craving in the luteal phase is a result of the lead-in milieu set up during the follicular phase, due to current luteal phase milieu, and the change between the two phases. Also, the craving inventory that was administered during the luteal phase was used to ask participants about their cravings during the two-week period leading up to the luteal phase test day. This is an approximate two-week period immediately following the follicular phase testing. This adds further support for our hypothesis that the follicular phase milieu could set-up the cravings experienced during the luteal phase. Hence, luteal phase craving for sweet-, carbohydrate- and fat-rich foods were predicted independently using follicular phase and luteal phase endocannabinoids, clinical, eating behavior and, ovarian and satiety hormones.
The SEM predicts a dependent variable, in this case craving, using latent variables or factors modeled from the measured inputs. SEM includes a confirmatory factor analysis that identifies latent variables or factors that best represent measured variables, combined with a path analysis to identify potential causal relationships between the identified latent variables/factors and the outcome variable. The following latent variables were generated for our craving model: Endocannabinoids representing ECs and ECLs; Clinical variables representing clinical fasting and postprandial variables; Eating behaviors representing - cognitive restraint, hunger score and disinhibition; Ovarian/Satiety hormones representing estradiol, sex hormone binding globulin (SHBG), progesterone, DHEAS and leptin. Model fit, power and robustness were evaluated rigorously using several indices (22–24). The Tucker-Lewis Index (TLI) and Comparative Fit Index (CFI) are model fit estimators, ranging between 0 and 1, with a number closer to 1 considered a better fit for the model. However, if the CFI is 1, the TLI can be >1 indicating a very good model fit. The modification index provides a measure of model sensitivity to variable inclusion. Modification indices of <3.84 for individual correlation and covariance is considered a good fit. The Root Mean Square Error of Approximation (RMSEA) is habitually used in SEM to evaluate overall model power and robustness. An RMSEA of < 0.02, 0.02–0.08 and >0.08 are used to designate excellent, fair and poor model fits respectively. Finally, a chi-square (χ2) test was used to compare the model outcome to the FCI measured craving scores. A p-value of > 0.05 indicates the predicted scores are not significantly different from those measured, and therefore represent a well fit SEM model.
In the Figures that depict the SEM models (Figures 2 - 4), measured variables are represented by a rectangle or square box, and latent (or unmeasured) factors by a circle or ellipse (24). Single headed arrows or ‘paths’ are used to define causal relationships in the model and positive or negative coefficients are indicated by (+) and (−) symbols next to the arrows. Double-headed arrows indicate covariances or correlations (solid black lines indicate positive, and dashed black lines indicate inverse) that are a priori declared while building the model, without a causal interpretation. Statistically, the single headed arrows or paths represent regression coefficients, and double-headed arrows covariances. There is no assumption that the latent factors completely explain observed variation in the outcome variable. In addition, the associations between each variable (measured and latent) take into consideration the covariance and inter-correlation matrix of the data array. Hence, each figure represents a holistic picture of their inter-relationships, as opposed to independent correlations.
Figure 2:
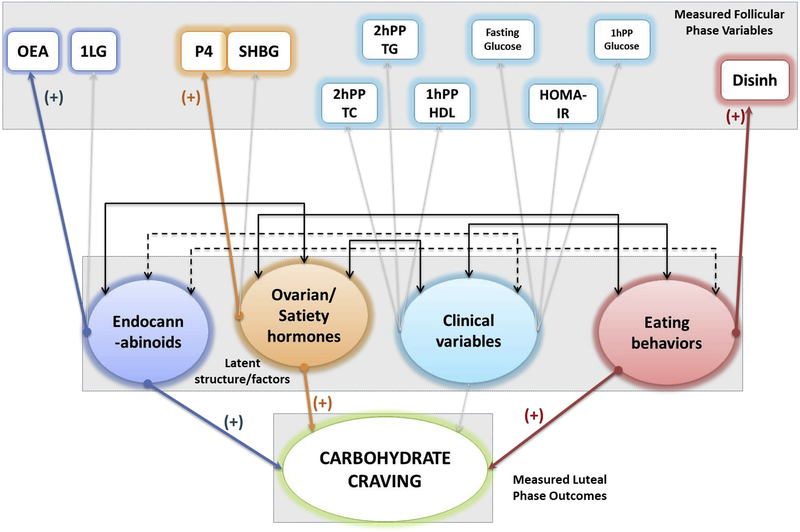
Follicular phase SEM path diagram, depicting role of endocannabinoids ovarian and satiety hormones, clinical variables and eating behaviors in craving sweet-tasting foods in the luteal phase in n = 17 women. The boxes are measured variables, circles are latent variables, and the final predictor variable is craving (in this case - carbs). The width of the colored arrows indicate the strength of the association, grayed out arrows indicate no association, and the double ended black arrows between the (latent) circles indicate positive (solid lines) or inverse (dotted lines) covariance. This model was identified with endocannabinoid like chemicals (OEA), ovarian hormones (progesterone), and eating behaviors (disinhibition) positively contributing to craving carbohydrates. A <0.001 RMSEA, with 1.000 CFI and 3.340 TLI, and p value of 0.919 indicates excellent model fit.
Figure 4:
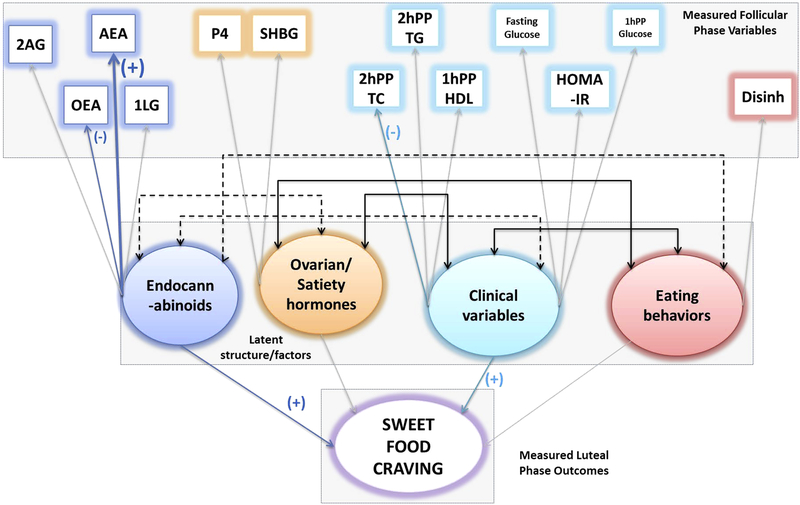
Follicular phase SEM path diagram, depicting role of endocannabinoids, ovarian and satiety hormones, clinical variables and eating behavior in craving sweet-tasting foods in the luteal phase in n = 17 women. The boxes are measured variables, circles are latent variables, and the final predictor variable is craving (in this case - sweets).The width of the colored arrows indicate the strength of the association, grayed out arrows indicate no association, and the double ended black arrows between the (latent) circles indicate positive (solid lines) or inverse (dotted lines) covariance. This model was identified with endocannabinoid like chemicals (OEA), endocannabinoids (AEA), and clinical parameters (2h PP TC) contributing positively to craving sweet-foods. A <0.001 RMSEA, with 1.000 CFI and 1.394 TLI, and p value of 0.727 indicates excellent model fit.
3. Results
3.1. Meal challenge and endocannabinoids between the phases:
Insulin was significantly higher at 2 h postprandial in the follicular phase compared to the luteal phase (p<0.001). No phase differences were found in fasting or postprandial triglycerides, total cholesterol, LDL or HDL cholesterol, and glucose following the standard meal challenge (Supplementary Table 2). There were no differences in the ECs or ECLs between the two phases (Supplementary Table 3).
3.2. Correlational analysis:
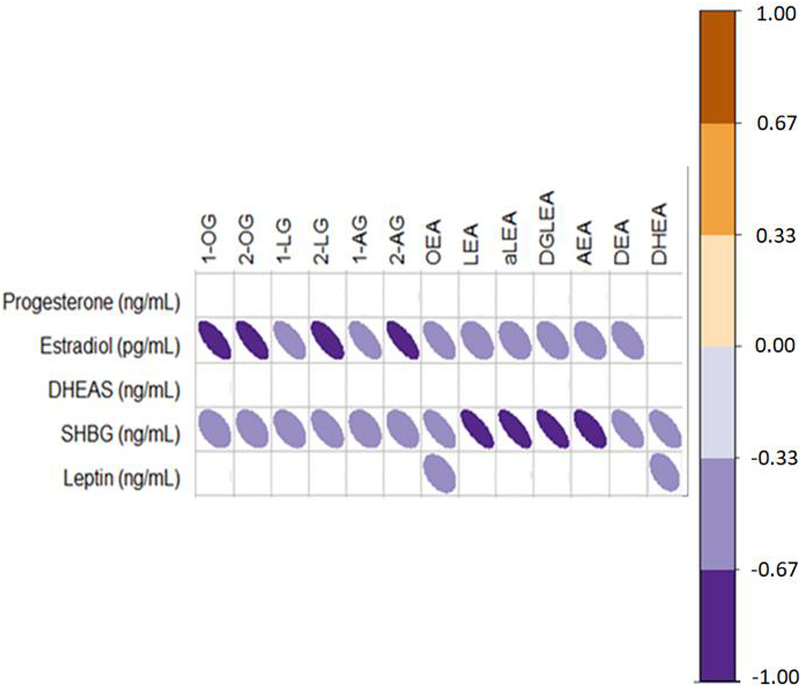
Estradiol was inversely associated with MAGs (2-OG: r =−0.753, p = 0.02, 2-AG: r = −0.677, p = 0.09) (Figure 1). Additionally, SHBG was inversely associated with ECLs (2-LG: r = −0.681, p = 0.09, aLEA: r = −0.841, p = 0.01, LEA: r = −0.770, p = 0.02), as was leptin (OEA: r = −0.451, p = 0.08, DHEA: r = −0.512, p = 0.06)
Figure 1:
The Spearman’s rank correlation analyses irrespective of phase for hormones and endocannabinoids (i.e. using data from both phases) are presented. The color scale orange and purple are used to indicate positive and inverse associations respectively that show a trend (0.1>p>0.05, rho: (+/−) 0.29–0.33) or are significant (p<0.05, rho: > (+/−) 0.34) in an n = 17 women.
3.3. Feature selection:
The cluster analysis grouped the clinical variables into six clusters in the follicular and luteal phases. ECLs were split into two clusters in both phases, while ovarian and satiety hormones were split into two clusters in follicular phase and 3 clusters in the luteal phase (Supplementary Figure 1).
3.4. Structural Equation Modeling:
Table 1 outlines the evaluation of the model fit, for each phase, for predicting cravings for sweet-tasting and carbohydrate-rich foods that were a fair, good or excellent fit, and were able to predict craving. Supplemental Table 4 outlines the models that were poorly fit. The models were built to predict craving experienced during the luteal phase, as a result of either follicular phase or luteal phase milieu. It is of interest to note that both AEA and 2AG were used in building the ECs + ECLs models, but 2-AG consistently weakened the model fit within this paradigm. Three models that matched the criteria to accept the fit based on RMSEA, TLI, CFI, chi square p value and modification indices are graphically represented in Figures 2–4.
Table 1:
SEM model fit parameters predicting sweet food, and fat-rich food craving during the luteal phase, using the luteal phase milieu, and the lead-in follicular phase milieu in n = 17 women.
| Independent variables | Dependent variable | RMSEA | Tucker Lewis Index | Comparative Fit Index | Chi square p-value for fit | Modification indices <3.84 | Model acceptability based on all validation parameters (Excellent, fair, poor) |
|---|---|---|---|---|---|---|---|
|
FOLLICULAR PHASE CRAVING MODELS TESTED | |||||||
| ECLs Clinical Variables Dietary Restraint Ovarian and Satiety Hormones | Craving Carbs | 0.058 | 0.904 | 0.935 | 0.374 | Yes | Fair |
| Craving sweets | <0.001 | 1.394 | 1.000 | 0.727 | Yes | Excellent | |
| ECLs Clinical Variables Dietary Restraint Ovarian and Satiety Hormones | Craving Carbs | <0.001 | 3.340 | 1.000 | 0.919 | Yes | Excellent |
| Craving sweets | 0.041 | 0.959 | 0.943 | 0.416 | Yes | Good | |
The model depicted in Figure 2 evaluates the impact of ECLs, ovarian hormones, fasting and postprandial metabolic parameters and eating behaviors in the follicular phase on craving carbohydrate rich foods in the luteal phase. ECLs (OEA), ovarian hormones (progesterone) and eating behavior (disinhibition) were positively associated with craving behavior as indicated by the positive estimates. This association indicates that higher OEA and progesterone in the follicular phase and overall higher disinhibition are associated with increased craving for carbohydrates. Clinical parameters following the standard meal challenge in the follicular phase did not appear to play a significant role in craving carbohydrate-rich foods in the luteal phase.
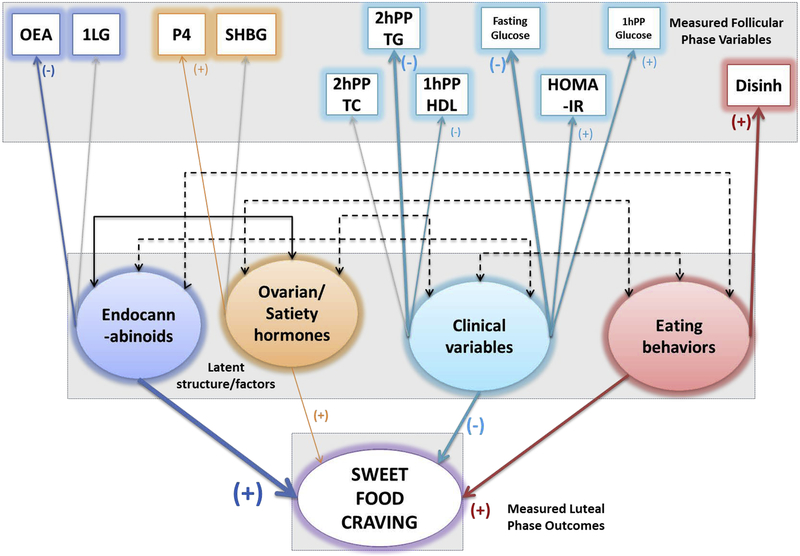
The model depicted in Figure 3 evaluates the effect of ECLs, ovarian hormones, fasting and postprandial metabolic parameters and eating behaviors in the follicular phase on craving sweet foods during the luteal phase. ECLs, ovarian/satiety hormones (progesterone) and eating behaviors (disinhibition) were positively associated, clinical parameters (2hPP TG, fasting glucose, 1hPP HDL) were inversely associated with craving. Curiously, while lipid clinical parameters appear to contribute inversely to the latent clinical variable construct, some of the glucose and insulin parameters contribute positively to it, despite the overall contribution to craving by clinical parameters being negative. Similarly, OEA appears to contribute inversely to the latent endocannabinoids factor/variable construct, even though ECLs as a whole positively contributes to sweet-craving.
Figure 3:
Follicular phase SEM path diagram, depicting role of endocannabinoids ovarian and satiety hormones, clinical variables and eating behaviors in craving sweet-tasting foods in the luteal phase in n = 17 women. The boxes are measured variables, circles are latent variables, and the final predictor variable is craving (in this case - sweets).The width of the colored arrows indicate the strength of the association, grayed out arrows indicate no association, and the double ended black arrows between the (latent) circles indicate positive (solid lines) or inverse (dotted lines) covariance. This model was identified with endocannabinoids (OEA) and eating behaviors (disinhibition) positively contributing to craving sweet foods, while ovarian hormones (progesterone) and clinical parameters (2hPP TG, 1hPP HDL, 1hPP Glucose, Fasting glucose and HOMA-IR) contributed inversely to craving sweet-foods. A 0.041 RMSEA, with 0.959 CFI and 0.943 TLI, and p value of 0.416 indicates good model fit.
The model depicted in Figure 4 is very similar to the one in Figure 3, with the only exception that it includes ECs as measured follicular phase variables. Hence, it evaluates the effect of ECs, ECLs, ovarian hormones, fasting and postprandial metabolic parameters and eating behaviors in the follicular phase on craving sweet foods in the luteal phase. ECLs (OEA), ECs (AEA) and clinical metabolic parameters (2h PP TC), were associated with craving sweet tasting foods. Curiously, 2hPP TC and OEA inversely contributed to the latent variable.
Finally, none of the models predicting fat craving were fit well and did not pass our robustness and rigor tests, as seen in Supplementary Table 4.
4. Discussion
In this report we examined circulating ovarian and satiety hormones, eating behaviors, an array of endocannabinoids and endocannabinoid-like-compounds as well as clinical metabolic parameters to gain insight into the metabolic underpinnings of food cravings. In our model testing, ECs alone were not adequate predictors of craving sweets and carbohydrates; but the combination of ECs and ECLs were best able to predict craving. An important result of the modeling building demonstrates that ECs and ECLs, as well as progesterone during the follicular phase appear to be better at predicting craving sweet- and carbohydrate-rich foods in the luteal phase. AEA and certain MAGs (1-LG, 1-OG) are positively associated with craving sweet-rich and carbohydrate rich foods. OEA appears to be inversely associated with craving sweets, while being positively associated with craving carbohydrates in the luteal phase.
Our SEM analyses suggest that endocannabinoids are positively associated with craving, both carbohydrate-rich and sweet-tasting foods, primarily driven by AEA, NAEs (represented by OEA) and MAGs (represented by 1-LG). AEA being positively associated with craving sweet-rich foods is not surprising, since several studies have reported the increase in sweet-taste liking, and consumption of palatable foods by ECs (25–27). Endocannabinoids induce hyperphagic responses via activation of CB1 receptors (28, 29), and both MAGs and NAEs express pharmacological effects via CB1/CB2 receptors as well (30).
Among ECLs, OEA was found to be positively associated with carbohydrate-rich food craving, while being inversely associated with sweet food craving. ECLs are much less understood relative to ECs (29). Several NAEs, especially OEA, modulate activation of GPR119 (10, 11), a fat sensor in enteroendocrine and pancreatic beta cells (31). This modulation of GPR119 has been suspected to impact activation of GLP-1, an incretin satiety hormone (32), reducing overall food intake in rodents. It is likely that this pathway plays a role in the inverse association of OEA with sweet-food craving. However, the dichotomy between carbohydrate and sweet-food craving and OEA warrants further study, possibly due to different neuro-endocrine mechanisms at play that are yet to be understood.
Metabolic pathways impacting food intake and cravings change from the fasted to the fed state (33). Lipids in the postprandial state can reduce desire to eat and cravings in a fed state, especially via cholecystokinin (34) as well as by mechanoreceptors activated by abdominal distension (35). Our SEM models agree with this ideology, and display their (2hPP TG, 1hPP HDL, 2hPP TC) inverse association with craving sweet-tasting foods.
The addition of ECs to the model predicting sweet-food craving (that originally was tested with ECLs only) reversed or negated the association of the latent clinical and eating behavior variables with craving sweet foods (Figure 3 vs 4). Since ECs can strongly influence craving sweet-rich foods, we theorize that ECs and ECLs together may be potent enough that other physiological factors may be relatively less potent.
Ovarian/satiety hormones, represented by progesterone and SHBG in the follicular phase appear to be associated with carbohydrate craving and sweet-food craving. Progesterone concentrations peak during the luteal phase (36) and have been shown to be associated with increased cravings (37). Interestingly, while progesterone appears to be positively associated with craving carbohydrate and sweet-rich foods in the presence of ECLs alone, adding ECs to the mix results in progesterone no longer being associated with craving sweet-rich foods. This further strengthens our theory that ECs and ECLs together are capable of influencing craving behaviors, likely even more than ovarian hormones. Future studies, however, will be needed to confirm this in larger samples, as well as by altering circulating ECs and ECLs and observing their downstream effect on cravings.
To our knowledge, the current study is the first to compare the postprandial insulinemic and glycemic responses between the two phases in healthy young women. In the present study, even though postprandial insulin was higher in the follicular phase, there were no differences in glucose or HOMA-IR. If minor differences between the two phases do exist in insulin sensitivity, as reported previously (38), the present study did not identify them.
The concurrent luteal phase milieu was not successful at predicting craving, but the follicular phase milieu was. There could be several reasons for this. It may be likely that the milieu during the follicular phase sets up women’s physiology in the luteal phase that dictates eating behavior. Alternatively, it could be due to the fact that we captured women during their ovulation in the follicular phase, but the luteal phase test occurred anytime in the mid to late luteal phase. Estradiol peaks during the late follicular phase (Supplementary figure 2), drops during ovulation, and then peaks again in the mid-luteal phase, while circulating progesterone concentrations during late-follicular/ovulatory phase of the cycle match those of the last few days of the late-luteal phase (39). Studying women closer to the late-luteal phase may identify stronger associations between circulating milieus and craving behaviors, since most pre-menstrual syndrome symptoms (which may include craving) are experienced during the latter part of the luteal phase (40). However, evidence about craving during the luteal phase spans the entire luteal phase, and not only the late luteal phase (41), especially since we did not target women with pre-menstrual syndrome (PMS) in the present study. Future studies could choose women with PMS and evaluate the same criteria.
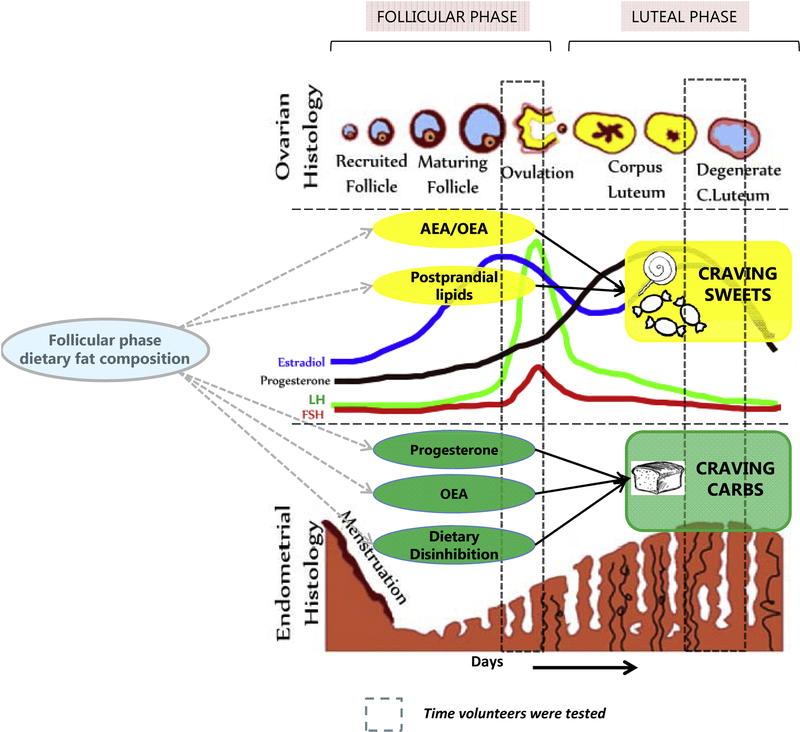
4.1. Clinical implications of endocannabinoids, cravings and dietary fatty acid composition
ECLs are structurally similar to ECs and they utilize the same biosynthesis and degradation pathways (30). This enables an “entourage” effect (42), an enhancement of the effect of ECs, by the presence of these analogues (ECLs). They also inhibit degradation of ECs by competitively binding with the degradation enzymes, or allosterically modify binding of ECs to CB1/2 (43). These could explain why our models were best fit when using ECs and ECLs together. Furthermore, both ECs and ECLs can be modulated by dietary fats. A dietary intervention altering omega-3 and omega-6 PUFA recently has shown to increase circulating ECs (44). Diets high in palm, olive, safflower fish and arachidonic acid have shown to differentially increase MAGs and NAEs (44). NAE’s are synthesized from phosphatidylethanolamines (phospholipids) using available fatty acids (30). Dietary fatty acids largely determine circulating NAEs, and increased oleic acid or linoleic acid intake has shown downstream increases in OEA and LEA respectively (45). Hence, ECLs could be potential dietary targets to modulate craving behaviors.
In the current study, NAEs, specifically OEA, was strongly associated with carbohydrate craving, but inversely with sweet-food craving. Since a mechanism exists for OEA to be satiety inducing, it is likely that in women who struggle with body weight maintenance due to sweet-food craving (46), modulating their dietary fat composition during the follicular phase (i.e. increase olive oil intake, but decrease long chain PUFA intake) may help modulate cravings.
4.2. Limitations
Our study sample size was small, and a larger study is needed to confirm the findings from this report. Similarly, even though we thoroughly evaluated the quality and robustness of the SEM models, it is still likely that the small sample size contributed to potential fallacies in model development. SEM makes assumptions about linearity of cause-effect associations between observed and predicted variables, as well as between covariate relationships amongst them (47). Simple regression models assume this for a relationship between just one independent and dependent variable, while SEMs do this for all variables involved, with increased “power” to predict a certain path. However, as the objective was to probe different relationships under these assumptions to find out what may be hypothesized as potential causal pathways to further study, SEM was the ideal choice.
We also did not closely monitor the study participants’ diet intake, and knowledge of their intake of carbohydrates and sweet foods during the luteal phase could have provided more insight into whether their craving behaviors affected their intake. Our testing times for the menstrual cycle phases could also have been repeated several times instead of just twice, to give a more comprehensive view of the underlying physiology. However, this is a first report on the associations between circulating ECs, ECLs and craving behaviors in healthy women experienced during the luteal phase of the menstrual cycle, and the observations are meaningful, albeit in a small group of women.
4.3. Conclusions
Our previous report identified luteal phase SHBG and estradiol/leptin ratio to be positively associated with craving sweet-rich foods, and leptin to be inversely associated with habitual sweet-rich food intake. Our current manuscript elaborates on these relationships, suggesting associations between progesterone and craving while looking at a more comprehensive picture by including the neuroendocrine system’s role (see Figure 5). It is not surprising that including more variables shed light on complex relationships that were previously undetected, not to mention that SEM models benefit from more measured variables representing their latent variables to better predict the outcome variable (24). In this study, the follicular phase metabolic and endocrine milieu appears to be a better predictor of craving than the luteal phase milieu. If we had measured craving during the follicular phase, we may have been able to predict that based on luteal phase milieu. A future study can evaluate whether there exists a cyclic feedback loop between these phases with food intake affecting metabolic milieu that further impacts craving and so on. OEA, progesterone and disinhibition are positively associated with craving carbohydrates, while OEA and lipids are inversely associated with craving sweet-rich foods, even though progesterone retains its positive association. Finally, the influence of these biological factors in craving indulging behaviors to result in increased carbohydrate and sweet-rich food intake needs to be evaluated further.
Figure 5:
A graphical representation of the proposed hypothetical metabolic-neuroendocrine framework involved in craving behaviors during the luteal phase of the menstrual cycle in normal healthy women.
Supplementary Material
Highlights.
What is already known about this subject?
Women experience craving for carbohydrate, fat-rich and sweet-tasting foods during the luteal phase of their menstrual cycle
Women consume more food during the luteal phase compared to the follicular phase, leading to weight gain over time
Ovarian hormones have primarily been considered responsible for these cravings, however, well designed controlled studies have not been conducted to confirm this consideration.
What does this study add?
Endocannabinoids and endocannabinoid like chemicals appear to be relatively stronger predictors of craving than ovarian hormones
N-arachidonoylethanolamide (AEA) and Oleoylethanolamide (OEA) are significantly associated with cravings during the luteal phase of the menstrual cycle
Our theoretical model suggests that altering dietary fatty acid intake during the follicular phase in the menstrual cycle could help relieve carbohydrate cravings in the luteal phase.
Acknowledgements:
This study was funded by USDA-ARS-CRIS Project 2032-51530-022, NIH-NIGMS Grant Number T32-GM008799, and Jastro Shields Award, UC Davis.
Footnotes
The authors declare no conflict of interest.
Clinical Trial Registration Number: NCT01407692
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Watkins BA, Kim J. The endocannabinoid system: directing eating behavior and macronutrient metabolism. Frontiers in psychology 2014;5:1506. doi: 10.3389/fpsyg.2014.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maccarrone M, Finazzi-Agro A. Endocannabinoids and their actions. Vitam Horm 2002;65:225–55. [DOI] [PubMed] [Google Scholar]
- 3.Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology 1999;143(3):315–7. [DOI] [PubMed] [Google Scholar]
- 4.Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. British journal of pharmacology 2002;136(4):550–7. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001;410(6830):822–5. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 6.Compilation Journal - The Bristish Pharmacological Society. Endocannabinoid-metabolising enzymes. British Journal of Pharmacology 2009; 158: S220–S221. [Google Scholar]
- 7.Fezza F, Bari M, Florio R, Talamonti E, Feole M, Maccarrone M. Endocannabinoids, related compounds and their metabolic routes. Molecules 2014;19(11):17078–106. doi: 10.3390/molecules191117078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosier B, Muccioli GG, Hermans E, Lambert DM. Functionally selective cannabinoid receptor signalling: therapeutic implications and opportunities. Biochemical pharmacology 2010;80(1):1–12. doi: 10.1016/j.bcp.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Maccarrone M, Finazzi-Agro A. The endocannabinoid system, anandamide and the regulation of mammalian cell apoptosis. Cell Death Differ 2003;10(9):946–55. doi: 10.1038/sj.cdd.4401284. [DOI] [PubMed] [Google Scholar]
- 10.Syed SK, Bui HH, Beavers LS, Farb TB, Ficorilli J, Chesterfield AK, Kuo MS, Bokvist K, Barrett DG, Efanov AM. Regulation of GPR119 receptor activity with endocannabinoid-like lipids. American journal of physiology Endocrinology and metabolism 2012;303(12):E1469–78. doi: 10.1152/ajpendo.00269.2012. [DOI] [PubMed] [Google Scholar]
- 11.Overton HA, Fyfe MC, Reynet C. GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. British journal of pharmacology 2008;153 Suppl 1:S76–81. doi: 10.1038/sj.bjp.0707529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorczyca AM, Sjaarda LA, Mitchell EM, Perkins NJ, Schliep KC, Wactawski-Wende J, Mumford SL. Changes in macronutrient, micronutrient, and food group intakes throughout the menstrual cycle in healthy, premenopausal women. European journal of nutrition 2015. doi: 10.1007/s00394-015-0931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kammoun I, Ben Saada W, Sifaou A, Haouat E, Kandara H, Ben Salem L, Ben Slama C. Change in women’s eating habits during the menstrual cycle. Annales d’endocrinologie 2017;78(1):33–7. doi: 10.1016/j.ando.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan S, Tryon RR, Horn WF, Welch L, Keim NL. Estradiol, SHBG and leptin interplay with food craving and intake across the menstrual cycle. Physiology & behavior 2016;165:304–12. doi: 10.1016/j.physbeh.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Hays NP, Roberts SB. Aspects of eating behaviors “disinhibition” and “restraint” are related to weight gain and BMI in women. Obesity 2008;16(1):52–8. doi: 10.1038/oby.2007.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of psychosomatic research 1985;29(1):71–83. [DOI] [PubMed] [Google Scholar]
- 17.La Frano MR, Fahrmann JF, Grapov D, Fiehn O, Pedersen TL, Newman JW, Underwood MA, Steinhorn RH, Wedgwood S. Metabolic perturbations of postnatal growth restriction and hyperoxia-induced pulmonary hypertension in a bronchopulmonary dysplasia model. Metabolomics 2017;13(4):32. doi: 10.1007/s11306-017-1170-6. [Google Scholar]
- 18.Walsh P, Behrens N, Carvallo Chaigneau FR, McEligot H, Agrawal K, Newman JW, Anderson M, Gershwin LJ. A Randomized Placebo Controlled Trial of Ibuprofen for Respiratory Syncytial Virus Infection in a Bovine Model. PLOS ONE 2016;11(4):e0152913. doi: 10.1371/journal.pone.0152913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal K, Hassoun LA, Foolad N, Pedersen TL, Sivamani RK, Newman JW. Sweat lipid mediator profiling: a noninvasive approach for cutaneous research. Journal of Lipid Research 2017;58(1):188–95. doi: 10.1194/jlr.M071738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team. R:A language and environment for statistical computing, Vienna, Austria, 2013. [Google Scholar]
- 21.van den Berg RA, Hoefsloot HC, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC genomics 2006;7:142. doi: 10.1186/1471-2164-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermida R, Luchman JN, Nicolaides V and Wilcox C. The issue of statistical power for overall model fit in evaluating structural equation models. Computational Methods in Social Sciences 2015;3: 25–42. [Google Scholar]
- 23.Hooper D, Coughlan J, Mullen M Structural Equation Modelling: Guidelines for Determining Model Fit. Electronic Journal of Business Research Methods 2008;6(1):53–60. [Google Scholar]
- 24.Hox JJ and Bechger TM. An introduction to Structural Equation Modeling. Family Science Review 2000;11: 354–373. [Google Scholar]
- 25.De Luca MA, Solinas M, Bimpisidis Z, Goldberg SR, Di Chiara G. Cannabinoid facilitation of behavioral and biochemical hedonic taste responses. Neuropharmacology 2012;63(1):161–8. doi: 10.1016/j.neuropharm.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida R, Ohkuri T, Jyotaki M, Yasuo T, Horio N, Yasumatsu K, Sanematsu K, Shigemura N, Yamamoto T, Margolskee RF, et al. Endocannabinoids selectively enhance sweet taste. Proc Natl Acad Sci U S A 2010;107(2):935–9. doi: 10.1073/pnas.0912048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinohara Y, Inui T, Yamamoto T, Shimura T. Cannabinoid in the nucleus accumbens enhances the intake of palatable solution. Neuroreport 2009;20(15):1382–5. doi: 10.1097/WNR.0b013e3283318010. [DOI] [PubMed] [Google Scholar]
- 28.Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. British journal of pharmacology 2001;134(6):1151–4. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirkham TC. Cannabinoids and appetite: food craving and food pleasure. Int Rev Psychiatry 2009;21(2):163–71. doi: 10.1080/09540260902782810. [DOI] [PubMed] [Google Scholar]
- 30.Kleberg K, Hassing HA, Hansen HS. Classical endocannabinoid-like compounds and their regulation by nutrients. Biofactors 2014;40(4):363–72. doi: 10.1002/biof.1158. [DOI] [PubMed] [Google Scholar]
- 31.Hansen HS, Rosenkilde MM, Holst JJ, Schwartz TW. GPR119 as a fat sensor. Trends Pharmacol Sci 2012;33(7):374–81. doi: 10.1016/j.tips.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Sihag J, Jones PJH. Oleoylethanolamide: The role of a bioactive lipid amide in modulating eating behaviour. Obesity reviews : an official journal of the International Association for the Study of Obesity 2017. doi: 10.1111/obr.12630. [DOI] [PubMed] [Google Scholar]
- 33.Berg JM TJ, Stryer L. Biochemistry. In: Freeman WH, ed. Food Intake and Starvation Induce Metabolic Changes. %th ed. New York, 2002:303. [Google Scholar]
- 34.VanderWeele DA. Insulin is a prandial satiety hormone. Physiology & behavior 1994;56(3):619–22. [DOI] [PubMed] [Google Scholar]
- 35.Covasa M, Ritter RC. Adaptation to high-fat diet reduces inhibition of gastric emptying by CCK and intestinal oleate. AS 2000;278(1):R166–70. [DOI] [PubMed] [Google Scholar]
- 36.Mesen TB, Young SL. Progesterone and the luteal phase: a requisite to reproduction. Obstetrics and gynecology clinics of North America 2015;42(1):135–51. doi: 10.1016/j.ogc.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox HC, Sofuoglu M, Morgan PT, Tuit KL, Sinha R. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology 2013;38(9):1532–44. doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeung EH, Zhang C, Mumford SL, Ye A, Trevisan M, Chen L, Browne RW, Wactawski-Wende J, Schisterman EF. Longitudinal study of insulin resistance and sex hormones over the menstrual cycle: the BioCycle Study. The Journal of clinical endocrinology and metabolism 2010;95(12):5435–42. doi: 10.1210/jc.2010-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reed BG CB. The Normal Menstrual Cycle and the Control of Ovulation South Dartmouth (MA): Endotext(Internet], 2015. [Google Scholar]
- 40.Both-Orthman B, Rubinow DR, Hoban MC, Malley J, Grover GN. Menstrual cycle phase-related changes in appetite in patients with premenstrual syndrome and in control subjects. The American journal of psychiatry 1988;145(5):628–31. doi: 10.1176/ajp.145.5.628. [DOI] [PubMed] [Google Scholar]
- 41.Davidsen L, Vistisen B, Astrup A. Impact of the menstrual cycle on determinants of energy balance: a putative role in weight loss attempts. International journal of obesity 2007;31(12):1777–85. doi: 10.1038/sj.ijo.0803699. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Shabat S, Fride E, Sheskin T, Tamiri T, Rhee MH, Vogel Z, Bisogno T, De Petrocellis L, Di Marzo V, Mechoulam R. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. European journal of pharmacology 1998;353(1):23–31. [DOI] [PubMed] [Google Scholar]
- 43.Costa B, Comelli F, Bettoni I, Colleoni M, Giagnoni G. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB(1), TRPV1 and PPARgamma receptors and neurotrophic factors. Pain 2008;139(3):541–50. doi: 10.1016/j.pain.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Ramsden CE, Zamora D, Makriyannis A, Wood JT, Mann JD, Faurot KR, MacIntosh BA, Majchrzak-Hong SF, Gross JR, Courville AB, et al. Diet-induced changes in n-3- and n-6-derived endocannabinoids and reductions in headache pain and psychological distress. J Pain 2015;16(8):707–16. doi: 10.1016/j.jpain.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Artmann A, Petersen G, Hellgren LI, Boberg J, Skonberg C, Nellemann C, Hansen SH, Hansen HS. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochimica et biophysica acta 2008;1781(4):200–12. doi: 10.1016/j.bbalip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Wurtman RJ, Wurtman JJ. Brain serotonin, carbohydrate-craving, obesity and depression. Obesity research 1995;3 Suppl 4:477S–80S. [DOI] [PubMed] [Google Scholar]
- 47.VanderWeele TJ. Invited commentary: structural equation models and epidemiologic analysis. American journal of epidemiology 2012;176(7):608–12. doi: 10.1093/aje/kws213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.