Abstract
This study sought to evaluate the impact of prophylactic cranial irradiation (PCI) on the pattern of brain recurrence after radical treatment in patients with limited-disease small-cell lung cancer (LD-SCLC). Patients treated with radiotherapy and chemotherapy between January 2006 and December 2014 at a single institution were retrospectively examined. Radiotherapy was performed using accelerated hyperfractionated radiotherapy (twice daily, 45 Gy in 30 fractions) or conventional fractionated radiotherapy (once daily, 50 Gy in 25 fractions). The chemotherapy regimen consisted of intravenous platinum–etoposide. A total of 162 patients were included and the median follow-up duration was 38 months. Ninety-three patients underwent PCI, and the 3-year overall survival (OS) rates were 14% among patients without PCI and 41% among those with PCI (P < 0.001). The frequency of brain metastases as a first recurrence site (BMFR) was significantly lower among patients who underwent PCI, compared with those who did not (P = 0.002). The median time to the l of BMFR was significantly shorter among patients without PCI than among those with PCI (P = 0.012). In addition, 68% of the BMFR patients who did not undergo PCI exhibited five or more lesions, while only 12% of BMFR patients who did undergo PCI exhibited five or more lesions (P < 0.001). PCI had a significant positive impact on patient prognosis after radical treatment for LD-SCLC, and the difference in the number of, and time to the appearance of, BMFR between patients treated with PCI and those treated without PCI might affect the clinical outcomes.
Keywords: LD-SCLC, PCI, brain metastasis, recurrence
INTRODUCTION
Small-cell lung cancer (SCLC) accounts for ~13% of all lung cancer cases [1] and is characterized by aggressive growth and early dissemination, despite a favorable response to chemotherapy and radiotherapy [2]. The brain is a common site of metastasis for SCLC, and >50% of SCLC patients are at risk of developing brain metastases by 2 years [3]. Because the blood–brain barrier has been considered to protect the central nervous system from most cytotoxic agents, and SCLC is very radiosensitive, the role of prophylactic cranial irradiation (PCI) has been studied in several trials [4]. Auperin et al. [5] performed a meta-analysis analyzing clinical trials examining PCI and reported that PCI reduced the lifetime risk of brain metastases and improved the survival of patients with limited-disease SCLC (LD-SCLC). Since the publication of this meta-analysis, PCI has been considered the standard of care for LD-SCLC patients who exhibit a complete response after their initial treatment [6]. This standard has been specified in the National Comprehensive Cancer Network guidelines [7].
In recent clinical practice, PCI has been performed in patients who respond to combined chemotherapy and thoracic radiotherapy [8]. However, despite its proven survival benefit, PCI is often declined by patients and caregivers because of fears regarding long-term neurocognitive dysfunction [9]. Furthermore, the recurrence and progression of brain metastases after PCI is not uncommon, and the survival of these patients generally remains poor [10]. PCI has been shown by reduce the frequency of brain metastases to 16–25% among LD-SCLC patients [5, 11], but the pattern of brain metastases after PCI has not been sufficiently discussed. Therefore, in this study, we evaluated the differences in the patterns of brain recurrence among patients with LD-SCLC who did or did not receive PCI after radical chemoradiotherapy.
MATERIALS AND METHODS
Patients
This retrospective study included patients with LD-SCLC who responded to treatment, involving combined radical thoracic radiotherapy and chemotherapy, between January 2006 and December 2014 at our institute. Patients who received induction chemotherapy were included. The tumor diagnosis had been confirmed histologically in all of the patients. Clinical staging was performed according to the 7th Union for International Cancer Control TNM staging system using a computed tomography (CT) scan or brain magnetic resonance imaging (MRI). 18F-fluorodeoxyglucose positron emission tomography/computed tomography scans were performed as necessary. Limited-disease consisted of patients with disease confined to a single hemithorax with or without contralateral mediastinal and ipsilateral supraclavicular lymph node involvement.
All of the study participants provided informed, written consent. The study protocol was approved by the Research Ethics Committee of our institution (Reference number: 2017–289). The research was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments.
Treatment
Thoracic radiotherapy was performed using accelerated hyperfractionated radiotherapy (AHF, twice daily, 45 Gy in 30 fractions over 3 weeks) or conventional-fractionated radiotherapy (CF, once daily, 50 Gy in 25 fractions over 5 weeks). For concurrent chemoradiotherapy, either AHF or CF was adopted as a fractionated schema, but CF was performed in patients who received sequential chemoradiotherapy. Regarding the selection of AHF or CF in patients with concurrent chemotherapy, the regimen was selected based on the general condition of the patients and/or the size of the radiation field.
The initial plan was delivered with 6 MV photons using anterior–posterior opposed fields that included the primary tumor, and metastatic lymph nodes, including the mediastinum and supraclavicular region, but excluded the contralateral hilar nodes. A boost dose was delivered to the primary tumor and metastatic lymph nodes. The chemotherapy regimen consisted of intravenous platinum–etoposide. PCI was indicated for patients whose response was greater than a partial response based on the RECIST v.1.1 criteria [12]. Radiation therapy was administered once daily at a total dose of 25 Gy in 10 fractions over 2 weeks using 10 MV photons. Two opposed lateral beams were used by shielding the orbits with a customized block. The target volume was the whole brain, extending to the lower border of the second cervical vertebra.
Outcome and statistical analysis
Overall survival (OS) was defined as the time from the date of the start of chemotherapy until the date of death from any cause and was censored at the date of the last follow-up for surviving patients. Treatment efficacy and the first recurrence site were evaluated radiologically. Additional disease sites found on subsequent imaging within 1 month of this event were also defined as initial recurrences.
All the statistical analyses were performed using R software, version 3.4.0 (The R Foundation, Vienna, Austria). The survival curves were estimated using the Kaplan–Meier product limit method and were compared using a univariate log-rank analysis and a multivariate Cox regression analysis. The patient characteristics and patterns of recurrence were compared using a chi-square test and the Wilcoxon signed-rank test. All the tests were two-sided, and P values of <0.05 were considered statistically significant.
RESULTS
Survival and prognostic factors
A total of 162 patients were included in this study. The patient characteristics are shown in Table 1. The median follow-up duration for the surviving patients was 38 months (range, 6–105 months). Among the 123 patients who died, 104 patients died because of disease progression, 11 died because of unknown causes, and 8 died because of other causes. The initial response to treatment was a complete response in 38 patients, a good partial response in 33 patients, and a partial response in 91 patients. The prognostic factors for OS are shown in Table 2. Age, TNM stage, presence of pulmonary effusion, thoracic radiotherapy dose, time from the start of any treatment until the end of chest irradiation (SER), and use of PCI were significant factors affecting OS in the univariate log-rank analyses. TNM stage, thoracic radiotherapy dose, and use of PCI were significant factors affecting OS in the multivariate Cox regression analysis.
Table 1.
Patient characteristics
| Total (n = 162) | PCI (n = 93) | No PCI (n = 69) | P value | |
|---|---|---|---|---|
| Age | ||||
| Median(range) | 67.5 (23–85) | 65 (23–76) | 72 (28–85) | <0.001 |
| Gender | ||||
| Male | 130 (80%) | 74 (80%) | 56 (81%) | 0.802 |
| Female | 32 (20%) | 19 (20%) | 13 (19%) | |
| ECOG PS | ||||
| 0 | 71 (44%) | 40 (43%) | 31 (45%) | 0.808 |
| 1 or 2 | 91 (56%) | 53 (57%) | 38 (55%) | |
| Stage | ||||
| IIA | 13 (8%) | 13 (14%) | 10 (14%) | 0.926 |
| IIB | 10 (6%) | |||
| IIIA | 63 (39%) | 80 (86%) | 59 (86%) | |
| IIIB | 76 (47%) | |||
| Radiation dose | ||||
| 45 Gy AHF | 94 (58%) | 68 (73%) | 26 (38%) | <0.001 |
| 50 Gy CF | 68 (42%) | 25 (27%) | 43 (62%) | |
| SER | ||||
| ≥30 days | 95 (59%) | 43 (46%) | 52 (75%) | <0.001 |
Bold values mean statistical significance (P value <0.05).
ECOG PS = Eastern Cooperative Oncology Group Performance Status, SER = start of any treatment until the end of chest irradiation, AHF = accelerated hyperfractionated radiotherapy, CF = conventional fractionated radiotherapy, PCI = prophylactic cranial irradiation.
Table 2.
Prognostic factors on overall survival
| P value | HR (95% CI) | ||
|---|---|---|---|
| univariate | multivariate | ||
| Age ≥70 vs <70 | 0.019 | 0.241 | 1.27 (0.85–0.1.88) |
| Male vs female | 0.212 | ||
| ECOG PS 0 vs 1–2 | 0.222 | ||
| Stage II vs III | 0.024 | 0.031 | 0.51 (0.27–0.94) |
| Pulmonary effusion yes vs no | 0.012 | 0.515 | 1.21 (0.68–2.16) |
| AHF vs CF | <0.001 | 0.016 | 0.49 (0.27–0.88) |
| SER <30 vs >30 | 0.012 | 0.266 | 0.72 (0.40–1.29) |
| PCI yes vs no | <0.001 | 0.004 | 0.54 (0.36–0.82) |
Bold values mean statistical significance (P value <0.05).
ECOG PS = Eastern Cooperative Oncology Group Performance Status, AHF = accelerated hyperfractionated radiotherapy, CF = conventional fractionated radiotherapy, SER = start of any treatment until the end of chest irradiation, PCI = prophylactic cranial irradiation, 95% CI = 95% confidence interval.
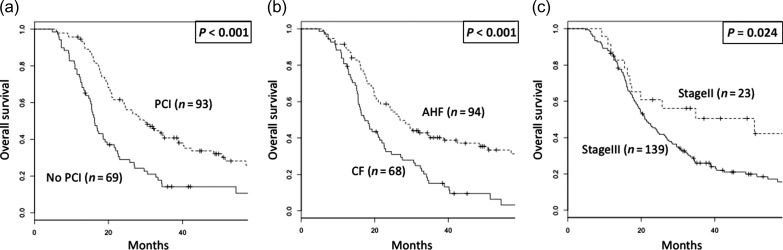
Ninety-three patients (57%) underwent PCI. The reasons for patients not undergoing PCI in the cohort of this study were as follows (21: deterioration of performance status, 15: patient refusal, 15: comorbidity or adverse event, 12: low cognitive function, 5: unknown, 1: history of brain irradiation). The 3-year OS rates were 14% (95% CI: 8–26%) in patients without PCI and 40% (95% CI: 31–52%) in those with PCI (P < 0.001, Fig. 1a). The 3-year OS rates were 15% (95% CI: 8–27%) in patients with CF and 40% (95% CI: 31–52%) in those with AHF (P < 0.001, Fig. 1b). The 3-year OS rates were 51% (95% CI: 33–77%) in patients with Stage II disease and 26% (95% CI: 19–35%) in those with Stage III disease (P = 0.024, Fig. 1c).
Fig. 1.
Overall survival of all patients stratified by (a) usage of prophylactic cranial irradiation, (b) fractionation schema of thoracic radiotherapy and (c) TNM stage. PCI = prophylactic cranial irradiation, AHF = accelerated hyperfractionated radiotherapy, CF = conventional fractionated radiotherapy.
One hundred and thirty-one (81%) patients underwent brain MRI at clinical staging (before initial chemoradiotherapy). One hundred and three (64%) patients were confirmed to have no brain metastases via MRI after initial chemoradiotherapy. The patients who underwent brain MRI both before and after initial chemoradiotherapy numbered 93 (57%), so additional analyses were carried out among these three patient groups (pre CRT MRI group, pre PCI MRI group, and pre CRT/PCI MRI group), and patients with PCI had significantly better OS than patients without PCI in all groups (P < 0.001, P < 0.001, P < 0.001, respectively).
Impact of recurrence pattern on prognosis
All sites of recurrence were observed in 125 patients (77%). In-field recurrence was observed in 47 patients (29%), and brain metastasis as a first recurrence site (BMFR) was observed in 45 patients (28%). Regarding the relationship between significant prognostic factors and the recurrence pattern, in-field recurrence was significantly higher among patients who were treated with CF compared with those treated with AHF, and the BMFR rate was significantly higher among patients who did not undergo PCI, compared with those who did (Table 3).
Table 3.
Pattern of recurrence
| PCI | No PCI | P value | AHF | CF | P value | Stage II | Stage III | P value | |
|---|---|---|---|---|---|---|---|---|---|
| (n = 93) | (n = 69) | (n = 94) | (n = 68) | (n = 23) | (n = 139) | ||||
| All recurrence | 68 (73%) | 57 (83%) | 0.155 | 65 (65%) | 60 (88%) | 0.004 | 14 (61%) | 111 (80%) | 0.045 |
| In-field recurrence | 30 (32%) | 17 (25%) | 0.291 | 21 (22%) | 26 (38%) | 0.028 | 3 (13%) | 44 (32%) | 0.068 |
| BMFR | 17 (18%) | 28 (41%) | 0.002 | 22 (23%) | 23 (34%) | 0.144 | 5 (22%) | 40 (29%) | 0.485 |
Bold values mean statistical significance (P value <0.05).
BMFR = brain metastasis as a first recurrence site, PCI = prophylactic cranial irradiation, AHF = accelerated hyperfractionated radiotherapy, CF = conventional fractionated radiotherapy.
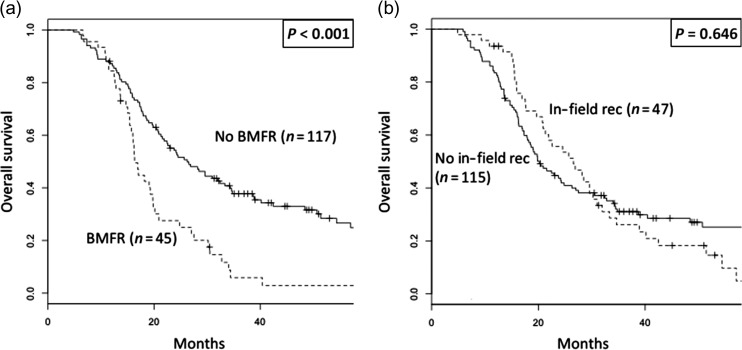
Regarding the impact of the recurrence pattern on OS, patients with BMFR exhibited a significantly lower OS than those without BMFR (3-year OS, 6% vs 38%, P < 0.001, Fig. 2a). However, the OS in patients who developed in-field recurrences was not significantly different from those who did not (3-year OS, 26% vs 31%, P = 0.646, Fig. 2b).
Fig. 2.
Overall survival of all patients stratified by (a) appearance of brain metastasis as a first recurrence site and (b) appearance of in-field recurrence. BMFR = brain metastasis as a first recurrence site, rec = recurrence.
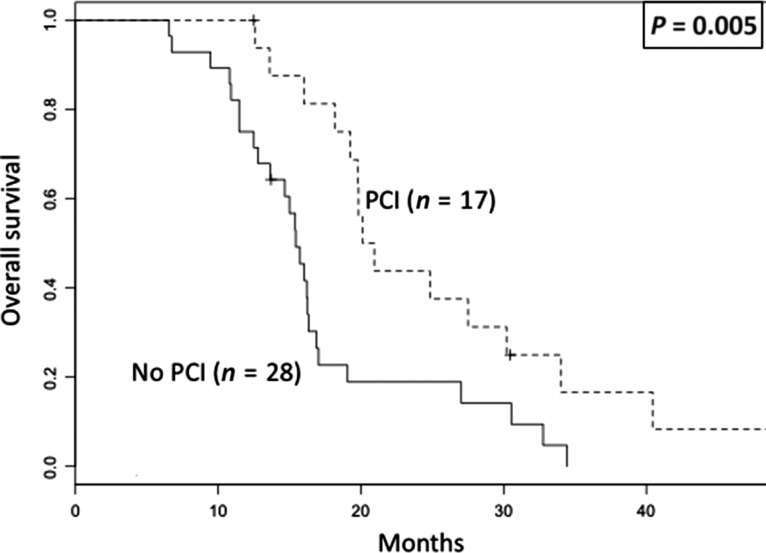
Among patients who developed BMFR, patients without PCI exhibited a significantly lower OS than those treated with PCI (3-year OS, 0% vs 17%, P = 0.005, Fig. 3). The BMFR pattern is shown in Table 4. The median time to the occurrence of BMFR was significantly shorter among patients who did not undergo PCI than among those who did (7.5 months vs 10 months, P = 0.012). Regarding the BMFR pattern, 68% of the BMFR patients who did not undergo PCI exhibited five or more lesions, while only 12% of BMFR patients who did undergo PCI exhibited five or more lesions (P < 0.001). The maximum lesion size was not different between the patients who underwent PCI and those who did not.
Fig. 3.
Overall survival of patients with brain metastasis as a first recurrence site stratified by usage of prophylactic cranial irradiation. PCI = prophylactic cranial irradiation.
Table 4.
Pattern of brain metastasis as a first recurrence site
| PCI (n = 17) | No PCI (n = 28) | P value | |
|---|---|---|---|
| Time to appearance (range), month | 10 (6–34) | 7.5 (4–31) | 0.012 |
| Number of metastatic lesions <5 | 15 (88%) | 9 (32%) | <0.001 |
| Median size (range), mm | 16 (4–43) | 19 (4–66) | 0.193 |
Bold values mean statistical significance (P value <0.05).
PCI = prophylactic cranial irradiation.
As a salvage radiotherapy after BMFR, stereotactic radiosurgery (SRS) was performed in 16 patients (12: patients with PCI, 4: patients without PCI). Whole-brain radiotherapy (WBRT) was performed in 29 patients, and all of them were patients who did not undergo PCI.
DISCUSSION
This retrospective study performed at a single institution investigated the difference in the pattern of brain recurrence in patients treated with or without PCI after radical chemoradiotherapy for LD-SCLC. The results showed that patients who underwent PCI had a statistically significant better prognosis and a lower frequency of BMFR than those who did not. Furthermore, significant differences in the number of metastatic lesions and the time to the occurrence of BMFR were observed between patients who underwent PCI and those who did not. The efficacy of cytotoxic chemotherapy for brain metastases has been unclear because of the inability of chemotherapy drugs to penetrate the blood–brain barrier. Therefore, the main treatment modalities for brain metastases are surgical resection or radiotherapy, including WBRT and SRS. Several reports have demonstrated that PCI reduces the frequency of [5, 11] and prolongs the time until the occurrence of brain metastases [8], similar to the results of the present study. However, the correlation between the pattern of BMFR and the use of PCI has not been previously reported. This is the first report to show that PCI reduces the number of BMFR after radical treatment for LD-SCLC.
In the present study, the significant prognostic factors consisted of not only the use of PCI, but also the total dose of thoracic radiotherapy and the TNM stage. In addition, BMFR was a significant negative predictor affecting OS. However, while the BMFR rates differed between patients who underwent PCI and those who did not, similar trends were not observed in terms of the dose of the thoracic radiotherapy and the TNM stage. The difference in the survival curves, especially the early phase of the survival curve, for OS was prominent when stratified according to the use of PCI (Fig. 1a), compared with that stratified according to the fractionation schema (CF vs AHF, Fig. 1b) or the TNM stage (Stage II vs Stage III, Fig. 1c). One explanation for this difference might be the difference in OS between patients with BMFR and those without BMFR (Fig. 2a), since PCI delayed the median time to occurrence of BMFR (7.5 months vs 10 months, P = 0.012) in addition to the incidence of BMFR. The Graded Prognostic Assessment (GPA) is a prognostic index for brain metastases from malignant tumors, and the GPA includes the number of brain lesions as a factor in this prognostic index [13]. The rationale for this index was to analyze corrected data from a prospective randomized clinical trial (RTOG 9508) that randomized patients to receive WBRT alone or WBRT plus SRS, and a statistically significant prognostic advantage was observed for patients with a single brain metastasis treated with WBRT plus SRS [14]. These results demonstrated that there was a statistically significant advantage for patients with a single brain metastasis who were treated with WBRT plus SRS. In addition, a disease-specific GPA reported by Sperduto et al. demonstrated that the number of brain metastases was a significant prognostic factor for patients with SCLC, non-small-cell lung cancer, melanoma, or renal cell cancer [15]. Considering the results of this retrospective study, a reduced number of BMFR may explain, at least in part, the improved prognosis enabled by the use of PCI in patients with LD-SCLC.
Forty-one of 69 patients without PCI didn’t develop BMFR in our study, so there may be patients who can avoid PCI. A recently published report suggested that PCI may not have a survival benefit in patients confirmed to have no brain metastases after initial therapy [16]. However, our study showed different results. Eze et al. [17] described a prognostic role of PCI in LS-SCLC and described a similarly significant magnitude of this benefit regardless of brain MRI after initial treatment, and this supported our results. There may be limits to imaging-guided evaluation for these conditions. Recently, research into liquid biopsy has been actively performed for lung cancer. Detection of tumor-associated mutations in cell-free DNA could potentially be used to identify the patients most likely to benefit from PCI, while sparing other patients from the toxicities associated with brain radiotherapy [18].
In this study, BMFR after PCI was observed in 18% (17/93) of all the patients and was a significant predictor of a poor prognosis. Salvage therapy for brain recurrence after PCI is important for maintaining the quality of life of patients. While WBRT is an option for brain recurrence after PCI, late toxicities associated with WBRT have been documented [9, 19]. Chang et al. reported that patients randomly assigned to receive SRS plus WBRT were significantly more likely to show a decline in learning and memory function at 4 months than those assigned to receive SRS alone (mean posterior probability of decline, 52% vs 24%) [20]. Several studies have also suggested that SRS could be a potentially effective and minimally invasive treatment option for brain metastasis after WBRT [21, 22]. Wegner et al. reported that the local control at 6 months among patients with SCLC who received salvage SRS after WBRT was 90%, and only 2.2% of these patients developed treatment-related toxicity [22]. In the present study, 88% of the patients who developed BMFR after PCI exhibited four or less metastatic lesions. This result suggests that SRS is the optimal treatment of choice and can enable satisfactory local control.
The present study had several limitations. First, the retrospective nature of this study may have, to some degree, caused a selection bias among the patients analyzed in the study. Second, the number of patients was limited because the study was performed at a single institution. In addition, the second-line chemotherapy regimens varied from one patient to another. However, we believe that the results of the present study provide useful information regarding the management of patients with LD-SCLC who develop brain metastases after PCI, since no previous reports have suggested that the use of PCI can reduce the number of metastatic lesions in patients with BMFR.
In conclusion, the results of this study indicated that PCI has a significant positive impact on patient prognosis after radical treatment for ld-SCLC, and the difference in the number of, and time to the appearance of, BMFR between patients treated with PCI and those treated without PCI might affect the clinical outcomes.
ACKNOWLEDGEMENTS
Part of this study was presented at the International Association for the Study of Lung Cancer 18th World Conference on Lung Cancer in Yokohama, Japan, 15–18 October 2017 (Abstract number: 9648).
CONFLICT OF INTEREST
The authors have stated that they have no conflicts of interest.
FUNDING
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan [JSPS KAKENHI; grant numbers 15K19838 and 16K10412], and a Health Science Research grant from the Ministry of Health and Welfare and the National Cancer Center Research and Development Fund [grant number 28-A-14].
REFERENCES
- 1. Govindan R, Page N, Morgensztern D et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the Surveillance, Epidemiologic, and End Results database. J Clin Oncol 2006;24:4539–44. [DOI] [PubMed] [Google Scholar]
- 2. Jackman DM, Johnson B. Small-cell lung cancer. Lancet 2005;366:1385–96. [DOI] [PubMed] [Google Scholar]
- 3. Seute T, Leffers P, ten Velde GPM et al. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer 2004;100:801–6. [DOI] [PubMed] [Google Scholar]
- 4. Meert AP, Paesmans M, Berghmans T et al. Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer 2001;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Auperin A, Arriagada R, Pignon JP et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476–84. [DOI] [PubMed] [Google Scholar]
- 6. Zhang W, Jiang W, Luan L et al. Prophylactic cranial irradiation for patients with small-cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer 2014;14:793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology. Small Cell Lung Cancer v2 2017 https://www.nccn.org/professionals/physician_gls/default.aspx (27 July 2018, date last accessed).
- 8. Tai P, Assouline A, Joseph K et al. Prophylactic cranial irradiation for patients with limited-stage small-cell lung cancer with response to chemoradiation. Clin Lung Cancer 2013;14:40–4. [DOI] [PubMed] [Google Scholar]
- 9. Le Pechoux C, Laplanche A, Favivre-Finn C et al. Clinical neurological outcome and quality of life among patients with limited small-cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99–01, EORTC 22003–08004, RTOG 0212 and IFCT 99–01). Ann Oncol 2011;22:1154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramlov A, Tietze A, Khalil AA et al. Prophylactic cranial irradiation in patients with small cell lung cancer. a retrospective study of recurrence, survival and morbidity. Lung Cancer 2012;77:561–6. [DOI] [PubMed] [Google Scholar]
- 11. Arriagada R, Le Chevalier T, Riviere A et al. Patterns of failure after prophylactic cranial irradiation in small-cell lung cancer: analysis of 505 randomized patients. Ann Oncol 2002;13:748–54. [DOI] [PubMed] [Google Scholar]
- 12. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 13. Sperduto PW, Berkey B, Gaspar LE et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008;70:510–4. [DOI] [PubMed] [Google Scholar]
- 14. Andrews DW, Scott CB, Sperduto PW et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665–72. [DOI] [PubMed] [Google Scholar]
- 15. Sperduto PW, Chao ST, Sneed PK et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 2010;77:655–61. [DOI] [PubMed] [Google Scholar]
- 16. Mamesaya N, Wakuda K, Omae K et al. Efficacy of prophylactic cranial irradiation in patients with limited-disease small-cell lung cancer who were confirmed to have no brain metastasis via magnetic resonance imaging after initial chemoradiotherapy. Oncotarget 2018;9:17664–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eze C, Roengvoraphoj O, Niyazi M et al. Treatment response and prophylactic cranial irradiation are prognostic factors in a real-life cohort comprehensively staged with cranial magnetic resonance imaging. Clin Lung Cancer 2016;18:243–9. [DOI] [PubMed] [Google Scholar]
- 18. Almodovar K, Iams WT, Meador CB et al. Longitudinal cell-free DNA analysis in patients with small cell lung cancer reveals dynamic insights into treatment efficacy and disease relapse. J Thorac Oncol 2018;13:112–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kundapur V, Elichuk T, Ahmed S et al. Risk of hippocampal metastases in small cell lung cancer patients at presentation and after cranial irradiation: a safety profile study for hippocampal sparing during prophylactic or therapeutic cranial irradiation. Int J Radiat Oncol Biol Phys 2015;91:781–6. [DOI] [PubMed] [Google Scholar]
- 20. Chang EL, Wefel JS, Hess KR et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole brain irradiation: a randomized controlled study. Lancet Oncol 2009;10:1037–44. [DOI] [PubMed] [Google Scholar]
- 21. Harris S, Chan MD, Lovato JF et al. Gamma Knife stereotactic radiosurgery as salvage therapy after failure of whole-brain radiotherapy in patients with small-cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wegner RE, Oslon AC, Kondziolka D et al. Stereotactic radiosurgery for patients with brain metastases from small cell lung cancer. Int J Radiat Oncol Biol Phys 2011;81:21–7. [DOI] [PubMed] [Google Scholar]