Abstract
Objectives
The aim of this study was to investigate the mechanism of linezolid resistance and evaluate the risk factors for linezolid-resistant Enterococcus faecalis (LZR-Efa) infections.
Methods
A total of 730 E. faecalis isolates were collected, and whole-genome sequencing and bioinformatics analysis were performed. Meanwhile, risk factors related to linezolid resistance were analyzed by binary logistic regression.
Results
Twenty-six LZR-Efa were isolated from various clinical samples, and 24 isolates were multidrug resistant. Four isolates were daptomycin nonsusceptible, while all LZR-Efa were susceptible to vancomycin. Thirteen different sequence types (STs) were identified, and the most prevalent type was ST16 (23.1%). The genes dfrE, lsaA, and emeA were identified in all isolates. A total of 23 E. faecalis were positive for optrA gene, and six amino acids mutations were identified among 18 LZR-Efa in OptrA. The 23S rRNA mutation was found in 16 LZR-Efa isolates. However, the presence of cfr was not identified. Furthermore, there were 41 virulence genes detected, and 10 genes (ace, bopD, cpsA, cpsB, ebpB, ebpC, efaA, fss1, fss2, and srtC) were found in all isolates. A total of nine isolates were positive for multiple virulent factors (ace, asa1, cylA, efaA, esp, and gelE). There was no difference in the number of virulence factors among different specimens (P=0.825). It is of note that all patients had not been prescribed linezolid or traveled abroad previously. Moreover, previous use of carbapenems was a risk factor for LZR-Efa infections.
Conclusion
The main trends of LZR-Efa, with lower level of resistance, were sporadic mainly in the department of surgery. optrA and 23S rRNA were the main resistance mechanisms. In addition, carbapenems use was an independent predictor of LZR-Efa infections.
Keywords: linezolid, resistance mechanism, virulent factors, risk factors
Introduction
Enterococcus has become one of the most important opportunistic pathogens leading to nosocomial infections, posing significant challenge to clinicians. The vast majority of clinical enterococcal infections in humans are caused by Enterococcus faecalis and E. faecium. E. faecium, much less frequently isolated than E. faecalis, has a higher incidence of resistance to multiple antimicrobial agents.1 According to the Zyvox Annual Appraisal of Potency and Spectrum (ZAAPS) program data, ampicillin, erythromycin, levofloxacin, and teicoplanin resistance rates for E. faecalis and E. faecium were 0% and 91.8%, 53.7% and 88.4%, 25.4% and 90.8%, and 0.4% and 14.7%, respectively.2 In 2016, the European Union and European Economic Area population-weighted mean percentage for high-level gentamicin resistance in E. faecalis was 30.5%, with national percentages ranging from 12.5% to 56.3%.3 It is of note that the number of vancomycin-resistant Enterococcus (VRE) has been increasing, resulting in the limited selection for treatment. Vega and Dowzicky showed that 40.8% (235/576) E. faecium and 1.6% (33/2,004) E. faecalis isolates were vancomycin-resistant.4
Linezolid was approved for management of VRE infections by the American Food and Drug Administration in 2000. Then linezolid-resistant Enterococcus was reported 1 year later. According to the ZAAPS and Linezolid Experience and Accurate Determination of Resistance program, the proportion of linezolid resistance in Enterococcus was 0.22% (21/9,417) and 0.78% (67/8,604), respectively. The main resistance mechanisms included alterations in the ribosomal proteins L3 and/or L4, mutations in domain V of the 23S rRNA, and/or presence of cfr or optrA gene. Resistance to linezolid in VRE is a result of decreased binding due to mutations at the 23S rRNA or acquisition of a cfr gene through horizontal transmission.5,6
The treatment and clinical outcomes regarding linezolid-resistant Enterococcus were limited. Older age, male gender, renal impairment, liver disease, diabetes, solid organ, and hematologic transplant have all been identified as risk factors for enterococcal blood stream infections in nonselected observational cohort studies. In addition, Billington et al7 found that E. faecalis infections were associated with a urinary focus, genitourinary malignancy, and abnormal genitourinary anatomy. However, risk factors for linezolid resistance reported in the literature have been inconsistent.8 The aim of this study was to evaluate the epidemiological, virulence factors, antibiotic resistance mechanism, and clinical profiles of LZR-Efa infections, gaining insights into control and prevention of resistance transmission.
Methods
Bacterial strains and clinical data
A total of 730 E. faecalis isolates were collected from patients hospitalized at The First Affiliated Hospital of Zhejiang University from January 2011 to December 2015. Species identification was conducted by API20 (bioMérieux, Durham, NC, USA) and MALDI-TOF technique (Bruker Diagnostics, Bremen, Germany). Linezolid susceptibility was screened via agar dilution method to determine minimal inhibitory concentration (MIC). The isolates with MIC of linezolid ≥8 mg/L were frozen at −80°C for further analysis.
The LZR-Efa infection group was compared with the linezolid-sensitive E. faecalis (LZS-Efa) infection group to evaluate the risk factors for linezolid resistance. The two groups were matched for age, same sex, specimen sources, and department (ratio: 1:2) from the same collection year. The medical records of patients were reviewed by two researchers. The included data were as follows: demographics, clinical characteristics (underlying diseases, comorbidities, invasive procedures, surgical procedures), laboratory examination, treatment history, hospitalization, and clinical outcomes. Antimicrobial drug exposure referred to the use of antibiotics for more than 72 hours at any point 2 weeks prior to diagnosis.
The work was in accordance with the Declaration of Helsinki. This study was approved by the recommendations of the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University, with reference number 2018502. To protect personal privacy, identifying information of each patient in the electronic database was encrypted. Informed consent was waived by the Clinical Research Ethics Committee because no intervention was involved and no patient-identifying information was included.
Antibiotic susceptibility test
Antibiotic agents (oxacillin, cefazolin, cefuroxime, clindamycin, erythromycin, levofloxacin, moxifloxacin, tetracycline, tigecycline, amikacin, fosfomycin, trimethoprim-sulfamethoxazole, linezolid, vancomycin, daptomycin, rifampin) were purchased from Dalian Meilun Biotech (Dalian, China). Glucose-6-phosphate was obtained from Sigma-Aldrich Co. (St Louis, MO, USA). Tigecycline and daptomycin susceptibility test were done via broth dilution method according to CLSI recommendations. The MICs of the other 15 antibiotics were determined using the twofold serial agar dilution method.9 E. faecalis ATCC 29212 were used as quality-control strains.
Multilocus sequence typing (MLST)
MLST was performed on all LZR-Efa isolates using the scheme of Institute Pasteur, and the sequence types (STs) were assigned using the MLST database (http://pubmlst.org/ecloacae). A minimal spanning tree was generated by using BioNumerics v7.0 to provide a graphical representation of the clonal distribution of LZR-Efa.
Whole-genome sequencing (WGS) and data analysis
WGS was carried out for 26 LZR-Efa with further analysis of gene environment. Genomic DNA was extracted by QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) and sequenced using HiSeq 2000 (Illumina, San Diego, CA, USA) with constructing 2×125 bp paired-end libraries. De novo assembly was done using the CLC Workbench v8.0 (QIAGEN, Hilden, Germany). The resistance genes were identified by BLAST against the ResFinder 2.1 database (https://cge.cbs.dtu.dk/services/ResFinder/). The bioinformatics tools used in this study were available at the following web platforms: National Center for Biotechnological Information, Sequence Manipulation Suite, and European Bioinformatics Institute.
This Whole Genome Shotgun BioProject for LZR-Efa has been deposited at GenBank under the accession QNGG00000000–QNHF00000000 (Table S1).
Statistical analysis
Continuous variables were presented as mean ± SD (x±SD), and independent samples t-tests were used for comparisons. Data with non-normal distribution were expressed as medians with corresponding IQRs and compared using the Wilcoxon rank-sum test. Categorical variables presented as numbers and percentages and compared percentages using the chi-squared test. For multivariate analysis, binary logistic regression was used to identify risk factors. A two-tailed P-values <0.05 were considered to be statistically significant. Data analysis was performed using SPSS Statistics for Windows, version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Detection of linezolid-resistant clinical isolates and their clinical characteristics
Among 730 clinical E. faecalis isolates over the study period, 26 (3.5%, 26/730) were resistant to linezolid. The incidences of LZR-Efa were 0%–1.2%, 4.4%–5.07%, and 2.5% from 2011 to 2015. The patients ranged in age from 18 to 70 years (Table 1). There were 11 males and 15 females, 4 of whom were outpatients. These samples obtained from culture included urine (n=13), ascites (n=5), bile (n=3), fester (n=3), and blood (n=2). The patients were from multiple cities in the Zhejiang Province, China. Medical records were reviewed, and patient histories confirmed that they had not been prescribed linezolid or traveled abroad previously. With the exception of one patient who died of multiple organ dysfunction syndrome, all the other patients recovered.
Table 1.
Clinical features of LZR-Efa
| Isolates | Sources | Ward | Age, y | Sex | Diagnosis | Prognosis | Region | Job | Year |
|---|---|---|---|---|---|---|---|---|---|
| 6,888 | Urine | Outpatients | 24 | F | Urinary infection | Improvement | Hangzhou | Worker | 01/2012 |
| 8,714 | Blood | 6B-17 | 62 | F | Hepatolithiasis | Improvement | Jinhua | Retired | 01/2013 |
| 10,938 | Fester | 5-1A | 62 | M | Metastatic carcinoma of bladder | Improvement | Huzhou | Worker | 05/2013 |
| 11,340 | Ascites | 6B-18 | 68 | F | Hepatocholangiocarcinoma | Improvement | Huzhou | Retired | 06/2013 |
| 11,382 | Urine | Outpatients | 41 | F | Urinary infection | Improvement | Hangzhou | Worker | 06/2013 |
| 12,654 | Bile | 6B-18 | 59 | M | Hepatolithiasis | Improvement | Huzhou | Farmer | 08/2013 |
| 13,470 | Blood | 3-4 | 67 | M | Nephrostomy | Improvement | Jiaxing | Farmer | 09/2013 |
| 13,484 | Fester | 7-2 | 65 | F | Mammary cancer | Improvement | Hangzhou | Farmer | 09/2013 |
| 14,980 | Urine | 3-4 | 62 | M | Prostatic cancer | Improvement | Hangzhou | Retired | 11/2013 |
| 15,224 | Urine | 9-5 | 40 | F | Nephrotic syndrome | Improvement | Shaoxing | No | 12/2013 |
| 15,407 | Bile | 6B-18 | 51 | F | Hepatolithiasis | Improvement | Hangzhou | Worker | 12/2013 |
| 15,814 | Urine | 3-3 | 70 | M | Prostatic hyperplasia | Improvement | Wenzhou | Retired | 01/2014 |
| 17,838 | Urine | 10-5 | 64 | F | Renal calculus | improvement | Hangzhou | Retired | 03/2014 |
| 18,026 | Urine | 6B-7 | 32 | M | IgA nephropathy | Improvement | Hangzhou | Worker | 04/2014 |
| 19,663 | Urine | Outpatients | 23 | F | Urinary infection | Improvement | Hangzhou | Worker | 06/2014 |
| 19,910 | Fester | 3-2 | 18 | M | Sacroiliac disease | Improvement | Hangzhou | Student | 07/2014 |
| 23,903 | Ascites | 6B-12 | 60 | F | Pancreatic cancer | Improvement | Jiaxing | Retired | 07/2014 |
| 23,967 | Bile | 6A-4 | 63 | M | Acute obstructive suppurative cholangitis | Improvement | Shaoxing | Worker | 07/2014 |
| 24,393 | Urine | 10-5 | 57 | F | Ureteral calculus | Improvement | Shaoxing | Farmer | 08/2014 |
| 26,167 | Urine | 5-2 | 37 | F | Renal calculus | Improvement | Hangzhou | Worker | 11/2014 |
| 27,149 | Ascites | 9-3 | 65 | M | HIV | Died | Shaoxing | Farmer | 12/2014 |
| 27,451 | Urine | Outpatients | 46 | F | Urinary infection | Improvement | Hangzhou | Worker | 01/2015 |
| 31,890 | Urine | 10-1 | 69 | M | Urethral stricture | Improvement | Shaoxing | Retired | 07/2015 |
| 32,142 | Urine | 6A-8 | 64 | F | Renal calculus | Improvement | Jinhua | Retired | 07/2015 |
| 32,633 | Ascites | 7-2 | 68 | F | Malignant tumor of abdominal wall | Improvement | Ningbo | Farmer | 08/2015 |
| 33,710 | Ascites | 5-1 | 41 | M | Colonic tumor | Improvement | Hangzhou | Worker | 09/2015 |
Abbreviations: F, female; LZR-Efa, linezolid-resistant Enterococcus faecalis; M, male.
Characteristics of the antibiotic susceptibility and sequence types (STs)
The susceptibility test results for the 26 strains are shown in Table 2. All isolates were resistant to cefazolin, cefuroxime, clindamycin, and amikacin, whereas 46.1% (12/26), 69.2% (18/26), 69.2% (18/26), 73.1% (19/26), 84.6% (22/26), 88.5% (23/26), and 92.3% (24/26) of strains were resistant to oxacillin, trimethoprim-sulfamethoxazole, moxifloxacin, levofloxacin, tetracycline, erythromycin, and tigecycline, respectively. Rifampin resistance was seen in 30.8% (8/26) of the isolates. Only one strain showed intermediate sensitivity to fosfomycin. However, 15.4% (4/26) isolates were daptomycin nonsusceptible. In total, all these linezolid-resistant isolates retained susceptibility to vancomycin. Besides 12,654 and 24,393, other isolates were multidrug-resistant LZR-Efa.
Table 2.
The antibiotic susceptibility and resistance mechanism of 26 LZR-Efa
| Isolates | OXA | CFZ | CXM | ERY | CLI | LVX | MXF | TC | TGC | AMK | FM | SXT | LZ | VAN | DAP | RIF | MLST | optrA | 23S rRNA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6,888 | 8 | 32 | 32 | >32 | >32 | 1 | 0.5 | >32 | 1 | >32 | 64 | >8/152 | 8 | 1 | 8 | 2 | 16 | Ile86Arg, Glu238Lys, Pro463Thr | – |
| 8,714 | 8 | 16 | 8 | 1 | 8 | 2 | 1 | 1 | 0.25 | >32 | 32 | 0.25/4.75 | 8 | 1 | 8 | >4 | 674 | NA | Glu26Gln, Leu221Val, Glu260Lys |
| 10,938 | 8 | 32 | 32 | >32 | >32 | >32 | 32 | >32 | 1 | >32 | 32 | >8/152 | 8 | 1 | 4 | 2 | 16 | – | – |
| 11,340 | 8 | 32 | >32 | >32 | >32 | >32 | 32 | >32 | 0.5 | >32 | 64 | >8/152 | 16 | 1 | 4 | 1 | 476 | – | – |
| 11,382 | 8 | 32 | 16 | >32 | >32 | >32 | 16 | >32 | 1 | >32 | 64 | <0.015/0.25 | 8 | 1 | 2 | 1 | 856a | Asp158Tyr, Pro463Thr | Leu156Phe, Arg172Gln |
| 12,654 | 8 | 16 | 16 | 2 | 32 | 2 | 1 | 1 | 0.5 | >32 | 32 | 2/38 | 8 | 2 | 2 | >4 | 857a | NA | Val30Leu, Gly57Ser, Pro236Ser, Ile269Val |
| 13,470 | 16 | 32 | >32 | >32 | >32 | >32 | 16 | >32 | 0.5 | >32 | 64 | >8/152 | 8 | 1 | 4 | 1 | 480 | Asp158Tyr, Pro463Thr | Thr95Ile, Glu260Lys |
| 13,484 | 16 | 32 | >32 | >32 | >32 | 4 | 1 | >32 | 1 | >32 | 32 | >8/152 | 8 | 2 | 8 | 2 | 21 | Asp158Tyr, Pro463Thr | Val30Leu, Gly57Ser, Pro236Ser, Ile269Val |
| 14,980 | 16 | 32 | >32 | >32 | >32 | >32 | 16 | >32 | 0.5 | >32 | 64 | >8/152 | 8 | 1 | 4 | 0.5 | 585 | – | Arg172Gln, Pro236Ser |
| 15,224 | 8 | 16 | >32 | >32 | >32 | 32 | 16 | 32 | 0.5 | >32 | 32 | >8/152 | 8 | 1 | 2 | 1 | 16 | Asp158Tyr, Pro463Thr | – |
| 15,407 | 8 | 16 | 32 | >32 | >32 | 1 | 0.25 | 1 | 0.25 | >32 | 64 | 0.25/4.75 | 8 | 0.5 | 4 | 2 | 479 | NA | Pro81Ser, Ala177Ser |
| 15,814 | 8 | 32 | 16 | >32 | >32 | 1 | 0.5 | >32 | 1 | >32 | 32 | >8/152 | 8 | 1 | 4 | 1 | 16 | Ile86Arg, Glu238Lys, Pro463Thr | – |
| 17,838 | 16 | 32 | >32 | >32 | >32 | 32 | 8 | >32 | 0.5 | >32 | 8 | 0.25/4.75 | 8 | 1 | 4 | 2 | 480 | – | Thr95Ile, Glu260Lys |
| 18,026 | 16 | 32 | >32 | >32 | >32 | 32 | 16 | >32 | 0.5 | >32 | 32 | 0.0315/0.59375 | 8 | 1 | 4 | 4 | 476 | – | – |
| 19,663 | 16 | 32 | >32 | >32 | >32 | 32 | 8 | >32 | 0.5 | >32 | 64 | >8/152 | 8 | 1 | 2 | 2 | 480 | Asp158Tyr, Pro463Thr | Thr95Ile, Glu260Lys |
| 19,910 | 16 | 32 | >32 | >32 | >32 | 16 | 4 | >32 | 0.5 | >32 | 32 | >8/152 | 8 | 2 | 4 | >4 | 116 | Asp158Tyr, Pro463Thr | Arg172Gln |
| 23,903 | 16 | 32 | >32 | >32 | >32 | >32 | 16 | >32 | 0.5 | >32 | 64 | >8/152 | 8 | 1 | 4 | 0.5 | 585 | Pro463Thr, Ile604Met | Arg172Gln, Pro236Ser |
| 23,967 | 16 | 32 | >32 | >32 | >32 | >32 | 16 | >32 | 0.5 | >32 | 64 | >8/152 | 8 | 1 | 4 | 0.5 | 585 | Pro463Thr, Ile604Met | Arg172Gln, Pro236Ser |
| 24,393 | 8 | 16 | 16 | 2 | 32 | 1 | 0.5 | >32 | 0.5 | >32 | 32 | 0.0625/1.1875 | 16 | 1 | 4 | 4 | 81 | Asp158Tyr, Pro463Thr | Glu154Gln, Pro236Ser, Ile269Val |
| 26,167 | 8 | 16 | 16 | >32 | >32 | 1 | 0.5 | >32 | 1 | >32 | 64 | <0.015/0.25 | 8 | 1 | 2 | 2 | 619 | Asp158Tyr, Pro463Thr | Glu154Gln, Pro236Ser, Ile269Val |
| 27,149 | 8 | 32 | 32 | >32 | >32 | >32 | 32 | 1 | 0.5 | >32 | 32 | >8/152 | 8 | 1 | 4 | 2 | 858a | Asp158Tyr, Pro463Thr | – |
| 27,451 | 16 | 32 | >32 | >32 | >32 | >32 | 16 | >32 | 0.5 | >32 | 32 | >8/152 | 8 | 1 | 4 | 0.25 | 585 | – | Arg172Gln, Pro236Ser |
| 31,890 | 16 | 32 | >32 | >32 | >32 | >32 | 32 | >32 | 0.5 | >32 | 32 | >8/152 | 8 | 1 | 8 | 4 | 585 | Pro463Thr, Ile604Met | Arg172Gln, Pro236Ser |
| 32,142 | 8 | 32 | 16 | >32 | >32 | >32 | 32 | >32 | 0.5 | >32 | 128 | >8/152 | 8 | 1 | 4 | 1 | 16 | Asp158Tyr, Pro463Thr | – |
| 32,633 | 16 | 32 | 32 | >32 | >32 | >32 | 32 | >32 | 0.5 | >32 | 32 | >8/152 | 8 | 1 | 4 | 1 | 476 | Asp158Tyr, Pro463Thr | – |
| 33,710 | 8 | 32 | 32 | >32 | >32 | >32 | 32 | >32 | 0.5 | >32 | 32 | >8/152 | 8 | 1 | 4 | 1 | 16 | Asp158Tyr, Pro463Thr | – |
Note:
New ST types.
Abbreviations: AMK, amikacin; CFZ, cefazolin; CLI, clindamycin; CXM, cefuroxime; DAP, daptomycin; ERY, erythromycin; FM, fosfomycin; LVX, levofloxacin; LZ, linezolid; LZR-Efa, linezolid-resistant Enterococcus faecalis; MLST, multilocus sequence typing; MXF, moxifloxacin; NA, not available; OXA, oxacillin; RIF, rifampin; SXT, trimethoprim-sulfamethoxazole; TC, tetracycline; TGC, tigecycline; VAN, vancomycin.
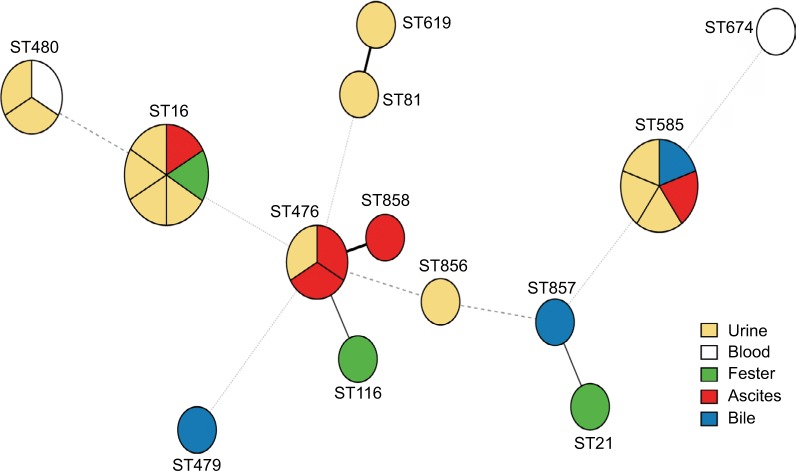
A total of 13 different STs were identified from 26 LZR-Efa isolates. The most prevalent type was ST16 (six isolates, 23.1%), followed by ST585 (five isolates, 19.2%), ST476 (three isolates, 11.5%), and ST480 (three isolates, 11.5%). Three new STs were identified (ST856–ST858) (Figure 1).
Figure 1.
Minimum spanning tree of LZR-Efa. Solid line indicates one allele difference and dashed line indicates differences in two alleles.
Abbreviation: LZR-Efa, linezolid-resistant Enterococcus faecalis.
Antibiotic resistance mechanism of LZREfa
In an attempt to link the phenotypic resistance data with genotypic evidence of resistance, we searched for mutations known to confer antimicrobial resistance. The genes dfrE, lsaA, and emeA were identified in all isolates (Table S2). Among the 26 LZR-Efa isolates examined in this study, 23 E. faecalis were positive for optrA gene. Six amino acids mutations were identified among the 18 LZR-Efa isolates, and of which Pro463Thr (463 tryptophan acid was changed to be threonine) was most frequently detected in isolates (Table 2). Both Pro463Thr and Asp158Tyr mutations of OptrA were the most abundant in LZR-Efa (12/18). Besides other two sites, Pro463Thr and Ile604Met mutations in three ST585 isolates, three mutational loci (Pro463Thr, Glu238Lys, and Ile86Arg) were found in two ST16 isolates. In addition, the 23S rRNA mutation was found in 16 LZR-Efa isolates (Table 2). It is of note that 10 isolates had both OptrA and 23S rRNA mutations. Mutations of specific amino acids were found in same type ST or ST with one allele difference. However, the presence of plasmid-borne ribosomal methyltransferase gene, cfr, was not identified in 26 LZR-Efa.
Characteristics of virulence genes
There were 41 virulence genes detected and 10 genes (ace, bopD, cpsA, cpsB, ebpB, ebpC, efaA, fss1, fss2, and srtC) were found in all isolates (Table S3). Of all the LZR-Efa isolates, 96.2% (25/26) were positive for ebpA, 88.5% (23/26) were positive for EF3023, 69.2% (18/26) were positive for nine genes (asa1, cpsC, cpsD, cpsE, cpsG, cpsH, cpsI, cpsJ, and cpsK), 69.2% (18/26) were positive for five genes (esp, fsrB, fsrC, gelE, and prgB/asc10), and 69.2% (18/26) were positive for three genes (cpsF, fsrA, and sprE). The hyl and agg genes were not detected in any LZR-Efa isolates. The bsh gene was found in 15,224 isolated from bile. A total of nine isolates were positive for multiple virulent factors (ace, asa1, cylA, efaA, esp, and gelE). There was no relationship between the number of virulence factors and clinical specimens (P=0.825).
Risk factors associated with the development of LZR-Efa
There were a total of 20 patients with LZR-Efa infections, and 40 patients with LZS-Efa were enrolled by reference to the matching criteria in the Methods section. Compared with patients with LZS-Efa, univariate analysis showed that those with LZR-Efa were more likely to exposure to carbapenem (P=0.007) (Table 3). The logistic regression analysis indicated that the previous use of carbapenems may be an independent risk factor for LZR-Efa infection (OR=6.631; 95% CI=1.489–29.522; P=0.013).
Table 3.
Risk factors for patients with LZR-Efa at univariate analysis
| LZR-Efa (n=20) | LZS-Efa (n=40) | P-value | |
|---|---|---|---|
| CP | 4 (20%) | 7 (17.5%) | 0.813 |
| CEP | 5 (25%) | 14 (35%) | 0.432 |
| BL/BLI | 7 (35%) | 8 (20%) | 0.206 |
| CAR | 7 (35%) | 3 (7.5%) | 0.007 |
| NIT | 2 (10%) | 6 (15%) | 0.591 |
| QUI | 6 (30%) | 6 (15%) | 0.171 |
| AMI | 2 (10%) | 4 (10%) | 1 |
| GLY | 2 (10%) | 1 (2.5%) | 0.209 |
| FM | 1 (5%) | 0 | 0.154 |
| SXT | 0 | 2 (5%) | 0.309 |
| Bowel preparation | 7 (35%) | 15 (37.5%) | 0.85 |
| Operation | 11 (55%) | 20 (50%) | 0.715 |
| Indwelling catheter | 9 (45%) | 20 (50%) | 0.715 |
| ICU | 2 (10%) | 1 (2.5%) | 0.209 |
Abbreviations: AMI, aminoglycoside; BL/BLI, β-lactams and β-lactamase inhibitors; CAR, carbapenems; CEP cephalosporin; CP, cephamicins; FM, fosfomycin; GLY, glycopeptide; ICU, intensive care unit; LZR-Efa, linezolid-resistant E. faecalis; LZS-Efa, linezolid-sensitive E. faecalis; NIT, nitromidazoles; QUI, quinolones; SXT, trimethoprimsulfamethoxazole.
Discussion
Since linezolid launched on the market, the detection rate of LZR-Efa and even the incidence of MDR are increasing.8 Although the prevalence of linezolid resistance is still low, emergence and dissemination of linezolid-resistant enterococci limit the therapeutic option for successful treatment of VRE infections. In the present study, with the analysis of LZR-Efa, we found MDR LZR-Efa were not spread by clonal strains. In addition, optrA and 23S rRNA were the main resistance mechanisms. Notably, all linezolid-resistant vancomycin-susceptible E. faecalis emerged without linezolid treatment. The virulence factors, associated with bacterial adherence, evasion of phagocytosis, and biofilm formation, were identified, while the stress response proteins were not found in any isolates. Furthermore, data from 60 patients were evaluated, and the results showed previous carbapenems exposure to be an independent risk factor for LZR-Efa infections.
The majority of LZR-Efa in our study was from urinary source, in keeping with prior observations.7 With an intrinsic and acquired resistance to some antimicrobial agents, enterococci have become important nosocomial pathogens.10 Although the prevalence of linezolid-resistant enterococci is currently very low, the incidence of LZR-Efa in the hospital setting, which was 3.6% (26/730) in this study, was higher than that in Europe.3 Of concern, 15.4% (4/26) isolates were daptomycin nonsusceptible. Note that, 1.8% (7/389) vancomycin-resistant E. faecium isolates were found to be resistant to linezolid in South Korea, which severely restricted the treatment options.11 Fortunately, all LZR-Efa isolates in our study were susceptible to vancomycin without creating a worse crisis in clinical treatment.
Overall, the clones of 26 LZR-Efa isolated in different time periods were diverse. The dominance of ST16 among our samples is somewhat consistent with prior studies in Malaysia and China.12 The same types of E. faecalis, ST16, ST27, ST116, ST256, ST476, ST480, and ST593, were detected in samples from animals and meat as well.13,14 The data presented here indicated that animal origin seemed to constitute a reservoir of LZR-Efa. Further investigations are needed to assess whether these LZR-Efa was indeed associated with the isolates from animals.
In the present study, optrA and 23S rRNA were the main resistance mechanisms for linezolid. The gene optrA could be horizontally transmitted among enterococci.5 In parallel, these surveillance data suggest that optrA may be disseminating in E. faecalis more rapidly than cfr in Staphylococcus aureus, implying a greater transferability of optrA.15 In addition, the optrA-positive incidence increased over time from 0.4% (1/262) in 2004–2005 to 3.9% (26/668) in 2013–2014.16 Previous studies found that optrA gene was more frequently seen in E. faecalis than in E. faecium and in food-producing animals than in humans (15.9% and 2%–2.9%, respectively).14,17 Interestingly, the optrA gene was detected in an E. faecium isolate 2 years before linezolid approval applications in China.16 Therefore, the species and geographies cross transmission might be one of the major reasons.
In general, the 23S rRNA mutation was considered as the most common mechanism of linezolid resistance. The 23S rRNA mutation rate of LZR-Efa was 61.5% (16/26), which is consistent with previous study.5 It has been demonstrated that 23S rRNA mutations could revert to a susceptible phenotype when selective drug pressure was removed, while rapid resistance selection emerged when selective pressure returned.18 Meanwhile, Alonso et al19 validated that the isolates with high linezolid MIC value were strongly related to the high number of 23S rRNA mutation copies. However, all LZR-Efa with 23S rRNA mutations showed low-level resistance in our study. And no isolates carried cfr gene in LZR-Efa. Although linezolid nonsusceptible clinical isolates almost exclusively harbored 23S rRNA alterations in the early 2000s, the detection rates of optrA and cfr were increasing in recent years. Therefore, the changes associated with linezolid resistance mechanisms are worthy of great concern.
Previous studies have demonstrated that some virulence factors play important roles in the pathogenesis of E. faecalis. Our present study found 10 virulence genes (ace, bopD, cpsA, cpsB, ebpB, ebpC, efaA, fss1, fss2, and srtC) in all isolates, and 9 isolates were positive for multiple virulent factors (ace, asa1, cylA, efaA, esp, and gelE). These virulence factors were mainly associated with bacterial adherence, evasion of phagocytosis, and biofilm formation. Several proteins, such as CYL and GelE, secreted into the extracellular medium have been implicated in enterococcal virulence. Meanwhile, cell-surface determinants, including the family of aggregation substance proteins (AS proteins), pilin, polysaccharides, polysaccharide antigen, have been reported to contribute to the large bacterial aggregations and biofilm formation. Esp, as one of AS proteins, are anchored to the cell wall, affect biofilm formation, and have a role in experimental urinary tract infections (UTIs) and/or endocarditis model.20,21 Capsular polysaccharide (cps) has a crucial role in pathogenesis, mediating evasion of phagocytosis by polymorphonuclear neutrophils, and stimulating cytokine production.22 Ebp pili are important for biofilm formation and for the pathogenesis of experimental endocarditis and UTIs.23 Zheng et al12 demonstrated a positive association between linezolid resistance in E. faecalis and robust biofilm formation. They found that the cylA gene was associated with weak biofilm formation, while the esp gene was only associated with strong or medium biofilm formation. It is of note that some stress response proteins (Gls, Npr, Ahp, Tpx), being important for virulence, were not identified in this present study. In addition, various putative enterococcal virulence determinants can also be found in strains colonizing the gastrointestinal tract of healthy individuals.24 The evidence of involvement of pathogenic factors still needs to be confirmed by further studies.
Usually, the emergence of linezolid-resistant enterococci was consistently related to prior linezolid exposure.8 Of the 55 studies on LZR-Efa, 14 (25.5%) reported the duration of linezolid treatment, with a mean of 29.8±48.8 days.5 Paradoxically, LZR-Efa could also develop in patients without prior exposure to linezolid in our study and previous studies. The similar studies suggested the presence of risk factors that predispose the linezolid resistance of the vancomycin-susceptible Enterococcus, such as exposure to other antibiotics, long-term hospitalization, and prolonged stay in the intensive care unit.5,25 Notably, we identified previous use of carbapenems as the only independent risk factor associated with LZR-Efa infections. After applying a logistic regression model, an independent risk factor for E. faecium acquisition was the previous use of carbapenems (OR=10.24; 95% CI=1.35–77.66).26 Other reports showed that the use of carbapenems is an independent predictor of E. faecium bacteremia.27 Empirical treatment with broad spectrum antibiotic may facilitate colonization and infection by depleting the gastrointestinal tract of its normal anaerobic flora. Therefore, we speculate that the acquisition of linezolid resistance among E. faecalis clinical isolates in the present study was not associated with the consumption of linezolid. It is possible that LZR-Efa colonization without symptoms in the intestinal tract served as a reservoir to be transmitted to other patients or changed into pathogenic bacteria.
The study has several limitations, including its retrospective form and the relatively small number of LZR-Efa. However, this is a real-life clinical experience providing useful suggestions to clinicians about the clinical characteristics of infections caused by LZR-Efa.
Conclusion
The results of our study showed that LZR-Efa were sporadic with low-level linezolid resistance, while multidrug resistance was quite serious. In addition, carbapenems use was an independent predictor of LZR-Efa infections. Therefore, it is important to implement infection-control practices against these resistant strains, with an emphasis on contact precautions and more careful use of antibiotics to prevent selection of resistance.
Supplementary materials
Table S1.
Essential information of whole-genome sequencing for 26 LZR-Efa
| Isolates | Accession | Clean reads | Clean bases | Length | Q30% | GC% |
|---|---|---|---|---|---|---|
| 6,888 | QNGG00000000 | 8,086,306 | 1,212,945,900 | 150 | 97.23 | 38.54 |
| 8,714 | QNGH00000000 | 8,063,042 | 1,209,456,300 | 150 | 97.22 | 38.57 |
| 10,938 | QNGI00000000 | 8,083,052 | 1,212,457,800 | 150 | 97.38 | 38.29 |
| 11,340 | QNGJ00000000 | 8,081,886 | 1,212,282,900 | 150 | 97.32 | 38.26 |
| 11,382 | QNGK00000000 | 8,087,010 | 1,213,051,500 | 150 | 97.46 | 38.54 |
| 12,645 | QNGL00000000 | 8,067,526 | 1,210,128,900 | 150 | 97.14 | 38.92 |
| 13,470 | QNGM00000000 | 8,084,470 | 1,212,670,500 | 150 | 97.49 | 38.73 |
| 13,484 | QNGN00000000 | 8,079,076 | 1,211,861,400 | 150 | 97.40 | 38.69 |
| 14,980 | QNGO00000000 | 8,084,456 | 1,212,668,400 | 150 | 97.25 | 38.12 |
| 15,224 | QNGP00000000 | 8,048,514 | 1,207,277,100 | 150 | 97.36 | 37.96 |
| 15,407 | QNGQ00000000 | 8,070,056 | 1,210,508,400 | 150 | 97.17 | 38.31 |
| 15,814 | QNGR00000000 | 8,076,626 | 1,211,493,900 | 150 | 97.33 | 38.58 |
| 17,838 | QNGS00000000 | 8,051,770 | 1,207,765,500 | 150 | 96.89 | 38.51 |
| 18,026 | QNGT00000000 | 8,060,562 | 1,209,084,300 | 150 | 97.45 | 38.67 |
| 19,663 | QNGU00000000 | 8,081,042 | 1,212,156,300 | 150 | 97.50 | 38.41 |
| 19,910 | QNGV00000000 | 8,094,698 | 1,214,204,700 | 150 | 96.19 | 38.03 |
| 23,903 | QNGW00000000 | 8,061,246 | 1,209,186,900 | 150 | 96.02 | 37.90 |
| 23,967 | QNGX00000000 | 8,103,376 | 1,215,506,400 | 150 | 96.26 | 38.14 |
| 24,393 | QNGY00000000 | 8,109,796 | 1,216,469,400 | 150 | 96.00 | 38.20 |
| 26,167 | QNGZ00000000 | 8,097,538 | 1,214,630,700 | 150 | 96.20 | 38.21 |
| 27,149 | QNHA00000000 | 8,116,074 | 1,217,411,100 | 150 | 96.26 | 38.84 |
| 27,451 | QNHB00000000 | 8,111,306 | 1,216,695,900 | 150 | 96.16 | 38.37 |
| 31,890 | QNHC00000000 | 8,085,996 | 1,212,899,400 | 150 | 96.18 | 38.19 |
| 32,142 | QNHD00000000 | 8,069,526 | 1,210,428,900 | 150 | 96.02 | 37.82 |
| 32,633 | QNHE00000000 | 8,104,740 | 1,215,711,000 | 150 | 96.22 | 38.21 |
| 33,710 | QNHF00000000 | 8,104,866 | 1,215,729,900 | 150 | 96.22 | 38.01 |
Abbreviation: LZR-Efa, linezolid-resistant Enterococcus faecalis.
Table S2.
Characteristics of resistance genes
| Isolates | dfrE (n=26) | lsaA (n=26) | emeA (n=26) | fexA (n=23) | ANT(9) -Ia (n=12) | tetO (n=20) | dfrG (n=19) | ErmB (n=19) | tetK (n=18) | APH(3′)-IIIa (n=17) | AAC(6′)-Ie-APH(2″)-Ia (n=17) | cat (n=17) | satNA4 (n=16) | ErmA (n=18) | lnuB (n=12) | lsaE (n=12) | aad(6) (n=9) | tetS (n=2) | tetM (n=2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6,888 | 99.3% | 98.8% | 97.7% | 98.9% | 100.0% | NA | 100.0% | NA | 99.8% | 100.0% | 100.0% | 97.2% | 99.4% | 85.2% | 100.0% | 99.8% | 100.0% | 99.6% | 95.7% |
| 8,714 | 100.0% | 99.2% | 97.7% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 10,938 | 99.3% | 98.8% | 97.7% | 98.9% | 100.0% | 95.6% | 100.0% | 100.0% | NA | 100.0% | 100.0% | NA | 98.9% | 85.2% | 100.0% | 100.0% | 100.0% | NA | NA |
| 11,340 | 100.0% | 98.8% | 97.7% | 98.9% | NA | 95.3% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | 100.0% | 99.4% | 85.2% | NA | NA | 100.0% | NA | NA |
| 11,382 | 100.0% | 99.4% | 97.7% | 98.9% | NA | 95.5% | NA | 100.0% | 99.8% | NA | NA | 97.2% | NA | 85.2% | NA | NA | NA | NA | NA |
| 12,645 | 99.3% | 98.2% | 97.7% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 13,470 | 99.3% | 99.6% | 97.7% | 98.9% | NA | 95.5% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | NA | 99.4% | 85.2% | NA | NA | 100.0% | NA | NA |
| 13,484 | 100.0% | 98.2% | 97.7% | 98.9% | NA | 90.9% | 100.0% | 100.0% | 95.2% | NA | 99.7% | 97.2% | NA | 85.2% | NA | NA | NA | NA | NA |
| 14,980 | 100.0% | 99.4% | 97.7% | 98.9% | 100.0% | 95.5% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | 97.2% | 99.4% | 85.2% | 100.0% | 100.0% | NA | NA | NA |
| 15,224 | 99.3% | 98.8% | 97.7% | 98.9% | NA | 95.6% | 100.0% | 100.0% | NA | NA | NA | 100.0% | NA | 85.2% | NA | NA | NA | NA | NA |
| 15,407 | 99.3% | 98.8% | 97.5% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 15,814 | 99.3% | 98.8% | 97.7% | 98.9% | 100.0% | NA | 100.0% | 99.2% | 99.8% | 100.0% | 100.0% | 97.2% | 99.4% | 85.2% | 100.0% | 100.0% | 100.0% | 99.6% | 95.7% |
| 17,838 | 99.3% | 99.6% | 97.7% | 98.9% | 100.0% | 95.5% | 99.4% | 100.0% | 99.8% | 100.0% | 100.0% | 100.0% | 99.4% | 85.2% | 100.0% | 100.0% | 100.0% | NA | NA |
| 18,026 | 100.0% | 98.8% | 97.7% | 98.9% | NA | 95.5% | NA | 99.6% | 99.8% | 100.0% | 98.0% | 97.2% | 99.4% | 85.2% | NA | NA | NA | NA | NA |
| 19,663 | 99.3% | 99.6% | 97.7% | 98.9% | 100.0% | 95.5% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | 100.0% | 99.4% | 85.2% | 100.0% | 99.8% | 100.0% | NA | NA |
| 19,910 | 100.0% | 99.0% | 97.7% | 98.9% | NA | 95.5% | 100.0% | NA | 99.8% | 100.0% | NA | NA | NA | 85.2% | NA | NA | NA | NA | NA |
| 23,903 | 100.0% | 99.4% | 97.7% | 98.9% | 100.0% | 95.5% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | 97.2% | 99.4% | NA | 100.0% | 100.0% | NA | NA | NA |
| 23,967 | 100.0% | 99.4% | 97.7% | 98.9% | 100.0% | 95.5% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | 97.2% | 99.4% | NA | 100.0% | 100.0% | NA | NA | NA |
| 24,393 | 99.3% | 99.6% | 97.7% | 98.9% | NA | 95.5% | NA | NA | 99.8% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 26,167 | 99.3% | 99.6% | 97.7% | 98.9% | NA | 95.5% | NA | 100.0% | 99.8% | 100.0% | 100.0% | NA | 99.4% | 85.2% | NA | NA | 100.0% | NA | NA |
| 27,149 | 100.0% | 98.8% | 97.7% | 98.9% | 100.0% | NA | 100.0% | NA | NA | 100.0% | 100.0% | 100.0% | 99.4% | 85.2% | 100.0% | 100.0% | NA | NA | NA |
| 27,451 | 100.0% | 99.4% | 97.7% | 98.9% | 100.0% | 95.5% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | 97.2% | 99.4% | NA | 100.0% | 100.0% | NA | NA | NA |
| 31,890 | 100.0% | 99.4% | 97.7% | 98.9% | 100.0% | 95.5% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | 97.2% | 99.4% | NA | 100.0% | 100.0% | NA | NA | NA |
| 32,142 | 99.3% | 98.8% | 97.5% | 98.9% | NA | 95.6% | 100.0% | 100.0% | NA | NA | NA | 100.0% | NA | 85.2% | NA | NA | NA | NA | NA |
| 32,633 | 100.0% | 98.8% | 97.7% | 98.9% | 100.0% | 95.3% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | NA | 99.4% | 85.2% | 100.0% | 100.0% | 100.0% | NA | NA |
| 33,710 | 99.3% | 98.8% | 97.5% | 98.9% | NA | 95.6% | 100.0% | 100.0% | NA | NA | NA | 100.0% | NA | 85.2% | NA | NA | NA | NA | NA |
Abbreviation: NA, not available.
Table S3.
Characteristics of virulence genes
| Genes | 6,888 (n=33) | 8,714 (n=28) | 10,938 (n=38) | 11,340 (n=24) | 11,382 (n=18) | 12,645 (n=17) | 13,470 (n=16) | 13,484 (n=18) | 14,980 (n=39) | 15,407 (n=21) | 15,224 (n=40) | 15,814 (n=34) | 17,838 (n=16) | 18,026 (n=25) | 19,663 (n=24) | 19,910 (n=27) | 23,903 (n=37) | 23,967 (n=37) | 24,393 (n=22) | 26,167 (n=22) | 27,149 (n=22) | 27,451 (n=37) | 31,890 (n=37) | 32,142 (n=37) | 32,633 (n=24) | 33,710 (n=35) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ace (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| asa1 (n=18) | + | − | + | + | − | − | + | − | + | − | + | + | + | + | + | − | + | + | − | + | − | + | + | + | + | + |
| bopD (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| bsh (n=1) | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| cpsA (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| cpsB (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| cpsC (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cpsD (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cpsE (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cpsF (n=17) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | − |
| cpsG (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cpsH (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cpsI (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cpsJ (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cpsK (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cylA (n=14) | + | − | + | − | − | − | − | − | + | − | + | + | − | − | + | − | + | + | + | + | − | + | + | + | − | + |
| cylB (n=11) | + | − | + | − | − | − | − | − | + | − | + | + | − | − | + | − | + | + | − | − | − | + | + | + | − | − |
| cylI (n=14) | + | − | + | − | − | − | − | − | + | − | + | + | − | − | + | − | + | + | + | + | − | + | + | + | − | + |
| cylL (n=10) | + | − | + | − | − | − | − | − | + | − | + | + | − | − | + | − | + | + | − | − | − | + | + | − | − | − |
| cylM (n=13) | + | − | + | − | − | − | − | − | + | − | + | + | + | − | + | − | + | + | − | − | − | + | + | + | − | + |
| cylR1 (n=12) | + | − | + | − | − | − | − | − | + | − | + | + | − | − | + | − | + | + | − | − | − | + | + | + | − | + |
| cylR2 (n=12) | + | − | + | − | − | − | − | − | + | − | + | + | − | − | + | − | + | + | − | − | − | + | + | + | − | + |
| cylS (n=12) | + | − | + | − | − | − | − | − | + | − | + | + | − | − | + | − | + | + | − | − | − | + | + | + | − | + |
| ebpA (n=25) | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| ebpB (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| ebpC (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| EF0149 (n=3) | − | − | − | − | − | − | − | − | + | − | − | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − |
| EF0485 (n=14) | + | − | + | + | − | − | − | − | + | − | + | + | − | + | + | + | + | − | − | − | + | + | + | − | + | |
| EF0818 (n=8) | − | + | − | − | − | + | + | + | − | + | + | − | − | − | − | − | − | − | + | + | − | − | − | − | − | − |
| EF3023 (n=23) | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | − | + | + | + | + | − | + | + | + | + |
| efaA (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| esp (n=17) | + | − | + | − | + | − | − | − | + | − | + | + | + | + | + | − | + | + | + | − | − | + | + | + | + | + |
| fsrA (n=16) | − | + | + | − | + | + | − | + | + | − | + | − | − | − | − | + | + | + | + | + | − | + | + | + | − | + |
| fsrB (n=17) | − | + | + | − | + | + | + | + | + | − | + | − | − | − | − | + | + | + | + | + | − | + | + | + | − | + |
| fsrC (n=17) | − | + | + | − | + | + | − | + | + | − | + | − | − | − | − | + | + | + | + | + | − | + | + | + | + | + |
| fss1 (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| fss2 (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| gelE (n=17) | − | + | + | − | + | + | + | + | + | − | + | − | − | − | − | + | + | + | + | + | − | + | + | + | − | + |
| prgB/asc10 (n=17) | + | + | + | + | − | − | − | − | + | − | + | + | + | − | + | + | + | − | + | + | + | + | − | + | − | + |
| sprE (n=16) | − | + | + | − | + | + | − | + | + | − | + | − | − | − | − | + | + | + | + | + | − | + | + | + | − | + |
| srtC (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
Acknowledgments
This work was supported by Department of Health of Zhejiang province (No. 2019RC003).
Footnotes
Author contributions
WY and YQ developed the concept and designed the experiments. MC, WY, YL, and ZW isolated bacteria and performed the laboratory measurements. HP and JZ collected and analyzed the epidemiological and clinical data from the patient records. HP and YQ gave conceptual advice. WY and YH wrote the paper. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Teixeira LM, Carvalho MGS, Shewmaker PL, Facklam RR. Enterococcus. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. Manual of Clinical Microbiology. 10th ed. Wahington, DC: ASM Press; 2011. p. 350e64. [Google Scholar]
- 2.Mendes RE, Deshpande L, Streit JM, et al. ZAAPS programme results for 2016: an activity and spectrum analysis of linezolid using clinical isolates from medical centres in 42 countries. J Antimicrob Chemother. 2018 Apr 6; doi: 10.1093/jac/dky099. Epub. [DOI] [PubMed] [Google Scholar]
- 3.Antimicrobial resistance surveillance in Europe. 2016. [Accessed October 26, 2018]. Available from: https://ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2016.
- 4.Vega S, Dowzicky MJ. Antimicrobial susceptibility among Gram-positive and Gram-negative organisms collected from the Latin American region between 2004 and 2015 as part of the Tigecycline Evaluation and Surveillance Trial. Ann Clin Microbiol Antimicrob. 2017;16(1):50. doi: 10.1186/s12941-017-0222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi R, Qin T, Fan W, Ma P, Gu B. The emerging problem of linezolid-resistant enterococci. J Glob Antimicrob Resist. 2018;13:11–19. doi: 10.1016/j.jgar.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Diaz L, Kiratisin P, Mendes RE, Panesso D, Singh KV, Arias CA. Transferable plasmid-mediated resistance to linezolid due to cfr in a human clinical isolate of Enterococcus faecalis. Antimicrob Agents Chemother. 2012;56(7):3917–3922. doi: 10.1128/AAC.00419-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billington EO, Phang SH, Gregson DB, et al. Incidence, risk factors, and outcomes for Enterococcus spp. blood stream infections: a population-based study. Int J Infect Dis. 2014;26:76–82. doi: 10.1016/j.ijid.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Smith TT, Tamma PD, Do TB, et al. Prolonged linezolid use is associated with the development of linezolid-resistant Enterococcus faecium. Diagn Microbiol Infect Dis. 2018;91(2):161–163. doi: 10.1016/j.diagmicrobio.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute Performance standards for antimicrobial susceptibility testing. 26th informational supplement. 2016. [Accessed January 2016]. Available from: http://www.clsi.org/
- 10.Miller WR, Munita JM, Arias CA. Mechanisms of antibiotic resistance in enterococci. Expert Rev Anti Infect Ther. 2014;12(10):1221–1236. doi: 10.1586/14787210.2014.956092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho SY, Kim HM, Chung DR, et al. Resistance mechanisms and clinical characteristics of linezolid-resistant Enterococcus faecium isolates: a single-centre study in South Korea. J Glob Antimicrob Resist. 2018;12:44–47. doi: 10.1016/j.jgar.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Zheng JX, Wu Y, Lin ZW, et al. Characteristics of and virulence factors associated with biofilm formation in clinical Enterococcus faecalis isolates in China. Front Microbiol. 2017;8:2338. doi: 10.3389/fmicb.2017.02338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammerum AM. Enterococci of animal origin and their significance for public health. Clin Microbiol Infect. 2012;18(7):619–625. doi: 10.1111/j.1469-0691.2012.03829.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Lv Y, Cai J, et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother. 2015;70(8):2182–2190. doi: 10.1093/jac/dkv116. [DOI] [PubMed] [Google Scholar]
- 15.Gawryszewska I, Żabicka D, Hryniewicz W, Sadowy E. Linezolid-resistant enterococci in Polish hospitals: species, clonality and determinants of linezolid resistance. Eur J Clin Microbiol Infect Dis. 2017;36(7):1279–1286. doi: 10.1007/s10096-017-2934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui L, Wang Y, Lv Y, et al. Nationwide surveillance of novel oxazolidinone resistance gene optrA in Enterococcus isolates in China from 2004 to 2014. Antimicrob Agents Chemother. 2016;60(12):7490–7493. doi: 10.1128/AAC.01256-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai J, Wang Y, Schwarz S, et al. Enterococcal isolates carrying the novel oxazolidinone resistance gene optrA from hospitals in Zhejiang, Guangdong, and Henan, China, 2010–2014. Clin Microbiol Infect. 2015;21(12):1095.e1–4. doi: 10.1016/j.cmi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Mendes RE, Deshpande LM, Jones RN. Linezolid update: stable in vitro activity following more than a decade of clinical use and summary of associated resistance mechanisms. Drug Resist Updat. 2014;17(1–2):1–12. doi: 10.1016/j.drup.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Alonso M, Marín M, Iglesias C, Cercenado E, Bouza E, García de Viedma D. Rapid identification of linezolid resistance in Enterococcus spp. based on high-resolution melting analysis. J Microbiol Methods. 2014;98:41–43. doi: 10.1016/j.mimet.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Heikens E, Singh KV, Jacques-Palaz KD, et al. Contribution of the enterococcal surface protein Esp to pathogenesis of Enterococcus faecium endocarditis. Microbes Infect. 2011;13(14–15):1185–1190. doi: 10.1016/j.micinf.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leendertse M, Heikens E, Wijnands LM, et al. Enterococcal surface protein transiently aggravates Enterococcus faecium-induced urinary tract infection in mice. J Infect Dis. 2009;200(7):1162–1165. doi: 10.1086/605609. [DOI] [PubMed] [Google Scholar]
- 22.Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10(4):266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh KV, Nallapareddy SR, Murray BE. Importance of the ebp (endocarditis- and biofilm-associated pilus) locus in the pathogenesis of Enterococcus faecalis ascending urinary tract infection. J Infect Dis. 2007;195(11):1671–1677. doi: 10.1086/517524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solheim M, Aakra A, Snipen LG, Brede DA, Nes IF. Comparative genomics of Enterococcus faecalis from healthy Norwegian infants. BMC Genomics. 2009;10:194. doi: 10.1186/1471-2164-10-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahim S, Pillai SK, Gold HS, Venkataraman L, Inglima K, Press RA. Linezolid-resistant, vancomycin-resistant Enterococcus faecium infection in patients without prior exposure to linezolid. Clin Infect Dis. 2003;36(11):E146–E148. doi: 10.1086/374929. [DOI] [PubMed] [Google Scholar]
- 26.Gudiol C, Ayats J, Camoez M, et al. Increase in bloodstream infection due to vancomycin-susceptible Enterococcus faecium in cancer patients: risk factors, molecular epidemiology and outcomes. PLoS One. 2013;8(9):e74734. doi: 10.1371/journal.pone.0074734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamada Y, Magarifuchi H, Oho M, et al. Clinical features of enterococcal bacteremia due to ampicillin-susceptible and ampicillin-resistant enterococci: an eight-year retrospective comparison study. J Infect Chemother. 2015;21(7):527–530. doi: 10.1016/j.jiac.2015.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Essential information of whole-genome sequencing for 26 LZR-Efa
| Isolates | Accession | Clean reads | Clean bases | Length | Q30% | GC% |
|---|---|---|---|---|---|---|
| 6,888 | QNGG00000000 | 8,086,306 | 1,212,945,900 | 150 | 97.23 | 38.54 |
| 8,714 | QNGH00000000 | 8,063,042 | 1,209,456,300 | 150 | 97.22 | 38.57 |
| 10,938 | QNGI00000000 | 8,083,052 | 1,212,457,800 | 150 | 97.38 | 38.29 |
| 11,340 | QNGJ00000000 | 8,081,886 | 1,212,282,900 | 150 | 97.32 | 38.26 |
| 11,382 | QNGK00000000 | 8,087,010 | 1,213,051,500 | 150 | 97.46 | 38.54 |
| 12,645 | QNGL00000000 | 8,067,526 | 1,210,128,900 | 150 | 97.14 | 38.92 |
| 13,470 | QNGM00000000 | 8,084,470 | 1,212,670,500 | 150 | 97.49 | 38.73 |
| 13,484 | QNGN00000000 | 8,079,076 | 1,211,861,400 | 150 | 97.40 | 38.69 |
| 14,980 | QNGO00000000 | 8,084,456 | 1,212,668,400 | 150 | 97.25 | 38.12 |
| 15,224 | QNGP00000000 | 8,048,514 | 1,207,277,100 | 150 | 97.36 | 37.96 |
| 15,407 | QNGQ00000000 | 8,070,056 | 1,210,508,400 | 150 | 97.17 | 38.31 |
| 15,814 | QNGR00000000 | 8,076,626 | 1,211,493,900 | 150 | 97.33 | 38.58 |
| 17,838 | QNGS00000000 | 8,051,770 | 1,207,765,500 | 150 | 96.89 | 38.51 |
| 18,026 | QNGT00000000 | 8,060,562 | 1,209,084,300 | 150 | 97.45 | 38.67 |
| 19,663 | QNGU00000000 | 8,081,042 | 1,212,156,300 | 150 | 97.50 | 38.41 |
| 19,910 | QNGV00000000 | 8,094,698 | 1,214,204,700 | 150 | 96.19 | 38.03 |
| 23,903 | QNGW00000000 | 8,061,246 | 1,209,186,900 | 150 | 96.02 | 37.90 |
| 23,967 | QNGX00000000 | 8,103,376 | 1,215,506,400 | 150 | 96.26 | 38.14 |
| 24,393 | QNGY00000000 | 8,109,796 | 1,216,469,400 | 150 | 96.00 | 38.20 |
| 26,167 | QNGZ00000000 | 8,097,538 | 1,214,630,700 | 150 | 96.20 | 38.21 |
| 27,149 | QNHA00000000 | 8,116,074 | 1,217,411,100 | 150 | 96.26 | 38.84 |
| 27,451 | QNHB00000000 | 8,111,306 | 1,216,695,900 | 150 | 96.16 | 38.37 |
| 31,890 | QNHC00000000 | 8,085,996 | 1,212,899,400 | 150 | 96.18 | 38.19 |
| 32,142 | QNHD00000000 | 8,069,526 | 1,210,428,900 | 150 | 96.02 | 37.82 |
| 32,633 | QNHE00000000 | 8,104,740 | 1,215,711,000 | 150 | 96.22 | 38.21 |
| 33,710 | QNHF00000000 | 8,104,866 | 1,215,729,900 | 150 | 96.22 | 38.01 |
Abbreviation: LZR-Efa, linezolid-resistant Enterococcus faecalis.
Table S2.
Characteristics of resistance genes
| Isolates | dfrE (n=26) | lsaA (n=26) | emeA (n=26) | fexA (n=23) | ANT(9) -Ia (n=12) | tetO (n=20) | dfrG (n=19) | ErmB (n=19) | tetK (n=18) | APH(3′)-IIIa (n=17) | AAC(6′)-Ie-APH(2″)-Ia (n=17) | cat (n=17) | satNA4 (n=16) | ErmA (n=18) | lnuB (n=12) | lsaE (n=12) | aad(6) (n=9) | tetS (n=2) | tetM (n=2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6,888 | 99.3% | 98.8% | 97.7% | 98.9% | 100.0% | NA | 100.0% | NA | 99.8% | 100.0% | 100.0% | 97.2% | 99.4% | 85.2% | 100.0% | 99.8% | 100.0% | 99.6% | 95.7% |
| 8,714 | 100.0% | 99.2% | 97.7% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 10,938 | 99.3% | 98.8% | 97.7% | 98.9% | 100.0% | 95.6% | 100.0% | 100.0% | NA | 100.0% | 100.0% | NA | 98.9% | 85.2% | 100.0% | 100.0% | 100.0% | NA | NA |
| 11,340 | 100.0% | 98.8% | 97.7% | 98.9% | NA | 95.3% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | 100.0% | 99.4% | 85.2% | NA | NA | 100.0% | NA | NA |
| 11,382 | 100.0% | 99.4% | 97.7% | 98.9% | NA | 95.5% | NA | 100.0% | 99.8% | NA | NA | 97.2% | NA | 85.2% | NA | NA | NA | NA | NA |
| 12,645 | 99.3% | 98.2% | 97.7% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 13,470 | 99.3% | 99.6% | 97.7% | 98.9% | NA | 95.5% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | NA | 99.4% | 85.2% | NA | NA | 100.0% | NA | NA |
| 13,484 | 100.0% | 98.2% | 97.7% | 98.9% | NA | 90.9% | 100.0% | 100.0% | 95.2% | NA | 99.7% | 97.2% | NA | 85.2% | NA | NA | NA | NA | NA |
| 14,980 | 100.0% | 99.4% | 97.7% | 98.9% | 100.0% | 95.5% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | 97.2% | 99.4% | 85.2% | 100.0% | 100.0% | NA | NA | NA |
| 15,224 | 99.3% | 98.8% | 97.7% | 98.9% | NA | 95.6% | 100.0% | 100.0% | NA | NA | NA | 100.0% | NA | 85.2% | NA | NA | NA | NA | NA |
| 15,407 | 99.3% | 98.8% | 97.5% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 15,814 | 99.3% | 98.8% | 97.7% | 98.9% | 100.0% | NA | 100.0% | 99.2% | 99.8% | 100.0% | 100.0% | 97.2% | 99.4% | 85.2% | 100.0% | 100.0% | 100.0% | 99.6% | 95.7% |
| 17,838 | 99.3% | 99.6% | 97.7% | 98.9% | 100.0% | 95.5% | 99.4% | 100.0% | 99.8% | 100.0% | 100.0% | 100.0% | 99.4% | 85.2% | 100.0% | 100.0% | 100.0% | NA | NA |
| 18,026 | 100.0% | 98.8% | 97.7% | 98.9% | NA | 95.5% | NA | 99.6% | 99.8% | 100.0% | 98.0% | 97.2% | 99.4% | 85.2% | NA | NA | NA | NA | NA |
| 19,663 | 99.3% | 99.6% | 97.7% | 98.9% | 100.0% | 95.5% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | 100.0% | 99.4% | 85.2% | 100.0% | 99.8% | 100.0% | NA | NA |
| 19,910 | 100.0% | 99.0% | 97.7% | 98.9% | NA | 95.5% | 100.0% | NA | 99.8% | 100.0% | NA | NA | NA | 85.2% | NA | NA | NA | NA | NA |
| 23,903 | 100.0% | 99.4% | 97.7% | 98.9% | 100.0% | 95.5% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | 97.2% | 99.4% | NA | 100.0% | 100.0% | NA | NA | NA |
| 23,967 | 100.0% | 99.4% | 97.7% | 98.9% | 100.0% | 95.5% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | 97.2% | 99.4% | NA | 100.0% | 100.0% | NA | NA | NA |
| 24,393 | 99.3% | 99.6% | 97.7% | 98.9% | NA | 95.5% | NA | NA | 99.8% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 26,167 | 99.3% | 99.6% | 97.7% | 98.9% | NA | 95.5% | NA | 100.0% | 99.8% | 100.0% | 100.0% | NA | 99.4% | 85.2% | NA | NA | 100.0% | NA | NA |
| 27,149 | 100.0% | 98.8% | 97.7% | 98.9% | 100.0% | NA | 100.0% | NA | NA | 100.0% | 100.0% | 100.0% | 99.4% | 85.2% | 100.0% | 100.0% | NA | NA | NA |
| 27,451 | 100.0% | 99.4% | 97.7% | 98.9% | 100.0% | 95.5% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | 97.2% | 99.4% | NA | 100.0% | 100.0% | NA | NA | NA |
| 31,890 | 100.0% | 99.4% | 97.7% | 98.9% | 100.0% | 95.5% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | 97.2% | 99.4% | NA | 100.0% | 100.0% | NA | NA | NA |
| 32,142 | 99.3% | 98.8% | 97.5% | 98.9% | NA | 95.6% | 100.0% | 100.0% | NA | NA | NA | 100.0% | NA | 85.2% | NA | NA | NA | NA | NA |
| 32,633 | 100.0% | 98.8% | 97.7% | 98.9% | 100.0% | 95.3% | 100.0% | 100.0% | 99.8% | 100.0% | 100.0% | NA | 99.4% | 85.2% | 100.0% | 100.0% | 100.0% | NA | NA |
| 33,710 | 99.3% | 98.8% | 97.5% | 98.9% | NA | 95.6% | 100.0% | 100.0% | NA | NA | NA | 100.0% | NA | 85.2% | NA | NA | NA | NA | NA |
Abbreviation: NA, not available.
Table S3.
Characteristics of virulence genes
| Genes | 6,888 (n=33) | 8,714 (n=28) | 10,938 (n=38) | 11,340 (n=24) | 11,382 (n=18) | 12,645 (n=17) | 13,470 (n=16) | 13,484 (n=18) | 14,980 (n=39) | 15,407 (n=21) | 15,224 (n=40) | 15,814 (n=34) | 17,838 (n=16) | 18,026 (n=25) | 19,663 (n=24) | 19,910 (n=27) | 23,903 (n=37) | 23,967 (n=37) | 24,393 (n=22) | 26,167 (n=22) | 27,149 (n=22) | 27,451 (n=37) | 31,890 (n=37) | 32,142 (n=37) | 32,633 (n=24) | 33,710 (n=35) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ace (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| asa1 (n=18) | + | − | + | + | − | − | + | − | + | − | + | + | + | + | + | − | + | + | − | + | − | + | + | + | + | + |
| bopD (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| bsh (n=1) | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| cpsA (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| cpsB (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| cpsC (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cpsD (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cpsE (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cpsF (n=17) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | − |
| cpsG (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cpsH (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cpsI (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cpsJ (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cpsK (n=18) | + | + | + | + | − | − | − | − | + | + | + | + | − | + | − | + | + | + | − | − | + | + | + | + | + | + |
| cylA (n=14) | + | − | + | − | − | − | − | − | + | − | + | + | − | − | + | − | + | + | + | + | − | + | + | + | − | + |
| cylB (n=11) | + | − | + | − | − | − | − | − | + | − | + | + | − | − | + | − | + | + | − | − | − | + | + | + | − | − |
| cylI (n=14) | + | − | + | − | − | − | − | − | + | − | + | + | − | − | + | − | + | + | + | + | − | + | + | + | − | + |
| cylL (n=10) | + | − | + | − | − | − | − | − | + | − | + | + | − | − | + | − | + | + | − | − | − | + | + | − | − | − |
| cylM (n=13) | + | − | + | − | − | − | − | − | + | − | + | + | + | − | + | − | + | + | − | − | − | + | + | + | − | + |
| cylR1 (n=12) | + | − | + | − | − | − | − | − | + | − | + | + | − | − | + | − | + | + | − | − | − | + | + | + | − | + |
| cylR2 (n=12) | + | − | + | − | − | − | − | − | + | − | + | + | − | − | + | − | + | + | − | − | − | + | + | + | − | + |
| cylS (n=12) | + | − | + | − | − | − | − | − | + | − | + | + | − | − | + | − | + | + | − | − | − | + | + | + | − | + |
| ebpA (n=25) | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| ebpB (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| ebpC (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| EF0149 (n=3) | − | − | − | − | − | − | − | − | + | − | − | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − |
| EF0485 (n=14) | + | − | + | + | − | − | − | − | + | − | + | + | − | + | + | + | + | − | − | − | + | + | + | − | + | |
| EF0818 (n=8) | − | + | − | − | − | + | + | + | − | + | + | − | − | − | − | − | − | − | + | + | − | − | − | − | − | − |
| EF3023 (n=23) | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | − | + | + | + | + | − | + | + | + | + |
| efaA (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| esp (n=17) | + | − | + | − | + | − | − | − | + | − | + | + | + | + | + | − | + | + | + | − | − | + | + | + | + | + |
| fsrA (n=16) | − | + | + | − | + | + | − | + | + | − | + | − | − | − | − | + | + | + | + | + | − | + | + | + | − | + |
| fsrB (n=17) | − | + | + | − | + | + | + | + | + | − | + | − | − | − | − | + | + | + | + | + | − | + | + | + | − | + |
| fsrC (n=17) | − | + | + | − | + | + | − | + | + | − | + | − | − | − | − | + | + | + | + | + | − | + | + | + | + | + |
| fss1 (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| fss2 (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| gelE (n=17) | − | + | + | − | + | + | + | + | + | − | + | − | − | − | − | + | + | + | + | + | − | + | + | + | − | + |
| prgB/asc10 (n=17) | + | + | + | + | − | − | − | − | + | − | + | + | + | − | + | + | + | − | + | + | + | + | − | + | − | + |
| sprE (n=16) | − | + | + | − | + | + | − | + | + | − | + | − | − | − | − | + | + | + | + | + | − | + | + | + | − | + |
| srtC (n=26) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |