Abstract
Purpose
Soft tissue sarcoma (STS) is rare but aggressive neoplasm. Interstitial brachytherapy (ISBT) alone or combined with external beam radiotherapy (EBRT) as post-operative treatment improves loco-regional (LRC) and distant control.
Material and methods
Out of twenty-nine non-metastatic STS (lower limb 64%) patients (median age 37 yrs), treated with surgery and post-operative ISBT during February 2011 – December 2016, 27 patients with > 6 months follow-up were analyzed. Spindle cell sarcoma was the commonest (24%) histology. Eleven patients (44%) received EBRT (45-50 Gy), where ISBT was used as boost (16-20 Gy). Fourteen patients (56%) received ISBT alone (4 Gy per fractio). Treatment was done with a 60 Cobalt (60Co) source high-dose-rate system.
Results
With a median follow-up of 20 months (17-51 months), LRC rate was 85.7% (with EBRT 90.5% and ISBT 83.2% alone). Median disease-free survival (DFS) was 39.7 ±3.9 months (32-47.2 months). Median loco-regional failure-free survival (LRRFS) was 43.8 ±3.6 months (36.8-50.9 months). Distant failure-free survival (DFFS) was 18 months (15.5-26.6 months). Overall survival was 42.4 ±3.4 months (35.7-48.1 months). Tumor grade was a significant factor for DFFS. Total radiation dose (including EBRT) has significant influence on DFS and LRRFS. 14.8% patients developed ≥ grade 2 late toxicity (skin atrophy, hypo-pigmentation, and telangiectasia).
Conclusions
Combination of surgery and ISBT with/out EBRT improves local and distant control with acceptable late toxicities. 60Co-based ISBT is safe and gives a good outcome.
Keywords: Cobalt 60, interstitial brachytherapy, soft tissue sarcoma
Purpose
Soft tissue sarcomas (STS) are comparatively rare heterogeneous group of tumors with different distinct histopathological subtypes. It accounts for 21% of all pediatric non-hematological malignancies, and less than 1% of all adult solid neoplasm [1]. Due to rarity of the incidence and heterogeneous pathological varieties, STS have aggressive biological behavior and high potential for local recurrence after surgery. Adjuvant radiation therapy (RT), either preoperative or post-operative, improves local control (LC) rates after local resection/ limp sparing surgery, eliminating the need for amputation in limb sarcoma [2,3,4,5]. Use of RT as single modality treatment option gives a similar outcome in comparison to surgery without any major functional impairment [5].
Brachytherapy (BT) delivers high-dose of radiation precisely to the target tissue with high conformity sparingly the nearby normal tissues, thereby escalating the dose and eliminating the possibility of normal tissue toxicities. Role of adjuvant post-operative BT with or without EBRT showed increased LC rate [6,7,8,9,10,11,12,13]. These retrospective series included patients from different age groups and tumor grades. BT was used either as single modality or as boost to EBRT in either radical or post-operative setting. Most common mode of BT delivery was interstitial brachytherapy (ISBT).
Despite the fact that Cobalt 60 (60Co) and Iridium 192 (192Ir) as high-dose-rate brachytherapy (HDR BT) source have different physical characteristics, they have identical dose distributions and clinical impact as supported by different dosimetric and clinical studies [14,15,16,17]. Longer half-life of 60Co sources (5 years vs. 73 days) makes it logistically more advantageous in low resource countries. However, higher energy emitting 60Co requires more radiation protection measures. Evidence of ISBT using 60Co HDR source is very little [17,18]. Clinical evidences on 60Co HDR source-based BT are mostly on cervical cancer intracavitary applications.
Initially, we reported phase 2 feasibility analysis of 60Co HDR-based ISBT in different sites [18]. In the current study, we analyzed our 5 years institutional experience of 60Co-based HDR ISBT in STS (radical BT or BT boost).
Material and methods
Study design and study population
In this retrospective institutional trial, twenty-nine non-metastatic pathologically proven STS patients, irrespective of site and location of tumor (treated between January 2011 to December 2016 with multimodality approach) were included. Patients < 6 months follow-up and patients having unplanned surgery were excluded from the study.
Staging evaluation
Before the treatment, all patient underwent either magnetic resonance imaging (MRI) or contrast enhanced computer tomography (CECT) of the local and regional, CECT thorax, and histopathological examination (± immunohistochemistry) from biopsy specimen before planned surgery.
Surgery
All surgeries were planned and discussed in multidisciplinary team. Basic surgical principles were to acquire a R0 resection [19]. Minimum 2 cm free margins were maintained. Dissections were done through uncontaminated normal tissue planes. Biopsy scars (tattooed) were also removed and drains were placed as close as possible to incision lines.
Brachytherapy
Any high-grade tumor (≥ grade 2) with size ≥ 5 cm lesion was included as a candidate for post-operative radiotherapy [20]. In most of the cases, we attempted intra-operative catheter placement, especially in deep seated tumors. For selective cases, post-operative ISBT was completed (superficial tumor, R1/R2 resection after primary surgery). In situations, where tumor bed was directly related to neurovascular bundles or bones (where periosteum is removed), ISBT was not attempted.
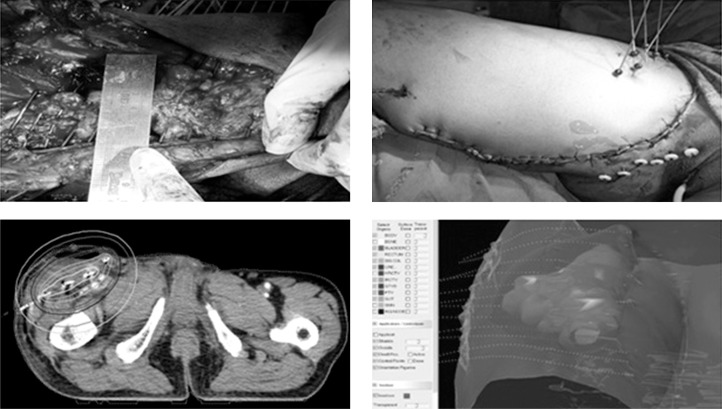
In majority of cases, radiation oncologist team attended the surgery with surgical oncologist team. Surgical clips were placed after removal of the tumor to localize the clinical target volume (CTV). In most of patients, we placed BT catheter intra-operatively. Hollow plastic flexible catheters were placed with help of flexible steel needles through skin over the tumor bed either along or perpendicular to the incision line depending on the location and size of the tumor bed. Aimed distance between catheters was 1.5 cm. Parallelism was maintained between catheters. Entry and exit points of the catheters were kept 2 cm away from incision line. We used catheters with one end open. Buttons/balls were placed in both ends of the catheters. Adequate gap of 5 mm was kept between end buttons and skin to encompass post-operative tissue edema. Catheters were finally inspected after closure of the skin to ensure parallelism and rule out any kinking of the catheters. We did planning based on CECT, placing dummy ribbons through catheters (to aid catheter reconstruction) with slice thickness of 2.5 mm on 5th post-operative day. Clinical target volume was contoured with help of surgical clips, surgical photographs, preoperative imaging, and pathological information. Organs at risk (OAR) were also contoured, mostly bone and skin (Figure 1). According to our institutional protocol, we contoured 2 mm of skin thickness from external contour over 5 cm of the CTV margin. Catheter reconstructions were done by library method. This method is time saving and reduces the probability of catheter misidentification, specially dealing with large number of catheters [21]. CT-based planning and optimizations were completed using HDR plus treatment planning system (version 2.6, Eckert & Ziegler BEBIG GmbH, Berlin, Germany).
Fig. 1.
ISBT process (steps are depicted sequentially from upper left, upper right lower left, and lower right figure). Upper left figure demonstrates per operative catheter placement in tumor bed maintaining parallelism equidistantly. Upper right figure shows the site after closure of wound with the BT catheters in situ. Lower right figure shows two dimensional isodose distribution. Lower left figure shows three dimensional isodose distribution with respect to reconstructed catheters and CTV
For post-operative cases, post-operative CECT was done to localize surgical clips and tumor bed. Brachytherapy catheters insertions were done under regional anesthesia. Catheter geometry was planned considering surgical note, position of surgical clips, and pathological findings. Planning scans were taken on day 2 after insertion.
Dose prescription was 3.5-4 Gy per fraction. We evaluated CTV D90 (defined as the minimum dose covering 90% of the CTV volumes), CTV V100%, V300% (defined as percentage volumes of CTV receiving 100% and 300% of prescription doses, respectively), and respective OAR doses. For skin, we evaluated maximum point doses and D10 cc dose (defined as maximum dose received by minimum 10 cc volumes of skin) and V100% doses. We calculated EQD2 (defined as equivalent dose in 2 Gy/fraction). For BT boost cases, provisional EBRT doses were also summarized. For plan evaluation, our protocol was to keep CTV D90 does to 100%. For OAR, we followed published cut off guidelines. For skin, as there is no such reported guideline, we tried to keep maximum dose as low as possible and d10 cc to < 2/3rd of D90 doses.
Adjuvant radical BT (without EBRT) was given for small (≤ T2), superficial tumors, with R0/R1 resection. In other cases, EBRT was added. For radical ISBT, 12-13 fractions (of 4 Gy/fraction, 2 fractions daily, 6 hours apart) were prescribed (EQD2 around 60 Gy). For BT boost, 4-5 fractions (EQD2 around 16-20 Gy) were prescribed. Treatment delivery was given using MultiSource® (Eckert & Ziegler BEBIG GmBH, Berlin, Germany), HDR remote after-loader system with 60Co source.
External beam radiation therapy
In ISBT boost cases, EBRT was administered after 3 weeks of ISBT. For cases where intra-operative catheter placement was not done, we attempted earlier initiation of EBRT. Planning scan was done with 2.5 mm slice thickness. CTV was defined considering preoperative imaging, ISBT CTV, and pathological findings. Approximately 5 cm CTV margins were applied. At least 1.5-2.0 cm of limb circumference was spared from radiotherapy portals. We tried to spare half circumference of uninvolved bone whenever possible and kept uninvolved compartment out of radiation port as far as possible. Three-dimensional conformal RT (3D CRT) planning was done. Dose prescriptions were in the range of 45-50 Gy (1.8-2 Gy/fraction).
Chemotherapy
Anthracyclin and ifosfamide-based chemotherapy (ChT) was applied to chemo-sensitive histological subtypes. Total, 4-6 cycles of ChT was given. Injection filgrastim was administered routinely. Dose modification was done in patients with ≥ grade 2 hematological toxicities. In patients, where ChT was added as neo-adjuvant prior to surgery, patient was restaged by clinical and MRI findings. In adjuvant setting, ChT was given 2-3 weeks after completion of RT.
Follow-up
Patients were followed-up 2-3 monthly in initial 2 years and 6 months thereafter. During follow-up, patients were examined clinically. Chest X-rays were done in every follow-up. In suspicion of lung metastasis, CECT thorax was completed. Imaging of local sites were done depending on locations of the primary tumors. For R2 resection cases, in first follow-up visit, MRI or CECT was routinely done. MRI with or without contrast (or CECT) of the local site (every 6 months for 2 years, then annually) were completed for cases where loco-regional areas cannot be properly assessed by physical examination. In other cases, loco-regional imaging was done on clinical suspicion of recurrences. Toxicities, if occurred, were noted and managed accordingly. Progressive disease cases were managed as per institutional protocols. Late toxicities were measured using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [22]. Grade 2 and higher-grade toxicities were considered as quantal end points for toxicity reporting.
Statistical principle
Statistical analysis was done using Statistical Package for the Social Sciences (SPSS) software, version 20 (IBM Corporation, USA). Descriptive statistics was done using frequency tables. Univariate analysis was completed to find out the impact of individual risk factors. Kaplan-Meier survival (KMS) plots were used to estimate overall survival (OS), disease-free survival (DFS), loco-regional failure-free survival (LRFFS), and distant failure-free survival (DFFS). Cox regression analysis was done to estimate of influence of individual factors on survival plots. P value < 0.05 was considered as statistically significant.
Results
Total twenty-nine eligible non-metastatic STS patients were treated between 2011-2016. Two patients were excluded from the analysis due to short follow-up (< 6 months), therefore final sample size was 27 patients.
Descriptive statistic
Baseline characteristics of the patients are summarized in Table 1. Histopathologic subtypes are summarized in Table 2.
Table 1.
Baseline characteristics
| Baseline profile, n (%) | |
|---|---|
| Median age | 38 years (10-73) |
| Sex | |
| Male | 15 (53.6) |
| Female | 12 (42.9) |
| Stage | |
| I | 6 (21.4) |
| II | 17 (60.7) |
| III | 4 (14.3) |
| Grade | |
| 1 | 4 (14.3) |
| 2 | 13 (46.4) |
| 3 | 10 (35.7) |
| Site | |
| Lower limb | 17 (62.9) |
| Trunk | 7 (26) |
| Upper limb | 3 (11.1) |
Table 2.
Histological subtypes
| Histological types, n (%) (n = 27) | |
|---|---|
| Chondrosarcoma | 2 (7.2) |
| Dermatofibrosarcoma protuberance (DFSP) | 3 (10.7) |
| Fibrosarcoma | 2 (7.2) |
| Leiomyosarcoma | 2 (7.1) |
| Malignant fibrous histiocytoma (MFH) | 1 (3.6) |
| Malignant peripheral nerve sheath tumor (MPNST) | 2 (7.1) |
| Myxofibrosarcoma | 1 (3.6) |
| Pleomorphic sarcoma | 4 (14.3) |
| Spindle cell ca | 6 (21.4) |
| Synovial cell ca | 2 (7.2) |
| STS NOS (no other specified) | 3 (10.7) |
Treatment
Chemotherapy was given to 17 (60.7%) patients. Nineteen (70.4%) patients received radical ISBT, whereas 8 (29.7%) patients received ISBT boost along with EBRT. Majorities (17, 62.9%) were post-operative ISBT implants. In ten cases (37.1%), we did intra-operative catheter insertions. 19 (70.4%) patients were treated by radical adjuvant ISBT (without EBRT) and in 8 (29.6%) cases, ISBT boost was given along with adjuvant EBRT. In 15 (53.7%), 2 (7.2%), and 1 (3.6%) cases we performed single, double, and triple plane catheter insertions, respectively.
Dosimetric analysis
Median EBRT dose was 50 Gy (30-50 Gy). Median ISBT prescription dose (per fraction) was 4 Gy (3-5 Gy). In radical ISBT (without EBRT), median fraction size was 12 (9-16) and in ISBT boost cases, it was 5 (3-6). Other dosimetric analyses are presented in Table 3.
Table 3.
Dosimetric analysis
| ISBT/dosimetric type | Parameters | Median (range) | Mean ± SD | EQD2α/β = 10 ± EBRT, Median (Gy) | EQD2α/β = 10 ± EBRT, Mean ± SD (Gy) |
|---|---|---|---|---|---|
| CTV dosimetry | |||||
| Radical ISBT | D90 | 4 Gy (3-5) | 4.1 ±0.5 Gy | 60.7 (33.4-67.4) | 55.3 ±9.4 |
| V100 | 84.8% (61.5-98) | 83.1 ±11.3% | – | – | |
| V300 | 7% (1.5-33.5) | 11.6 ±9.3% | |||
| Boost ISBT | D90 | 4 Gy (3-5.5) | 3.3 ±0.7 Gy | 66.6 (42-78) | 66.1 ±11.4 |
| V100 | 91% (83.8-98.8) | 92.4 ±4.1% | – | – | |
| V300 | 7.6% (0.2-18) | 7.2 ±4.5% | – | – | |
| Skin dosimetry | |||||
| D10cc | 2.8 Gy (0.5-8.4) | 5.9 ±1.5 Gy | – | – | |
| V100 | 17.8% (0-74.4) | 22.9 ±10.5% | – | – |
ISBT – interstitial brachytherapy; EQD2α/β = 10 – equivalent dose in 2 Gy/fraction; SD – standard deviation; CTV – clinical target volume; D90 – defined as the minimum dose covering 90% of the CTV volumes; V100%, V300% – defined as percentage volumes of CTV receiving 100% and 300% of prescription doses, respectively; D10cc dose – defined as maximum dose received by minimum 10 cc volumes of skin
Outcome (survival) analysis
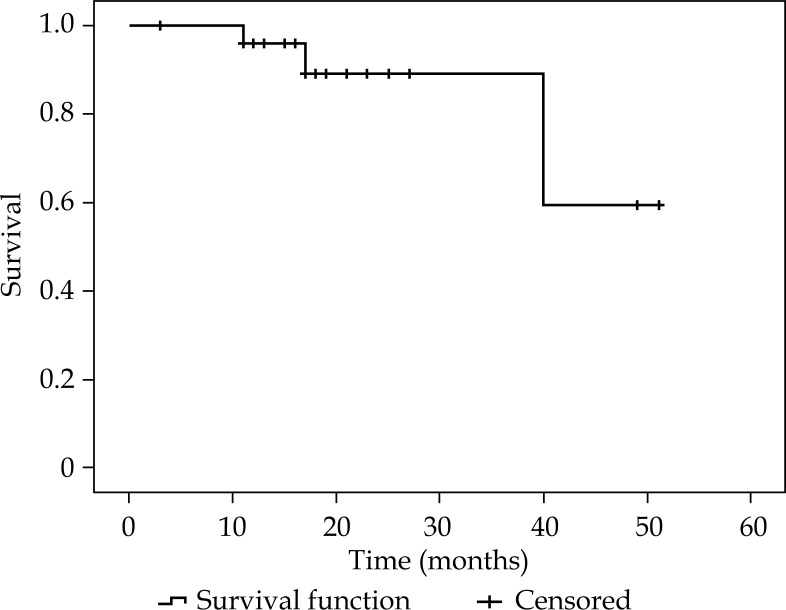
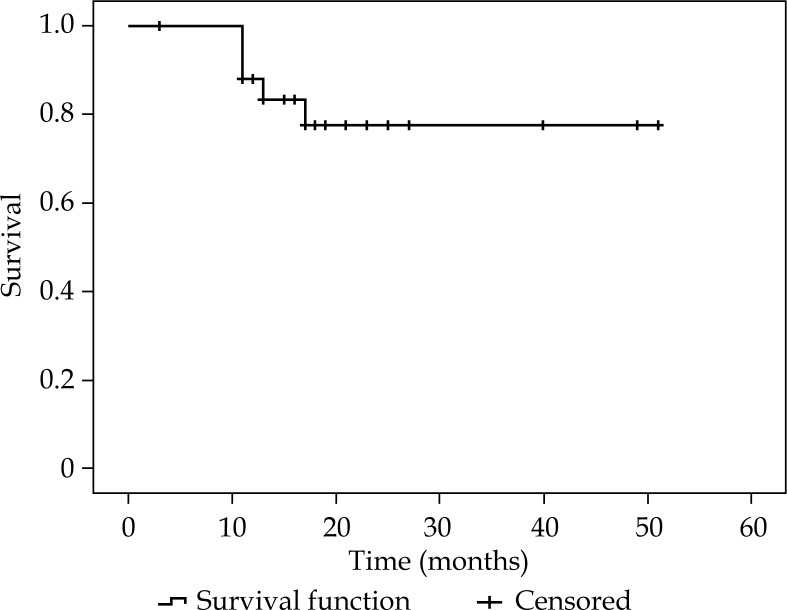
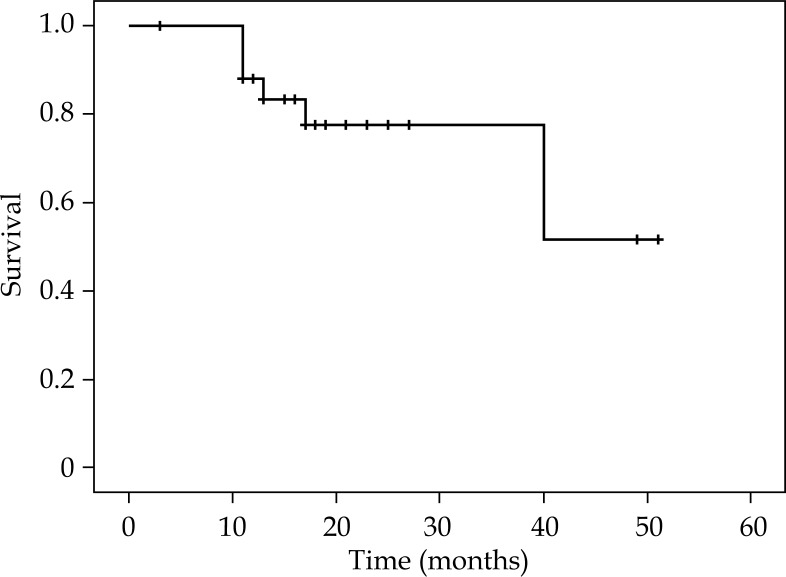
Survival analysis are summarized in Table 4. Figures 2-4 show KMS survival plots of LRFFS, DFS, and OS, respectively. With a median follow-up of 20 months (17-51 months), overall LC rates were 85.7% (BT boost: 90.5%/radical BT: 83.2%). LRRFS was 43.8 ±3.6 months (36.8-50.9 months). DFS was 39.7 ±3.9 months (32-47.2 months). Median DFFS and OS were 21.5 ±2.7 months (15.9-26.6 months) and 42.4 ±3.4 months (35.7-48.1 months), respectively.
Table 4.
Outcome (survival) analysis
| Median follow-up | 20 months (17-51) |
|---|---|
| Progression | 1 (3.7%) |
| LR recurrence | 3 (10.7%) |
| Lung metastasis | 4 (14.9%) |
| Liver metastasis | 1 (3.7%) |
| Over all RR (5 yr) | 75% (BT boost: 83.3%/radical BT: 76.2%) |
| Local control (5 yr) | 85.7% (BT boost: 90.5%/radical BT: 83.2%) |
| Survival (months) | Mean ± SE (95% CI) |
| LRFFS | 43.8 ±3.6 (36.8-50.9) |
| DFS | 39.7 ±3.9 (32-47.2) |
| Distant FFS | 21.5 ±2.7 (15.9-26.6) Median 18 (15.5-26.6) |
| OS | 42.4 ±3.4 (35.7-48.1) |
RR – response rate; SE – standard error of mean; LRFFS – loco-regional failure-free survival; DFS – disease-free survival; distant FFS – distant failure-free survival; OS – overall survival; yr – years
Fig. 2.
Kaplan-Meier survival plot showing loco-regional failure-free survival
Fig. 4.
Kaplan-Meier survival plot showing overall survival
Fig. 3.
Kaplan-Meier survival plot showing disease-free survival
On Cox regression analysis, tumor grade was found to be a significant factor on DFS and DFFS. Total EQD2 dose was significant factor for LRFFS and DFS. None of the dosimetric data had influence overall OS.
Late toxicity analysis
Late toxicities were reported using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Twenty two (81.4%) patients did not suffer any significant (grade 2 and higher) late skin toxicity. One (3.7%) patient had grade 2 telangiectasia, 2 (7.4%) patients had grade 2 skin hypo-pigmentation, and 1 (3.7%) patient had grade 2 and grade 3 skin atrophy, respectively. No other late toxicity occurred during the follow-up period.
Discussion
This study is probably the first report of ISBT of STS using 60Co HDR sources. We did this clinical audit to find out dosimetric and clinical outcome (in terms of local control, survival, and toxicities) of this HDR source.
HDR BT using 60Co HDR sources has similar dosimetric and clinical outcome with commonly used 192Ir sources [14,15,16,17]. Clinical outcome data using 60Co HDR sources in intracavitary BT has been published [17]. Our center is using 60Co HDR-based BT systems since 2011. In 2012, we published initial experience of ISBT in different sites using this system [18]. It was phase I feasibility study and first of its kind. In this study, we summarized first three months follow-up (from October 2010 to December 2011) of 31 patients with carcinoma of different sites, treated at our center with 60Co HDR BT with interstitial implants or surface molds with or without EBRT. We showed that HDR BT interstitial implants or surface molds using 60Co sources for different sites is clinically feasible and safe with favorable short-term response and toxicity. It is also a very viable option for economically strained regions as for the half-life of 60Co.
Adjuvant BT is an important component of multimodality management of non-metastatic STS. ISBT using 192Ir sources (± EBRT) as adjunct to radical surgery improves LC [6,7,8,9,10,11,12,13]. In STS, ISBT has many advantages over EBRT. ISBT catheters can be directly placed over respected tumor bed during operation. Therefore, high-dose can be precisely delivered to the area containing microscopic disease. Sharp and rapid dose fall off in BT helps relatively spare the nearby normal tissue. Successful results of ISBT depends on proper imaging, preferably R0 surgery, placement of surgical clips over margin, and proper technique of ISBT catheter insertion covering surgical bed with adequate margin.
Experience of ISBT was accumulated over the years worldwide, with excellent outcome results and acceptable toxicities. Use of ISBT as single modality RT technique for STS in children is well established [11,12]. Post-operative ISBT with small margin avoiding EBRT has a good local control rate, with proper sparing of growing bones and soft tissues. Outcome and toxicity results of some of recent published works of ISBT in STS are detailed in Table 5. In this table, we also compared outcome of our study. All the patients in our study (n = 27) are of primary STS. We found a comparable LC rate (after 5 years follow-up) with previous reported series.
Table 5.
Literature review
| Author (year) [reference number] Institute | Inclusion criteria (n) | EBRT dose (Gy) Median (range) | BT dose (Gy) Median (range) | Local control (%) | Survival | Complication rate (%) |
|---|---|---|---|---|---|---|
| Shiu et al. (1991) [7], Memorial Sloan-Kettering Cancer Centre, USA | Locally advanced/candidate for amputation (33) | – | LDR 44 (25-54) | At 3 yr, 88% At 5 yr, 70% |
– | 39% (overall) |
| Harrison et al. (1993) [8], Memorial Sloan-Kettering Cancer Centre, USA | After gross tumor resection BT (intra-operative insertion) vs. no BT, randomized control trial (166) |
– | LDR 42-45 | At 5 yrs, 82% vs. 67% | 5 yr DFS 81% vs. 80%(NS) |
14% vs. 10% (NS) (wound complication) |
| Chaudhary et al. (1998) [9], Tata Memorial Hospital, India | Non-metastatic STS in adults (177) | 45 (12-70) | HDR alone 30 (29-50) | At 30 months, 70%, After salvage, 86% | – | < 1% |
| Rosenblatt et al. (2003) [10], Haifa, Israel | Non-metastatic STS (32) | 39.2 (16.2-45) | LDR 33 (18-49), HDR 16 | At 3 yrs, 87.5% | At 5 yrs DFS 56%, OS 70% | Wound complication 16%, Late local toxicities 19% |
| Laskar et al. (2007) [11], Tata Memorial Hospital, India | Non-metastatic STS, children, (median age 13 yrs) (50) | 45 (30.6-45) | HDR alone 36 (30-40) EBRT + HDR BT 21 (15-36) |
At 51 months, 82% | At 51 months, DFS 68%, OS 71% | Wound complication 4%, Late toxicities 20% |
| Laskar et al. (2014) [12], Tata Memorial Hospital, India | Non-metastatic STS, children, (Median age 15 yrs) (76) | – | – | At 70 months, 85% | At 70 months, DFS 74%, OS 77% | Wound complication 8%, Late local toxicities 31% |
| Cortesy et al. (2017) [13], Italy | Primary or recurrent STS (107) | 46 (mean) | LDR and PDR 20 (mean?) | At 5 yrs, 80.5% | At 5 yrs, DFS 58.6% OS 87.4% | |
| Current study | Primary STS (27) | 50 (30-50) | HDR alone 60.7 (33.4-67.4) EBRT + HDR BT 66.6 (42-78) | At 5 yrs, 85.7% | DFS 39.7 ±3.9 months OS 42.4 ±3.4 months |
≥ grade 2 skin toxicity in 14.8% patients |
EBRT – external beam radiation therapy; BT – brachytherapy; HDR – high-dose-rate; PDR – pulsed-dose-rate; LDR – low-dose-rate; DFS – disease-free survival; OS – overall survival; yrs – years; NS – not significant
Like other studies [11,12,13], tumor grade influenced the LC, DFS, and DFFS with grade 1 tumors having better prognosis than grade 2 and 3. However, there was no difference in OS. Groups receiving ISBT alone versus groups receiving ISBT + EBRT had comparable LC rates (90.5% vs. 83.2%, respectively; p value = 0.21) corroborating results published by Laskar et al. [11]. Similarly, total EQD2 dose influenced LRRFS and DFFS like all previous reports. In our study, any of the co-factors had an effect over OS. It may be due to heterogeneity in patient population (age, grade, site, use of adjuvant ChT).
In our series, most of the patients had mild erythema and pain over ISBT sites for 7-10 days after RT, which resolved subsequently. 18.5% of patients had late toxicities, of which majorities were skin related (14.5% had > grade 2). This finding is comparable to the earlier reports of 192Ir source-based ISBT [10,11,12,13].
There are few limitations of this study. This study is retrospective assessment in nature with small sample size. There is lack of homogeneity of radical/boost ISBT approach, chemotherapy use, and age among patients in this study. Nevertheless, all the patients were treated with approximately uniform doses (radical ISBT/EBRT + ISBT combination group-wise). Moreover, all the patients were followed-up by same team of radiation oncologists of our BT unit.
Conclusions
Excellent local control and disease-free survival with acceptable toxicities have been observed in ISBT ± EBRT in STS patients using 60Co-based HDR system. Survival and toxicity outcomes are comparable with published series of HDR ISBT using 192Ir sources. Results were persistent irrespective of age groups, histopathology, and grades. Tumor grade and total dose in terms of EQD2 are the two major factors influencing outcome. 60Co-based HDR ISBT is feasible, safe, and can be a good option for resource constraint countries for its longer half-life than 192Ir.
Acknowledgement
We are grateful to all staffs of brachytherapy unit of the study hospital for their kind contribution. We also express our gratitude to all our patients whose cooperation made the study complete.
Disclosure
Authors report no conflict of interest.
Source of support: R. G. Kar Medical College and Hospital, Kolkata, India.
Presented (e-poster) at Annual Conference of Association of Radiation Oncologists of India 2016, November 2016, Bhubaneswar, India.
References
- 1.Burningham Z, Hashibe M, Spector L, Schiffman JD. The epidemiology of sarcoma. Clin Sarcoma Res. 2012;2:14.. doi: 10.1186/2045-3329-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindberg RD, Martin RG, Romsdahl MM, Barkley HT., Jr Conservative surgery and postoperative radiotherapy in 300 adults with soft-tissue sarcomas. Cancer. 1981;47:2391–2397. doi: 10.1002/1097-0142(19810515)47:10<2391::aid-cncr2820471012>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Suit HD, Proppe KH, Mankin HJ, Wood WC. Preoperative radiation therapy for sarcoma of soft tissue. Cancer. 1981;47:2269–2274. doi: 10.1002/1097-0142(19810501)47:9<2269::aid-cncr2820470928>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Eilber FR, Mirra JJ, Grant TT, et al. Is amputation necessary for sarcomas? A seven-year experience with limb salvage. Ann Surg. 1980;192:431–438. doi: 10.1097/00000658-198010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilaris BS, Shiu MH, Nori D, et al. Limb-sparing therapy for locally advanced soft-tissue sarcomas. Endocuriether/Hyperthermia Oncol. 1985;1:17–24. [Google Scholar]
- 7.Shiu MH, Hilaris BS, Harrison LB, Brennan MF. Brachytherapy and function-saving resection of soft tissue sarcoma arising in the limb. Int J Radiat Oncol Biol Phys. 1991;21:1485–1492. doi: 10.1016/0360-3016(91)90323-v. [DOI] [PubMed] [Google Scholar]
- 8.Harrison LB, Franzese F, Gaynor JJ, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in the management of completely resected soft tissue sarcomas of the extremity and superficial trunk. Int J Radiat Oncol Biol Phys. 1993;27:259–265. doi: 10.1016/0360-3016(93)90236-o. [DOI] [PubMed] [Google Scholar]
- 9.Choudhury AJ, Laskar S, Badhwar R. Interstitial brachytherapy in soft tissue sarcomas The Tata Memorial Hospital experience. Strahlenther Onkol. 1998;174:522–528. doi: 10.1007/BF03038985. [DOI] [PubMed] [Google Scholar]
- 10.Rosenblatt E, Meushar N, Bar-Deroma R, et al. Interstitial brachytherapy in soft tissue sarcomas: The Rambam experience. Isr Med Assoc J. 2003;5:547–551. [PubMed] [Google Scholar]
- 11.Laskar S, Bahl G, Muckaden MA, et al. Interstitial brachytherapy for childhood soft tissue sarcoma. Pediatr Blood Cancer. 2007;49:649–655. doi: 10.1002/pbc.21118. [DOI] [PubMed] [Google Scholar]
- 12.Laskar S, Khanna N, Puri A, et al. Interstitial brachytherapy for childhood soft tissue sarcomas: Long-term disease outcome and late effects. Int J Radiat Oncol Biol Phys. 2014;90:S113–114. [Google Scholar]
- 13.Cortesi A, Galuppi A, Frakulli R, et al. Adjuvant radiotherapy with brachytherapy boost in soft tissue sarcoma. J Contemp Brachytherapy. 2017;9:256–262. doi: 10.5114/jcb.2017.68215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richter J, Baier K, Flentje M. Comparison of 60Co and 192Ir sources in high dose rate afterloading brachytherapy. Strahlenther Onkol. 2008;184:187–192. doi: 10.1007/s00066-008-1684-y. [DOI] [PubMed] [Google Scholar]
- 15.Strohmaier S, Zwierzchowski G. Comparison of 60Co and 192Ir sources in HDR brachytherapy. J Contemp Brachytherapy. 2011;3:199–208. doi: 10.5114/jcb.2011.26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zakaria GA, Schütte W, Azhari HA. Dosimetry of HDR afterloading machines with Ir-192 and Co-60 sources: comparisons of different international protocols. Z Med Phys. 2010;20:215–224. doi: 10.1016/j.zemedi.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Ntekim Al, Adenipekun AA, Akinlade Bl, et al. High-dose-rate brachytherapy in the treatment of uterine cervical cancer using cobalt-60 radionuclide source: Three years treatment outcome. West Afri J Radiol. 2014;21:21–25. [Google Scholar]
- 18.Basu S, Basu A, Ghosh K, Dutta S. A phase I feasibility study for HDR interstitial Brachytherapy using 60Co for different sites. Radiother Oncol. 2012;103(Supplement 2):S166.. [Google Scholar]
- 19.Wittekind C, Compton C, Quirkey P, et al. A uniform residual (R) tumor classification. Cancer. 2009;115:3483–3488. doi: 10.1002/cncr.24320. [DOI] [PubMed] [Google Scholar]
- 20.Yang JC, Chang AE, Baker AR, et al. Randomised prospective study of benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 21.Otal A, Richart J, Rodriguez S, et al. A method to incorporate interstitial components into the TPS gynecologic rigid applicator library. J Contemp Brachytherapy. 2017;9:59–65. doi: 10.5114/jcb.2017.65290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50Assessed on 5th September 2018.