Abstract
Background and Objectives:
Surgery provokes inflammatory and immune responses, so efforts have been made to reduce host response by using less invasive techniques. The purpose of this experimental study was to investigate the surgical stress induced by skin incision and the role of liver response in this process.
Methods:
Seventy male anesthetized Wistar rats were subjected to a midline incision confined strictly to the skin (dermis) of either 1 cm long (n = 20), 10 cm long (n = 20), or no incision (n = 20) or served as controls (n = 10). Skin trauma was left open for a 20-minutes period, and then was meticulously sutured. At 3 and 24 hours later, laparotomy was performed on half the rats of each group, for blood and liver sampling. In serum and liver homogenates, cytokine-induced neutrophil chemoattractant (CINC)1/interleukin (IL)-8 and tumor necrosis factor (TNF)-α levels were measured with enzyme-linked immunosorbent assays and nitric oxide (NO) using a Griess reaction.
Results:
Skin trauma was found to significantly (P < .01) increase all inflammatory mediators tested (CINC1/IL-8, TNF-α, NO) in serum of operated rats versus controls, the increase being proportionally dependent on the length of skin incision. In liver homogenates, CINC1/IL-8 was significantly (P < .01) increased in operated animals versus controls, similarly to serum levels. In contrast, liver TNF-α levels were inversely related to serum levels, and a significant (P < .01) decrease in TNF-α was observed in liver homogenates of operated animals compared with the controls, indicating that the increased TNF-α in blood reflects liver TNF-α secretion.
Conclusion:
Our findings suggest that inflammatory and immune reactions induced by skin-only surgical trauma are closely correlated to the length of skin incision.
Keywords: Surgical skin trauma, Surgical incision, Skin stress response, Postoperative immune reaction
INTRODUCTION
It has long been recognized that tissue injury, as a result of a surgical procedure, accelerates a neurophysiological reflex response – namely surgical stress response – which involves the hypothalamic-pituitary-adrenal axis activation and results in a complex cascade of neuroendocrine, inflammatory, metabolic, and immunological responses.1–5 The rapid evolution of laparoscopic surgery led to its establishment as the “must” technique for a plethora of surgical interventions, because, in addition to its other advantages, such as less pain and better cosmetic results, to list a few, it has been considered as a minimally invasive procedure, in the aspect of surgical stress induction.5–9
There is now increased experimental and clinical evidence that laparoscopic, compared with open, surgery reduces the inflammatory response and preserves the immune function of the host, due to reduction of the tissue trauma.10–13 On the other hand, there is an augmented volume of data suggesting that the explanation of “less trauma, less response” is not as simple as we would like to believe, for at least two reasons: the first one is the peritoneal trauma, where the small incision for laparoscopic surgery does not necessary mean less surgical stress, taking into account the additional influence of the gas itself, its temperature, its pressure, and so on,14–16 and the second one is the knowledge that the skin is an independent neuroendocrine organ.17–21 Based on these reasons, it has been suggested that the skin incision related processes lead to inflammation and sensitization of the peripheral and central neurons, thus accelerating a concomitant neurophysiologic stress response.22,23
Because this hypothesis is solely based on data related to studies on local anesthetics for pain control after a skin incision,24,2 we decided to investigate (1) whether the length of skin trauma/surgical incision would affect the magnitude of inflammatory and immune responses presented in the systemic circulation and (2) the role of the liver in this process.
MATERIALS AND METHODS
Animals
Seventy male Wistar rats weighing 267 ± 11.4 g were included in this study. The experiment was performed at the Surgical Research Laboratory of the AHEPA University Hospital. The experimental protocol was approved by the Department of Animal Care and Use Committee of the Greek Ministry of Agriculture and adhered to the European Community Guiding Principles for the Care and Use of Animals. Rats were individually housed in Plexiglas cages, under stable laboratory conditions, with a 12-hour dark/light cycle and free access to standard rat chow, for a 7-day environmental adaptation period. Water was available ad libitum throughout the experiment.
Experimental Settings
At the end of the environmental adaptation period, the rats were randomly allocated into 3 experimental groups of 20 animals per group, plus 10 rats used for baseline measurements only. These 10 rats (Ctr) were killed and then, immediately, blood and liver tissue specimens were taken and stored for further processing, as analyzed later. Regarding the remaining 60 experimental animals, 20 rats were allocated into the short incision (S) group, 20 rats were allocated into the long incision (L) group, and 20 rats served as anesthesia controls (C), to assess the influence of anesthesia-related stress (Figure 1).26
Figure 1.
Flowchart of the in vivo experiments.
After intramuscular ketamine-induced anesthesia (50 mg/kg) and under strictly sterile conditions, all 60 animals were subjected to a midline incision, confined strictly to the superficial skin layer (dermis), at a length of 1 cm for the S group, 10 cm for the L group, and sham incision for the C group. The skin trauma site was left open for a 20-minute period, to simulate the operation time, and then was meticulously sutured with a 5.0 polypropylene suture. Rats were then placed in clean cages with no bedding and allowed to recover from anesthesia. At 3 and 24 hours later, 10 rats from each group were similarly anesthetized to be subjected to an abdominal opening for blood and liver tissue sampling, and then the rats were killed with an anesthetic overdose. Blood serum and liver tissue were stored at –80°C for subsequent analysis.
Cytokine-Induced Neutrophil Chemoattractant 1/Interleukin-8 and Tumor Necrosis Factor-α Assessment
Cytokine-induced neutrophil chemoattractant (CINC)1/interleukin (IL)-8 and tumor necrosis factor (TNF)-α concentrations in rat serum and liver homogenates were measured with use of sandwich enzyme-linked immunosobent assay (ELISA), according to the manufacturer's instructions (Duoset ELISA Development System, R&D Systems, UK, Abingdon, England).
Nitric Oxide Measurement
Nitric oxide (NO) production was determined spectrophotometrically by measuring the total concentration of nitrites/nitrates (NOx) representing the end products of NO metabolism.27 To determine local production of NO, total NOx of culture supernatants were assayed using a modification of the Griess reaction, as previously described.26,28,29
Protein Assay
Homogenates of liver tissue were examined for estimation of total protein per well using the Bio-Rad protein microassay. The protein assay was based on Bradford's dye-binding procedure,30 as previously described.26,28,29
Statistical Analysis
Data were expressed as the mean ± standard deviation (SD) of the samples (n = 10) of each group. Statistical analyses were performed by using the 1-way repeated-measures analysis of variance (ANOVA) for multiple comparisons between groups. Statistical significance was established at the level of P ≤ .05 for all tests.
RESULTS
Assessment of serum from control animals (Ctr) revealed a basal amount of CINC1/IL-8 levels (175.19 ± 24.62 pg/mL, n = 10). Anesthesia itself, at 3 hours (C3), was found to accelerate only a small increase in CINC1/IL-8 levels in rat serum (207.54 ± 24.23 pg/mL, n = 10, P = NS). In contrast, skin trauma was found to rapidly (within 3 hours) provoke a significant increase in CINC1/IL-8 serum levels mainly in L3 rats (from 207.54 ± 24.23 pg/mL, n = 10, to 438.65 ± 79.84 pg/mL, n = 10, P < .01) as well as in S3 rats (from 207.54 ± 24.23, n = 10, to 285.25 ± 35.69 pg/mL, n = 10, P < .05) compared with the anesthesia group (C3). Interestingly, the increase in CINC1/IL-8 serum levels (at 3 hours) in the L3 group was significant higher (P < .05) compared with the increase in the S3 group. After 24 hours, both L24 and S24 groups demonstrated an expected slow decline of CINC1/IL-8 serum levels that continued to be statistically higher (P < .05) compared with the anesthesia group (C24), but there was not any statistical significant difference between the L24 and S24 groups (Figure 2).
Figure 2.
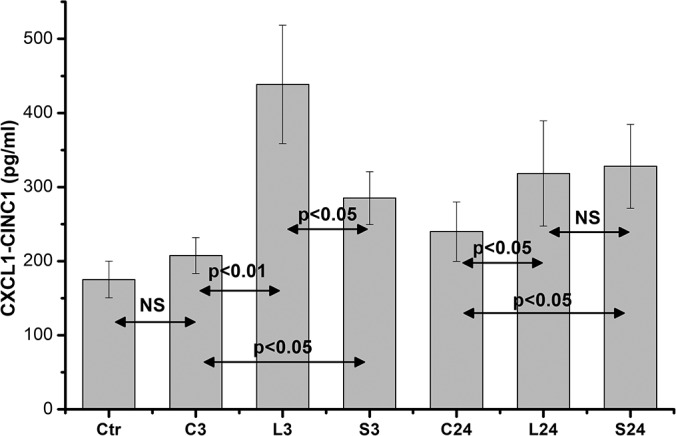
Serum CINC1/IL-8 levels. Data are presented as the mean ± standard error (SE) of 10 samples per group. C0 represents baseline measurements; C3 and C24 represent anesthesia-only subjected rats, at 3 and 24 hours; L3 and L24 represent the long-incision–subjected rats at 3 and 24 hours; and S3 and S24 represent the short-incision–subjected rats at 3 and 24 hours.
A similar increase was observed in serum TNF-α and NO levels. In particular, TNF-α levels were found slightly but significantly increased at 3 hours in both L3 and S3 groups in relation to C3 (P < .05), while the lower increase in the S3 levels did not reach any statistical significance compared with L3 values. At 24 hours, both L24 and S24 groups exhibited a highly significant increase in relation to C24 (from 3.36 ± 0.53 pg/mL, n = 10, to 36.76 ± 0.53 pg/mL, n = 10, and 20.62 ± 7.23 pg/mL, n = 10, respectively, P < .001) with the L24 levels also being highly significantly increased (P < .001) compared with the S24 levels, thus confirming that the TNF-α serum levels are clearly related to the length of skin incision (Figure 3).
Figure 3.

Serum TNF-α levels. Data are presented as the mean ± standard error (SE) of 10 samples per group. C0 represents baseline measurements; C3 and C24 represent anesthesia-only subjected rats, at 3 and 24 hours; L3 and L24 represent the long-incision–subjected rats at 3 and 24 hours; and S3 and S24 represent the short-incision–subjected rats at 3 and 24 hours.
NO serum levels were found highly significantly increased at 24 hours postincision in both L24 and S24 groups in relation to C24 (from 75.68 ± 6.59 μmol/mL, n = 10, to 299.52 ± 27.98 μmol/mL, n = 10, and 227.66 ± 16.93 μmol/mL, n = 10, respectively, P < .01), while the increased NO serum levels in the long-incision rats were significantly higher (P < .05) compared with S24 rats' NO levels (Figure 4).
Figure 4.
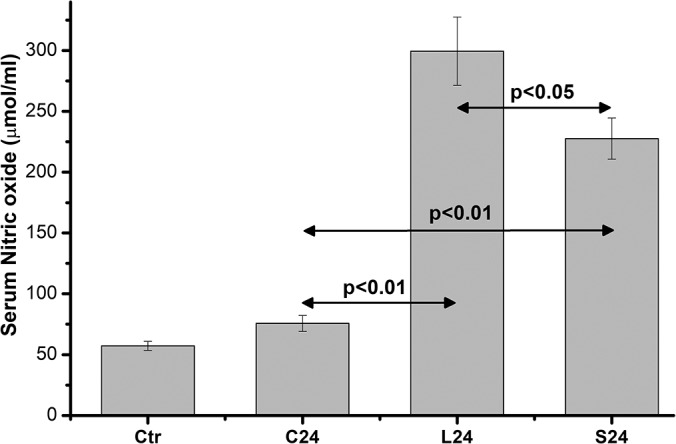
Serum NO levels. Data are presented as the mean ± standard error (SE) of 10 samples per group. C0 represents baseline measurements; C3 and C24 represent anesthesia-only subjected rats, at 3 and 24 hours; L3 and L24 represent the long-incision–subjected rats at 3 and 24 hours; and S3 and S24 represent the short-incision–subjected rats at 3 and 24 hours.
Regarding measurements in liver homogenates, only CINC1/IL-8 and TNF-α levels were analyzed. The CINC1/IL-8 levels in liver homogenates were increased in a similar pattern to serum levels, confirming the direct effect of the incision's length on the inflammatory response. Anesthesia itself, at 3 hours (C3), was found to accelerate a significant increase of CINC1/IL-8 levels in rat liver (P < .01), compared with controls, while the CINC1/IL-8 liver levels were significantly increased in L3 (from 22.34 ± 2.53 pg/mL, n = 10, to 52.032 ± 2.86 pg/mL, n = 10, P < .01) and S3 rats (from 22.34 ± 2.53, n = 10, to 40.54 ± 2.1 pg/mL, n = 10, P < .05) compared with the C3 group. This increase was significantly higher (P < .05) in the L3 group compared with the S3 group. After 24 hours, both L24 and S24 groups demonstrated a decrease in liver CINC1/IL-8 levels, but they continued to be statistically higher (P < .01 and P < .05, respectively) compared with the C24 group. Despite the higher levels of the L24 compared with the S24 group, this difference did not reach any statistically significant difference (Figure 5).
Figure 5.
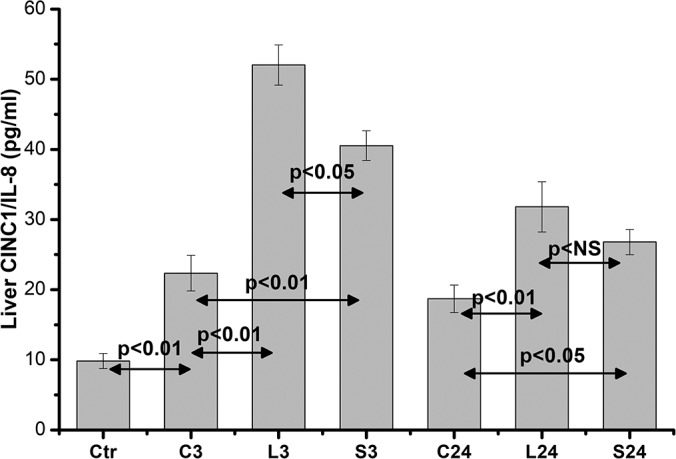
Liver homogenates CINC1/IL-8 levels. Data are presented as the mean ± standard error (SE) of 10 samples per group. C0 represents baseline measurements; C3 and C24 represent anesthesia-only subjected rats, at 3 and 24 hours; L3 and L24 represent the long-incision–subjected rats at 3 and 24 hours; and S3 and S24 represent the short-incision–subjected rats at 3 and 24 hours.
In contrast, liver TNF-α levels were inversely those of the serum. Anesthesia induced a significant increase (P < .01) in TNF-α levels in liver homogenates compared with controls. A highly significant decrease in TNF-α was observed in both L3 and S3 groups (P < .01) compared with both control and anesthesia levels, while the difference between L3 and S3 was negligible. At 24 hours, a further reduction was observed in both L24 and S24 groups that was statistically significant in relation to C24 levels (P < .01), but, of interest, this reduction was significantly lower for both L24 and S24 groups (P < .01), compared with the 3-hour liver homogenates levels (from 284.78 ± 27.89 pg/mL, n = 10, to 216.42 ± 21.19 pg/mL, n = 10, and from 292.75 ± 28.67 pg/mL, n = 10, to 198.92 ± 19.48 pg/mL, n = 10, respectively, for the L24 and S24 groups), probably indicating that the increased serum TNF-α after skin incision in blood reflects a liver TNF-α secretion (Figure 6).
Figure 6.
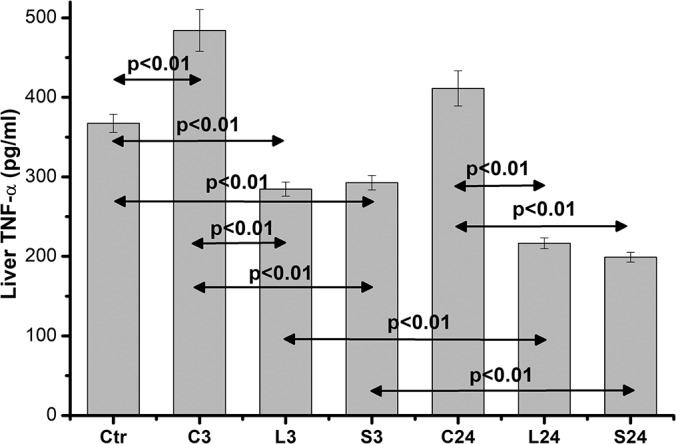
Liver homogenate TNF-α levels. Data are presented as the mean ± standard error (SE) of 10 samples per group. C0 represents baseline measurements; C3 and C24 represent anesthesia-only subjected rats, at 3 and 24 hours; L3 and L24 represent the long-incision–subjected rats at 3 and 24 hours; and S3 and S24 represent the short-incision–subjected rats at 3 and 24 hours.
DISCUSSION
In the present experimental study in Wistar rats, we demonstrated that skin-incision trauma promotes the release of inflammatory mediators – such as CINC1/IL-8, TNF-α and NO – in the rat serum compared with control animals without trauma, the magnitude of which is directly related to the length of the skin incision. Furthermore, by analyzing liver homogenates, we revealed a similar increase of CINC1/IL-8 levels, proportionate to the length of the skin incision. On the contrary, liver TNF-α values were found to be decreased in skin-trauma animals compared with the constitutive levels of the controls, probably indicating that the increased TNF-α in serum reflects liver TNF-α secretion. Although it is known that the skin-only trauma markedly affects the immune system and thus is implicated in the overall inflammatory and immune response following any surgical procedure,1 our findings further support this hypothesis and add new knowledge on the role of inflammatory mediators released after surgical incision and during the healing process. Furthermore, our results clearly support our initial hypothesis that the immune-inflammatory response induced by skin-only surgical trauma is proportionally correlated to the length of skin incision.
In recent years, many clinical studies have reported less postoperative pain, shorter hospital stay, and faster recovery in patients undergoing laparoscopic, compared with those who underwent open surgery. Thus, the hypothesis was born, that the advantages of laparoscopy are due to less mobilization of systemic inflammatory reaction (ie, the acute phase responses of the entire organism to any external stimuli, including surgical trauma). This hypothesis was verified in the early 1990s by numerous clinical and experimental studies and came back up to the forefront 15 years later, after the introduction of laparoscopic as well as the single-port surgery.10,14,25
It was thus assumed that the extent of surgical trauma causes a proportional degree of systemic inflammatory reaction, that is reliable to be considered as an indicator of tissue injury,31 although some studies argue that the main reason for the inflammatory reaction is derived from the peritoneal mesothelial cells.14–16,32,33 According to Walter Cannon's historic hypothesis, the stress response is an evolutionarily adaptive psychophysiological survival mechanism that allows the individual to react to an acute stressor34; in the case of trauma, this response appeared to be a complex process involving the immune, neuroendocrine, and metabolic factors, teleologically targeting wound healing.7,35,36
In the context of this knowledge, the idea of minimizing skin surgical trauma, from that of conventional laparotomy to laparoscopy and to single-port surgery, went further to that of natural orifice transluminal endoscopic surgery (NOTES), which means to perform operations without the need of skin incisions and instead exchange the need of skin incision with internal viscerotomies. Although it sounds reasonable, results are rather inconsistent – not because the theory is wrong but because of other factors that are involved, such as the inflammatory reaction of the peritoneum caused by visceral port-site trauma, possible enteral content spillage, and longer operative time.37–41
Our data support this concept: Serum IL-8 – found to be significantly elevated in the long-incision– versus the short-incision–subjected rats – is a neutrophil chemoattractant peptide released in the early phase of immune response to recruit neutrophils in the inflammation site.42 Specifically, open surgery was found to cause a neutrophil chemotaxis, the earliest and an essential event of the host defense system against infection, via chemokine-induced alterations43 in lymphocyte subpopulations.44 In addition, the natural killer cell–induced interactions between macrophages and lymphocytes and the increase in inflammatory mediators, such as proinflammatory cytokines and NO, have been reported as consequences of major abdominal surgery.36,45–46
Nevertheless, the surgical incision for intraperitoneal access typically involves multitissue injury, including skin, muscle, fascia, peripheral nerves, and vasculature, which add to the complexity of understanding the underlying mechanisms of damaged tissues response during surgery; however, recent in-depth research in the fields of skin neuroimmunology and neuroendocrinology strongly supports the old issue that the skin can serve as a peripheral neuroendocrine organ.17–21 Its strategic location as the barrier between the environment and the internal milieu determines its critical neuroendocrine activities, which are predominantly regulated by local cutaneous factors.19,21 Moreover, recent research portrays the skin and its appendages as both a prominent target of key stress mediators (eg, corticotropin-releasing hormone, adrenocorticotropin, cortisol, catecholamines, prolactin, substance P, and nerve growth factor) and a potent source of these immunomodulatory mediators of the stress responses.17,18,20,21
In the present study, we decided to use a skin-only trauma model for the study of acute stress response, thus avoiding the interference of all the other different tissues of the abdominal wall, which are, more or less, involved in the process of inflammatory reaction. This model, which has already been used in rodents for the investigation of the mechanisms behind postoperative pain, has demonstrated that skin-only incisions induce mechanical and heat hypersensitivity similar to levels observed with incisions that extend farther than the skin48–50; thus, cutaneous injury might drive the majority of postoperative pain and, consequently, the majority of inflammatory processes, or vice versa.42,51
Ishibashi et al52 were the only ones who experimented on a 3-cm skin incision with or without laparotomy, besides the other different types of laparotomies (1, 2, and 3 cm; 1 × 3cm; 3 cm transverse; 3 cm with rapid closure). The authors reported statistically (P < .05) lower IL-6 levels in skin-only incision compared with full-thickness laparotomy of the same length. Although these findings are not comparable to ours, because we have to evaluate the inflammatory effect of different-length skin-only incisions, the discrimination between skin incision and laparotomy adds to our findings: skin makes the difference and all the other structures follow.
Finally, another point to be mentioned is the knowledge that effective afferent neural blockade with continuous epidural local anesthetics inhibits a major part of the endocrine metabolic responses, but no important effects have been demonstrated on inflammatory or immunologic responses.53,54 These data must be taken into account when the fast-track multimodal recovery management is applied55 in laparoscopy-subjected individuals; that means epidural anesthesia should not be omitted, even in short-duration interventions, although Veenhof et al56 reported in a small study group undergoing laparoscopic surgery with fast-track care that immune function assessed by means of systemic HLA-DR expression remained at highest levels versus controls.
Existing knowledge indicates that various factors are involved in wound trauma besides the length of surgical skin incision. Regarding postoperative pain, Barabas et al51 showed that although mechanical hypersensitivity induced by skin incision is independent from transient receptor potential ankyrin 1 (TRPA1) expression, heat hypersensitivity is mainly affected by the expression of transient receptor potential vanilloid 1 (TRPV1). Fujita et al57 showed that the progression of postoperative pain was parallel with the development of inflammation at the site of the incision, suggesting that inflammatory mediators contribute to the underlying molecular mechanisms of pain. Indeed, administration of high doses of nonsteroidal anti-inflammatory drugs had an analgesic effect in a postoperative pain model in rats.58 Previous studies in mice with persistent inflammatory pain have shown that the proinflammatory cytokines IL-8 and TNF-α are involved in prefrontal synaptic transmission.59,60 In our work, studying immunological factors that are related to the skin incision postoperative inflammatory reaction, we show that IL-8 and TNF-α are significantly upregulated, in a length of incision–dependent manner. These data further support the hypothesis that proinflammatory mediator production is significant in the postoperative pain progression that is related to the length of the skin lesion.
CONCLUSION
Our findings suggest that the surgical stress induced by skin-only surgical trauma increases the production of inflammatory mediator. This increase is dependent on the length of the skin incision, while the liver was finally found to play an important role in this inflammatory reaction.
Contributor Information
Aristidis Ioannidis, Department of Surgery, Aristotle University of Thessaloniki, Thessaloniki, Greece..
Konstantinos Arvanitidis, Laboratory of Pharmacology, Democritus University of Thrace, Alexandroupolis, Greece..
Eirini Filidou, Laboratory of Pharmacology, Democritus University of Thrace, Alexandroupolis, Greece..
Vassilis Valatas, Gastroenterology Laboratory, Medical Department, University of Crete, Heraklion, Greece..
George Stavrou, Department of Surgery, Aristotle University of Thessaloniki, Thessaloniki, Greece..
Antonios Michalopoulos, Department of Surgery, Aristotle University of Thessaloniki, Thessaloniki, Greece..
George Kolios, Laboratory of Pharmacology, Democritus University of Thrace, Alexandroupolis, Greece..
Katerina Kotzampassi, Department of Surgery, Aristotle University of Thessaloniki, Thessaloniki, Greece..
References:
- 1. Menger MD, Vollmar B. Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg. 2004;389:475–484. [DOI] [PubMed] [Google Scholar]
- 2. Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–117. [DOI] [PubMed] [Google Scholar]
- 3. Kohl BA, Deutschman CS. The inflammatory response to surgery and trauma. Curr Opin Crit Care. 2006;12:325–332. [DOI] [PubMed] [Google Scholar]
- 4. Aller MA, Arias JL, Nava MP, Arias J. Posttraumatic inflammation is a complex response based on the pathological expression of the nervous, immune, and endocrine functional systems. Exp Biol Med (Maywood). 2004;229:170–181. [DOI] [PubMed] [Google Scholar]
- 5. Watt DG, Horgan PG, McMillan DC. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery. 2015;157:362–380. [DOI] [PubMed] [Google Scholar]
- 6. Buunen M, Gholghesaei M, Veldkamp R, Meijer DW, Bonjer HJ, Bouvy ND. Stress response to laparoscopic surgery: a review. Surg Endosc. 2004;18:1022–1028. [DOI] [PubMed] [Google Scholar]
- 7. Grande M, Tucci GF, Adorisio O, et al. Systemic acute-phase response after laparoscopic and open cholecystectomy. Surg Endosc. 2002;16:313–316. [DOI] [PubMed] [Google Scholar]
- 8. Sista F, Schietroma M, Santis GD, et al. Systemic inflammation and immune response after laparotomy versus laparoscopy in patients with acute cholecystitis, complicated by peritonitis. World J Gastrointest Surg. 2013;5:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pascual M, Alonso S, Pares D, et al. Randomized clinical trial comparing inflammatory and angiogenic response after open versus laparoscopic curative resection for colonic cancer. Br J Surg. 2011;98:50–59. [DOI] [PubMed] [Google Scholar]
- 10. Novitsky YW, Litwin DE, Callery MP. The net immunologic advantage of laparoscopic surgery. Surg Endosc. 2004;18:1411–1419. [DOI] [PubMed] [Google Scholar]
- 11. Evans C, Galustian C, Kumar D, et al. Impact of surgery on immunologic function: comparison between minimally invasive techniques and conventional laparotomy for surgical resection of colorectal tumors. Am J Surg. 2009;197:238–245. [DOI] [PubMed] [Google Scholar]
- 12. Ito Y, Oda M, Tsunezuka Y, et al. Reduced perioperative immune response in video-assisted versus open surgery in a rat model. Surg Today. 2009;39:682–688. [DOI] [PubMed] [Google Scholar]
- 13. Kim TK, Yoon JR. Comparison of the neuroendocrine and inflammatory responses after laparoscopic and abdominal hysterectomy. Korean J Anesthesiol. 2010;59:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moehrlen U, Schwoebel F, Reichmann E, Stauffer U, Gitzelmann CA, Hamacher J. Early peritoneal macrophage function after laparoscopic surgery compared with laparotomy in a mouse mode. Surg Endosc. 2005;19:958–963. [DOI] [PubMed] [Google Scholar]
- 15. Luk JM, Tung PH, Wong KF, Chan KL, Law S, Wong J. Laparoscopic surgery induced interleukin-6 levels in serum and gut mucosa: implications of peritoneum integrity and gas factors. Surg Endosc. 2009;23:370–376. [DOI] [PubMed] [Google Scholar]
- 16. Sammour T, Kahokehr A, Soop M, Hill AG. Peritoneal damage: the inflammatory response and clinical implications of the neuro-immuno-humoral axis. World J Surg. 2010;34:704–720. [DOI] [PubMed] [Google Scholar]
- 17. Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. [DOI] [PubMed] [Google Scholar]
- 19. Slominski A. Neuroendocrine system of the skin. Dermatology. 2005;211:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013;34:827–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends Immunol. 2006;27:32–39. [DOI] [PubMed] [Google Scholar]
- 22. Hill CE, Harrison BJ, Rau KK, et al. Skin incision induces expression of axonal regeneration-related genes in adult rat spinal sensory neurons. J Pain. 2010;11:1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rivest S. How circulating cytokines trigger the neural circuits that control the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 2001;26:761–788. [DOI] [PubMed] [Google Scholar]
- 24. Stenberg C, Ovlisen K, Svendsen O, Lauritzen B. Effect of local anaesthesia on neuronal c-fos expression in the spinal dorsal horn and hypothalamic paraventricular nucleus after surgery in rats. Basic Clin Pharmacol Toxicol. 2005;96:381–386. [DOI] [PubMed] [Google Scholar]
- 25. Lykkegaard K, Lauritzen B, Tessem L, Weikop P, Svendsen O. Local anaesthetics attenuates spinal nociception and HPA-axis activation during experimental laparotomy in pigs. Res Vet Sci. 2005;79:245–251. [DOI] [PubMed] [Google Scholar]
- 26. Kotzampassi K, Kolios G, Manousou P, et al. Oxidative stress due to anesthesia and surgical trauma: importance of early enteral nutrition. Mol Nutr Food Res. 2009;53:770–779. [DOI] [PubMed] [Google Scholar]
- 27. Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science 1992;258:1898–1902. [DOI] [PubMed] [Google Scholar]
- 28. Kolios G, Valatas V, Manousou P, Xidakis C, Notas G, Kouroumalis E. Nitric oxide and MCP-1 regulation in LPS activated rat Kupffer cells. Mol Cell Biochem. 2008;319:91–98. [DOI] [PubMed] [Google Scholar]
- 29. Kolios G, Kotzampassi K, Manousou P, et al. Enteral nutrition affects nitric oxide production in peripheral blood and liver after a postoperative lipopolysaccharide-induced endotoxemia in rats. Nutrition. 2007;23:575–581. [DOI] [PubMed] [Google Scholar]
- 30. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 31. Okholm C, Goetze JP, Svendsen LB, Achiam MP. Inflammatory response in laparoscopic versus. open surgery for gastric cancer. Scand J Gastroenterol. 2014;49:1027–1034. [DOI] [PubMed] [Google Scholar]
- 32. Jesch NK, Kuebler JF, Nguyen H, et al. Laparoscopy versus minilaparotomy and full laparotomy preserves circulatory but not peritoneal and pulmonary immune responses. J Pediatr Surg. 2006;41:1085–1092. [DOI] [PubMed] [Google Scholar]
- 33. Suematsu T, Hirabayashi Y, Shiraishi N, Adachi Y, Kitamura H, Kitano S. Morphology of the murine peritoneum after pneumoperitoneum versus laparotomy. Surg Endosc. 2001;15:954–958. [DOI] [PubMed] [Google Scholar]
- 34. Cannon WB. The emergency function of the adrenal medulla in pain and the major emotions. Am J Physiol 1914;33:356–372. [Google Scholar]
- 35. Roupe KM, Nybo M, Sjobring U, Alberius P, Schmidtchen A, Sorensen OE. Injury is a major inducer of epidermal innate immune responses during wound healing. J Invest Dermatol. 2010;130:1167–1177. [DOI] [PubMed] [Google Scholar]
- 36. Belizon A, Balik E, Feingold DL, et al. Major abdominal surgery increases plasma levels of vascular endothelial growth factor: open more so than minimally invasive methods. Ann Surg. 2006;244:792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vieira JP, Linhares MM, Caetano EM, Jr, et al. Evaluation of the clinical and inflammatory responses in exclusively NOTES transvaginal cholecystectomy versus laparoscopic routes: an experimental study in swine. Surgical endoscopy. 2012;26:3232–3244. [DOI] [PubMed] [Google Scholar]
- 38. Bergstrom M, Azadani A, Falk P, Park PO. Stress response and well-being after open, laparoscopic, and NOTES transgastric uterine horn resection in a randomized porcine model. Surgical endoscopy. 2014;28:2421–2427. [DOI] [PubMed] [Google Scholar]
- 39. Arroyo Vazquez J, Bergstrom M, Dot J, et al. Surgical trauma caused by different abdominal access routes: comparison of open surgical, laparoscopic, and NOTES transgastric techniques in a porcine model. J Laparoendosc Adv Surg Techn Part A. 2016;26:511–516. [DOI] [PubMed] [Google Scholar]
- 40. Sood V, Collins C, Harrington S, et al. Transgastric endoscopic pneumoperitoneum versus laparoscopy: effects on host systemic and peritoneal inflammatory responses in a porcine model. Surg Endosc. 2012;26:189–196. [DOI] [PubMed] [Google Scholar]
- 41. Guarner-Argente C, Martinez-Palli G, Navarro-Ripoll R, et al. Inflammatory impact of NOTES peritoneoscopy is not different from that of laparoscopy: a randomized comparative study in a survival porcine model. Surg Endosc. 2012;26:374–380. [DOI] [PubMed] [Google Scholar]
- 42. Kolios G, Robertson DAF, Jordan NJ, et al. Interleukin-8 production by the human colon epithelial cell line HT-29: modulation by interleukin −13. Br J Pharmacol 1996;119:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gessler P, Pretre R, Hohl V, Rousson V, Fischer J, Dahinden C. CXC-chemokine stimulation of neutrophils correlates with plasma levels of myeloperoxidase and lactoferrin and contributes to clinical outcome after pediatric cardiac surgery. Shock. 2004;22:513–520. [DOI] [PubMed] [Google Scholar]
- 44. Vaughan-Shaw PG, Rees JR, King AT. Neutrophil lymphocyte ratio in outcome prediction after emergency abdominal surgery in the elderly. Int J Surg. 2012;10:157–162. [DOI] [PubMed] [Google Scholar]
- 45. Tai LH, de Souza CT, Belanger S, et al. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res. 2013;73:97–107. [DOI] [PubMed] [Google Scholar]
- 46. Novitsky YW, Czerniach DR, Kaban GK, et al. Immunologic effects of hand-assisted surgery on peritoneal macrophages: comparison to open and standard laparoscopic approaches. Surgery. 2006;139:39–45. [DOI] [PubMed] [Google Scholar]
- 47. Nakamoto T, Yoshimura H, Honda T, et al. Treatments for the activating macrophages that reduces surgical stress and postoperative mortalities from bacterial infections and tumor metastases. In Vivo. 2007;21:357–364. [PubMed] [Google Scholar]
- 48. Pogatzki EM, Raja SN. A mouse model of incisional pain. Anesthesiology. 2003;99:1023–1027. [DOI] [PubMed] [Google Scholar]
- 49. Xu J, Brennan TJ. Comparison of skin incision versus. skin plus deep tissue incision on ongoing pain and spontaneous activity in dorsal horn neurons. Pain. 2009;144:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xu J, Brennan TJ. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology. 2010;112:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barabas ME, Stucky CL. TRPV1, but not TRPA1, in primary sensory neurons contributes to cutaneous incision-mediated hypersensitivity. Mol Pain. 2013;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ishibashi S, Takeuchi H, Fujii K, Shiraishi N, Adachi Y, Kitano S. Length of laparotomy incision and surgical stress assessed by serum IL-6 level. Injury. 2006;37:247–251. [DOI] [PubMed] [Google Scholar]
- 53. Kehlet H. Manipulation of the metabolic response in clinical practice. World J Surg. 2000;24:690–695. [DOI] [PubMed] [Google Scholar]
- 54. Ahlers O, Nachtigall I, Lenze J, et al. Intraoperative thoracic epidural anaesthesia attenuates stress-induced immunosuppression in patients undergoing major abdominal surgery. Br J Anaesth. 2008;101:781–787. [DOI] [PubMed] [Google Scholar]
- 55. Kehlet H. Fast-track colorectal surgery. Lancet. 2008;371:791–793. [DOI] [PubMed] [Google Scholar]
- 56. Veenhof AA, Vlug MS, van der Pas MH, et al. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg. 2012;255:216–221. [DOI] [PubMed] [Google Scholar]
- 57. Fujita I, Okumura T, Sakakibara A, Kita Y. Involvement of inflammation in severe post-operative pain demonstrated by pre-surgical and post-surgical treatment with piroxicam and ketorolac. J Pharmacy Pharmacol. 2012;64:747–755. [DOI] [PubMed] [Google Scholar]
- 58. Yamamoto T, Sakashita Y, Nozaki-Taguchi N. Anti-allodynic effects of oral COX-2 selective inhibitor on postoperative pain in the rat. Can J Anaesth. 2000;47:354–360. [DOI] [PubMed] [Google Scholar]
- 59. Jia D, Gao GD, Liu Y, et al. TNF-alpha involves in altered prefrontal synaptic transmission in mice with persistent inflammatory pain. Neurosci Lett. 2007;415:1–5. [DOI] [PubMed] [Google Scholar]
- 60. Cui GB, An JZ, Zhang N, Zhao MG, Liu SB, Yi J. Elevated interleukin-8 enhances prefrontal synaptic transmission in mice with persistent inflammatory pain. Mol Pain. 2012;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]



