Abstract
Ascorbate peroxidase (APX) is a redox enzyme of the trypanothione pathway that converts hydrogen peroxide (H2O2) into water molecules. In the present study, the APX gene was overexpressed in Leishmania braziliensis to investigate its contribution to the trivalent antimony (SbIII)-resistance phenotype. Western blot results demonstrated that APX-overexpressing parasites had higher APX protein levels in comparison with the wild-type line (LbWTS). APX-overexpressing clones showed an 8-fold increase in the antimony-resistance index over the parental line. In addition, our results indicated that these clones were approximately 1.8-fold more tolerant to H2O2 than the LbWTS line, suggesting that the APX enzyme plays an important role in the defence against oxidative stress. Susceptibility tests revealed that APX-overexpressing L. braziliensis lines were more resistant to isoniazid, an antibacterial agent that interacts with APX. Interestingly, this compound enhanced the anti-leishmanial SbIII effect, indicating that this combination represents a good strategy for leishmaniasis chemotherapy. Our data demonstrate that APX enzyme is involved in the development of L. braziliensis antimony-resistance phenotype and may be an attractive therapeutic target in the design of new strategies for leishmaniasis treatment.
Key words: Leishmania braziliensis, ascorbate peroxidase, antimony resistance, oxidative defence
Leishmaniasis is an important neglected tropical disease caused by different species of unicellular protozoan parasites belonging to the Leishmania genus. The three main clinical manifestations of this illness are cutaneous (CL), mucocutaneous (MCL) and visceral (VL). 1 Leishmania (Viannia) braziliensis, which is broadly distributed in the Americas, is the aetiological agent of both CL and MCL. 2 It is estimated that 700,000 to one million new cases of leishmaniasis and 20,000 to 30,000 deaths occur annually. 3
Chemotherapy is the main form of disease control, since there is no human vaccine available for use. 4 Thus, pentavalent antimony (SbV)-based compounds (meglumine antimoniate - Glucantime®, and sodium stibogluconate - Pentostam®) for several decades have been the principal drugs employed to treat all disease forms in many countries. 5 Nevertheless, the mode of antimony action has not been completely elucidated. It is accepted that SbV is a prodrug that is reduced to the trivalent (SbIII) form, which has leishmanicidal effects against amastigote and promastigote forms of the parasite. 6 Some studies have indicated that SbV inhibits glycolysis and fatty acid oxidation. 7
Many cases of antimony resistance have been reported in different countries, especially in Bihar (India), where the treatment failure rates for antimonials reached 65%. 8 Several mechanisms of resistance to these drugs have been proposed in the literature, such as a lower rate of drug reduction/activation, a decreased uptake or an increased efflux/sequestration of active molecules, gene amplification and higher activity of repair mechanisms due to the drug-induced damage. 9
Ascorbate peroxidase (APX) is a redox enzyme of the trypanothione pathway that converts hydrogen peroxide (H2O2) into water molecules, thus regulating oxidative stress in Leishmania and avoiding damage to the parasite cells. 10 Previous studies demonstrated that Trypanosoma cruzi extracts contained ascorbate-dependent peroxidase activity. 11 , 12 , 13 Nogueira et al. 14 showed that the APX level was increased in benznidazole-resistant T. cruzi populations. APX is an important factor that controls metacyclogenesis and apoptosis in L. major. 15 Interestingly, Mukherjee et al. 16 demonstrated an intra-chromosomal amplification of a sub-telomeric locus on chromosome 34, a region coding for APX, in antimony-resistant L. major. Since APX is absent in humans and it presents an important role in the antioxidant defence of the trypanosomatids, this enzyme may be considered an excellent drug target for chemotherapy of these parasites. 17
Considering a variety of resistance mechanisms to antimonials in Leishmania, it has become necessary to discover new targets to develop other therapeutic strategies to control the disease. Thus, the aim of this work was to overexpress the APX gene in L. braziliensis to investigate the contribution of this enzyme to the antimony-resistance phenotype of this parasite.
Promastigote forms of L. braziliensis (MHOM/BR/75/M2904) were grown at 26ºC in M199 medium supplemented as previously described. 18 All analyses were performed with parasites in the exponential growth phase.
To generate APX-overexpressing lines, a 918-bp fragment corresponding to the APX-coding region (TriTrypDB accession number LbrM.20.0150) was amplified with Pfx DNA polymerase (Invitrogen) from L. braziliensis genomic DNA using the forward primer:5’-TGGATCCCCACCATGACCGGTACCTCGCGG-3’ and the reverse primer: 5’-TTGGATCCTTAGCATTCCACTGCCGGTG-3’. The underlined sequences correspond to the BamHI restriction site. The next steps were performed as previously reported. 19 Briefly, the APX amplicons were cloned into the pGEM-T Easy® vector (Promega, Madison, WI, USA), digested with BamHI enzyme and introduced into the dephosphorylated pIR1BSD expression vector (kindly provided by Dr Stephen Beverley, Washington University, USA). After that, the pIR1BSD (empty vector) and pIR1BSD-APX constructs were linearised by SwaI digestion, electroporated into wild-type L. braziliensis, and the colonies were obtained on semisolid M199 medium containing 10 µg/mL blasticidin (BSD). After two weeks, genomic DNA from transfected clonal lines was subjected to PCR tests with primers specific for the BSD marker that confers resistance to blasticidin. The results indicated the presence of a 399-bp fragment in all blasticidin-resistant clones (data not shown), confirming successful transfection.
Western blot assays were carried out to test whether the clones overexpressed APX protein. Protein extracts from parasites were obtained according to the protocol previously described. 19 Total proteins (20 µg) were separated by electrophoresis on 12% SDS-polyacrylamide gel, electrotransferred onto nitrocellulose membrane (Bio-Rad, Hercules, CA, USA), blocked, washed and probed with rabbit polyclonal T. cruzi anti-APX antibody (1:20) 14 , for 12 h at 4ºC in blocking solution. The blots were washed and incubated with horseradish peroxidase-conjugated anti-rabbit IgG antibody (1:1,000) (GE Healthcare), washed, exposed to ECL Plus chemiluminescent substrate (GE Healthcare) and revealed by ImageQuant LAS 4000 (GE Healthcare). It is important to note that the APX amino acid sequences of L. braziliensis and T. cruzi had 63% identity (data not shown). Western blot results showed that in all Leishmania samples evaluated, the T. cruzi anti-APX antibody recognized a 34 kDa polypeptide, corresponding to the expected size of APX protein (Fig. 1A). Normalisation of the results with the monoclonal anti-α-tubulin antibody (1:15,000) (Sigma, St. Louis, USA) revealed that the APX protein level was 5-fold higher in transfected clones 4 and 13 from L. braziliensis than in the wild-type or transfected with empty vector (controls) (Fig. 1A).
Fig. 1: ascorbate peroxidase (APX) protein levels and trivalent antimony (SbIII) susceptibility assay of Leishmania braziliensis clonal lines untransfected or transfected with the APX gene. (A) Proteins (20 µg) were separated on a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto nitrocellulose membranes. The blots were probed with rabbit polyclonal Trypanosoma cruzi anti-APX antibody (1:20) and developed using the ECL Plus kit. The blots were normalised using the anti-α-tubulin monoclonal antibody (1:15,000). The band intensities were quantified using GelAnalyzer 2010 software. The ratio shown is relative to the L. (V.) braziliensis wild-type (LbWTS) band (clones/WTS). (B) Parasites were cultured in the absence or presence of increasing SbIII concentrations (0.6 to 149.7 µM) for 48 h, and the percentage of relative growth was determined using a Z1 Coulter Counter. Mean values ± standard deviations from three independent experiments performed in triplicate are indicated. Data were analysed by two-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test using GraphPad Prism 5.0 software. Statistically different values are denoted as follows: *p < 0.05; and ***p < 0.001.
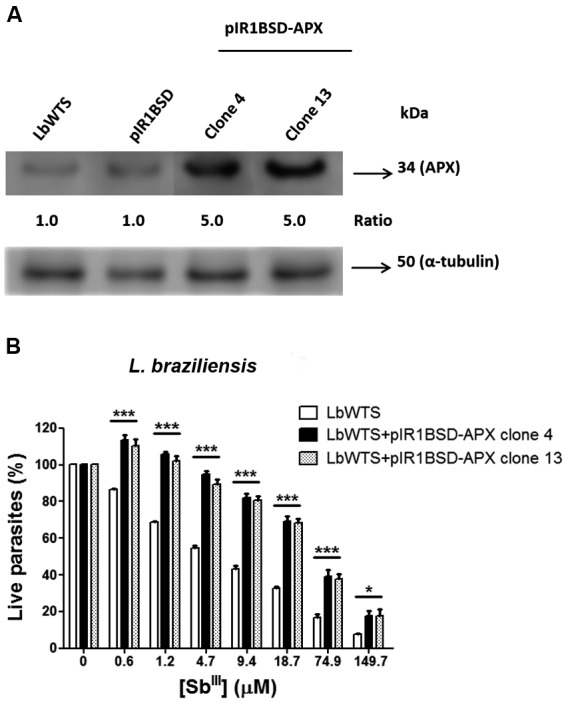
Promastigotes of wild-type L. braziliensis and APX-overexpressing cell lines were incubated in M199 medium at 2 x 106 cells/mL in 24-well plates in the absence or presence of increasing concentrations (0.6-149.7 μM) of potassium antimonyl tartrate (SbIII) (Sigma-Aldrich, St. Louis, MO, USA) for 48 h. The effective concentration required to decrease the growth by 50% (EC50) was determined using a model Z1 Coulter Counter (Beckman Coulter, Fullerton, CA, USA). The EC50 values were obtained from three independent measurements in triplicate, using the linear interpolation method. 19 The data indicated that the SbIII EC50 of the untransfected L. braziliensis line (LbWTS) was 7.3 μM, whereas clones 4 and 13 had EC50 values of 58 μM and 55.6 μM, respectively (Fig. 1B). This result demonstrates that these clones were approximately 8-fold more resistant to trivalent antimony in comparison with the untransfected control (LbWTS).
APX-overexpressing L. braziliensis clones were also subjected to susceptibility assays with H2O2 to analyse their tolerance to oxidative stress produced by several concentrations (100-400 μM) of this compound during a 48 h incubation. Our results revealed that the LbWTS line had an H2O2 EC50 of 184 μM, whereas LbAPX clones 4 and 13 had EC50 values of 324 μM and 304 μM, respectively (Fig. 2A). These data indicate that the resistance index for these clones was approximately 1.8-fold higher than for the wild-type line, suggesting that APX enzyme plays an important role in the defence against oxidative stress in L. braziliensis.
Fig. 2: in vitro tolerance to exogenous hydrogen peroxide, isoniazid EC50 for wild-type and ascorbate peroxidase (APX)-overexpressing Leishmania braziliensis lines, and the effect of isoniazid on the growth of L. braziliensis lines upon trivalent antimony (SbIII) exposure. Parasites were incubated in M199 medium in the absence or presence of different concentrations of (A) H2O2 (100 to 400 µM) and (B) isoniazid (200 to 10,000 µM). For competition tests (C), cells were exposed to the EC50 of SbIII 7.3, 58 and 55.6 μM for the L. (V.) braziliensis wild-type (LbWTS) and APX-overexpressing clones 4 and 13, respectively and the EC50 of isoniazid (563, 838 and 707 μM for the LbWTS and APX-overexpressing clones 4 and 13, respectively) independently or in combination, followed by incubation for 48 h. The percentage of relative growth was determined using a Z1 Coulter Counter. Mean values ± standard deviations from three independent experiments performed in triplicate are indicated. Data were analysed by one-way or two-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test using GraphPad Prism 5.0 software. Statistically different values are denoted as follows: *p < 0.05; **p < 0.01; and ***p < 0.001.

The amino acid sequence of APX was used for a search of possible drugs against this enzyme in DrugBanK (www.drugbank.ca), which returned the antibacterial agent isoniazid (DB00951). This drug, a synthetic derivative of isonicotinic acid, is used in the treatment of tuberculosis. Isoniazid interferes with the mycolate-synthetase enzyme, which is important in the synthesis of mycolic acid, a fundamental component of the mycobacteria cell wall. 20 Other mechanisms of action have been uncovered, such as chelation of metallic ions necessary for mycobacterial metabolism and interference in glucose metabolism and cellular respiration of mycobacteria. 21 Another study reported the first crystal structures of isoniazid complexes with APX. 22 Isoniazid is also part of the drug cocktail used by HIV-positive patients. It is used as a prophylaxis against tuberculosis, since these patients are frequently exposed to M. tuberculosis and their immune systems are deficient. 23 Thus, we incubated the wild-type L. braziliensis line and parasites transfected with the pIR1BSD-APX construct with increasing concentrations of isoniazid (200-10,000 μM) for 48 h and determined the isoniazid EC50 for these parasites. Clones 4 and 13 overexpressing APX had EC50 values of 838 and 707 μM, respectively (Fig. 2B). These values were 1.5- and 1.3-fold higher in comparison with the LbWTS line, which presented an isoniazid EC50 of 563 μM, demonstrating that both clones were more resistant to this drug. This result shows that overexpression of APX enzyme protects parasites from a lethal effect of the inhibitor isoniazid. Furthermore, the isoniazid effect on the growth of L. braziliensis lines exposed to SbIII was also evaluated in this study. Interestingly, the combination of these two drugs increased the leishmanicidal activity against LbWTS and APX-overexpressing clones in comparison to those lines incubated with SbIII or isoniazid alone (Fig. 2C). The anti-leishmanial effect was more pronounced in APX-overexpressing L. braziliensis clones 4 and 13, which exhibited a growth inhibition of 82% and 86%, respectively. On the other hand, the growth inhibition in the LbWTS line was 73%. These data indicate that the combination of SbIII and isoniazid produced a higher lethal effect in parasites overexpressing the APX enzyme, which are more resistant to SbIII and isoniazid, than the wild-type parasites. Therefore, the combination of these two drugs might represent a good strategy to be further evaluated for chemotherapy against leishmaniasis.
Trypanosomatids are frequently exposed to different reactive oxygen species (ROS). These parasites have a peculiar mechanism of antioxidant defence based on the trypanothione reductase system, which maintains an intracellular reducing environment. 17 This defence machinery is composed of many enzymes distributed in diverse cellular compartments and activated against various oxidants. 24 APX is a relevant mitochondrial enzyme involved in detoxification of H2O2 into water molecules. In the present study, transfection of the APX gene in L. braziliensis generated clones overexpressing APX protein, as shown by western blot analyses. In addition, functional assays demonstrated that APX overexpression rendered L. braziliensis clones 4 and 13 more resistant to SbIII. This result indicates that greater amounts of APX enzyme are necessary to reduce the toxic effects produced by the drug and to prevent parasite death due to perturbations to its redox potential. Thus, Wyllie et al. 25 suggested that SbIII causes alterations in the thiol redox potential of Leishmania, which can lead to cell death by oxidative stress. Interestingly, APX overexpression in L. major provoked the depletion of mitochondrial ROS burden and resistance to cardiolipin oxidation. 26 Kumar et al. 10 showed that APX overexpression in the amphotericin B-resistant L. donovani line rescues cells from the deleterious effect of oxidative stress.
We also investigated whether APX overexpression in L. braziliensis protects the parasite from the damage caused by increasing concentrations of exogenous H2O2. Our results indicated that clonal lines overexpressing the APX enzyme were less susceptible to H2O2 than the wild-type L. braziliensis line. Dolai et al. 26 demonstrated that overexpression of this enzyme in L. major decreased H2O2-induced lethality, corroborating our data. Additionally, APX overexpression protects this Leishmania species against apoptosis induced by oxidative stress generated by H2O2 or camptothecin treatment. 27 Interestingly, Pal et al. 15 showed that deletion of APX in L. major renders cells more susceptible to H2O2. Nogueira et al. 14 revealed that benznidazole-resistant populations of T. cruzi presented higher tolerance to exogenous H2O2 than their susceptible counterparts. Andrade and Murta 28 showed that L. braziliensis lines overexpressing tryparedoxin peroxidase (TXNPx), an enzyme that is also involved in antioxidant defence, were more tolerant to H2O2 when compared with the untransfected parental line. These data reinforce the notion that the APX and TXNPx enzymes are needed for detoxifying peroxidase activity, indicating their essential role in the defence against oxidative stress in trypanosomatids.
Isoniazid was found to be a potential inhibitor of APX enzyme during our search in DrugBank. This compound is a bactericidal agent that is active against organisms of the genus Mycobacterium and is used to treat all forms of tuberculosis (www.drugbank.ca/drugs/DB00951). A previous study reported that isoniazid can become an inhibitor of peroxidase activity in mutant soybean APX, demonstrating that point mutations in the enzyme active site can contribute to drug resistance. 22 Our results demonstrated that overexpression of APX enzyme confers resistance to isoniazid. Surprisingly, this drug enhanced the anti-leishmanial effect of SbIII, mainly against L. braziliensis clones overexpressing APX. This combination of drugs might represent a good strategy to be further elaborated for leishmaniasis chemotherapy. Interestingly, Amorim et al. 29 showed that pentacyano(isoniazid)ferrate(II), an organometallic compound analogue of isoniazid, is efficient at inhibiting proliferation of L. braziliensis promastigote and amastigote forms, suggesting that it is a possible safe drug for treatment of infection caused by this parasite.
This study is the first to show that isoniazid has an effect against L. braziliensis. In summary, our study evidences that overexpression of APX enzyme is involved in the mechanism of L. braziliensis SbIII-resistance. Importantly, earlier studies reported by our research group also indicated that other enzymes, e.g., tryparedoxin peroxidase and iron superoxide dismutase-A, have significant functions in the antioxidant defence and in the maintenance of antimony resistance in Leishmania. 28 , 30 Thus, our data contribute to understanding the participation of APX enzyme in the SbIII-resistance mechanism and direct the development of new strategies for leishmaniasis chemotherapy.
ACKNOWLEDGEMENTS
To the Program for Technological Development in Tools for Health-PDTIS-FIOCRUZ for use of its facilities and Farmanguinhos (FIOCRUZ-RJ, Brazil) for kindly providing isoniazid.
Footnotes
Financial support: CNPq, FAPEMIG (CBB-PPM00610/15). SMFM and DSM are research fellows supported by CNPq (150804/2017-2).
REFERENCES
- 1.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW. Visceral leishmaniasis what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5(11):873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 2.David CV, Craft N. Cutaneous and mucocutaneous leishmaniasis. Dermatol Ther. 2009;22(6):491–502. doi: 10.1111/j.1529-8019.2009.01272.x. [DOI] [PubMed] [Google Scholar]
- 3.Organization World Health. Leishmaniasis. 2018. http://www.who.int/news-room/fact-sheets/detail/leishmaniasis [Google Scholar]
- 4.Kumar R, Engwerda C. Vaccines to prevent leishmaniasis. Clin Transl Immunology. 2014;3(3):e13. doi: 10.1038/cti.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh OP, Singh B, Chakravarty J, Sundar S. Current challenges in treatment options for visceral leishmaniasis in India a public health perspective. Infect Dis Poverty. 2016;5:19–19. doi: 10.1186/s40249-016-0112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frézard F, Demicheli C, Ribeiro RR. Pentavalent antimonials new perspectives for old drugs. Molecules. 2009;14(7):2317–2336. doi: 10.3390/molecules14072317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman JD, Gallalee JV, Best JM. Sodium stibogluconate (Pentostam) inhibition of glucose catabolism via the glycolytic pathway and fatty acid beta-oxidation in Leishmania mexicana amastigotes. Biochem Pharmacol. 1987;36(2):197–201. doi: 10.1016/0006-2952(87)90689-7. [DOI] [PubMed] [Google Scholar]
- 8.Stauch A, Duerr HP, Dujardin JC, Vanaerschot M, Sundar S, Eichner M. Treatment of visceral leishmaniasis model-based analyses on the spread of antimony-resistant L. donovani in Bihar, India. PLoS Negl Trop Dis. 2012;6(12):e1973. doi: 10.1371/journal.pntd.0001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19(1):111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Das S, Purkait B, Sardar AH, Ghosh AK, Dikhit MR. Ascorbate peroxidase, a key molecule regulating amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob Agents Chemother. 2014;58(10):6172–6184. doi: 10.1128/AAC.02834-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Docampo R, de Boiso JF, Boveris A, Stoppani AO. Localization of peroxidase activity in Trypanosoma cruzi microbodies. Experientia. 1976;32(8):972–975. doi: 10.1007/BF01933918. [DOI] [PubMed] [Google Scholar]
- 12.Boveris A, Sies H, Martino EE, Docampo R, Turrens JF, Stoppani AO. Deficient metabolic utilization of hydrogen peroxide in Trypanosoma cruzi. Biochem J. 1980;188(3):643–648. doi: 10.1042/bj1880643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark D, Albrecht M, Arevalo J. Ascorbate variations and dehydroascorbate reductase activity in Trypanosoma cruzi epimastigotes and trypomastigotes. Mol Biochem Parasitol. 1994;66(1):143–145. doi: 10.1016/0166-6851(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 14.Nogueira FB, Rodrigues JF, Correa MM, Ruiz JC, Romanha AJ, Murta SM. The level of ascorbate peroxidase is enhanced in benznidazole-resistant populations of Trypanosoma cruzi and its expression is modulated by stress generated by hydrogen peroxide. Mem Inst Oswaldo Cruz. 2012;107(4):494–502. doi: 10.1590/s0074-02762012000400009. [DOI] [PubMed] [Google Scholar]
- 15.Pal S, Dolai S, Yadav RK, Adak S. Ascorbate peroxidase from Leishmania major controls the virulence of infective stage of promastigotes by regulating oxidative stress. PLoS One. 2010;5(6):e11271. doi: 10.1371/journal.pone.0011271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee A, Boisvert S, Monte-Neto RL, Coelho AC, Raymond F, Mukhopadhyay R. Telomeric gene deletion and intrachromosomal amplification in antimony-resistant Leishmania. Mol Microbiol. 2013;88(1):189–202. doi: 10.1111/mmi.12178. [DOI] [PubMed] [Google Scholar]
- 17.Turrens JF. Oxidative stress and antioxidant defenses a target for the treatment of diseases caused by parasitic protozoa. Mol Aspects Med. 2004;25(1-2):211–220. doi: 10.1016/j.mam.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Liarte DB, Murta SMF. Selection and phenotype characterization of potassium antimony tartrate-resistant populations of four New World Leishmania species. Parasitol Res. 2010;107(1):205–212. doi: 10.1007/s00436-010-1852-8. [DOI] [PubMed] [Google Scholar]
- 19.Moreira DS, Murta SMF. Involvement of nucleoside diphosphate kinase b and elongation factor 2 in Leishmania braziliensis antimony resistance phenotype. Parasit Vectors. 2016;9(1):641–641. doi: 10.1186/s13071-016-1930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bisaglia JB, Santussi WM, Militao AG, Gomes AP, Oliveira PC, Batista RS. Atualização terapêutica em tuberculose principais efeitos adversos dos fármacos. Bol Pneumol Sanit. 2003;11(2):53–59. [Google Scholar]
- 21.Saúde/FIOCRUZ Farmanguinhos/Ministério da. Isoniazida. http://www.far.fiocruz.br/wp-content/uploads/2016/09/Isoniazida
- 22.Metcalfe C, Macdonald IK, Murphy EJ, Brown KA, Raven EL, Moody PC. The tuberculosis prodrug isoniazid bound to activating peroxidases. J Biol Chem. 2008;283(10):6193–6200. doi: 10.1074/jbc.M707412200. [DOI] [PubMed] [Google Scholar]
- 23.Ayele HT, Mourik MSMV, Debray TPA, Bonten MJM. Isoniazid prophylactic therapy for the prevention of tuberculosis in HIV infected adults a systematic review and meta-analysis of randomized trials. PLoS One. 2015;10(11):e0142290. doi: 10.1371/journal.pone.0142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castro H, Romao S, Carvalho S, Teixeira F, Sousa C, Tomás AM. Mitochondrial redox metabolism in trypanosomatids is independent of tryparedoxin activity. PLoS One. 2010;5(9):e12607. doi: 10.1371/journal.pone.0012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wyllie S, Cunningham ML, Fairlamb AH. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J Biol Chem. 2004;279(38):39925–39932. doi: 10.1074/jbc.M405635200. [DOI] [PubMed] [Google Scholar]
- 26.Dolai S, Yadav RK, Pal S, Adak S. Leishmania major ascorbate peroxidase overexpression protects cells against reactive oxygen species-mediated cardiolipin oxidation. Free Radic Biol Med. 2008;45(11):1520–1529. doi: 10.1016/j.freeradbiomed.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 27.Dolai S, Yadav RK, Pal S, Adak S. Overexpression of mitochondrial Leishmania major ascorbate peroxidase enhances tolerance to oxidative stress-induced programmed cell death and protein damage. Eukaryot Cell. 2009;8(11):1721–1731. doi: 10.1128/EC.00198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrade JM, Murta SM. Functional analysis of cytosolic tryparedoxin peroxidase in antimony-resistant and -susceptible Leishmania braziliensis and Leishmania infantum lines. Parasit Vectors. 2014;7:406–406. doi: 10.1186/1756-3305-7-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amorim CF, Galina L, Carvalho NB, Sperotto NDM, Pissinate K, Machado P. Inhibitory activity of pentacyano(isoniazid)ferrate(II), IQG-607, against promastigotes and amastigotes forms of Leishmania braziliensis. PLoS One. 2017;12(12):e0190294. doi: 10.1371/journal.pone.0190294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tessarollo NG, Andrade JM, Moreira DS, Murta SM. Functional analysis of iron superoxide dismutase-A in wild-type and antimony-resistant Leishmania braziliensis and Leishmania infantum lines. Parasitol Int. 2015;64(2):125–129. doi: 10.1016/j.parint.2014.11.001. [DOI] [PubMed] [Google Scholar]


