Abstract
Objective:
To determine whether a relationship was evident between gliosis in the mediobasal hypothalamus (MBH) and plasma testosterone concentrations in men
Methods:
41 adult men (ages 18–50 years) from 23 twin pairs underwent fasting morning blood draw and brain MRI. T2 relaxation time was used to quantify gliosis in the MBH and control areas in the putamen and amygdala. Plasma concentrations of testosterone and 17β-estradiol were measured by LC-MS/MS. Body composition including visceral adiposity was measured by dual X-ray absorptiometry.
Results:
A negative association was found between MBH T2 relaxation time and plasma concentrations of both free and total testosterone (r = −0.29, P<0.05 and r = −0.37, P<0.01, respectively). Visceral adiposity exhibited a negative correlation with plasma total testosterone concentration (r = −0.45, P=0.001) but a positive correlation with MBH T2 relaxation time (r = 0.24, P=0.03). The negative correlation between plasma total testosterone and MBH T2 relaxation time remained significant after adjustment for visceral adiposity, age, BMI, and insulin resistance.
Conclusions:
In healthy men across a range of BMIs, MBH gliosis was associated with higher visceral adiposity but lower endogenous testosterone. These findings suggest that MBH gliosis could provide novel mechanistic insights into gonadal dysfunction in men with obesity.
Keywords: testosterone; men, obesity; mediobasal hypothalamus; gliosis
Introduction
A striking interdependence exists between obesity and hypogonadism in men. Men with low circulating levels of testosterone may be at increased risk of developing obesity, insulin resistance, and type 2 diabetes (1–3). In turn, obesity increases risk for gonadal dysfunction in both men and women, which manifests as alterations in plasma sex steroid concentrations and reduced fertility (4, 5). Such findings suggest a close, potentially bidirectional relationship between obesity and hypogonadism, but the mechanisms underlying the observed interdependence remain poorly understood.
Among men with obesity, a well-established association exists between higher body mass index (BMI) and increased risk of hypogonadotropic hypogonadism (HH) (6). HH in men with obesity is characterized by low free plasma testosterone concentrations but intact anterior pituitary function; low gonadotropin and sex steroid production in these men rather reflects a defect in gonadotropin-releasing hormone (GnRH) release by the hypothalamus (7). Thus, obesity-associated changes in hypothalamic function may be a critical facet of the evolution of hypogonadism in men with obesity.
Our group and others have previously demonstrated that in rodent models, high-fat diet feeding rapidly leads to a reactive inflammatory process within the mediobasal hypothalamus (MBH) called hypothalamic gliosis that precedes the onset of diet-induced obesity (8–10). Gliosis is a physiologic, adaptive response to injury in the central nervous system; however, if sustained, gliosis can lead to hypothalamic injury and loss of pro-opiomelanocortin (POMC) neurons in the MBH (10), which are key regulators of body weight and energy homeostasis (10, 11). The MBH is the anatomical locus of the infundibular nucleus (also known as the arcuate nucleus in rodents) which, importantly, houses several neuron populations including those that regulate gonadal function and reproduction [kisspeptin/neurokinin B/dynorphin (KNDy) neurons], as well as neurons that regulate energy homeostasis [POMC and neuropeptide Y/agouti-related protein (NPY/AgRP) neurons]. Neurons that produce GnRH also reside within the MBH; although GnRH neurons are found in the infundibular nucleus in humans, they reside outside of the arcuate nucleus in rodents. Gliosis in the MBH therefore could prove a novel mechanism contributing to comorbid obesity and hypogonadism in men.
Gliosis is detectable by magnetic resonance imaging (MRI). Clinical studies use visual identification of increased T2 signal intensity (brightness) to qualitatively detect gliosis in brain regions including the hypothalamus (12, 13). In contrast, quantitative MRI methods can be used as a research strategy to measure T2 signal through a standardized approach. Thus, longer T2 relaxation time (a measure of T2 signal) in the MBH is a validated index of gliosis in this region and correlates with findings of gliosis on histology in both rodents and humans (8, 9, 14). Longer MBH T2 relaxation times positively associate with both BMI and insulin resistance in clinical studies (14, 15). Corroborating these findings, BMI also was shown to correlate with post-mortem evidence of MBH gliosis (16). MRI is therefore an important technology to assess hypothalamic gliosis in vivo in humans and thereby better understand relationships among hypothalamic gliosis, obesity, and its associated co-morbidities.
Using quantitative MRI, we measured MBH T2 relaxation time (the index of hypothalamic gliosis) and plasma free and total testosterone concentrations in a cohort of men. We hypothesized that MBH gliosis would be associated with lower plasma testosterone concentrations and further predicted that this relationship would be independent of BMI.
Methods
Subjects
Subjects were otherwise healthy men age 18–50 years with normal body weight, overweight, or obesity (BMI 19–45 kg/m2) recruited from the Washington State Twin Registry for a study of genetic influences on obesity in which subjects with obesity were oversampled (17) [detailed recruitment methods previously published (18)]. Subjects for the present analyses included male subjects who had both baseline plasma samples available for sex steroid measurement and brain MRI images. A total of 41 adult men from 23 twin pairs met these inclusion criteria and were included in the current analyses. One subject was subsequently excluded from analyses involving visceral adiposity for an improbably low value for visceral fat mass; data for this subject was included in the remaining analyses. The University of Washington Human Subjects Division approved all study procedures. All subjects provided written informed consent for participation.
Study Protocol
The study protocol has been published previously (18). Briefly, each subject presented in the morning for anthropomorphic measures including height and body weight, and a fasting blood draw was performed at 0800. Subjects underwent brain MRI and body composition assessment via dual x-ray absorptiometry (DXA) scan.
Laboratory Analyses
Blood samples were collected in EDTA tubes containing protease inhibitor cocktail (Sigma–Aldrich, St. Louis, MO), aprotinin (Sigma-Aldreich, St. Louis, MO) and dipeptidyl peptidase-4 inhibitor (Millipore, Billerica, MA). Following collection, each sample was cold centrifuged at 4°C, aliquoted and then stored at −80°C until analysis. Plasma glucose concentration was measured using an automated assay (Roche Module P Chemistry auto-analyzer, based on the hexokinase method; Roche Diagnostics Inc., Indianapolis, IN). Plasma insulin concentration was measured using automated assay (Tosoh 2000 auto-analyzer, a two-site immune-enzymometric assay; Tosoh Bioscience Inc., San Francisco, CA). Insulin resistance was determined using homeostasis model assessment of insulin resistance (HOMAIR) (19). All assays were performed in duplicate and samples from each twin pair were processed in the same batch. Plasma total testosterone and 17β-estradiol concentrations were measured by liquid chromatography tandem mass spectrometry (LC-MS/MS) using an AB Sciex 5500 QTRAP tandem quadrupole mass spectrometer (AB Sciex; Foster City, CA) through modified methods described previously (20). The intra-assay coefficients of variation were <5% for measurement of both testosterone and 17β-estradiol. Sex hormone binding globulin (SHBG) concentrations were quantified by ELISA (R&D Systems; Minneapolis, MN) and used to calculate free testosterone using a standard albumin concentration of 43 g/L (21).
Body composition
Dual x-ray absorptiometry (DXA; on either General Electric Lunar Prodigy or iDXA using a correction factor) was used to measure body composition. Estimates of total, subcutaneous and abdominal visceral adipose tissue mass were determined using EnCore™ software (platform version 16.2, General Electric Medical Systems) (22).
MRI Image Acquisition and Analyses
Methodology for MRI image acquisition and analysis has been previously published (14). All magnetic resonance imaging was performed on a 3-Tesla Philips Achieva MR scanner (version 3.2, Philips Medical Systems, Best, The Netherlands) using a 32-channel head radiofrequency coil. Sequences included T1-weighted scan and quantitative multi-slice/multi-echo T2-weighted sequence with 16 echoes (inter-echo spacing 10 ms) (TR/TE/NSA: 2000/20–170/1). Images were acquired to span the area from the optic chiasm through the mammillary bodies. Nine to twelve slices were acquired for each subject (slice thickness=2.5 mm, inter-slice gap=0.2 mm). With an in-plane pixel resolution of 0.7–0.75 mm this resulted in a voxel size of 1.313 mm3. Calculations of T2 relaxation time were determined using the signal decay curve of the 16 echoes on a pixel-by-pixel basis and then displayed on a parametric map as quantitative T2 relaxation time in order to evaluate tissue T2 relaxation time by region. As in previous studies, the coronal slice immediately posterior to the optic chiasm encompassing the rostral arcuate/infundibular nucleus was identified for each subject (23). CVs for the left and right hypothalamus are 7.6% and 7.9%, respectively. Reference regions of interests (ROIs) in the putamen and amygdala were identified within this same coronal slice. ROIs were initially placed on high-resolution coronal images for ease of identifying anatomical structures and then transferred to a T2 parametric map. Mean +/− SD T2 relaxation time per ROI was recorded (OsiriX Imaging Software, version 5.6).
Statistical Analysis
Because twins were analyzed as individuals for the current study, generalized estimating equations were used to account for the non-independence of the twin sample. Robust standard errors were used to account for heteroskedasticity in the data. T2 relaxation time measurements in control regions (amygdala and putamen) were included as covariates in all models of MBH T2 relaxation time to control for scan-related variability in T2 signal. Scatterplots were generated using a mean-centering approach to reflect adjusted statistical models. Data are presented as mean ± standard deviation (SD).
Statistical analyses were performed using STATA version 12.1 (College Station, TX) and SPSS 25 (IBM Corporation; Armonk, New York), and graphs were created using GraphPad Prism 5 (GraphPad Inc., La Jolla, CA).
Results
Subject characteristics
Subjects ranged in age from 18–48 years and BMI from 20.0–40.0 kg/m2. Mean free and total testosterone concentrations were in the normal range (Table 1). Mean HOMA-IR was consistent with mild insulin resistance (24), and mean percent body fat was 31.1 ± 7.4%.
Table 1.
Cohort characteristics.
| Characteristic | Mean ± Standard Deviation (N=41) |
Body mass index <30 (N=20) |
Body mass index >30 (N=21) |
|---|---|---|---|
| Age (years) | 29.7 ± 10.3 | 26.7 ± 9.3 | 32.4 ± 10.6 |
| Body mass index (kg/m2) | 29.7 ± 5.8 <25 (13) 25–30 (7) 30–35 (13) >35 (8) |
24.6 ± 3.1 | 34.5 ± 2.6 |
| Body weight (kg) | 97.1 ± 23.8 | 76.4 ± 12.2 | 116.7 ± 12.5 |
| Free testosterone (nmol/L) (ref 0.32–0.49 nmol/L) |
0.44 ± 0.13 | 0.47 ± 0.14 | 0.40 ± 0.13 |
| Total testosterone (nmol/L) (ref 8.4–29.4 nmol/L) |
16.5 ± 5.7 | 18.4 ± 5.2 | 14.6 ± 5.6 |
| SHBG (nmol/L) | 20.6 ± 9.6 | 23.2 ± 9.7 | 18.1 ± 9.1 |
| Estradiol (pmol/L) (ref 57.6–156.4 pmol/L) |
102.7 ± 33.2 | 89.2 ± 21.8 | 115.6 ± 37.3 |
| HOMA-IR | 2.2 ± 1.7 | 1.2 ± 0.7 | 3.1 ± 1.9 |
| Total body fat mass (kg) | 30.5 ± 13.3 | 19.5 ± 6.8 | 41.0 ± 8.6 |
| Visceral fat mass (g) | 1382 ± 968 | 661 ± 615 | 2035 ± 739 |
| T2 relaxation time, mediobasal hypothalamus (msec) | 95.2 ± 6.0 | 94.2 ± 6.3 | 96.3 ± 5.6 |
| T2 relaxation time, putamen (msec) | 59.8 ± 4.3 | 60.9 ± 4.0 | 58.7 ± 4.3 |
| T2 relaxation time, amygdala (msec) | 82.6 ± 3.8 | 83.6 ± 3.7 | 81.7 ± 3.7 |
T2 relaxation time in the MBH exhibits a negative correlation with plasma testosterone concentrations in men
Longer T2 relaxation time in the MBH was negatively associated with plasma concentrations of both total and free testosterone (Figures 1A and B). These relationships remained and were minimally attenuated when models were adjusted for age (total testosterone r = −0.36, P<0.01; free testosterone r = −0.26, P=0.06). A significant association also was found between longer MBH T2 relaxation time and lower plasma SHBG concentration (Figure 1C). In contrast, no correlation was found between MBH T2 relaxation time and plasma 17β-estradiol concentration (Figure 1D).
Figure 1. Radiologic evidence that hypothalamic gliosis is associated with lower concentrations of both free and total testosterone.

Scatter plot and regression line showing correlations between mean bilateral MBH T2 relaxation time and plasma concentrations of total (A) and free (B) testosterone, SHBG (C), and17β-estradiol (D). Correlation P-values were determined through generalized estimating equations. Data are adjusted for T2 relaxation times in control regions in the putamen and amygdala. N=41.
BMI was negatively correlated with plasma concentrations of both total testosterone (r = −0.33, P<0.01) and free testosterone (r = −0.27, P<0.05) and positively associated with MBH T2 relaxation time (r = 0.30, P=0.02). Notably, the negative correlation between MBH T2 relaxation time and plasma total testosterone concentration remained significant after adjustment for BMI (r = −0.33, P=0.02), and the correlation between MBH T2 relaxation and plasma free testosterone persisted as a strong trend (r = −0.26, P=0.058). A correlation between higher BMI and lower plasma SHBG concentration was evident as a trend (r = −0.23, P=0.07), and, after adjustment for BMI, the negative correlation between MBH T2 relaxation time and plasma SHBG was attenuated (r = −0.27, P=0.08).
Similarly, the negative correlation between MBH T2 relaxation time and plasma total testosterone concentration remained significant after adjustment for insulin resistance as quantified by HOMA-IR (r = −0.32, P=0.02). The negative correlation between MBH T2 relaxation time and plasma free testosterone concentration again was evident as a trend after adjustment for insulin resistance (r = −0.24, P=0.09).
Visceral adiposity is inversely related to MBH gliosis and plasma testosterone concentrations Longer T2 relaxation time in the MBH was associated with greater visceral fat mass (Figure 2A). A positive correlation also was evident between MBH T2 relaxation time and total body fat mass, although this association did not achieve statistical significance (Figure 2B). Visceral adiposity was negatively associated with plasma concentrations of both total testosterone (Figure 2C) and free testosterone (Figure 2D), and these relationships were independent of age (total testosterone r = −0.45, P<0.001; free testosterone r = −0.39, P=0.001). Nonetheless, after adjustment for visceral fat mass, a significant, negative correlation between plasma total testosterone concentration and MBH T2 relaxation persisted and was minimally attenuated (r = −0.34, P=0.01). The negative correlation between MBH T2 relaxation time and plasma free testosterone concentration similarly remained evident (r = −0.28, P=0.03). Strikingly, the negative correlation between plasma total testosterone concentration and MBH T2 relaxation time remained significant after adjustment for BMI, insulin resistance, and visceral fat mass (P=0.01).
Figure 2. Higher visceral adipose mass is associated with both greater hypothalamic gliosis and lower plasma testosterone concentrations.
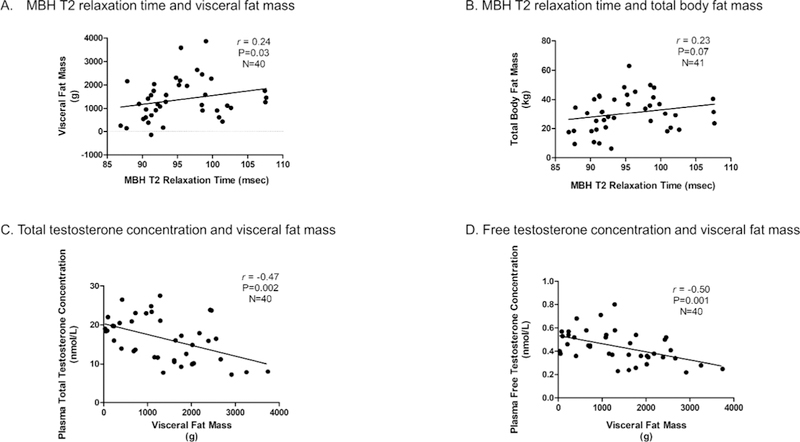
Scatter plot and regression lines showing correlations between mean bilateral MBH T2 relaxation time and visceral fat mass (A) and total body fat mass (B), as well as correlations between visceral fat mass and plasma concentrations of both total (C) and free (D) testosterone. Correlation P-values were determined through generalized estimating equations. Data shown in panels A and B are adjusted for T2 relaxation times in control regions in the putamen and amygdala.
Discussion
Our findings demonstrate associations of MBH gliosis with lower plasma testosterone and higher visceral adiposity in otherwise healthy men across a wide spectrum of BMI. The tendency for men exhibiting greater evidence of MBH gliosis to have lower endogenous circulating testosterone was not explained by other factors including age, higher BMI, or greater visceral adiposity. In contrast, no relationship was seen between MBH gliosis and circulating 17β-estradiol concentration. Notably, a negative correlation between MBH gliosis and circulating SHBG concentration also was evident but appeared attributable at least in part to BMI. These data therefore offer novel, preliminary evidence that MBH gliosis may be related to the high prevalence of hypogonadism in men with obesity, although its possible roles as a cause or effect of hypogonadism cannot be discerned from these cross-sectional analyses. Further, our findings suggest that visceral adipose deposition may be a marker of not only cardiometabolic risk but gonadal dysfunction in men.
No prior clinical studies to our knowledge have examined the relationship between MBH gliosis and gonadal function. However, rapidly accumulating evidence derived from both pre-clinical and clinical studies indicates that MBH gliosis plays a role in the pathogenesis of obesity. In rodent models of diet-induced obesity, MBH gliosis arises early after exposure to high-fat feeding and precedes the evolution of obesity (10, 25–27). Moreover, genetic or pharmacologic inhibition of MBH gliosis reduces obesity progression, suggesting a causal role for MBH gliosis in obesity pathogenesis (27, 28). Clinical studies to date have demonstrated positive correlations between MBH gliosis and BMI as well as insulin resistance, independent of BMI (14, 15). Radiographic evidence of hypothalamic gliosis was shown to correlate not only with BMI but also with genetic factors and host microbiome composition (15). In postmortem analyses, subjects with obesity exhibited greater microglial activation in the hypothalamus relative to subjects of normal body weight, and this degree of activation correlated with BMI (16). Interestingly, in a parallel rodent model of diet-induced obesity, the same authors demonstrated that the phenotype of activated microglia in the hypothalamus is highly dynamic and changes over time in response to high-fat feeding (16). Thus, clinical evidence supports a possible pathogenic role for MBH gliosis in human obesity and insulin resistance. Importantly, the negative correlation found between MBH gliosis and plasma testosterone concentration in the current study was independent of adiposity, suggesting that MBH gliosis may be an independent risk factor for male hypogonadism, which is plausible given the established role of neurons within the MBH in regulation of reproduction.
The MBH houses the infundibular nucleus (known as the arcuate nucleus in rodents), the center of appetite and energy expenditure regulation as well as reproductive function. In rodents with susceptibility to diet-induced obesity, chronic exposure to high-fat feeding led to gliosis in the arcuate nucleus which, in turn, was associated with decreased neurogenesis, loss of synapses, and dysregulation of POMC and NPY/AgRP neuron signaling due to disruption of the blood-brain barrier (29). Thus, one mechanism through which reactive gliosis could lead to HH is impairment of POMC and NPY/AgRP neuron function, both of which have been implicated in the regulation of GnRH neurons (30). Alternatively, gliosis could disrupt GnRH and/or KNDy neuron function directly, through parallel processes to those described for POMC and NPY/AgRP neurons, such as perikaryal ensheathment and reduced neurogenesis. However, these potential effects of gliosis have yet to be shown specifically for GnRH or KNDy neurons. GnRH neurons also receive direct, regulatory signals from glial cells (31), so changes in glial activation state further could impede normal, pulsatile GnRH secretion through altered signaling via these regulatory inputs. Finally, peripheral metabolic signals including leptin, ghrelin, and insulin are key regulators of GnRH neuron function, and changes in the production of these peripheral signals due to diet and/or obesity also could contribute to impaired gonadal function (30). Similarly, the central action of these mediators could be altered in the setting of obesity - irrespective of their peripheral production – due to insulin resistance, gliosis, or other obesityrelated changes. Notably, this regulation is likely mediated indirectly, as GnRH neurons exhibit negligible expression of the insulin and leptin receptors (30). Consistent with this idea, neuron-specific deletion of the insulin receptor led to a phenotype of diet-induced obesity and infertility in both male and female mice (32). Thus, multiple potential mechanisms could explain either the co-occurrence of obesity, MBH gliosis, and hypogonadism or a direct, causal relationship between MBH gliosis and gonadal dysfunction.
As our findings reflect cross-sectional data, prospective studies will be essential for establishing whether MBH gliosis precedes and predicts gonadal dysfunction with progressive obesity. Obesityassociated HH is reversible, as surgical weight loss interventions result in improved gonadal function with regard to both normalized plasma testosterone and improved semen quality in men (6, 33, 34). MBH gliosis also may be reversible; in a rodent model of diet-induced obesity, MBH gliosis was reversed after animals were transitioned back to a regular chow diet (8). Thus, additional work also is needed to determine whether regression of MBH gliosis after weight loss in men predicts restoration of gonadal function. Finally, this line of work will require parallel pre-clinical studies to determine mechanistically how the magnitude and/or duration of gliosis in the MBH may lead to dysregulated function of GnRH-secreting neurons.
The current findings also demonstrate strong correlations between visceral fat mass – but not total body fat mass – and both MBH gliosis and low testosterone concentrations. Visceral adiposity has been implicated in the evolution of obesity-related sequela including insulin resistance, metabolic syndrome, and non-alcoholic fatty liver disease (35–37). Recently, decreases specifically in visceral fat were shown to correlate with improvements in gonadal function following metabolic surgery (38). Increased visceral adiposity, MBH gliosis, and gonadal dysfunction could result in parallel from common mechanisms, including hyperleptinemia, systemic cytokine production, dietary factors, and insulin resistance (14, 39–41). Alternatively, the possibility also exists that visceral adiposity may contribute directly to obesity-related hypogonadism, an effect that could be mediated, in part, through elicitation of hypothalamic gliosis. The current analyses also demonstrate an association between greater MBH gliosis and lower plasma SHBG concentration, although this association was partially attributable to BMI. Hepatic lipid accumulation, insulin resistance, cytokines, and a diet high in monosaccharides have been mechanistically linked to obesity-associated decreases in circulating SHBG concentration (42), which contribute to but do not fully explain obesity-associated hypogonadism (40). POMC neurons regulate hepatic insulin sensitivity (43), providing one plausible mechanism through which MBH gliosis could give rise to hepatic insulin resistance and, consequently, diminished SHBG production. The relevance particularly of altered insulin sensitivity and glucose metabolism to the evolution of HH in men with obesity is underscored by findings that men with obesity and diabetes had both a higher prevalence of HH and an accelerated decline in circulating free testosterone concentration relative to men with obesity but without diabetes (44). Thus, common pathways may underlie many of these obesity-associated traits, and the possible contribution of these pathways to the negative association between MBH gliosis and circulating testosterone concentration may account for our finding that this association was not explained by obesity alone. Future mechanistic work is needed to fully delineate the relationships among these diverse facets of reproductive and metabolic dysregulation.
The present analyses have several limitations. As this study was cross-sectional in design, the directionality of the relationship between MBH gliosis and circulating testosterone cannot be determined. The cohort size was relatively small, and findings require validation in additional, larger subject cohorts, as well as longitudinal analyses to establish the temporal relationships among obesity, MBH gliosis, and HH in men. Importantly, increased T2 relaxation time is not specific to gliosis and, for example, also increases consequent to edema or infection. However, as subjects were otherwise healthy men, these alternative pathologies are unlikely to be present. Moreover, increased T2 signal in the MBH previously has been shown to correlate with post-mortem evidence of gliosis (14). Nonetheless, although our MRI findings indicate tissue changes within the MBH, histologic analyses would be required to definitively show that these radiographic findings truly reflect gliosis and not an alternative form of tissue pathology. Although our analyses benefitted from the broad range of BMI and adiposity among subjects, additional work is needed to establish whether the relationship between MBH gliosis and plasma testosterone varies as a function of subject body weight, body fat mass, age, or other clinical variables. Most subjects in this cohort had testosterone concentrations in the eugonadal range, and the present findings must be extended to subjects with true obesity-related HH. Similarly, the relationship between MBH gliosis and HH will need to be verified for both biochemical evidence of hypogonadism and hypogonadism manifesting with clinical symptoms. Finally, these analyses were performed exclusively in men, and future work will be dedicated to establishing whether increases in MBH gliosis similarly are evident in women with obesity-associated reproductive dysfunction.
Conclusion
Our analyses demonstrate an association between hypothalamic gliosis and diminished endogenous testosterone concentrations in men. These data suggest a potential new mechanism whereby as yet undefined dietary exposures may evoke both excess adiposity and impairment in gonadal function. Insights gained from delineating the overlapping, central mechanisms of energy balance and gonadal dysregulation could inform novel clinical approaches to the management of obesity and its associated co-morbidities.
Study Importance:
What is already known about this subject? (up to 3 short bullet points)
Obesity is associated with hypogonadotropic hypogonadism in men.
The regulation of energy balance and gonadal function co-localize to the infundibular nucleus of the mediobasal hypothalamus.
Hypothalamic gliosis may play a key role in obesity pathogenesis, but its role in obesity-associated hypogonadism is wholly unexplored.
What does your study add?
We demonstrate novel evidence that hypothalamic gliosis quantified by MRI exhibits an independent, negative correlation with plasma testosterone concentrations in men.
This association persisted after adjustment for body mass index, age, insulin resistance, and visceral fat mass.
These findings suggest that hypothalamic gliosis may be a link between obesity and gonadal dysfunction in men.
Acknowledgements
We would like to thank Tyler A. Bosch for his expertise and assistance and the twins of the Washington State Twin Registry for their participation.
Funding:
This research was supported by the following: KEB: NIH/NICHD K12HD053984
KBR: American Heart Association 16GRNT30700006
STP: Eunice Kennedy Shriver National Institute of Child Health and Development U54 HD042454 and the University of Washington Robert McMillen Professorship in Lipid Research
EAS: NIH R01DK089036 and R01DK098466 and the American Diabetes Association (1–17-ICTS-085).
Additional assistance was provided by the University of Washington’s Nutrition Obesity Research Center (P30 DK035816), Diabetes Research Center (P30 DK017047), and Institute of Translational Health Sciences (UL1 TR000423).
Footnotes
Disclosure:
Dr. Matsumoto reports research support from AbbVie and GlaxoSmithKline and personal fees from AbbVie, Aytu, Partnership for Clean Competition, and UpToDate, outside the submitted work. The authors have no other conflicts of interest to disclose.
References
- 1.Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol 2013;9:479–93. [DOI] [PubMed] [Google Scholar]
- 2.Shahani S, Braga-Basaria M, Basaria S. Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab 2008;93:2042–9. [DOI] [PubMed] [Google Scholar]
- 3.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006;295:1288–99. [DOI] [PubMed] [Google Scholar]
- 4.Davidson LM, Millar K, Jones C, Fatum M, Coward K. Deleterious effects of obesity upon the hormonal and molecular mechanisms controlling spermatogenesis and male fertility. Hum Fertil (Camb) 2015;18:184–93. [DOI] [PubMed] [Google Scholar]
- 5.Best D, Avenell A, Bhattacharya S. How effective are weight-loss interventions for improving fertility in women and men who are overweight or obese? A systematic review and meta-analysis of the evidence. Hum Reprod Update 2017;23:681–705. [DOI] [PubMed] [Google Scholar]
- 6.Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella Carretero JI. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update 2017:1–19. [DOI] [PubMed] [Google Scholar]
- 7.George JT, Millar RP, Anderson RA. Hypothesis: kisspeptin mediates male hypogonadism in obesity and type 2 diabetes. Neuroendocrinology 2010;91:302–7. [DOI] [PubMed] [Google Scholar]
- 8.Berkseth KE, Guyenet SJ, Melhorn SJ, et al. Hypothalamic gliosis associated with high-fat diet feeding is reversible in mice: a combined immunohistochemical and magnetic resonance imaging study. Endocrinology 2014;155:2858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee D, Thaler JP, Berkseth KE, Melhorn SJ, Schwartz MW, Schur EA. Longer T(2) relaxation time is a marker of hypothalamic gliosis in mice with diet-induced obesity. Am J Physiol Endocrinol Metab 2013;304:E1245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012;122:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev 2011;91:389–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005;58:840–6. [DOI] [PubMed] [Google Scholar]
- 13.Poppe AY, Lapierre Y, Melancon D, et al. Neuromyelitis optica with hypothalamic involvement. Mult Scler 2005;11:617–21. [DOI] [PubMed] [Google Scholar]
- 14.Schur EA, Melhorn SJ, Oh SK, et al. Radiologic evidence that hypothalamic gliosis is associated with obesity and insulin resistance in humans. Obesity (Silver Spring) 2015;23:2142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreutzer C, Peters S, Schulte DM, Fangmann D, Türk K, Wolff S, et al. Hypothalamic Inflammation in Human Obesity is Mediated by Environmental and Genetic Factors. Diabetes 2017. Epub 2017/06/02. [DOI] [PubMed] [Google Scholar]
- 16.Baufeld C, Osterloh A, Prokop S, Miller KR, Heppner FL. High-fat diet-induced brain regionspecific phenotypic spectrum of CNS resident microglia. Acta Neuropathol 2016;132:361–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strachan E, Hunt C, Afari N, et al. University of Washington Twin Registry: poised for the next generation of twin research. Twin research and human genetics : the official journal of the International Society for Twin Studies 2013;16:455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melhorn SJ, Askren MK, Chung WK, et al. FTO genotype impacts food intake and corticolimbic activation. Am J Clin Nutr 2018;107:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–95. [DOI] [PubMed] [Google Scholar]
- 20.Mostaghel EA, Nelson PS, Lange P, et al. Targeted androgen pathway suppression in localized prostate cancer: a pilot study. J Clin Oncol 2014;32:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids 2009;74:512–9. [DOI] [PubMed] [Google Scholar]
- 22.Kaul S, Rothney MP, Peters DM, et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity 2012;20:1313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abel TW, Rance NE. Stereologic study of the hypothalamic infundibular nucleus in young and older women. J Comp Neurol 2000;424:679–88. [DOI] [PubMed] [Google Scholar]
- 24.Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord 2013;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorfman MD, Thaler JP. Hypothalamic inflammation and gliosis in obesity. Curr Opin Endocrinol Diabetes Obes 2015;22:325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douglass JD, Dorfman MD, Thaler JP. Glia: silent partners in energy homeostasis and obesity pathogenesis. Diabetologia 2017;60:226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valdearcos M, Douglass JD, Robblee MM, et al. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell Metab 2017;26:185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douglass JD, Dorfman MD, Fasnacht R, Shaffer LD, Thaler JP. Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol Metab 2017;6:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horvath TL, Sarman B, García-Cáceres C, et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A 2010;107:14875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manfredi-Lozano M, Roa J, Tena-Sempere M. Connecting metabolism and gonadal function: Novel central neuropeptide pathways involved in the metabolic control of puberty and fertility. Front Neuroendocrinol 2018;48:37–49. [DOI] [PubMed] [Google Scholar]
- 31.Ojeda SR, Prevot V, Heger S, Lomniczi A, Dziedzic B, Mungenast A. Glia-to-neuron signaling and the neuroendocrine control of female puberty. Ann Med 2003;35:244–55. [DOI] [PubMed] [Google Scholar]
- 32.Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science 2000;289:2122–5. [DOI] [PubMed] [Google Scholar]
- 33.Pham NH, Bena J, Bhatt DL, Kennedy L, Schauer PR, Kashyap SR. Increased Free Testosterone Levels in Men with Uncontrolled Type 2 Diabetes Five Years After Randomization to Bariatric Surgery. Obes Surg 2018;28:277–80. [DOI] [PubMed] [Google Scholar]
- 34.Håkonsen LB, Thulstrup AM, Aggerholm AS, Olsen J, Bonde JP, Andersen CY, et al. Does weight loss improve semen quality and reproductive hormones? Results from a cohort of severely obese men. Reprod Health 2011;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko YH, Wong TC, Hsu YY, Kuo KL, Yang SH. The Correlation Between Body Fat, Visceral Fat, and Nonalcoholic Fatty Liver Disease. Metab Syndr Relat Disord 2017;15:304–11. [DOI] [PubMed] [Google Scholar]
- 36.Faria G, Gonçalves A, Cunha R, et al. Beyond central adiposity: liver fat and visceral fat area are associated with metabolic syndrome in morbidly obese patients. Int J Surg 2015;14:75–9. [DOI] [PubMed] [Google Scholar]
- 37.Walker GE, Marzullo P, Ricotti R, Bona G, Prodam F. The pathophysiology of abdominal adipose tissue depots in health and disease. Horm Mol Biol Clin Investig 2014;19:57–74. [DOI] [PubMed] [Google Scholar]
- 38.Liu F, Tu Y, Zhang P, Bao Y, Han J, Jia W. Decreased visceral fat area correlates with improved total testosterone levels after Roux-en-Y gastric bypass in obese Chinese males with type 2 diabetes: a 12-month follow-up. Surg Obes Relat Dis 2018;14:462–8. [DOI] [PubMed] [Google Scholar]
- 39.Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013;93:359–404. [DOI] [PubMed] [Google Scholar]
- 40.Souteiro P, Belo S, Oliveira SC, et al. Insulin resistance and sex hormone-binding globulin are independently correlated with low free testosterone levels in obese males. Andrologia 2018:e13035 Epub 2018/05/09. [DOI] [PubMed] [Google Scholar]
- 41.La J, Roberts NH, Yafi FA. Diet and Men’s Sexual Health. Sex Med Rev 2018;6:54–68. [DOI] [PubMed] [Google Scholar]
- 42.Hammond GL. Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. J Endocrinol 2016;230:R13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berglund ED, Vianna CR, Donato J, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest 2012;122:1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010;33:1186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


