Abstract
Background
It has been previously shown that anesthesia and analgesia can affect outcomes in the rat burn model and that buprenorphine alleviated pain without drastically altering the outcomes of interest. Recently, the use of a sustained release (SR) formulation of buprenorphine has been promoted over conventional buprenorphine. In this study, we assessed whether buprenorphine SR altered hemodynamic parameters in our rat model of severe burn injury.
Materials and Methods
Adult male Sprague Dawley rats were randomized to receive either conventional buprenorphine (0.05mg/kg) or buprenorphine SR (1mg/kg). Buprenorphine-SR was administered 24 hours before the experiment. Buprenorphine was administered on the day of experiment. These groups were further randomized to control or scald burn (60% of total body surface area). Systolic and diastolic blood pressure (SBP, DBP) and heart rate (HR) were measured using a noninvasive blood pressure system prior to receiving analgesia and after 72 hours.
Results
As expected, HR was significantly higher after burn injury regardless of analgesic (p<0.0001). Both SBP and DBP were significantly decreased in burned animals receiving conventional buprenorphine (p<0.0001) but neither were altered in the buprenorphine SR treated burned animals. However, SBP, DBP, and HR were significantly increased after 72 hours in control animals receiving buprenorphine SR (p<0.0001).
Conclusions
These data indicate that buprenorphine SR alters the hemodynamic response to injury and may not be an appropriate choice for a model of severe burn injury. If this analgesic is used, investigators must cautiously form conclusions especially in experimental conditions that would be expected to alter cardiac hemodynamics.
Keywords: analgesia, blood pressure, cytokines, heart rate, pain management
INTRODUCTION
Animal models of disease are used to determine underlying mechanisms, identify novel therapeutic targets, and test the efficacy and safety of therapeutics. In order for these models to provide reliable translatable information, the model must mimic the human disease conditions as well as control for any variables that may confound results. Our institution has investigated the clinical implications of severe burn injury as well as used both small and large animal models to investigate the mechanisms of burn induced organ dysfunction (1–3). One such model is a rodent scald burn model where 60% of the total body surface area is injured. This model exhibits many of the key characteristics of human burn injury including elevated levels of cytokines, increased heart rate, and weight loss (3). Investigators at our institution previously showed that the choice of anesthesia, analgesia, and euthanasia method can profoundly affect outcomes in this model. From this study, it was determined that buprenorphine was the optimal analgesic choice as it alleviated pain without drastically altering the cytokine profile (4).
Buprenorphine acts as a partial agonist of μ-opioid receptors while antagonizing δ- and κ-opioid receptors (5, 6). Buprenorphine is highly effective for reducing pain in rodents and has been shown to be more effective than other common analgesics including carprofen and tramadol (7–9). Thus, for many years it has been the standard of care in many laboratory animal species. However, buprenorphine can depress cardiac function, cause unwanted sedation, and increase pica behavior (10–12).
Recently, the use of buprenorphine-SR, a polymer system that releases buprenorphine for 72 hours, has been promoted over conventional buprenorphine which is generally administered every 8-12 hours (7, 13). The longer duration of action reduces stress to the animal as a result of handling and repeated injections. Buprenorphine-SR has been shown to equivalently reduce pain in various rodent models of pain without the side effects often reported with conventional buprenorphine (14, 15). However, there are no reports to date of whether buprenorphine-SR alleviates pain in animal models of severe burn injury. Thus, the aim of this study was to determine whether buprenorphine-SR altered outcomes in our rodent model of severe burn injury.
MATERIALS AND METHODS
Animal Model of Burn Injury
The protocol for this study was approved by the University of Texas Medical Branch-Galveston Institutional Animal Care and Use Committee. Animals were cared for and handled according to the Guide for the Care and Use of Laboratory Animals. As previously described, a full-thickness 60% of total body surface area scald burn was performed on male Sprague-Dawley rats (3, 4). Animals were allocated to either burn or control. General anesthesia was accomplished using isoflurane (1–3% in air). Toe pinch was used to ascertain the level of anesthesia. Following the burn, Lactated Ringer’s solution (40ml/kg) was administered for resuscitation. Analgesia was given as described below. Control animals were treated similarly to the burn animals with the exception of the burn procedure and resuscitation. All animals were allowed to recover from anesthesia under high-flow oxygen and then housed individually in wire bottom cages throughout the experiment.
Experimental Design
Experiment A
Adult male Sprague-Dawley rats (n=16, Charles River Laboratories, Inc.) received Buprenorphine-SR (1mg/kg subcutaneous (SC)) 24 hours prior to the beginning of the experiment. Animals were then randomized to control or burn. Experiment was terminated 72 hours post-burn per veterinarian recommendation with euthanasia via CO2.
Experiment B
Adult male Sprague-Dawley rats (n=20, 5/group) were randomized to receive either Buprenorphine SR or conventional buprenorphine. Buprenorphine-SR was administered at 1 mg/kg SC 24 hours prior to the experiment. Conventional buprenorphine was administered at 0.05 mg/kg SC on the day of the experiment. Animals were then further randomized to control or burn. Animals were euthanized via decapitation without anesthesia 72 hours post-burn. Plasma was collected for measurement of cytokines.
Hemodynamic Measurements
Rodent blood pressure measurements were recorded using the Kent Scientific CODA Non-Invasive Blood Pressure System (Kent Scientific, Torrington, CT, USA). Data were collected according to manufacturer specifications. Briefly, rats were put in weight appropriate animal holders and placed on a warming platform. Size appropriate occlusion and volume pressure recording (VPR) cuffs were placed on the tail of each animal and allowed to acclimatize for at least 5 minutes. Animals were considered ready for analysis once their tail measured at least 32°C. VRP sensor cuff readings were collected over 20 cycles consisting of occlusion cuff inflation (up to 250 mm Hg) followed by 20 seconds of deflation time while the blood pressure is measured. Measurements were determined valid if not rejected by the CODA NIBP software and these values were included in the final analysis.
Cytokine Analysis
Serum concentrations of pro-inflammatory cytokines were determined using readily available kits. Enzyme-linked immunosorbent assays used to analyze Interleukin 1β, interleukin 6, tumor necrosis factor-alpha, cytokine-induced neutrophil chemoattractant-1 and -2 were purchased from R&D Systems Inc. (Minneapolis, MN). Monocyte chemotactic protein-1 was purchased from Biosource™ International Inc. (Camarillo, CA). All assays were performed according to the manufacturer’s instructions.
Statistical Analysis
Hemodynamic parameters were modeled by mixed analysis of variance with relation to all combinations of treatment group and time point, blocking on animal to control for repeated measures. Differences among treatments and times were assessed by Tukey-adjusted contrasts.
Cytokine expression data were analyzed with one-way ANOVA followed by Tukey-Kramer’s post hoc test (GraphPad Prism 7.02). Data are expressed as means ± standard error of the mean. A p-value <0.05 was considered significant.
RESULTS
Experiment A
Following the UTMB IACUC recommendations, our established burn model was altered to use buprenorphine-SR as the analgesic agent instead of conventional buprenorphine. Within 24 hours post-burn, many of the animals began displaying symptoms of pica. A second dose of buprenorphine-SR was administered to animals at the recommendation of veterinary staff. Table 1 summarizes the adverse events that occurred in this cohort of animals. Thirty percent of the animals self-eviscerated and were euthanized. Another 20% of the animals were found dead in their cages. The remaining animals were euthanized 72 hours post-burn at the recommendation of the head veterinarian. Necropsy was performed on animals that were found dead as well as the euthanized animals. While the necropsy of one animal unveiled evidence of sepsis, there were no consistent changes in any of the other necropsies that would explain this phenomenon.
Table 1.
Summary of adverse events in Experiment A
| Event | N (%) |
|---|---|
| Evisceration | 5 (30) |
| Death | 3 (20) |
| Euthanized | 8 (50) |
Experiment B
In order to determine whether the buprenorphine-SR was the cause of the aforementioned adverse events in our animal model, we then compared hemodynamic parameters and cytokine levels in animals treated with buprenorphine-SR and conventional buprenorphine.
Buprenorphine-SR Alters the Hemodynamic Response
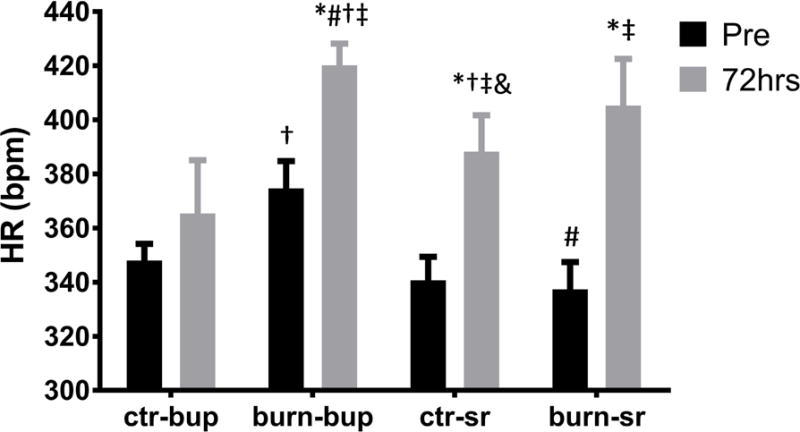
Systolic and diastolic blood pressure (SBP and DBP) were significantly decreased 72 hours post-burn in burned animals that received conventional buprenorphine with no differences noted in the respective control group (Fig. 1A, B; p<0.0001). However, no changes in SBP and DBP were observed in burned animals treated with buprenorphine-SR while both SBP and DBP were significantly increased in control animals receiving buprenorphine-SR (Fig. 1 A, B; p<0.0001). HR was significantly increased in both the conventional buprenorphine and the buprenorphine-SR groups following burn injury when compared to their respective controls (Fig. 2; p<0.0001). Heart rate increased significantly in the control animals treated with buprenorphine-SR after 72 hours (Fig. 2; p<0.0001). These data indicate that buprenorphine-SR can have profound effects on the hemodynamic response even in the absence of an additional stimulus such as burn injury.
Figure 1.
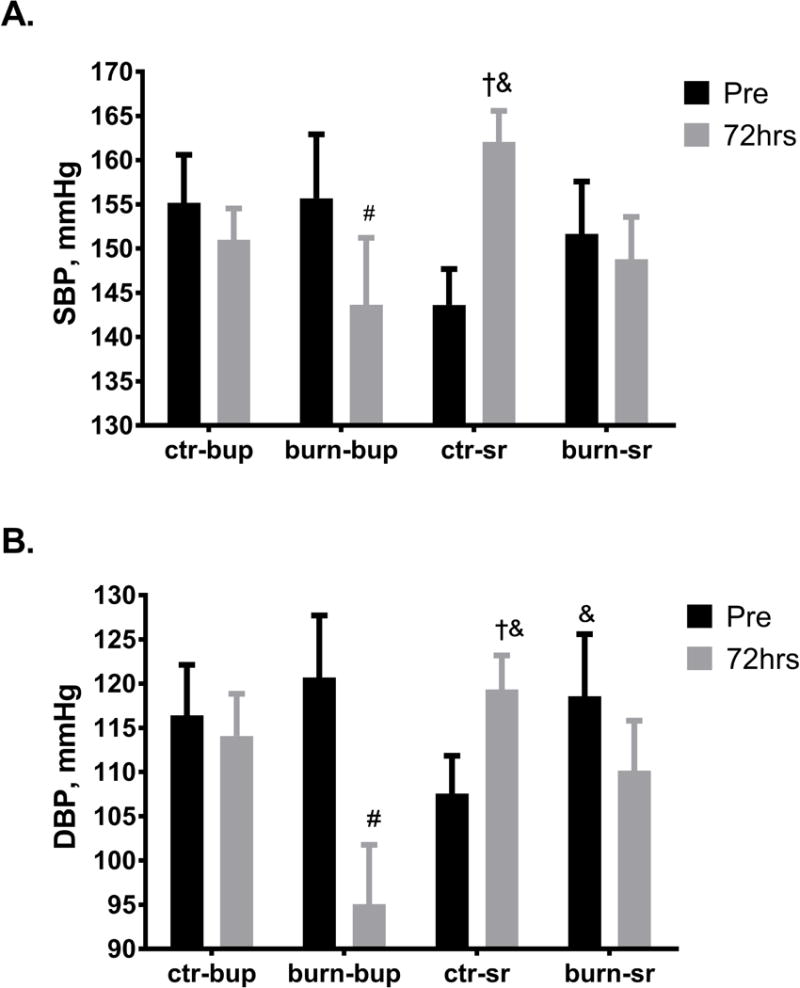
Average systolic (A) and diastolic (B) blood pressure (SBP, DBP) before analgesia administration and 72 hours after burn in buprenorphine and buprenorphine-SR treated animals. Ctr-bup, control animals treated with conventional buprenorphine; burn-bup, burned animals treated with conventional buprenorphine; ctr-sr, control animals treated with buprenorphine-SR, burn-sr, burned animals treated with buprenorphine-SR; #, p<0.05 vs burn-bup Pre; †, p<0.05 vs ctr-SR Pre; &, p<0.05 vs burn-bup 72hrs.
Figure 2.
Average heart rate blood pressure before analgesia administration and 72 hours post-burn in buprenorphine and buprenorphine-SR treated animals. Ctr-bup, control animals treated with conventional buprenorphine; burn-bup, burned animals treated with conventional buprenorphine; ctr-sr, control animals treated with buprenorphine-SR, burn-sr, burned animals treated with buprenorphine-SR; #, p<0.05 vs burn-bup Pre; †, p<0.05 vs ctr-SR Pre; &, p<0.05 vs burn-bup 72hrs; *, p<0.05 vs ctr-bup Pre; ‡, p<0.05 vs burn-SR Pre.
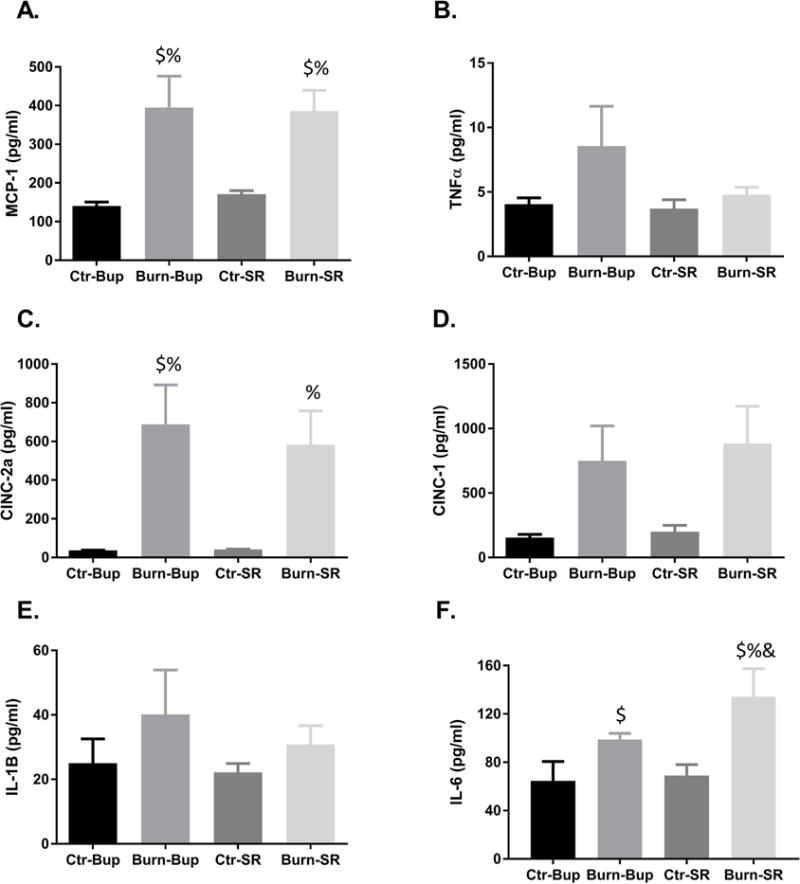
Cytokine Analysis
We measured the concentrations of 6 cytokines previously shown to be altered post-burn in rats (4). There were no significant differences in tumor necrosis factor-alpha, cytokine-induced neutrophil chemoattractant-1, or interleukin-1B serum expression among any of the groups (Fig 3.B, D, E). Monocyte chemoattractant protein 1, cytokine-induced neutrophil chemoattractant-2a and interleukin-6 were all significantly increased in burned animals compared to their respective control regardless of which analgesic they received (Fig 3.A, C, F; p=0.002, 0.007, p<0.0001, respectively). Additionally, serum interleukin-6 concentrations were significantly higher post-burn in the buprenorphine-SR group compared to the conventional buprenorphine group (Fig 3.F; p<0.026).
Figure 3.
Average serum monocyte chemoattractant protein 1 (A, MCP-1), tumor necrosis factor alpha (B, TNF-α), cytokine-induced neutrophil chemoattractant-2a (C, CINC-2a), cytokine-induced neutrophil chemoattractant-1 (D, CINC-1), interleukin-1β (E, IL-1β, and interleukin-6 (F, IL-6) concentrations 72 hours post-burn in buprenorphine and buprenorphine-SR treated animals. Ctr-bup, control animals treated with conventional buprenorphine; burn-bup, burned animals treated with conventional buprenorphine; ctr-sr, control animals treated with buprenorphine-SR, burn-sr, burned animals treated with buprenorphine-SR; $, p<0.05 vs ctr-Bup 72hrs; %, p<0.05 vs ctr-SR 72hrs; &, p<0.05 vs burn-Bup 72hrs.
DISCUSSION
In our first cohort, we observed a higher number of adverse events such as pica and self-evisceration after switching from conventional buprenorphine to buprenorphine-SR. Clark et al., reported that buprenorphine treated animals exhibited greater signs of pica compared to animals that did not receive buprenorphine (11). This behavior was evident even with administration of a single dose of buprenorphine. However, another study comparing buprenorphine and buprenorphine-SR at higher doses than were used in the present study did not observe any signs of pica behavior (14). These differences could be attributed to each study using a different rodent strain, although, the latter study used the same strain as our study.
Additionally, Foley et al. stated that, in their hands, buprenorphine-SR administered at 1.2 mg/kg was equivalent to dosing conventional buprenorphine at 0.2 mg/kg every 12 hours and effectively reduced pain (14). Similarly, 1.2 mg/kg buprenorphine-SR effectively reduced pain in a rodent model of incisional pain equivalent to 0.05 mg/kg buprenorphine (15). Neither of these studies reported any adverse events such as pica behavior or death. Of note, the majority of these studies were performed under conditions that do not cause wide-spread systematic disruptions of signaling and normal processes unlike our model of severe burn. Higher blood concentrations of buprenorphine are associated with hyperalgesia (16). Additionally, buprenorphine is a mixed agonist-antagonist, acting as an antagonist at the κ-opioid receptor and a partial agonist at the μ-opioid receptor. The opioid receptor-like receptor (ORL-1) is also involved in buprenorphine’s mechanism and may block its antinociceptive effects (17). Based on these studies, it appears that the current recommended dose of buprenorphine-SR is either insufficient to alleviate pain or results in concentrations that cause hyperalgesia and exacerbate pica behavior.
We observed that the nonburned buprenorphine-SR treated animals had significantly higher heart rate and blood pressure at the end of the study compared to prior to administration of the analgesic. While Foley et al. did not assess hemodynamic parameters, they noted significantly increased activity levels in buprenorphine-SR treated animals that did not undergo a surgical intervention indicating that buprenorphine-SR affects these animals in a manner unrelated to pain relief (14). Ilbäck et al. showed that conventional buprenorphine increased heart and blood pressure in a dose dependent manner. These effects were evident with one dose of buprenorphine, continued to be measurable long after administration, and were more prominent at higher doses (18). In the current study, we report a similar phenomenon with buprenorphine-SR administration.
Of the six cytokines that we measured, only interleukin-6 expression was significantly different between buprenorphine and buprenorphine-SR post-burn. In a study published in 2002, Maas et al. implicated interleukin-6 along with other pro-inflammatory cytokines as contributors to burn-induced cardiovascular dysfunction (19). While investigators from our institution previously reported that buprenorphine administration can affect the cytokine profile post-burn, others have shown that chronic buprenorphine treatment had limited effect on the rodent immune system (4, 20). There is not a defined mechanism by which buprenorphine-SR might affect interleukin-6 plasma levels. Thus, we postulate that the current recommended dosage of 1 mg/kg buprenorphine-SR is too high to achieve adequate analgesia without significant adverse effects in our rodent model of severe burn injury.
In summary, these data indicate that buprenorphine-SR alters the hemodynamic response to injury and may not be an appropriate choice for a model of severe burn injury at the current recommended dose. We further postulate that adverse events similar to what we are reporting with our severe burn model could result from the use of buprenorphine-SR in any experimental model characterized by a systemic stress response, inflammatory response, or extensive trauma and could confound study data. Therefore, the appropriate dose and utilization of this analgesic in trauma animal models requires further study.
Study Limitations
One limitation of this study is the small sample size. This may explain why we did not reach statistical significance in all of the cytokines after burn injury. However, even with this small sample size, we were able to determine significant differences in interleukin-6 between the conventional buprenorphine and the buprenorphine-SR treated animals. There might be other differences between the two groups that would become more apparent in a larger sample size. Another limitation is that we have altered the protocol for the model to use isoflurane as the anesthetic rather than the ketamine/xylazine mixture that we had previously published. This change in procedure was undertaken to reduce recovery time from anesthesia. As was previously published, isoflurane can alter the cytokine profile after burn injury (4). However, these changes were not significantly different from the changes seen with ketamine/xylazine. Finally, we were unable to determine hemodynamic data nearer the peak of the buprenorphine concentration (24–48 hours) due to the shock response in our model causing a reduction of tail blood flow.
Acknowledgments
The authors would like to thank Kasie Cole for professionally editing the manuscript as well as the veterinary staff of University of Texas Medical Branch-Galveston. We would also like to thank Clark R. Andersen for the statistical analysis of the hemodynamic data.
This work was supported by National Institutes of Health (P50GM060338, T32GM008256, and R01GM056687 [DNH]; and R01GM112936 [CCF]), and Shriners of North America (80500 and 80100 [DNH]; and 87300, 84202, and 84291 [CCF]). This study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award (UL1RR029876) from the National Center for Advancing Translational Sciences, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS: ANG, CCF, and DNH contributed to the conception and design of the study as well as the drafting of the manuscript. ANG, RPC, AP, JWJ, MW, and AA, contributed to the acquisition, analysis, and interpretation of data. All authors reviewed and approved the final manuscript prior to submission.
DISCLOSURE
The authors declare they have no competing interests as defined by Journal of Surgical Research, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
- 1.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, et al. Pathophysiologic response to severe burn injury. Annals of surgery. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PloS one. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herndon DN, Wilmore DW, Mason AD., Jr Development and analysis of a small animal model simulating the human postburn hypermetabolic response. The Journal of surgical research. 1978;25:394–403. doi: 10.1016/s0022-4804(78)80003-1. [DOI] [PubMed] [Google Scholar]
- 4.Al-Mousawi AM, Kulp GA, Branski LK, Kraft R, Mecott GA, et al. Impact of anesthesia, analgesia, and euthanasia technique on the inflammatory cytokine profile in a rodent model of severe burn injury. Shock. 2010;34:261–268. doi: 10.1097/shk.0b013e3181d8e2a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leander JD. Buprenorphine has potent kappa opioid receptor antagonist activity. Neuropharmacology. 1987;26:1445–1447. doi: 10.1016/0028-3908(87)90112-2. [DOI] [PubMed] [Google Scholar]
- 6.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine-and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. The Journal of pharmacology and experimental therapeutics. 1976;197:517–532. [PubMed] [Google Scholar]
- 7.McKeon GP, Pacharinsak C, Long CT, Howard AM, Jampachaisri K, et al. Analgesic effects of tramadol, tramadol-gabapentin, and buprenorphine in an incisional model of pain in rats (Rattus norvegicus) Journal of the American Association for Laboratory Animal Science: JAALAS. 2011;50:192–197. [PMC free article] [PubMed] [Google Scholar]
- 8.Christoph T, Kogel B, Schiene K, Meen M, De Vry J, et al. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. European journal of pharmacology. 2005;507:87–98. doi: 10.1016/j.ejphar.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 9.Matsumiya LC, Sorge RE, Sotocinal SG, Tabaka JM, Wieskopf JS, et al. Using the Mouse Grimace Scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. Journal of the American Association for Laboratory Animal Science: JAALAS. 2012;51:42–49. [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez EA, Hartsfield SM, Melendez LD, Matthews NS, Slater MR. Cardiovascular effects of buprenorphine in anesthetized dogs. American journal of veterinary research. 1997;58:1280–1284. [PubMed] [Google Scholar]
- 11.Clark JA, Jr, Myers PH, Goelz MF, Thigpen JE, Forsythe DB. Pica behavior associated with buprenorphine administration in the rat. Laboratory animal science. 1997;47:300–303. [PubMed] [Google Scholar]
- 12.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clinical pharmacology and therapeutics. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 13.Curtin LI, Grakowsky JA, Suarez M, Thompson AC, DiPirro JM, et al. Evaluation of buprenorphine in a postoperative pain model in rats. Comparative medicine. 2009;59:60–71. [PMC free article] [PubMed] [Google Scholar]
- 14.Foley PL, Liang H, Crichlow AR. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. Journal of the American Association for Laboratory Animal Science: JAALAS. 2011;50:198–204. [PMC free article] [PubMed] [Google Scholar]
- 15.Chum HH, Jampachairsri K, McKeon GP, Yeomans DC, Pacharinsak C, et al. Antinociceptive effects of sustained-release buprenorphine in a model of incisional pain in rats (Rattus norvegicus) Journal of the American Association for Laboratory Animal Science: JAALAS. 2014;53:193–197. [PMC free article] [PubMed] [Google Scholar]
- 16.Wala EP, Holtman JR., Jr Buprenorphine-induced hyperalgesia in the rat. European journal of pharmacology. 2011;651:89–95. doi: 10.1016/j.ejphar.2010.10.083. [DOI] [PubMed] [Google Scholar]
- 17.Lutfy K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol. 2004;2:395–402. doi: 10.2174/1570159043359477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilback NG, Siller M, Stalhandske T. Effects of buprenorphine on body temperature, locomotor activity and cardiovascular function when assessed by telemetric monitoring in rats. Laboratory animals. 2008;42:149–160. doi: 10.1258/la.2007.06002e. [DOI] [PubMed] [Google Scholar]
- 19.Maass DL, White J, Horton JW. IL-1beta and IL-6 act synergistically with TNF-alpha to alter cardiac contractile function after burn trauma. Shock. 2002;18:360–366. doi: 10.1097/00024382-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Van Loveren H, Gianotten N, Hendriksen CF, Schuurman HJ, Van der Laan JW. Assessment of immunotoxicity of buprenorphine. Laboratory animals. 1994;28:355–363. doi: 10.1258/002367794780745119. [DOI] [PubMed] [Google Scholar]


