Abstract
Background
Liver fibrosis is characterized as excessive deposition of the extracellular matrix (ECM) proteins, primarily by activated hepatic stellate cells (HSCs). NF-κB has been reported as one of major mediators of HSC activation. Previously, our team reported that oridonin exhibited anti-hepatic fibrogenetic activity in vitro. In this study, we examined the effects of its novel derivative CYD0618 on HSC viability, apoptosis and NF-κB signaling.
Methods
Cell proliferation of activated human and rat HSC cell lines LX-2 and HSC-T6 was measured using Alamar Blue Assay. Apoptosis was measured by Cell Death ELISA. Cellular proteins were determined by Western blots and immunofluorescence.
Results
CYD0618 significantly inhibited LX-2 and HSC-T6 cell proliferation in a dose-dependent manner. CYD0618 induced cell apoptosis in both cell lines. CYD0618 treatment increased cell cycle inhibitory protein p21, p27, and induced apoptosis marker c-PARP, while suppressing the expression of collagen type I. CYD0618 blocked LPS-induced NF-κB p65 nuclear translocation and DNA binding activity and prevented LPS-induced NF-kB inhibitory protein IκBα phosphorylation and degradation. LPS-stimulated NF-κB downstream target cytokines IL-6, MCP-1 were attenuated by CYD0618. Endogenous and LPS- stimulated NF-κB p65 S536 phosphorylation was inhibited by CYD0618 treatment.
Conclusions
The potent anti-hepatic fibrogenetic effect of CYD0618 may be mediated via suppression of the NF-κB pathway.
Keywords: Liver fibrosis, therapeutics, Oridonin, NF-κB pathway, hepatic stellate cells
Introduction
Hepatic fibrosis is a wound-healing response that is associated with the sustained inflammatory signals of chronic liver disease (CLD).1,2 Hepatic fibrosis typically evolves over decades and can ultimately lead to its most advanced form, cirrhosis.1 Hepatic cirrhosis is a significant global disease, estimated to have caused over a million deaths in 2010, and is caused by a wide range of etiologies.3 While traditional treatments have focused on reducing the exposure to etiological agents, more recent research has been focused on finding agents which prevent the progression of fibrosis to cirrhosis or induce regression of advanced fibrosis.4
NF-κB has been proposed to be the central link between hepatic injury, fibrosis, and hepatocellular carcinoma, and it represents a target for the prevention or treatment of liver fibrosis.5 NF-κB plays an important role in the suppression of apoptosis, and is necessary for normal hepatic development and homeostasis.6 Blockage of the NF-κB pathway is sufficient to increase the rate of apoptosis in hepatic stellate cells (HSCs).7 Initiation of the classic NF-κB pathway leads to activation of the IκB kinase (IKK) complex, which results in phosphorylation and proteasome-dependent degradation of NF-κB inhibitor IκB, nuclear translocation of NF-κB dimers (e.g. p50/p65) and ultimately synthesis of antiapoptotic factors.6,8 In an alternative pathway for NF-κB activation, phosphorylation of NF-κB subunit p65 plays an important role in modulating transcriptional activity of NF-κB independent of IkB proteins and phosphorylation specific to the serine 536 (S536) site has been shown to alter the association with basal components of the transcriptional machinery.9,10
Previous studies from our group have demonstrated that oridonin, an active compound isolated from Rabdosia rubescens, has potent anti-hepatic fibrogenetic effects by decreasing HSC viability and inducing HSC apoptosis.11 Rabdosia rubescens has been used as a traditional medicine for the treatment of various cancers and inflammatory disorders.12–15 In vivo studies have demonstrated that oridonin has protective effects against acute liver injury in mice.16 Additionally, several studies have shown that oridonin affects glutathione depletion, reactive oxygen species, and the NF-κB pathway.17,18 However, oridonin has poor aqueous solubility and bioavailability, and its short biological half-life presents obstacles for its clinical use.19 To this end, novel derivatives of oridonin have been developed and demonstrated enhanced anti-hepatic fibrogenetic effects.20,21 CYD0618, a novel oridonin analog, was chosen for further study due to its enhanced potency and aqueous solubility. We hypothesized that CYD0618 will potently inhibit hepatic fibrogenetic activity through suppression of both the classic and alternative NF-κB signaling pathways.
Methods
Reagents
All cell culture mediums and trypsin were purchased from Life Technology Corp. (Carlsbad, CA). Oridonin and Bay 11–7082 were purchased from Sigma-Aldrich Co. LLC. (St. Louis, MO). CYD0618 is a newly designed nitrogen-enriched oridonin analogue with a thiazole ring fused at C-1 and C-2 of the A-ring, resulting in significantly improved aqueous solubility (Figure 1). CYD0618 was synthesized following our previously reported protocols.22,23 All experiments were conducted within a biosafety level 2 laboratory in full compliance with the University of Texas Medical Branch Biological Safety Committee.
Figure 1:
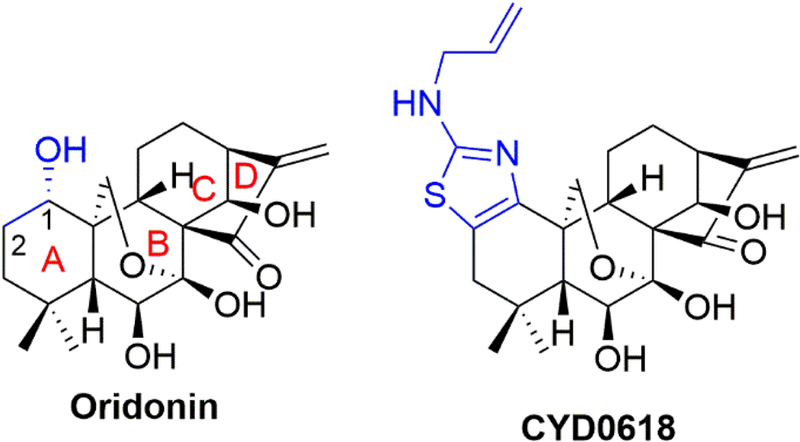
Chemical structures of oridonin and its new analogue CYD0618
Cell Culture
The human immortalized HSC line LX-2 and rat immortalized HSC line HSC-T6 were a gift from Dr. Scott Friedman (Mount Sinai Medical Center, New York) and cultured at 37°C with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) with a high glucose concentration (4.5 g/L) supplemented with 5% fetal bovine serum (FBS), 1% penicillin/streptomycin. All experiments were performed on cells within 6 weeks of culture from liquid nitrogen. Human and rat cell lines were used to ensure that these effects were not limited to a single cell line.
Cell Viability Assay
Cell viability was assessed using Alamar Blue assay (Cat#Dal1025) purchased from Life Technologies (Grand Island, NY) and by following the manufacturer’s instructions. Fluorescence intensity was monitored using a SpectraMax M5 microplate reader from Molecular Devices, LLC (Sunnyvale, CA) with excitation and emission wavelengths set at 544 and 590 nm, respectively. Assay was performed in triplicate and repeated at least 3 times.
Cell Death Detection ELISA Assay
8 × 103 cells/well were seeded into 96 well plates. The next day, after reaching 70–80% confluence, cells were replaced with fresh complete medium and treated as indicated. Apoptosis was determined using a Cell Death Detection ELISA Kit (product # 11 774 425 001) from Roche Diagnostic Corp. (Indianapolis, IN) following the manufacturer’s protocol. Assay was performed in duplicate and repeated twice.
Immunofluorescence staining
Immunofluorescence staining was performed as previously described24 with anti-NF-κΒ p65 (Cat#8242) from Cell Signaling Technology Inc. (Danvers, MA). After the indicated treatments and staining, the cells were visualized by Nikon Eclipse Ti confocal microscope at x20 magnification (Nikon Instruments Inc.).
p65 DNA Binding Assay
NF-κΒ p65 DNA binding activity was identified by TransAM NF-κΒ DNA-binding enzyme-link immunosorbent assay (ELISA) kit (Cat#40096) purchased from Active Motif (Carlsbad, CA) by following the manufacturer’s instructions.
Western Immunoblotting
Whole cell extracts were prepared as previously described.11 Anti-Collagen Type I polyclonal antibody (600–401-103) was purchased from Rockland Immunochemicals Inc. (Gilbertsville, PA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (10R-G109A) was purchased from Fitzgerald Industries (Concord, MA). Anti-p21 (Cat#556431) and p27 (Cat#610241) were from BD Biosciences (San Jose, CA). Anti-IL-1β (sc-7884) was obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX). Anti-IL-6 (ab9324) was obtained from Abcam (Cambridge, UK). Cleaved PARP (Cat#5625), phospho-NF-κΒ p65 Ser536 (Cat#3033), NF-κΒ p65 (Cat#8242), phospho-IκBα Ser32 (Cat#2859), and IκΒα (Cat#4814) antibodies were obtained from Cell Signaling Technology Inc. (Danvers, MA).
Measurement of cytokine secretion
The concentration of secreted IL-6 and MCP-1 in the cell culture supernatant were determined using a specific human IL-6 and MCP-1 ELISA kit according to the manufacturer’s instructions.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7.0 from GraphPad Software Inc. (La Jolla,CA). Data is presented as mean ± standard error of the mean, with significance defined as P<0.05. Errors bars represent the standard error of the mean.
Results
CYD0618 decreases HSC viability
CYD0618 inhibits LX-2 cell viability in a dose-dependent manner with a half maximal inhibitory concentration (IC50) of 0.42 μΜ. LX-2 cell viability was significantly decreased at concentrations greater than 0.5 μΜ (P<0.001) (Figure 2A). CYD0618 inhibits HSC-T6 cell viability in a dose-dependent manner with an IC50 of 0.50 μΜ. HSC-T6 cell viability was significantly decreased at concentrations greater than 75 μΜ (P<0.001) (Figure 2B). CYD0618 increases cell growth arrest markers p21 (Figure 2C) and p27 in LX-2 cells (Figure 2D).
Figure 2:
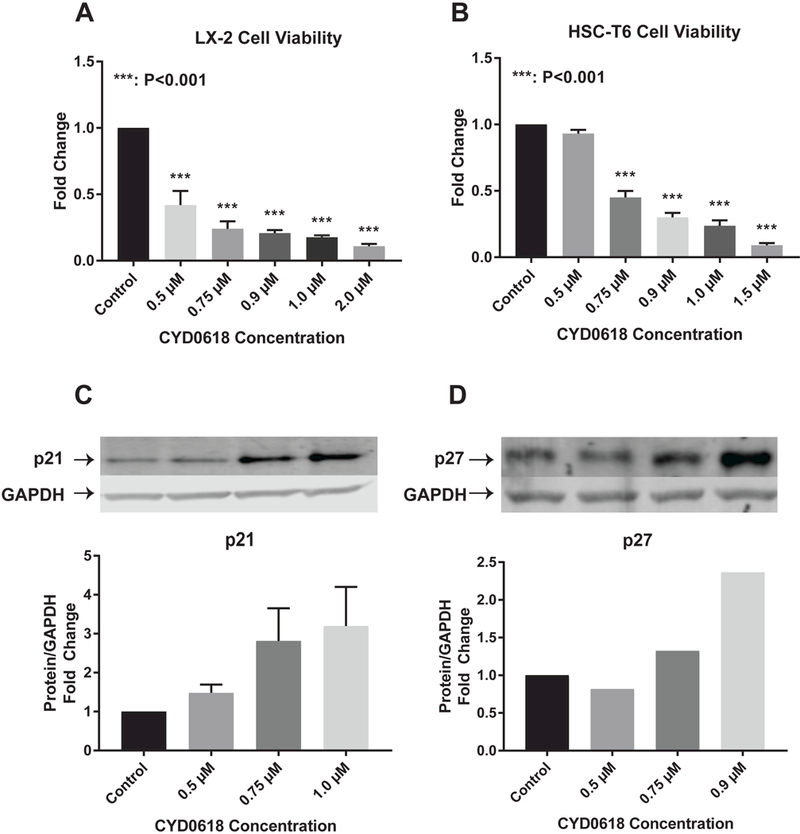
CYD0618 decreases HSC viability (A) LX-2 and (B) HSC-T6 cell viability after treatment with a series of concentrations of CYD0618 for 48 hours. Western blots for LX-2 whole cell lysate after incubation with CYD0618 for cell growth arrest markers (C) p21 and (D) p27. P-values shown compared to vehicle. Densitometric analyses of bands were quantified and data expressed as fold of control normalized to GAPDH. All P-values shown compared to vehicle.
C3A and HepG2 cells were used to test the cytotoxic effects of oridonin on normal human hepatocytes (Data not shown). C3A cells are a clonal derivative of the widely used human hepatocellular carcinoma HepG2 cell line and were selected for its better-differentiated hepatocyte phenotype.25 CYD0618 had no significant effect on C3A cell viability at concentrations up to 1.0 μΜ with an IC50 of 20.73 μΜ. CYD0618 had no significant effect on HepG2 cell viability at concentrations up to 0.75 μΜ with an IC50 of 0.96 μΜ. CYD0618 concentration of 0.5 μΜ or 0.75 μΜ was used in the following experiments to avoid cellular toxicity.
CYD0618 induces HSC apoptosis and decreases Collagen I levels
CYD0618 induces apoptosis in LX-2 cells in a dose dependent manner. Apoptosis was significantly induced at concentrations greater than 0.5 μΜ (P<0.01) (Figure 3A). CYD0618 induces apoptosis in HSC-T6 cells in a dose dependent manner. Apoptosis was significantly induced at concentrations greater than 0.75 μΜ (P<0.05) (Figure 3B). CYD0618 significantly increases cell death marker cleaved poly (ADP-ribose) polymerase (c-PARP) in LX-2 cells in a dose dependent manner (0.75 μΜ, P<0.05 and 1.0 μΜ P<0.01) (Figure 3C). CYD0618 significantly decreases HSC end product Collagen I in LX-2 cells at concentrations of 0.75 μΜ (P<0.05) and 1.0 μΜ (P<0.05) (Figure 3D).
Figure 3:
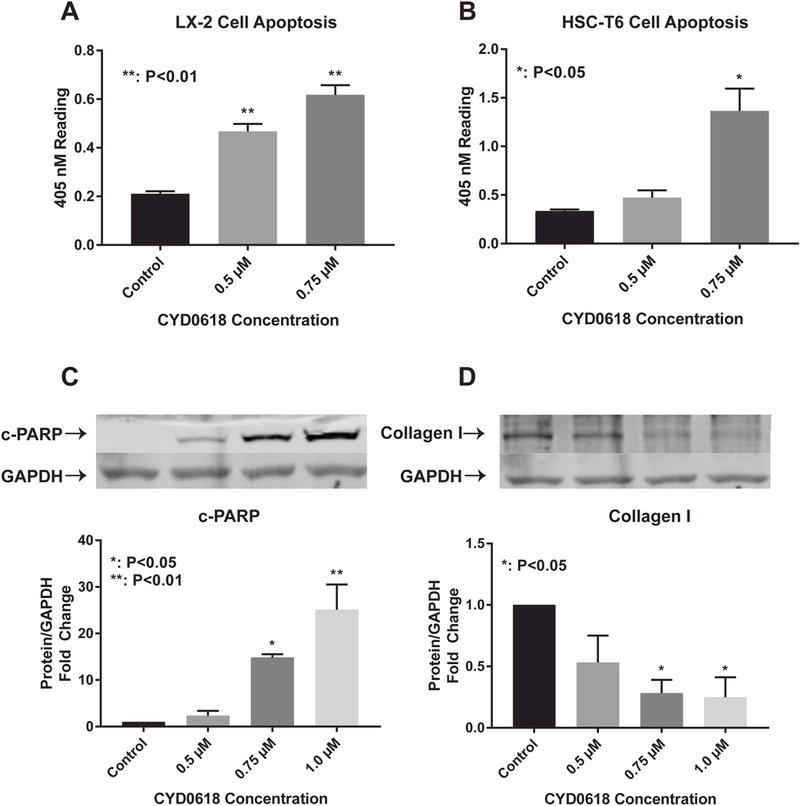
CYD0618 induces HSC apoptosis and decreases Collagen I levels Cell Death Detection ELISA for (A) LX-2 and (B) HSC-T6 cells after treatment with CYD0618 at indicated concentrations for 24 hours. Western blot for LX-2 whole cell lysate after incubation with CYD0618 for apoptosis maker (C) cleaved-PARP and HSC regulated (D) Collagen I. Densitometric analyses of bands were quantified and data expressed as fold of control normalized to GAPDH. All P- vales shown compared to vehicle.
CYD0618 suppresses classic NF-κΒ pathway
Immunofluorescence for NF-κB subunit p65 (Figure 4A) demonstrates the presence of p65 in the cytoplasm in a control state in LX-2 cells. Addition of LPS results in p65 translocation to the nucleus. Addition of CYD0618 decreases translocation of p65 into the nucleus. These results are confirmed using p65 protein levels in the nucleus, which are elevated with LPS exposure and return to near normal levels with addition of CYD0618 (Figure 4B). p65 DNA binding assay demonstrates significantly increased DNA binding with LPS exposure (P<0.01) with a return to near control levels with the addition of CYD0618 (Figure 4C). The LPS-induced increase (P<0.001) in the ratio of phosphorylated to total NF- kB regulatory protein IκBα is reversed by addition of CYD0618. LPS-exposure results in IκBα degradation but IκBα returns to near control levels after addition of CYD0618 (Figure 4D). Treatment with CYD0618 alone without LPS-stimulation demonstrated no change from the control state for nuclear NF-kB levels, phosphorylated IκBα levels, and total IκBα levels (Figures 4B and 4D).
Figure 4:
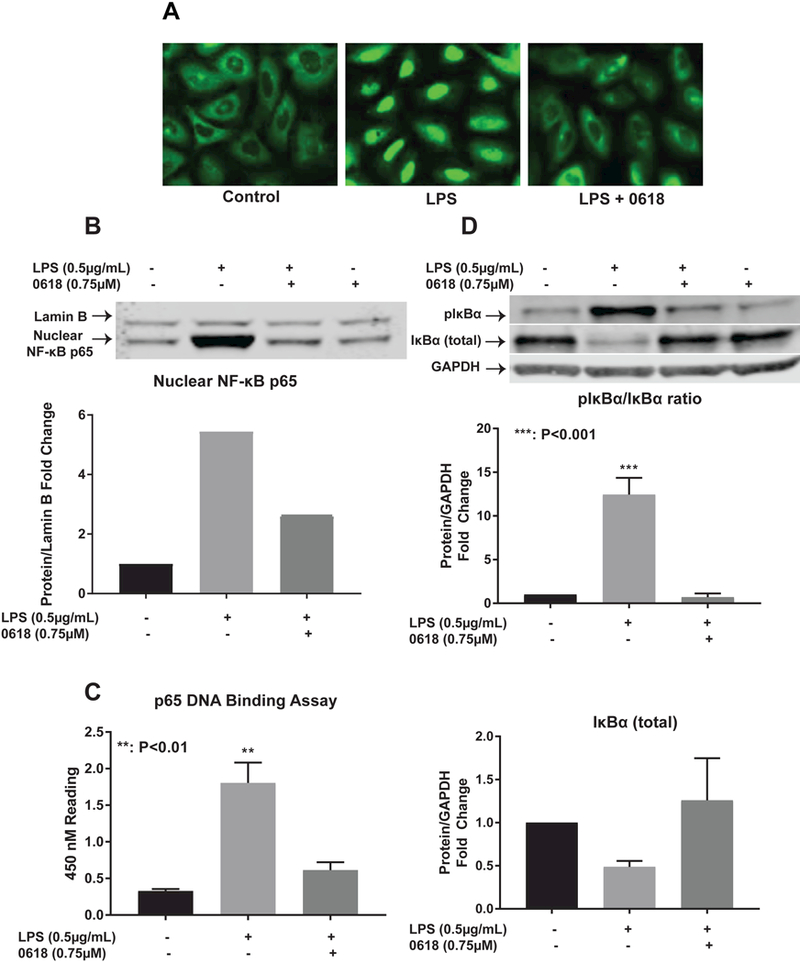
CYD0618 suppresses classic NF-kB pathway LX-2 cells treated with LPS, CYD0618, or both CYD0618 and LPS for 1 hour underwent (A) Immunofluorescence staining for p65, (B) nuclear fraction Western blot with antibodies for NF-κB p65, (C) p65 DNA binding ELISA assay with 10 μg of nuclear protein, and (D) whole cell lysate Western blot with antibodies for NF-κB inhibitory protein IκBα and phosphorylated-IκBα. P-values shown compared to vehicle. Densitometric analyses of bands were quantified and data expressed as fold of control normalized to GAPDH for cytosolic protein or Lamin A for nuclear protein.
CYD0618 inhibits alternative pathway for NF-kB activation
CYD0618 significantly decreased LPS-induced levels of phosphorylated p65 at S536 (P<0.001) with no significant change in total NF-κB p65 (Figure 5A). CYD0618 also significantly decreased endogenous phosphorylated p65 S536 (P<0.01) with no significant change in non-phosphorylated NF-kB p65 (Figure 5B). Treatment with CYD0618 alone without LPS-stimulation demonstrated no change from the control state for phosphorylated p65 levels (Data not shown).
Figure 5:
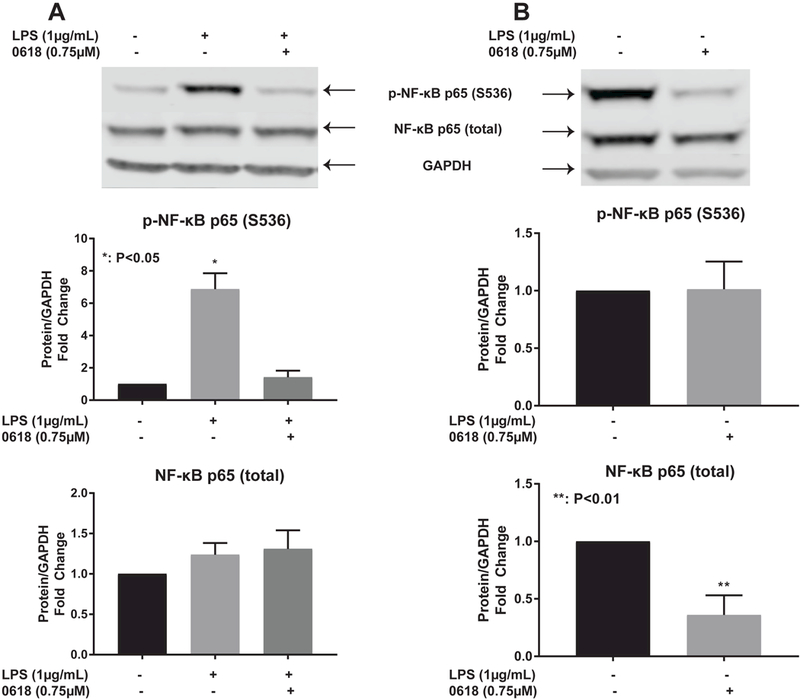
CYD0618 inhibits alternative pathway for NF-κB activation Western blots for LX-2 whole cell lysate after treatment with LPS alone, CYD0618 alone, or both for (A)LPS-stimulated and (B)endogenous NF-κB p65 and phosphorylated NF-κB p65. Densitometric analyses of bands were quantified and data expressed as fold of control normalized to GAPDH. P-values shown compared to vehicle.
CYD0618 inhibits expression of NF-κΒ target genes
Significant increase in IL-6 levels is seen with addition of LPS alone (P<0.001) and LPS with CYD0618 (P<0.05). CYD0618 significantly reduces IL-6 levels when compared to LPS alone (P<0.001) (Figure 6A). A significant increase in MCP-1 levels is seen with the addition of LPS alone (P<0.001). CYD0618 significantly reduces MCP-1 levels when compared to LPS alone (P<0.001) (Figure 6B). In addition, CYD0618 reduces IL-β expression to near control levels after LPS exposure (Figure 6C).
Figure 6:
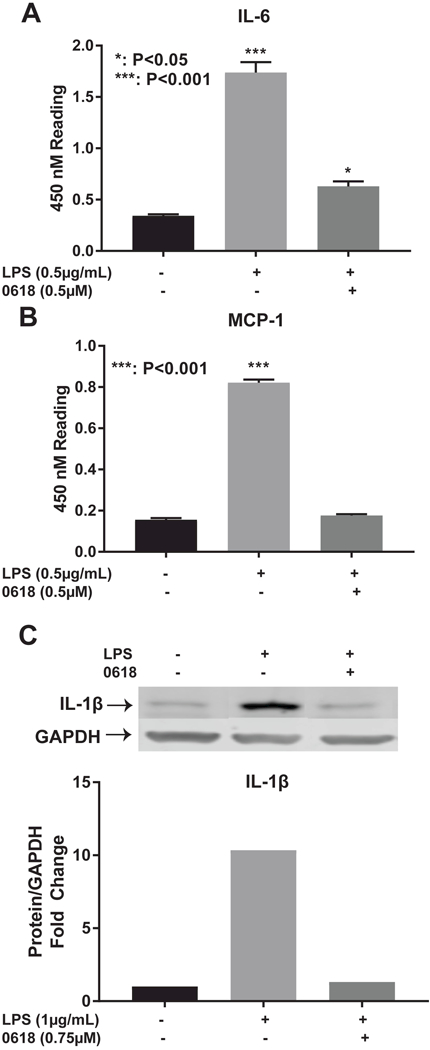
CYD0618 inhibits expression of NF-κB regulated genes ELISA for (A) IL-6 and (B)MCP-1 antibodies after treatment with LPS or both LPS and CYD0618. Western blot for LX-2 whole cell lysate after incubation with LPS or both LPS and CYD0618 for (C) IL- 1B. GAPDH was used as loading control. Densitometric analyses of bands were quantified and data expressed as fold of control normalized to GAPDH. P-values shown compared to vehicle.
Selective NF-κΒ inhibitor, Bay 11–7082, inhibits HSC proliferation and induces HSC apoptosis
Bay 11–7082 inhibits LX-2 cell viability in a dose-dependent manner with an IC50 of 4.65 μΜ. LX-2 cell viability was significantly decreased at concentrations greater than 4 μΜ (P<0.001) (Figure 7A). Bay 11–7082 inhibits HSC-T6 cell viability in a dose-dependent manner with an IC50 of 6.93 μΜ. HSC-T6 cell viability was significantly decreased at concentrations greater than 4 μM (P<0.05) (Figure 7B). Bay 117082 significantly induces apoptosis in LX-2 cells at a concentration of 7.5 μΜ (P<0.05) (Figure 7C). Bay 11–7082 significantly induces apoptosis in HSC-T6 cells at a concentration of 10 μΜ (P<0.01) (Figure 7D).
Figure 7:
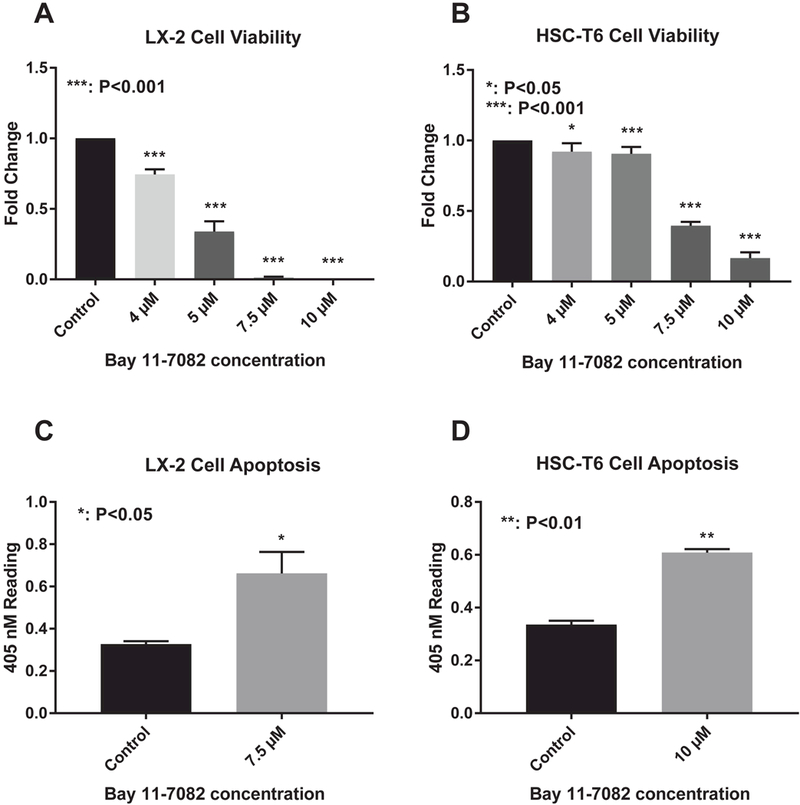
NF-kB inhibitor inhibits HSC proliferation and induces HSC apoptosis (A) LX-2 and (B) HSC-T6 cell viability after treatment with a series of concentrations of Bay-11–7082. Cell Death Detection ELISA for (C) LX-2 and (D) HSC-T6 cells after treatment with Bay-11–7082 for 24 hours. P-values shown compared to vehicle.
Discussion
Our results demonstrate that CYD0618 is an effective potential anti-hepatic fibrogenetic agent via suppression of the NF-κΒ pathway. CYD0618 significantly decreases cell viability and induces apoptosis in activated HSCs. This leads to a significant decrease in Collagen I, a major component of the extracellular matrix (ECM) responsible for fibrosis. In addition, our results demonstrate that the NF-κΒ pathway is significantly impaired by CYD0618, suggesting a possible mechanism for this anti-hepatic fibrogenetic activity. Both the classical and alternative pathways of NF-κΒ activation were significantly inhibited by CYD0618. Use of a selective NF-κΒ inhibitor confirms that NF-κΒ pathway inhibition results in the same anti-hepatic fibrogenetic effects as those seen with CYD0618.
HSC apoptosis is associated with reversal of hepatic fibrosis.8 Fibrosis is mediated by HSCs, which deposit excess ECM in the liver parenchyma when activated. The ECM is a hydrated gel composed of up to 30% collagens, as well as elastins, fibronectins, laminins, and proteoglycans.26 HSCs are activated by a variety of stimuli, including nuclear factor kappa-light-chain-enhancer of activated Β cells (NF-κΒ), transforming growth factor β1 (TGF β1), lipopolysaccharide (LPS)/toll-like receptors, tissue hypoxia, platelet-derived growth factors (PDGF), nicotinamide adenine dinucleotide phosphate-oxidase (NADPH), and the renin-angiotensin system.27 HSC activation results in over expression of α-smooth muscle actin (α-SMA), excessive production of collagens I and III, and secretion of tumor necrosis factor α (TNF-α), IL-6, IL-1β, and MCP-1 among other cytokines.28–32 In vivo studies have demonstrated that apoptosis of HSCs has resulted in accelerated recovery from liver fibrosis.8
CYD0618 decreases cell viability and induces apoptosis in HSCs in vitro. The potency of CYD0618 is approximately the same as other oridonin analogues and is much more potent than the parent compound.11
This finding is particularly important due to the low potency of unaltered oridonin.19 While other investigators have attempted to prepare targeted nanoparticles for the delivery of oridonin,19 a simpler and potentially more effective solution is to modify the parent compound itself so that it has greater pharmacologic activity. CYD0618 is non-toxic for C3A cells at concentrations which significantly decrease and induce apoptosis in HSCs, indicating that CYD0618’s potency is great enough to combat fibrosis without a significant impact on other hepatocytes. C3A cells are a clonal derivative of the widely used human hepatocellular carcinoma HepG2 cell line and were selected for their better-differentiated hepatocyte phenotype.
CYD0618 increases p21 and p27 concentration, which are cyclin dependent kinase inhibitors that are involved in cell growth arrest.33,34 Oridonin and its analogues are known to cause S-phase arrest in LX-2 cells, consistent with these results.11,20,21 CYD0618 also significantly increases levels of c-PARP.
Oridonin induces cell death through caspase-3 independent degradation of PARP,35 which is also consistent with our results in this study. Finally, CYD0618 decreases levels of Collagen 1, a key end product of activated HSCs that contributes to hepatic fibrosis.31
Previous studies have shown the potent anti-fibrotic properties of oridonin and its analogues,11,20,21 but the mechanism of action is unknown. NF-κB activation is essential for both HSC survival and activation5,7,36 and CYD0618 significantly inhibits the NF-κB pathway.
We observed a decrease in endogenous and LPS- induced phosphorylated p65, which decreases NF-κB activation as well as transcriptional activity potential.9 While oridonin is known to inhibit the classic NF- κB pathway,17,18 our study is the first to link oridonin or its analogues to alternative NF-kB activation pathways, specifically S536 p65 phosphorylation. Unlike its association with IkB proteins, which equally affects the expression of all NF-κB dependent genes, differential p65 phosphorylation exerts its effects in a gene-specific manner.9 Phosphorylation at S536 is the result of at least 5 kinases and is involved in the recruitment of components of the basal transcriptional machinery.10 These results suggest a novel mechanism for the anti-hepatic fibrogenetic activity of oridonin and its analogues.
In addition, the classic NF-κΒ pathway was inhibited by CYD0618. Our study is the first to demonstrate classic NF-κB pathway inhibition by an oridonin analog. Activation of the NF-κB pathway involves phosphorylation and proteasome-dependent degradation of NF-κΒ inhibitor IκBα and nuclear translocation of NF-κB dimers (e.g. p50/p65).6,8 Our results demonstrate inhibition of LPS-induced IκBα phosphorylation by CYD0618. Additionally, CYD0618 significantly reduces LPS-induced p65 DNA binding and LPS-induced p65 nuclear translocation via immunofluorescence and Western Blot. The pro- inflammatory cytokines IL-6, IL-1B and MCP-1 are upregulated by NF-κΒ pathway activation.37 CYD0618 administration led to a reduction in the levels of these cytokines after LPS-stimulation, demonstrating CYD0618’s anti-inflammatory properties.
Bay-11–7082 is a selective NF-κΒ inhibitor with strong anti-inflammatory, anti-cancer and neuroprotective effects.38 Bay-11–7082 is an inhibitor of κB kinase (IKK), which blocks the phosphorylation and proteasome mediated degradation of NF-κΒ inhibitory protein IκBα. Our results demonstrate that Bay-11–7082 decreases cell viability and induces apoptosis in the human and rat immortalized HSC lines LX-2 and HSC-T6. These results mirror those seen with CYD0618, as well as its parent compound oridonin,11 demonstrating that selective inhibition of the NF-κΒ pathway results in anti- hepatic fibrogenesis. This further strengthens the conclusion that NF-κΒ pathway inhibition is responsible for the potent anti-hepatic fibrogenetic effects of CYD0618.
Conclusion
In activated human and rat HSCs, the novel oridonin analogue CYD0618 has demonstrated a significant ability to inhibit hepatic fibrogenesis in vitro. The future direction of this research will involve in vivo models. These effects are mediated via inhibition of the NF-κΒ pathway, which further elucidates the mechanism of oridonin and its analogues. A better understanding of the mechanism of action will allow for the development of more potent and potentially safer therapeutics for liver fibrosis.
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health under award number T32DK007639. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25(2):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novo E, Cannito S, Paternostro C, Bocca C, Miglietta A, Parola M. Cellular and molecular mechanisms in liver fibrogenesis. Arch Biochem Biophys. 2014;548:20–37. [DOI] [PubMed] [Google Scholar]
- 3.Byass P The global burden of liver disease: a challenge for methods and for public health. BMC Med. 2014;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123(5):1887–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luedde T, Schwabe RF. NF-kappaB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8(2): 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta J, Fan Y, Gupta N, Fan G, Gelinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;25(51):6800–6816. [DOI] [PubMed] [Google Scholar]
- 7.Oakley F, Meso M, Iredale JP, et al. Inhibition of inhibitor of kappaB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis. Gastroenterology. 2005;128(1):108–120. [DOI] [PubMed] [Google Scholar]
- 8.Elsharkawy AM, Oakley F, Mann DA. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis. 2005;10(5):927–939. [DOI] [PubMed] [Google Scholar]
- 9.Hochrainer K, Racchumi G, Anrather J. Site-specific phosphorylation of the p65 protein subunit mediates selective gene expression by differential NF-kappaB and RNA polymerase II promoter recruitment. J Biol Chem. 2013;288(1):285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buss H, Dorrie A, Schmitz ML, Hoffmann E, Resch K, Kracht M. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-{kappa}B at serine 536 is mediated by multiple protein kinases including I{kappa}B kinase (IKK)-{alpha}, IKK{beta}, IKK{epsilon}, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31- mediated interleukin-8 transcription. J Biol Chem. 2004;279(53):55633–55643. [DOI] [PubMed] [Google Scholar]
- 11.Bohanon FJ, Wang X, Ding C, et al. Oridonin inhibits hepatic stellate cell proliferation and fibrogenesis. J Surg Res. 2014;190(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang HP, Li GQ, Guo WZ, et al. Oridonin synergistically enhances JQ1-triggered apoptosis in hepatocellular cancer cells through mitochondrial pathway. Oncotarget. 2017;8(63): 106833–106843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong AM, Zhang Y, Kesler K, et al. Genomic and in vivo evidence of synergy of a herbal extract compared to its most active ingredient: Rabdosia rubescens vs. oridonin. Exp Ther Med. 2010;1(6):1013–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Wold EA, Ding Y, Shen Q, Zhou J. Therapeutic Potential of Oridonin and Its Analogs: From Anticancer and Antiinflammation to Neuroprotection. Molecules. 2018;23(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Y, Ding C, Ye N, et al. Discovery and development of natural product oridonin- inspired anticancer agents. Eur J Med Chem. 2016;122:102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng Y, Chen C, Yu H, et al. Oridonin ameliorates lipopolysaccharide/D-galactosamine- induced acute liver injury in mice via inhibition of apoptosis. Am J Transl Res. 2017;9(9):4271–4279. [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Y, Wei W, Zhang N, et al. Oridonin stabilizes retinoic acid receptor alpha through ROS-activated NF-kappaB signaling. BMC Cancer. 2015;15:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo LM, Kuo CY, Lin CY, Hung MF, Shen JJ, Hwang TL. Intracellular glutathione depletion by oridonin leads to apoptosis in hepatic stellate cells. Molecules. 2014;19(3):3327–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C, Zhang D, Guo H, et al. Preparation and characterization of galactosylated bovine serum albumin nanoparticles for liver-targeted delivery of oridonin. Int J Pharm. 2013;448(1):79–86. [DOI] [PubMed] [Google Scholar]
- 20.Bohanon FJ, Wang X, Graham BM, et al. Enhanced effects of novel oridonin analog CYD0682 for hepatic fibrosis. J Surg Res. 2015;199(2):441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohanon FJ, Wang X, Graham BM, et al. Enhanced anti-fibrogenic effects of novel oridonin derivative CYD0692 in hepatic stellate cells. Mol Cell Biochem. 2015;410(1–2):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding C, Zhang Y, Chen H, et al. Oridonin ring A-based diverse constructions of enone functionality: identification of novel dienone analogues effective for highly aggressive breast cancer by inducing apoptosis. J Med Chem. 2013;56(21):8814–8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding C, Zhang Y, Chen H, et al. Novel nitrogen-enriched oridonin analogues with thiazole-fused A-ring: protecting group-free synthesis, enhanced anticancer profile, and improved aqueous solubility. J Med Chem. 2013;56(12):5048–5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunez Lopez O, Bohanon FJ, Wang X, et al. STAT3 Inhibition Suppresses Hepatic Stellate Cell Fibrogenesis: HJC0123, a Potential Therapeutic Agent for Liver Fibrosis. RSC Adv. 2016;6(102): 100652–100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly JH, Darlington GJ. Modulation of the liver specific phenotype in the human hepatoblastoma line Hep G2. In Vitro Cell Dev Biol. 1989;25(2):217–222. [DOI] [PubMed] [Google Scholar]
- 26.Roderfeld M Matrix metalloproteinase functions in hepatic injury and fibrosis. Matrix Biol. 2017. [DOI] [PubMed] [Google Scholar]
- 27.Tsukamoto H, Zhu NL, Wang J, Asahina K, Machida K Morphogens and hepatic stellate cell fate regulation in chronic liver disease. J Gastroenterol Hepatol. 2012;27 Suppl 2:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemoinne S, Cadoret A, El Mourabit H, Thabut D, Housset C. Origins and functions of liver myofibroblasts. Biochim Biophys Acta. 2013;1832(7):948–954. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Wang Z, Kwong SQ, et al. Inhibition of PDGF, TGF-beta, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J Hepatol. 2011;55(3):612–625. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, Seo GS, Park YN, Yoo TM, Sohn DH. Effects and regulation of osteopontin in rat hepatic stellate cells. Biochem Pharmacol. 2004;68(12):2367–2378. [DOI] [PubMed] [Google Scholar]
- 31.Ramm GA, Shepherd RW, Hoskins AC, et al. Fibrogenesis in pediatric cholestatic liver disease: role of taurocholate and hepatocyte-derived monocyte chemotaxis protein-1 in hepatic stellate cell recruitment. Hepatology. 2009;49(2):533–544. [DOI] [PubMed] [Google Scholar]
- 32.Fang S, Yuan J, Shi Q, et al. Downregulation of UBC9 promotes apoptosis of activated human LX-2 hepatic stellate cells by suppressing the canonical NF-kappaB signaling pathway. PLoS One. 2017;12(3):e0174374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui Q, Yu JH, Wu JN, et al. P53-mediated cell cycle arrest and apoptosis through a caspase-3- independent, but caspase-9-dependent pathway in oridonin-treated MCF-7 human breast cancer cells. Acta Pharmacol Sin. 2007;28(7):1057–1066. [DOI] [PubMed] [Google Scholar]
- 34.Cai M, Jin S, Deng L, et al. Lewis y antigen promotes p27 degradation by regulating ubiquitin-proteasome activity. Oncotarget. 2017;8(66):110064–110076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang CL, Wu LJ, Tashiro S, Onodera S, Ikejima T. Oridonin induces a caspase- independent but mitochondria- and MAPK-dependent cell death in the murine fibrosarcoma cell line L929. Biol Pharm Bull. 2004;27(10):1527–1531. [DOI] [PubMed] [Google Scholar]
- 36.Sun B, Karin M. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene. 2008;27(48):6228–6244. [DOI] [PubMed] [Google Scholar]
- 37.Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86(2):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J, Rhee MH, Kim E, Cho JY. BAY 11–7082 is a broad-spectrum inhibitor with anti-inflammatory activity against multiple targets. Mediators Inflamm. 2012;2012:416036. [DOI] [PMC free article] [PubMed] [Google Scholar]


