Abstract
Diet-induced obesity induces peripheral inflammation accompanied by a loss of myenteric neurons. Few studies, however, have investigated the effects of a high fat diet (HFD) on either the development of myenteric neurons or prior to the occurrence of obesity. The present study assessed the effects of maternal HFD on the density and neurochemical phenotype of myenteric ganglia in the upper gastrointestinal tract. Sprague-Dawley rats were fed either a control or HFD (14% or 60% kcal from fat, respectively) from embryonic day 13; the fundus, corpus and duodenum were fixed thereafter at postnatal 2, 4, 6 and 12 weeks of age for subsequent immunohistochemical studies. While myenteric ganglion size did not differ throughout the study, HFD exposure decreased the number of nitrergic neurons by 6 weeks of age in all regions. This decrease was accompanied by a loss of PGP-immunoreactive neurons, suggesting a decline in myenteric neuronal number. HFD also increased myenteric plexus glial cell density in all regions by 4 weeks of age. These changes occurred in the absence of an increase in serum or gastric inflammatory markers. The present study suggests that exposure to a HFD during the perinatal time period results in glial proliferation and loss of inhibitory nitrergic neurons prior to the onset of obesity, suggesting that dietary alterations may affect gastrointestinal functions independently of increased adiposity or glycemic dysregulation.
Keywords: perinatal, high fat diet, myenteric plexus, glial cells
Introduction
Maternal over-nutrition during the perinatal period is known to predispose offspring to develop metabolic syndrome, obesity and its associated comorbidities in later life (Levin, 2006; Biddinger & Fox, 2010; Levin, 2010b; a). The perinatal period, encompassing pre- and early postnatal time-points, is critical for the development of central and peripheral autonomic neurocircuits required for homeostatic regulation of visceral functions such as gastric motility and emptying, intestinal transit, glucose homeostasis, and appetite regulation (Levin, 2006; 2010b; a; Tamashiro & Moran, 2010). Energy homeostasis requires the appropriate integration of both peripheral and multiple central neurocircuits, at the level of the gastrointestinal (GI) tract, these involve both intrinsic (enteric) as well as extrinsic (principally vagal) inputs. Disruption or dysfunction of any of these neural inputs may dysregulate upper GI functions, leading to disordered meal patterns, altered food intake and energy balance, and contribute to the onset and severity of obesity (Berthoud, 2008; Furness, 2012; Browning & Travagli, 2014)
Despite a significant degree of influence from extrinsic, particularly vagal efferent inputs to the upper GI tract, the myenteric plexus innervating the circular and longitudinal intestinal smooth muscle, is capable of a large degree of intrinsic control of GI functions (Furness, 2012). Development of the myenteric plexus begins at embryonic day 12.5 (E12.5), when myenteric neurons are first observed in the stomach and small intestine (Tanano et al., 2005). Vagal efferent axons encircle myenteric neurons by E16.5, synaptic differentiation begins around E17 and synaptic vesicles are present around E19 (Ratcliffe et al., 2011). Structural and functional growth in the myenteric plexus, including synaptogenesis and changes in neurotransmitter expression, continues after birth, as does ganglion formation and the innervation of developing smooth muscle (Rodrigues et al., 2011).
Damage to, or plasticity within, either enteric or vagal neurocircuits, may result in gastrointestinal dysmotility, and is observed frequently in both obese patients and animal models (Madsen, 1992; Park & Camilleri, 2005; Gallagher et al., 2007). The loss of nitrergic myenteric neurons, in several studies of both prediabetic and Type II diabetic patients and animal models, a common comorbidity of obesity, offers a possible explanation for the commonly observed GI disturbances in these patients (Surendran & Kondapaka, 2005; Iwasaki et al., 2006; Chandrasekharan & Srinivasan, 2007; Honore et al., 2011; Stenkamp-Strahm et al., 2013; Rivera et al., 2014; Stenkamp-Strahm et al., 2015) but whether these alterations within the myenteric plexus occur prior to obesity and contribute to its development, or occur in response to increased adiposity and peripheral inflammation, have not been studied in great detail.
The aims of the present study, therefore, were to investigate whether early exposure to a HFD during enteric nervous system (ENS) development induces myenteric neuronal loss in rats even prior to the development of obesity.
Experimental Procedures
All experiments were conducted with the approval of the Institutional Animal Care and Use Committee and according to National Institute of Health regulations.
Animals
Timed pregnant Sprague-Dawley dams (Charles River, Kingston, NY, USA) were housed under a standard 12-hour light/dark cycle and had ad libitum access to water and either a control diet (14% kcal from fat, Purina Mills, Gray Summit, MO, USA) or HFD (60% kcal from fat, D12492; Research Diets Inc., NJ, USA) from embryonic day 13 (E13). The rat pups were weaned at postnatal day 21 and maintained on the same control (n=30) or HFD (n=28; termed ‘perinatal high fat diet’; PNHFD). Body weight and blood glucose were measured at the time of sacrifice, at 2, 4, 6, 8 & 12 weeks of age.
Tissue Processing for Immunohistochemistry
Briefly, rats were anesthetized with isoflurane (5% with air) and, in the absence of the foot pinch withdrawal reflex, were euthanized by administration of bilateral pneumothorax. A ventral midline incision was made to remove the stomach and proximal small intestine. The stomach was dissected along the major and minor curvatures while the duodenum was dissected along the mesenteric border. Tissues were cleaned and pinned, mucosal side uppermost, under light tension on Sylgard-lined dishes and fixed overnight in Zamboni’s fixative (see below for composition) at 4°C. Fixed tissues were subsequently cleared of fixative by repeated washes in 70% ethanol before being dehydrated and rehydrated using a graded series of alcohols and xylene. The tissue was then stored in 0.1M phosphate buffered saline (PBS) containing 0.05% sodium azide for later processing. Under magnification (6-50×), the mucosal, submucosal and circular layer of the tissue were removed, leaving the myenteric plexus adherent to the underlying longitudinal muscle.
Immunohistochemical staining
All tissue washing and antibody incubation procedures were done on a multi-purpose rocking shaker at room temperature. Tissues were washed (3 × 15min) in 0.1M PBS, followed by 90mins in 0.05% sodium borohydrate in 0.1M PBS, followed by washing (3 × 5min) in immunobuffer (IB; see below for composition). Tissues were then incubated in IB containing either 10% Normal Horse Serum (NHS) or Normal Goat Serum (NGS) for 1h before being incubated with primary antibodies (diluted in IB+10% NHS/NGS) for 3 days. The primary antibodies used included goat anti-choline acetyltransferase (ChAT; 1:250 dilution; EMD Millipore; Burlington, MA ), mouse anti nitric oxide synthase (NOS; 1:500 dilution; Sigma Chemical Co; St. Louis, MO), mouse anti HuC/D (1:500; Life Technologies/ThermoFisher Scientific; Waltham, MA), rabbit anti-glial fibrillary acidic protein (GFAP; 1:750; EMD Millipore), and both rabbit- or goat-anti protein gene product 9.5 (PGP9.5: both 1:500 dilution; EMD Millipore). Tissues were washed subsequently (3×5min, 3×10min) in PBS before being incubated in IB+1% normal donkey serum (NDS) or normal goat serum (NGS) for 1h prior to application of the 2° antibody diluted in IB+1% NDS/NGS for 24 hours. The secondary antibodies used included donkey anti-goat, donkey antimouse, goat anti-rabbit, and goat anti-guinea pig Alexa Fluor 488, and donkey anti-mouse, goat anti-rabbit, or donkey anti-rabbit Alexa Fluor 568 (all 1:500 dilution, Life Technologies/ThermoFisher Scientific). Tissues were rinsed in PBS (3 × 5 min), plated onto gelatin-subbed slides, and coverslipped with Fluoromount (Southern Biotechnology; Birmingham, AB)
Image analysis
Immunohistochemical tissues were examined using an Olympus Fluoview FV1000 confocal microscope (Center Valley, Pa, USA). Confocal images (1μm thick) throughout the depth of the myenteric plexus were taken at a total magnification of ×200 and complied into a z-stack. Confocal images for GFAP- and HuC/D-IR were taken at a total magnification of ×600.
Sequential scanning and adjusting appropriate High-Low settings avoided colour “bleed through” and ensured approximate equal intensities across all samples. The number of ChAT-IR and NOS-IR neurons were counted in 3 representative images per each fundus, corpus and duodenum samples, and were expressed as both a percentage of total PGP9.5-IR, as well as number of neurons per ganglia. The intensity of glial staining in myenteric ganglia and the neuronal vs cytoplasmic localization of HuC/D-IR were analyzed using Image J software (https://imagej.nih.gov/ij/). To determine the intensity of GFAP-IR, the outline of myenteric ganglia were traced, background staining was equalized, and the total pixel intensity calculated. The cytoplasmic vs neuronal localization of HuC/D was determined by examining pixel intensity in both the neuronal and nuclear profile of individual neurons.
Measurement of serum inflammatory markers
Blood was collected from the aorta at the time of euthanasia, stored at room temp for 1hr and centrifuged at 1500g for 3min or until separated; serum was extracted and stored at −80°C until use. Inflammatory markers, including IFN-λ, IL-10, IL-3, IL-1β, IL-4, IL-5, IL-6, KC-GRO and TNF-α, were measured using a multiplex cytokine assay kit (Meso Scale Discovery V-PLEX pro-inflammatory Panel 2 for rat; Rockville, MD) with samples run in duplicate according to the manufacturer’s protocol, with results expressed as pg/ml.
Measurement of gastrointestinal inflammatory markers
The gastric corpus was removed and freeze clamped in liquid nitrogen followed by storage at −80°C. Prior to use, samples were weighed and crushed via mortar and pestle. Total RNA was extracted from the samples by homogenizing in QIAzol Lysis Reagent (Qiagen, Hilden, Germany). Total RNA was purified using the RNeasy Lipid Tissue Mini Kit (Qiagen) with DNase treatment followed by spectrophotometry with Nanodrop 1000 (Wilmington, DE). 1μg of RNA was quantitatively converted into cDNA (High Capacity cDNA Reverse Transcription Kit with RNase Inhibitor, Applied Biosystems, Foster City, CA) using a Veriti 96-well thermal cycler (Applied Biosystems). cDNA was prepared for qPCR using TaqMan Universal Master Mix II and single-tube Taqman Gene Expression Assays (ThermoFisher, Leesport, PA) and cycled in a QuantStudio 12k flex thermal cycler (Applied Biosystems) according to the manufacturers instructions.
Samples were prepared in triplicate. Any sample that did not superimpose upon the triplicate was excluded from analysis. Data were normalized to α-actin (Actb) and expressed as fold-change. Data were analyzed using QuantStudio 12k Flex software (Applied Biosystems) and GraphPad Prism 6 (La Jolla, CA).
Statistical Analysis
Sample sizes had normal distributions and equal variances; data were evaluated within regions across age groups using a 1-way ANOVA with post-hoc Bonferroni tests to evaluate data between the diet groups at specific ages. Analyses were performed via GraphPad Prism. All data are represented as mean ± SEM. Differences were considered statistically significant at p < 0.05.
Solution composition
Phosphate Buffered Saline (mM): NaCl 115; NaH2PO4 75; KH2PO4 7.5 Zamboni’s fixative (mM): 1.5% (w/v) paraformaldehyde; KH2PO4 19; Na2HPO4100; 240ml saturated picric acid; 1600ml H2O adjusted to pH 7.4 Immunobuffer (mM):Tris base 10; NaCl 0.9%; phosphate buffer 10; thimerosal 0.05%, adjusted to pH 7.4
Results
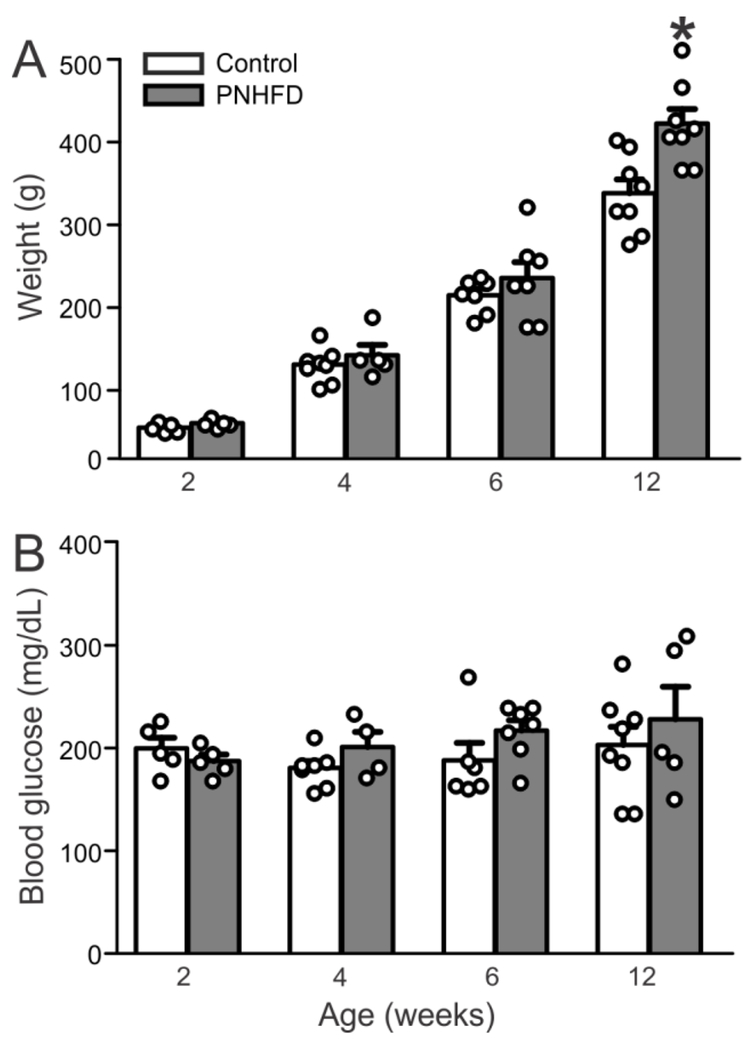
There was no significant difference in body weight between control and PNHFD rats until 12 weeks of age when PNHFD rats were considered obese, defined as 20% increase in weight relatively to control (321.1±16.5g vs 405±17.7g; P<0.05; Figure 1A). Blood glucose levels at time of sacrifice were not significantly different at any of the experimental time points in the present study (Figure 1B).
Figure 1. Rats exposed to a HFD exposure from the perinatal period onwards were obese, but not hyperglycemic, by 12 weeks of age.
A. Body mass (g) of control and PNHFD rats. By 12 weeks of age, PNHFD rats weight >20% more than age-matched control rats and were therefore classified as obese. The HFD-induced increase in body mass reached significance at the same time-point in both male and female rats; data were therefore combined (N=5-8 per data point)
B. Blood glucose levels at the time of sacrifice. Note that glycemic levels were not different at any time-point throughout the study (N=5-8 per data point).
PNHFD does not alter serum cytokine levels
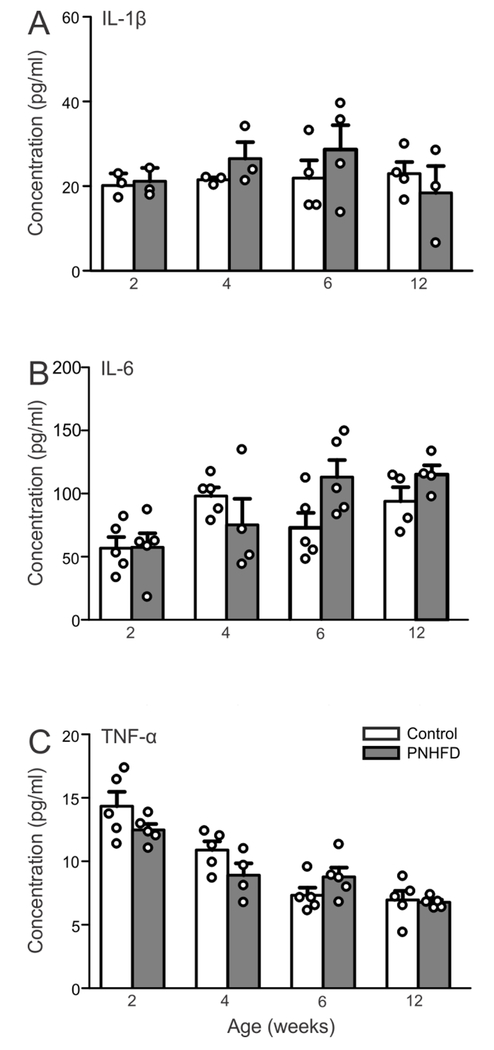
To quantify circulating inflammatory markers, serum IFN-λ, IL-10, IL-3, IL-1β, IL-4, IL-5, IL-6, KC-GRO and TNF-α levels were measured at 2,4,6, and 12 weeks of age in control and PNHFD rats (N=3-5 for each). Differences were not observed in any of the inflammatory markers measured, at any of the time points studied (P>0.05); for the sake of clarity, only IL-1β, IL-6 and TNF-α are illustrated (Figure 2).
Figure 2. PNHFD did not alter serum cytokine levels.
Serum levels (pg/ml) of IL-1β (A), IL-6 (B), and TNF-α (C) in control (white bars) and PNHFD (grey bars) rats at 2, 4, 6 and 12 weeks of age (N=3-5 per data point). Exposure to a PNHFD did not alter serum cytokine levels of any inflammatory marker (IFN-λ, IL-10, IL-3, IL-1β, IL-4, IL-5, IL-6, KC-GRO and TNF-α levels) at any time-point during this study. For the sake of clarity, only IL-1β, IL-6, and TNF-α are displayed graphically).
These results suggest that exposure to a HFD during the perinatal and subsequent weaning periods did not induce systemic inflammation during the time period of the present study.
PNHFD does not alter gastric cytokine levels
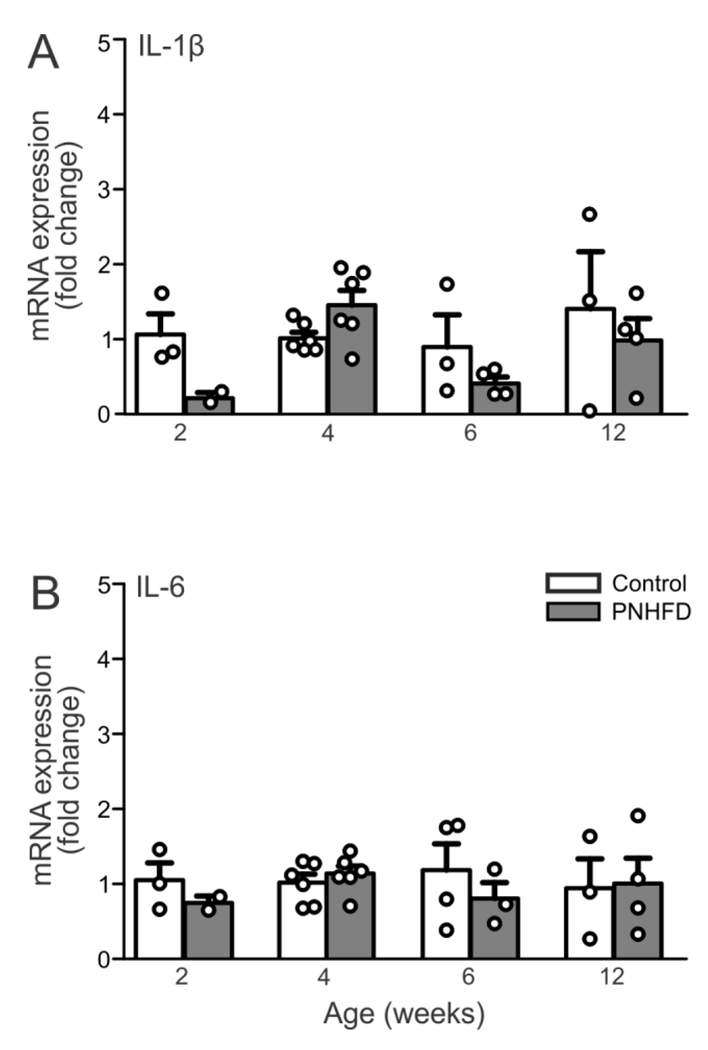
The mRNA levels of the cytokines IL-1 β and IL-6 were quantified in the gastric corpus at 2, 4, 6 and 12 weeks of age (n=3-7 per time point). Differences were not observed in any of the IL-1β and IL-6 at any of the time points studied (P>0.05; Figure 3)
Figure 3. PNHFD did not alter cytokine levels in gastric tissue.
mRNA levels for IL-1β (A) and IL-6 (B) levels in the gastric corpus in control (light bars) and PNHFD (grey bars) rats at 2,4,6, and 12 weeks of age (N=3-7 per time point). Note that PNHFD exposure had no effect on IL-1β or IL-6 mRNA levels in the gastric corpus at any time point in the present study.
These results suggest that exposure to a HFD during the perinatal and subsequent weaning periods did not induce gastrointestinal inflammation during the time period of the present study
Perinatal HFD induces neuronal loss in myenteric ganglia
While the size of myenteric ganglia increased with age in both control and PNHFD ganglia in the fundus, corpus and duodenum, it did not differ between control and PNHFD animals at any time point studied. In the corpus of control rats, for example, the ganglion size increased from 8,769±504mm2 to 9,367 ±677mm2, 12,875±1,018mm2, 17,741±741mm2 and 15,440±527mm2 at 2,4,6,8 and 12 weeks of age, respectively (F(4,10)=28.82; P<0.05), whereas in the corpus of PNHFD rats, the ganglion size increased in a similar manner from 9,311±876mm2 to 9,311±654mm2, 12,384±1,153mm2, 21,189±1,922mm2 and 15,586±606mm2 at 2,4,6,8 and 12 weeks of age, respectively F(4,10)=18.89; P<0.05). The size of ganglia were not different between control and PNHFD rats after 2 (8,769±504mm2 vs 9,311±876 mm2; t(9,20)=0.4007; P>0.05), 4 (9,367±677 mm2 vs 9331±654 mm2, t(9,20)=0.02634; P>0.05), 6 (12,875±1,018 mm2 vs 12,384±1,153 mm2t(9,20)=0.3621; P>0.05) or 12 weeks of age (15,440±527 mm2 vs 15,586±606 mm2; t(9,20)=0.1078; P>0.05; data not shown).
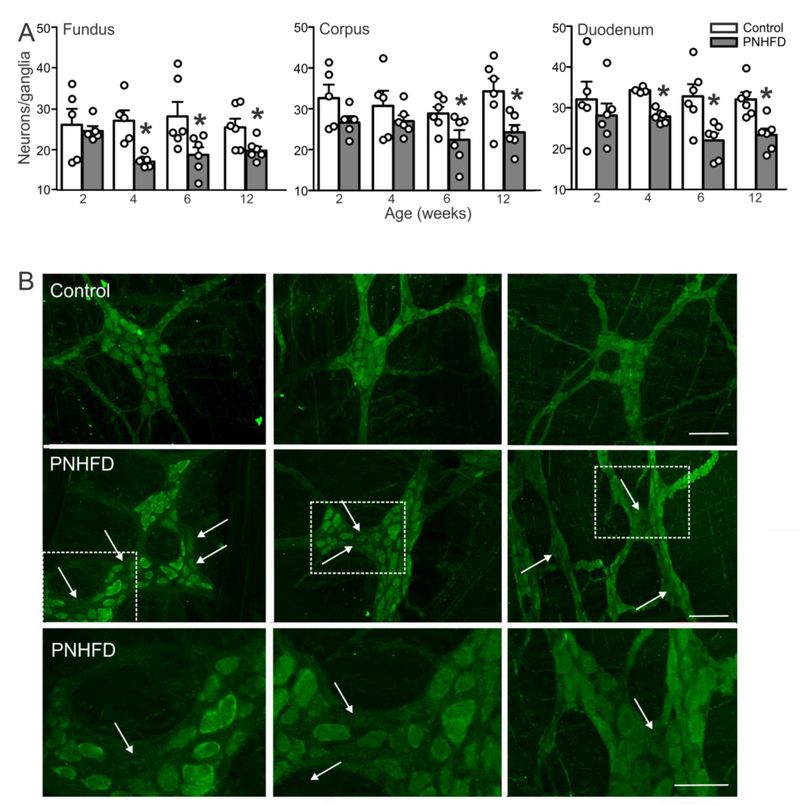
In contrast, however, by 4 weeks of age, PNHFD rats showed decreased numbers of PGP-IR myenteric neurons per ganglion in the fundus (F(3,63) = 6,312; P<0.05) and duodenum (F(3,66) = 5.194; P<0.05), with numbers in the corpus decreasing by 6 weeks of age (F(3,65) = 4.705; P<0.05); Figure 4). This decrease remained stable and consistent until the end of the study.
Figure 4. PNHFD decreased the number of PGP-IR neurons in myenteric ganglia.
A. Number of neurons per ganglia in the fundus (left), corpus (middle) and duodenum (right). Note that the number of PGP-IR neurons per ganglia were significant decreased by 4 weeks of age in the fundus and duodenum, and by 6 weeks of age in the corpus. This decrease in neuronal number was sustained throughout the remainder of the study (N=4-6 per data point; *P<0.05 vs control diet; one way ANOVA followed by post hoc Bonferroni comparison between groups).
B. Representative images of PGP-IR in the fundus (left), corpus (middle) and duodenum (right) of control (upper) and PNHFD (middle and lower) myenteric ganglia of 12 week old rats. Note that areas of neuronal loss (arrows) were apparent in all regions studied following PNHFD exposure. The lower panels represent higher magnification images of the areas highlighted in the corresponding panel above.
Scale bar = 100μM, upper and middle panels, 50μM lower panel
This data indicates that, while PNHFD did not affect myenteric ganglion size, it did decrease neuronal number, suggesting neuronal loss in response to PNHFD exposure.
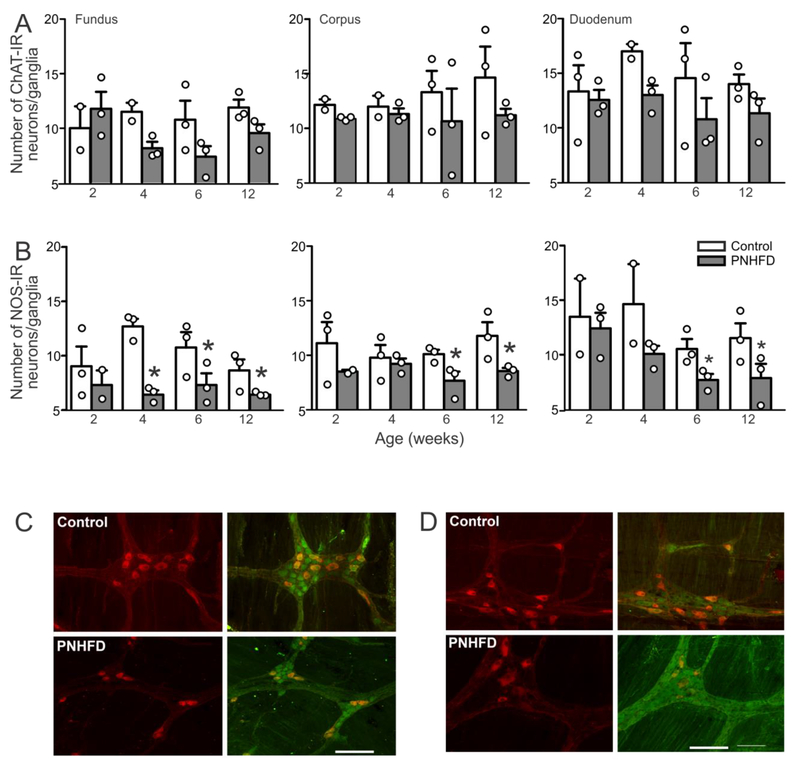
Perinatal HFD decreases the number, but not the proportion, of NOS-IR neurons in the myenteric plexus
Despite the apparent decrease in number of myenteric neurons, exposure to a PNHFD did not alter NOS-IR neuronal number when expressed as a percentage of total (PGP9.5-IR) neurons in the fundus (F(3,7) = 0.7329; P>0.05) corpus (F(3,7) = 0.3279; P>0.05) or duodenum (F(3,8) = 0.7387; P>0.05) at any of the time points investigated. Similarly, exposure to PNHFD did not alter the proportion of ChAT-IR neurons in the fundus (F(3,8) =3.421; P>0.05), corpus (F(3,6) = 0.313; P>0.05) or duodenum (F(3,8) = 0.5953; P>0.05) at any time point investigated (Table 1).
Table 1:
Effects of PNHFD on proportion of NOS- and ChAT-IR myenteric neurons
| Proportion of NOS-IR neurons | 2 weeks of age | 12 weeks of age | |
|---|---|---|---|
| Fundus | Control | 33±3.5% | 32±0.9% |
| PNHFD | 27±1.6% | 31±2.3% | |
| Corpus | Control | 32±1.1% | 30±1.1% |
| PNHFD | 29±2.5% | 31±0.8% | |
| Duodenum | Control | 35±1.4% | 34±1.5% |
| PNHFD | 40±4.9% | 32±1.0% | |
| Proportion of ChAT-IR neurons | 2 weeks of age | 12 weeks of age | |
| Fundus | Control | 44±1.4% | 51±3.7% |
| PNHFD | 49±5.9% | 51±2.9% | |
| Corpus | Control | 42±4.2% | 49±2.3% |
| PNHFD | 44±2.4% | 53±3.2% | |
| Duodenum | Control | 48±2.0% | 47±0.5% |
| PNHFD | 51±3.2% | 49±2.7% | |
In contrast to the lack of changes in proportion of nitrergic or cholinergic neurons, however, the number of NOS-IR neurons declined significantly in response to PNHFD exposure. By 4 weeks of age, the number of NOS-IR neurons in the fundus was reduced relative to control (t(7,59) = 6.239; P<0.05) and this reduced was consistent throughout the rest of the study ((t(7,59) = 2.747 and 3.191 at 6 and 12 weeks of age, respectively; P<0.05 for each; Figure 5). Similarly, the number of NOS-IR neurons was reduced in the corpus by 6 weeks of age (t(7,61) = 2.687; P<0.05) and this reduction was maintained until the end of the study at 12 weeks of age (t(7,59) = 3.891; P<0.05), as well as in the duodenum, which also showed a decline in NOS-IR at 4, 6 and 12 weeks of age (t(7,56) = 3.083, 2.815 and 2.926, respectively, P<0.05 for both).
Figure 5. PNHFD decreased the number of NOS-IR, but not ChAT-IR neurons in myenteric ganglia.
A Number of cholinergic (ChAT-IR) myenteric neurons in the fundus (left), corpus (middle), and duodenum (right) of control and PNHFD rats. Note that while there was a trend towards a decrease in number of cholinergic neurons, this did not reach significance during the time-period of the current study (N=3 per data point)
B. Number of nitrergic (NOS-IR) myenteric neurons in the fundus (left), corpus (middle), and duodenum (right) of control and PNHFD rats. A significant decrease in number of nitrergic neurons was observed in all regions studied, from 4 weeks of age in the fundus, and by 6 weeks of age in the copurs and duodenum. The loss of NOS-IR neurons remained consistent throughout the duration of the study. (N=3 per data point; *P<0.05; one way ANOVA followed by post hoc Bonferroni comparison between groups)
C. Representative images of ChAT-IR (red; left) and merged ChAT-IR and PGP9.5-IR (green; right) in control (upper) and PNHFD (lower) corpus myenteric ganglia of 12 week old rats. Scale bar = 50μm.
D: Representative images of NOS-IR (red; left) and merged NOS-IR and PGP9.5-IR (green; right) in control (upper) and PNHFD (lower) corpus myenteric ganglia of 12 week old rats. Note the decrease in number of NOS-IR following PNHFD. Scale bar = 50μm.
In contrast, there was no significant loss of ChAT-IR neuron, even by 12 weeks of age in either the fundus (t(7,55) = 1.562; P>0.05), the corpus (t(7,58) = 1.898; P>0.05) or the duodenum (t(7,61) = 1.149; P>0.05; Figure 5)
Taken together with the observed decrease in PGP9.5-IR neurons, these results suggest a PNHFD-induced loss of nitrergic neurons in the fundus, corpus and duodenum by 4-6 of age.
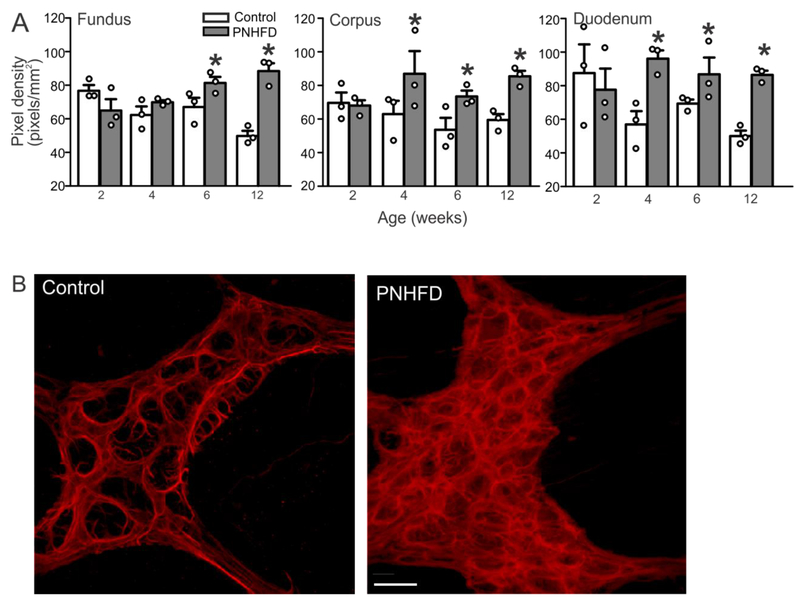
Perinatal HFD increases astrocyte-like cell density in the myenteric plexus
As measured by an increase in staining intensity, the density of GFAP-IR within myenteric ganglia increased following PNHFD exposure in the fundus by 6 and 12 weeks of age (t(7,62) = 3.182 and 7.825 respectively, P<0.05 for each) and in the corpus and duodenum by 4 weeks of age (corpus t(7,64) = 3.579, 2.954, and 3.628 for 4,6, and 12 weeks, respectively, P<0.05 for each; duodenum t(7,64) = 4.712, 2.628, and 4.386 for 4,5, and 12 weeks, respectively, P<0.05 for each; Figure 6).
Figure 6. PNHFD increased glial cell density in the myenteric plexus.
A. Increase in GFAP-IR pixel cell density in myenteric ganglia of the fundus (left), corpus (middle), and duodenum (right). This increase was observed by 4 weeks of age, and maintained throughout the duration of the study (N=3 per data point; *P<0.05; one way ANOVA followed by post hoc Bonferroni comparison between groups)
B. Representative images of the myenteric ganglia in the corpus of 6 week old rats showing increased glial cell density in a PNHFD (right) vs control (left) myenteric ganglion. Scale bar = 20μm
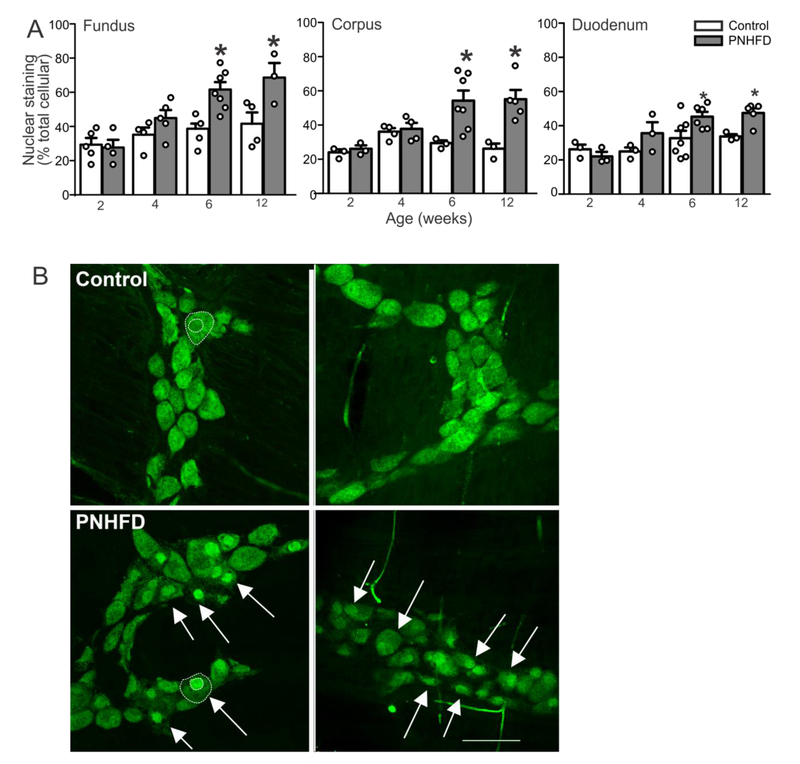
Perinatal HFD associated with changes in distribution of HuC/D immunoreactivity
HuC/D is a common pan-neuronal marker that recognizes mRNA binding proteins; the cellular location of HuC/D-IR can also reflect the oxidative stress of the neuron at the time of fixation (Desmet et al., 2014). In control myenteric neurons, HuC/D-IR was distributed evenly throughout the nucleus and cytoplasm of the cell body (Figure 7). In contrast, following PNHFD exposure, HuC/D-IR was concentrated in the nucleus of myenteric neurons of the fundus, corpus, and duodenum, suggesting HFD exposure induced neuronal stress and/or damage. In detail, when compared to control neurons, PNHFD animals presented a significant increase in nuclear localization of HuC/D at 6 and 12 weeks of age in the fundus (t(7,31) = 4.141 and 3.418, respectively, P<0.05 for both), the, corpus (t(7,31) = 3.617 and 3.970, respectively, P<0.05 for both), and duodenum (t(7,25) = 3.005 and 2.859, respectively, P<0.05 for both; Figure 7).
Figure 7. PNHFD increased nuclear expression of HuC/D in myenteric neurons.
A. Increased proportion of nuclear HuC/D expression (expressed as percentage of pixels) beginning at 6 weeks (N=3-5 per data point; *; P<0.05; one way ANOVA followed by post hoc Bonferroni comparison between groups)
B. Representative images of myenteric ganglia in the fundus of 12 week old rats in control (upper) and PNHFD (lower) rats. The outline of the neuron and nucleus are marked in a representative control and PNHFD neuron in white dotted lines (left panel). Note the increased nuclear aggregation of HuC/D following PNHFD (white arrows). Scale bar = 100μm
Discussion
The results of the present study suggest that exposure to a HFD from the perinatal period onwards alters myenteric neuronal number and phenotype and density of enteric glia, even in the absence of increased body weight, glycemic dysregulation, and local or systemic inflammation. Specifically, the present study demonstrated that a PNHFD is associated with: (i) loss of myenteric, principally nitrergic, neurons, in the fundus, corpus, and duodenum, (ii) nuclear concentration of HuC/D, suggestive of oxidative damage or neuronal stress, and (iii) increased enteric glial staining density suggestive of proliferation. We suggest, therefore, that exposure to a HFD diet during and after development of the GI tract exacerbates the deleterious effects of a HFD on the enteric nervous system and may possibly contribute to the onset and severity of GI dysfunction and dysregulation associated with the development of obesity.
The results demonstrated that, in PNHFD rats, myenteric neurons lose NOS-IR (or NOS-IR declines below a detectable threshold) accompanied by an increase in glial cell staining, even prior to nuclear aggregation of HuC/D, suggestive of neuronal damage, or the loss of PGP-IR, indicative of neuronal loss. In contrast, we did not observe any decrease in numbers of cholinergic neurons. nor was there an increase in proportion of cholinergic neurons, which may have been expected given the loss of NOS-IR neurons. Our results displayed a trend towards a decrease in the number of cholinergic neurons by the end of the study, which may imply that cholinergic neurons are vulnerable to the effects of obesity, which was apparent by 12 weeks of age in the present study, rather than the effects of HFD per se.
Previous studies have demonstrated that prolonged HFD exposure, diet induced obesity (Stenkamp-Strahm et al., 2013; Rivera et al., 2014), as well as both Type 1 and Type 2 diabetes (Spangeus et al., 2000; Spangeus & El-Salhy, 2001; Chandrasekharan & Srinivasan, 2007; Zandecki et al., 2008; Demedts et al., 2013) are associated with a loss of myenteric nitrergic neurons, and are accompanied by disrupted GI motility and gastric emptying patterns (Park & Camilleri, 2005; Little et al., 2007; Little & Feinle-Bisset, 2011; Mushref & Srinivasan, 2013). While the cause of myenteric neurodegeneration remains unknown, microbial factors, dyslipidemia, oxidative stress and increased inflammatory factors have been suggested to play important roles (Stenkamp-Strahm et al., 2013; Rivera et al., 2014; Stenkamp-Strahm et al., 2015). The question remains, however, whether enteric neuronal damage occurs prior to such pathophysiological changes, or in response to the glycemic and metabolic dysregulation associated with these diseases. In the present study, a clear PNHFD-induced loss of nitrergic myenteric neurons was observed long before any increase in body weight, and in the absence of glycemic disruption or alterations in either circulating or gastric cytokine levels. This implies, therefore, that the myenteric plexus disruption observed in the present study was not secondary to obesity, hyperglycemia, and systemic or local inflammation. Furthermore, this suggests that enteric neuronal damage, with the consequent potential for disrupted gastric and intestinal functions, may precede and potentially contribute to altered food intake, meal patterns, and energy homeostasis associated with the development of obesity.
Our previous studies (Bhagat et al., 2015; McMenamin et al., 2017) previous demonstrated alterations in central vagal neurocircuits in response to a PNHFD. In fact, the intrinsic membrane properties of central vagal neurons are dysfunctional following exposure to a PNHFD, even prior to the onset of obesity; dorsal motor nucleus of the vagus (DMV) neurons that innervate the stomach are less excitable, have a lower membrane input resistance, and fire fewer action potentials in response to depolarizing inputs (Bhagat et al., 2015). Furthermore PNHFD also induces dysregulation of the synaptic inputs these vagal efferent motoneurons receive, including an increased response to the GABAA receptor antagonist, bicuculline, suggesting an increase in tonic GABAergic synaptic input (McMenamin et al., 2017). It should be noted that, while DMV neurons are spontaneously active and display pacemaking properties, firing action potentials at approximately 1 Hz (Travagli & Gillis, 1994), their excitability is sculpted continuously by the variety of synaptic inputs they receive (Travagli et al., 1991; Davis et al., 2004; Babic et al., 2011) most notably from the adjacent nucleus of the tractus solitarius (NTS). Of these synaptic inputs, the inhibitory GABAergic input appears to be the most important in determining the basal activity of DMV neurons, hence vagal efferent outflow to the upper GI tract (Sivarao et al., 1998). The mechanism responsible for this increase in inhibitory synaptic input remains to be determined, but it is of interest to note that the perinatal period is critically important for the development of vagal neurocircuits (McMenamin et al., 2016) raising the possibility that exposure to a HFD during this critical developmental period alters central neurocircuit maturation. The resulting alteration in vagal efferent outflow will further exacerbate the likely dysregulation of GI functions resulting from the loss of inhibitory nitrergic neurons observed in the present study, although the extent of these alterations remains to be determined.
The ENS contains glial cells similar to those in the central nervous system (CNS) that are also stained by glial cell marker, glial fibrillary acidic protein, (GFAP). PNHFD rats expressed an increase in enteric glial cell density in the myenteric plexus, beginning at 4 weeks of age. Enteric glial cells function in a manner similar to astrocytes in the CNS, producing neurotransmitter precursors, expressing neurotransmitter receptors, and being involved in uptake and degradation of neurotransmitters in addition to their prominent role in neuroglial signaling (von Boyen et al., 2004; Gulbransen & Sharkey, 2009; 2012; Coelho-Aguiar et al., 2015) Obesity is associated with a low-level chronic inflammation associated with alterations of enteric glia (Stenkamp-Strahm et al., 2013; Neunlist et al., 2014); the present study, however, suggests that glial cell density may also be increased prior to weight gain and systemic or local inflammation. In the present study, local pro-inflammatory cytokine levels, including IL-1-β and IL-6 were unchanged at any postnatal timepoint measured, suggesting the observed changes in enteric glia did not occur in response to gastric inflammation. Further studies will be required to determine whether the observed changes in enteric glia contribute to the subsequent loss of nitrergic myenteric neurons observed shortly thereafter.
An increase in HuC/D nuclear localization in myenteric neurons has also been correlated with an increase in glial expression in pathological states, and may indicate compromised neuronal health (Desmet et al., 2014; Rivera et al., 2014). In the present study, nuclear localization of HuC/D was significant by 6 weeks of age, the time-point at which nitrergic neuronal loss was first apparent. Again, such alterations in presumed neuronal health were observed prior to weight gain or glycemic dysregulation, suggesting that neuronal stress occurs in the absence of obesity. Other studies investigating diet-induced effects on the enteric nervous system demonstrated that adult rats fed a diet with increased fat content (21% kcal from fat) for long periods of time (33 weeks) showed a similar nuclear distribution of HuC/D which was also accompanied by neuronal loss (Rivera et al., 2014). It remains to be determined, however, whether the increase in glial cell proliferation accompanied by an increase in nuclear HuC/D expression occurs in response to neuronal damage, or reflects a neuroprotective mechanism.
Obesity is a multifactorial disease involving both genetic influences to environmental factors, but is ultimately due to an imbalance in energy homeostasis(Levin, 2006; Berthoud, 2008; Berthoud & Morrison, 2008; Levin, 2010b; a). Vagal reflexes are vital to the control, integration and regulation of GI reflexes involved in energy homeostasis including food intake, satiety signaling and digestion (Schwartz et al., 2000; Berthoud, 2008). Several studies have shown that maternal and environmental stress during pre- and early post-natal developmental periods can alter autonomic neurocircuits permanently, which may induce long-term disruption to a variety of GI functions(Card et al., 2005; Banihashemi & Rinaman, 2010; Rinaman et al., 2011; Sullivan et al., 2011). In both humans and rodents, exposure to a HFD during critical perinatal developmental periods, predisposes offspring to an increased risk of developing metabolic disorders and their associated comorbid conditions (Levin, 2006; Levin, 2010a; Levin, 2010b). Future studies will be required to determine whether defined developmental periods are more susceptible to the adverse effects of HFD exposure than others, as well as determining whether such changes are permanent.
The perinatal period is known to be critical in the development of autonomic neurocircuits required for the regulation of many visceral functions including digestive processes, food intake regulation and energy homeostasis. The present study suggests that exposure to a HFD during this critical perinatal period as well as early adulthood is associated with glial cell proliferation, myenteric neuronal stress, and loss of inhibitory nitergic myenteric neurons and that these changes occur prior to weight gain, the development of obesity, or glycemic dysregulation. Since environmental factors, including maternal diet, are an important predictor of offspring metabolic outcomes (Levin, 2006; 2010b; a; Tamashiro & Moran, 2010), understanding how dietary manipulations during vulnerable developmental periods disrupts neurocircuit patterning will be an important step to understanding the physiology and pathophysiology of autonomic homeostatic control.
Highlights.
The effects of perinatal high fat diet (PNHFD) on myenteric neurons was studied
PNHFD increased glial cell density but did not induce serum or tissue inflammation
PNHFD induced a loss of NOS- but not ChAT-IR neurons
PNHFD induced myenteric plexus disruption prior to obesity or glycemic dysregulation
Acknowledgements
The authors acknowledge gratefully the help of Maithilli Navaratnarajah and Dr CH Lang, as well as Beth Worley and Drs Traci Czyzyk and Amy Arnold for their help and guidance on measurement of serum and tissue inflammatory markers. The authors also thank Dr R. Alberto Travagli for his input and discussion on earlier versions of this manuscript as well as thanking W. Nairn Browning for support and encouragement.
Funding
This work was supported by National Science Foundation grant IOS 1148978 (KNB)
Abbreviations
- ChAT
choline acetyltransferase
- DIO
diet induced obesity
- ENS
enteric nervous system
- GFAP
glial fibrillary acidic protein
- GI
gastrointestinal
- -IR
-immunoreactivity
- HFD
high fat diet
- NDS
normal donkey serum
- NGS
normal goat serum
- NOS
nitric oxide synthase
- PBS
phosphate buffered saline
- PGP9.5
protein gene product 9.5
- PNHFD
perinatal high fat diet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Babic T, Browning KN & Travagli RA (2011) Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am. J. Physiol Gastrointest. Liver Physiol, 300, G21–G32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihashemi L & Rinaman L (2010) Repeated brief postnatal maternal separation enhances hypothalamic gastric autonomic circuits in juvenile rats. Neuroscience, 165, 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR (2008) The vagus nerve, food intake and obesity. Regui. Pept, 149, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR & Morrison C (2008) The brain, appetite, and obesity. Annu. Rev. Psychol, 59, 55–92. [DOI] [PubMed] [Google Scholar]
- Bhagat R, Fortna SR & Browning KN (2015) Exposure to a high fat diet during the perinatal period alters vagal motoneurone excitability, even in the absence of obesity. J Physiol, 593, 285–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger JE & Fox EA (2010) Meal parameters and vagal gastrointestinal afferents in mice that experienced early postnatal overnutrition. Physiol Behav, 101, 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN & Travagli RA (2014) Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol, 4, 1339–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Levitt P, Gluhovsky M & Rinaman L (2005) Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. J. Neurosci, 25, 9102–9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharan B & Srinivasan S (2007) Diabetes and the enteric nervous system. Neurogastroenterol. Motil, 19, 951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho-Aguiar JM, Bon-Frauches AC, Gomes AL, Verissimo CP, Aguiar DP, Matias D, Thomasi BB, Gomes AS, Brito GA & Moura-Neto V (2015) The enteric glia: identity and functions. Glia, 63, 921–935. [DOI] [PubMed] [Google Scholar]
- Davis SF, Derbenev AV, Williams KW, Glatzer NR & Smith BN (2004) Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res, 1017, 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demedts I, Masaoka T, Kindt S, De HG, Geboes K, Farre R, Vanden Berghe P & Tack J (2013) Gastrointestinal motility changes and myenteric plexus alterations in spontaneously diabetic biobreeding rats. J Neurogastroenterol. Motil, 19, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet AS, Cirillo C & Vanden Berghe P (2014) Distinct subcellular localization of the neuronal marker HuC/D reveals hypoxia-induced damage in enteric neurons. Neurogastroenterol. Motil, 26, 1131–1143. [DOI] [PubMed] [Google Scholar]
- Furness JB (2012) The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol, 9, 286–294. [DOI] [PubMed] [Google Scholar]
- Gallagher TK, Geoghegan JG, Baird AW & Winter DC (2007) Implications of altered gastrointestinal motility in obesity. Obes. Surg, 17, 1399–1407. [DOI] [PubMed] [Google Scholar]
- Gulbransen BD & Sharkey KA (2009) Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology, 136, 1349–1358. [DOI] [PubMed] [Google Scholar]
- Gulbransen BD & Sharkey KA (2012) Novel functional roles for enteric glia in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol, 9, 625–632. [DOI] [PubMed] [Google Scholar]
- Honore SM, Zelarayan LC, Genta SB & Sanchez SS (2011) Neuronal loss and abnormal BMP/Smad signaling in the myenteric plexus of diabetic rats. Auton. Neurosci, 164, 51–61. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Kajimura M, Osawa S, Kanaoka S, Furuta T, Ikuma M & Hishida A (2006) A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol, 41, 1076–1087. [DOI] [PubMed] [Google Scholar]
- Levin BE (2006) Metabolic imprinting: critical impact of the perinatal environment on the regulation of energy homeostasis. Philos. Trans. R. Soc. Lond B Biol. Sci, 361, 1107–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE (2010a) Developmental gene x environment interactions affecting systems regulating energy homeostasis and obesity. Front Neuroendocrinol, 31, 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE (2010b) Interaction of perinatal and pre-pubertal factors with genetic predisposition in the development of neural pathways involved in the regulation of energy homeostasis. Brain Res, 1350, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TJ & Feinle-Bisset C (2011) Effects of dietary fat on appetite and energy intake in health and obesity--oral and gastrointestinal sensory contributions. Physiol Behav, 104, 613–620. [DOI] [PubMed] [Google Scholar]
- Little TJ, Horowitz M & Feinle-Bisset C (2007) Modulation by high-fat diets of gastrointestinal function and hormones associated with the regulation of energy intake: implications for the pathophysiology of obesity. Am. J Clin. Nutr, 86, 531–541. [DOI] [PubMed] [Google Scholar]
- Madsen JL (1992) Effects of gender, age, and body mass index on gastrointestinal transit times. Dig. Dis. Sci, 37, 1548–1553. [DOI] [PubMed] [Google Scholar]
- McMenamin CA, Anselmi L, Travagli RA & Browning KN (2016) Developmental regulation of inhibitory synaptic currents in the dorsal motor nucleus of the vagus in the rat. J. Neurophysiol, 116, 1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin CA, Travagli RA & Browning KN (2017) Perinatal high fat diet increases inhibition of dorsal motor nucleus of the vagus neurons regulating gastric functions. Neurogastroenterol. Motil. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushref MA & Srinivasan S (2013) Effect of high fat-diet and obesity on gastrointestinal motility. Ann. Transl. Med, 1, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Rolli-Derkinderen M, Latorre R, Van LL, Coron E, Derkinderen P & De GR (2014) Enteric glial cells: recent developments and future directions. Gastroenterology, 147, 1230–1237. [DOI] [PubMed] [Google Scholar]
- Park MI & Camilleri M (2005) Gastric motor and sensory functions in obesity. Obes. Res, 13, 491–500. [DOI] [PubMed] [Google Scholar]
- Ratcliffe EM, Farrar NR & Fox EA (2011) Development of the vagal innervation of the gut: steering the wandering nerve. Neurogastroenterol. Motil, 23, 898–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Banihashemi L & Koehnle TJ (2011) Early life experience shapes the functional organization of stress-responsive visceral circuits. Physiol Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera LR, Leung C, Pustovit RV, Hunne BL, Andrikopoulos S, Herath C, Testro A, Angus PW & Furness JB (2014) Damage to enteric neurons occurs in mice that develop fatty liver disease but not diabetes in response to a high-fat diet. Neurogastroenterol. Motil, 26, 1188–1199. [DOI] [PubMed] [Google Scholar]
- Rodrigues DM, Li AY, Nair DG & Blennerhassett MG (2011) Glial cell line-derived neurotrophic factor is a key neurotrophin in the postnatal enteric nervous system. Neurogastroenterol. Motil, 23, e44–e56. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Seeley RJ & Baskin DG (2000) Central nervous system control of food intake. Nature, 404, 661–671. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Krowicki ZK & Hornby PJ (1998) Role of GABAa receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterology and Motility, 10, 305–313. [DOI] [PubMed] [Google Scholar]
- Spangeus A & El-Salhy M (2001) Myenteric plexus of obese diabetic mice (an animal model of human type 2 diabetes). Histol. Histopathol, 16, 159–165. [DOI] [PubMed] [Google Scholar]
- Spangeus A, Suhr O & El-Salhy M (2000) Diabetic state affects the innervation of gut in an animal model of human type 1 diabetes. Histol. Histopathol, 15, 739–744. [DOI] [PubMed] [Google Scholar]
- Stenkamp-Strahm C, Patterson S, Boren J, Gericke M & Balemba O (2013) High-fat diet and age-dependent effects on enteric glial cell populations of mouse small intestine. Auton. Neurosci, 177, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp-Strahm CM, Nyavor YE, Kappmeyer AJ, Horton S, Gericke M & Balemba OB (2015) Prolonged high fat diet ingestion, obesity, and type 2 diabetes symptoms correlate with phenotypic plasticity in myenteric neurons and nerve damage in the mouse duodenum. Cell Tissue Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Smith MS & Grove KL (2011) Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology, 93, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran S & Kondapaka SB (2005) Altered expression of neuronal nitric oxide synthase in the duodenum longitudinal muscle-myenteric plexus of obesity induced diabetes mouse: implications on enteric neurodegeneration. Biochem. Biophys. Res. Commun, 338, 919–922. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL & Moran TH (2010) Perinatal environment and its influences on metabolic programming of offspring. Physiol Behav, 100, 560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanano A, Hamada Y, Takamido S, Kataoka Y, Watanabe J, Kamiyama Y & Yamada H (2005) Structural development of PGP9.5-immunopositive myenteric plexus in embryonic rats. Anat. Embryol. (Berl), 209, 341–348. [DOI] [PubMed] [Google Scholar]
- Travagli RA & Gillis RA (1994) Hyperpolarization-activated currents Ih and Ikir in rat dorsal motor nucleus of the vagus neurons, in vitro. J. Neurophysiol, 71, 1308–1317. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Rossiter CD & Vicini S (1991) Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am. J. Physiol, 260, G531–G536. [DOI] [PubMed] [Google Scholar]
- von Boyen GB, Steinkamp M, Reinshagen M, Schafer KH, Adler G & Kirsch J (2004) Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut, 53, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandecki M, Vanden Berghe P, Depoortere I, Geboes K, Peeters T, Janssens J & Tack J (2008) Characterization of myenteric neuropathy in the jejunum of spontaneously diabetic BB-rats. Neurogastroenterol. Motil, 20, 818–828. [DOI] [PubMed] [Google Scholar]