Abstract
Background and aim:
Adenosine A2B receptor (A2BAR) agonist reduces myocardial reperfusion injury by acting on inflammatory cells. Recently, a cardiosplenic axis was shown to mediate the myocardial post-ischemic reperfusion injury. This study aimed to explore whether the infarct-squaring effect of A2BAR agonist was primarily due to its action on splenic leukocytes.
Methods:
C57BL6 (wild-type, WT) mice underwent 40 min of left coronary artery occlusion followed by 60 min of reperfusion. A2BR knockout (KO) and interleukin (IL)- 10KO mice served as donors for splenic leukocytes. Acute splenectomy was performed 30 min prior to ischemia. The acute splenic-leukocyte adoptive transfer was performed by injecting 5 × 106 live splenic leukocytes into splenectomized mice. BAY60–6583, an A2BAR agonist, was injected by i.v. 15 min before ischemia. The infarct size (IS) was determined using TTC and Phthalo blue staining. The expression of p-Akt and IL-10 was estimated by Western blotting. Immunofluorescence staining assessed the localization of IL-10 expression.
Results:
BAY 60–6583 reduced the myocardial IS in intact mice but failed to reduce the same in splenectomized mice, which had a smaller IS than intact mice. BAY 60–6583 reduced the IS in splenectomized mice with the acute transfer of WT splenic leukocytes; however, it did not protect the heart of splenectomized mice with the acute transfer of A2bRKO splenic leukocytes. Furthermore, BAY 60–6583 increased the levels of p-Akt and IL-10 in WT spleen. Moreover, it did not exert any protective effect in IL-10KO mice.
Conclusion:
A2BAR activation prior to ischemia stimulated the IL-10 production in splenic leukocytes via a PI3K/Akt pathway, thereby exerting anti-inflammatory effects that limited the myocardial reperfusion injury.
Keywords: adenosine A2B receptor, myocardial reperfusion injury, PI3K/Akt, splenic leukocytes
INTRODUCTION
Adenosine receptor family has four subsets: A1, A2A, A2B, and A3 that are associated with cardiac tissue protection in different settings (1). For decades, all the four subtype receptors have been shown to mediate a cardioprotective effect, albeit via different mechanisms. The activation of A1AR has been known to protect the heart by introducing a preconditioning phenomenon (2-4). Previous studies found that A2AAR induced a cardioprotective effect via the CD4+ lymphocytes (5). A3AR protects the heart by acting on the bone marrow-derived cells (6, 7) via interaction with A2AAR (8). Unlike other subtypes, the mechanism of A2BAR-induced cardioprotection is more complicated, and conflicting results have been reported. Eckle et al. (9) demonstrated that ischemic preconditioning failed to protect the heart from reperfusion injury in A2BAR knockout (KO) mice, indicating that it mediated the cardioprotective effects of Ischemic Preconditioning (IPC). In contrast, the study by Auchampach et al. (10) and our previous study reported (11) that cardioprotection of IPC is independent of A2BAR. Furthermore, another previous study found that BAY 60–6583, an A2BAR agonist, attenuated the myocardial reperfusion injury by modulating the phenotypic switch of macrophages to an anti-inflammatory M2 subset and reduced the neutrophil infiltration after reperfusion. However, the precise mechanism underlying the protection of A2BAR agonist is yet to be elucidated.
Recently, a critical role of the cardiosplenic axis in myocardial reperfusion injury has gained attention. Swirski et al. (12) demonstrated that spleen released the monocytes after myocardial infarction that participated in the ventricular remodeling process. The study found that splenic monocytes significantly limited the ventricular remodeling and improved the cardiac function. Moreover, Ismahil et al. (13) reported that splenic mononuclear phagocyte network was involved in chronic heart failure post-myocardial infarction. Furthermore, the cardiosplenic axis was found to mediated the acute myocardial ischemia/reperfusion injury via the HMGB1-RAGE pathway (14). Splenic leukocytes mediated the myocardial infarction, exacerbating the effects of acute hyperglycemia (15). These results strongly suggested that splenic leukocytes, especially splenic mononuclear phagocyte network including the monocytes/macrophages, were potential targets to abrogate the myocardial reperfusion injury. Whether the splenic leukocytes mediate the cardioprotection of A2BAR agonist is still unclear.
The present study utilized an established in vivo mouse model of myocardial ischemia and reperfusion injury in order to test the hypothesis that A2BAR agonist acts on the splenic leukocytes and protects the heart from myocardial reperfusion injury.
MATERIALS and METHODS
This study conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Eighth edition, revised 2011) and was conducted using the protocols approved by the Institutional Animal Care and Use Committee, University of Virginia, USA.
Animals and experimental protocols
C57BL6 wild-type (WT) IL-10KO mice (9–13-weeks-old) were purchased from The Jackson Laboratory (ME, USA). A2BARKO mice were generated by Deltagen, Inc. (CA, USA) and colonized in the vivarium of the University of Virginia. WT mice underwent 40 min of left coronary artery occlusion, followed by 60 min reperfusion, which caused an identical myocardial infarct size (IS) as compared to the 24 h reperfusion in previous studies (15) (16). A2BARKO and IL-10KO mice were used as donors for splenic leukocytes. Acute splenectomy (SPLX) was performed 30 min before myocardial ischemia with or without acute splenic leukocyte reconstitution after splenectomy. The splenic leukocytes were isolated using the gentleMACS single splenocyte isolation system according to the manufacturer’s protocol (Miltenyi Biotec, CA, USA). Viability (>95%) of the splenocytes was determined using Trypan blue staining. As described previously, the splenic-leukocyte adoptive transfer (SPAT) was performed by injecting 5 × 106 isolated splenocytes in 100 μL phosphate-buffered saline (PBS) into splenectomized mice via the left external jugular vein 5 min after removal of the spleen. BAY60–6583, an A2BR agonist, was administered (100 μg/kg, i.v.) 15 min before ischemia (Figure 1).
Figure 1. Experimental protocol.

SPAT, splenic-leukocyte reconstituted mice; SPLX, splenectomy.
Splenectomy
As described previously (15), the mice were anesthetized with sodium pentobarbital (80 mg/kg i.p.) and intubated orally. Artificial respiration was maintained with 0.80 FiO2, 120 strokes/min, and a 0.2–0.5 mL stroke volume. A small abdominal incision was made, and the peritoneal cavity entered. The spleen was located and brought to the incision. The hilum was clamped and ligated with 3–0 silk sutures, following which, the spleen was removed. Subsequently, the incision was closed in two layers using 4–0 vicryl sutures.
Myocardial ischemia/reperfusion injury and measurement of IS
C57BL/6 mice were subjected to 40 min ligation of the left coronary artery (LCA), followed by 60 min of reperfusion as detailed previously(15). Briefly, after anesthesia and intubation, the heart was exposed by left thoracotomy. LCA was identified under a surgical microscope. A 7–0 silk suture was placed around LCA at 1 mm inferior to the left auricle. Ischemia was induced by tying down the suture over a piece of PE-60 tube, and reperfusion was induced by removing the tube. The successful ligation of LCA was indicated by a pale appearance in the ischemic zone and ST-segment elevation in electrocardiogram (ECG). ECG was monitored perioperatively using PowerLab (ADInstruments, CO, USA). The mice were euthanized 60 min after reperfusion, and the hearts were cannulated through the ascending aorta for perfusion with 3 mL of 1.0% TTC. Then, the LCA was re-occluded with the same suture that was used for coronary occlusion prior to 10% Phthalo blue perfusion for determining the risk region (RR). Subsequently, the left ventricle was cut into 5–7 transverse slices that were weighed and digitally photographed to determine IS as the percentage of RR.
Western blotting
Western blotting was used to detect the levels of p-Akt and IL-10 in the spleen and heart as described previously (11). Briefly, the total protein was extracted from the indicated experimental groups using RIPA buffer, and the concentration was determined using bicinchoninic acid protein assay kit (Thermo Scientific, IL, USA). All Western blot analyses were performed according to standard procedures. An equivalent of 20 μg protein was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Bio-Rad, CA, USA). The membranes were blocked with 1% milk in Tris-buffered saline with Tween 20 and probed with primary antibodies such as total-Akt, phospho-Akt S473, or IL-10 antibody (1:2000; Cell Signaling Technology, MA, USA) and appropriate secondary antibodies (1:5000; Promega, WI, USA). Subsequently, the proteins were visualized with the enhanced chemiluminescent substrate (Thermo Scientific), followed by densitometry analysis using the FluorChem 8900 imaging system (Alpha Innotech, CA, USA). Actin was used as an endogenous control protein.
Immunofluorescence staining
Immunofluorescence staining was performed as previously described (11). The spleens were fixed in 4% paraformaldehyde in PBS (pH 7.4) for 1 h at room temperature, followed by 30% sucrose overnight at 4 °C. The fixed spleens were frozen in OCT and sliced. The slides were permeabilized using 0.3% Triton X-100 in PBS. After blocking with 10% normal serum for 1 h, the specimens were co-labeled with anti-IL-10 and anti- CD11b antibodies overnight at 4 °C. After washing, the sections were incubated with Alexa Fluor 488- and Alexa Fluor 594-conjugated secondary antibodies for 1 h. ProLong Gold anti-fade reagent was used to mount the specimens. The images were acquired under same parameters for each fluorochrome using an Olympus BX-41 Microscope (Olympus, PA, USA) attached with a Retiga-2000R camera (Qlmaging, BC, USA). The images were further processed using Image J software.
Statistical analysis
All data were presented as the mean ± standard error of the mean (SEM). All data were compared using one-way analysis of variance (ANOVA), followed by the t-test with Bonferroni correction for unpaired data.
RESULTS
Adenosine A2B receptor agonist protected the heart against reperfusion injury by acting on splenic leukocytes
As shown previously, BAY 60–6583 significantly reduced the myocardial IS as compared to the IR group (50.2±1% vs 29±2.5%, p<0.05). However, BAY 60–6583 failed to reduce the IS in splenectomized mice, which had a smaller IS than the intact mice (34.7±2.1% vs 32.5±3.1%, p=NS). The reconstitution of splenic leukocytes from either WT or A2BRKO mice to splenectomized mice restored the IS to the level as that of the IR group. As expected, BAY 60–6583 reduced the IS of the WT splenic-leukocyte reconstituted mice (49.7±1.6% vs 27.9±2.0, p<0.05); however, it did not protect the heart of the A2BRKO splenic-leukocyte reconstituted mice (50.2±2.1% vs 49.7±1.8%, p<0.05) (Figure 2). The RR among all groups was similar (data not shown).
Figure 2. Myocardial IS measured using TTC and Phthalo blue staining.
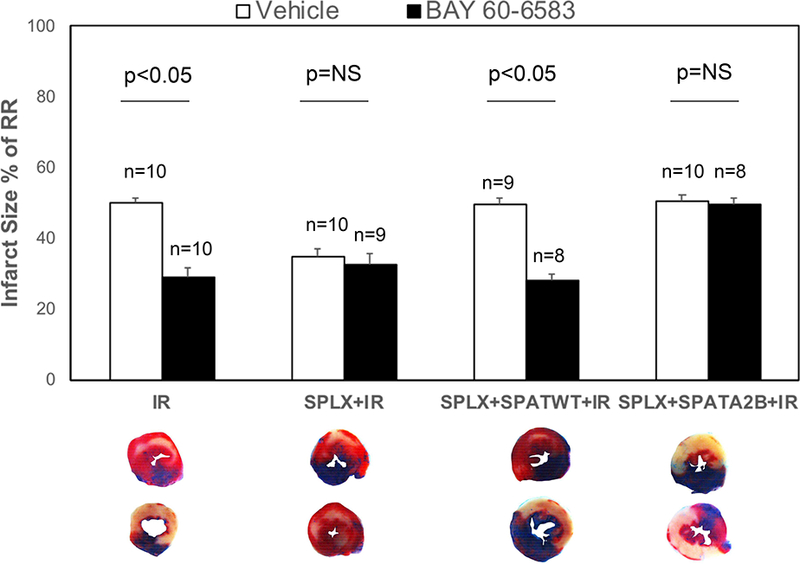
BAY 60–6583 significantly reduced the myocardial IS as compared to the IR group but failed to reduce that of the splenectomized mice, which had a smaller IS than the intact mice. The reconstitution of splenic leukocytes from either WT or A2bARKO mice to splenectomized mice restored the IS to the level of the IR group. BAY 60–6583 reduced the IS of the WT splenic-leukocyte reconstituted mice but failed to protect the heart of A2BARKO splenic- leukocyte reconstituted mice. The risk regions were not different among all groups. IR, myocardial ischemia and reperfusion injury; SPATA2B, A2BARKO splenic-leukocyte reconstituted mice; SPATWT, wild-type splenic-leukocyte reconstituted mice; SPLX, splenectomy.
BAY 60–6583 increased the p-Akt and IL-10 levels in the spleen but not in the myocardium of mice without IR injury
The non-injured mice were treated with BAY 60–6583, and the p-Akt levels were analyzed in the spleen and myocardium. Interestingly, BAY 60–6583 significantly increased the levels of p-Akt in the spleen but were not affected in the myocardium (Figure 3). Then, the IL-10 level was assessed, which has been reported to occur downstream of PI3K/Akt pathway. BAY 60–6583 significantly increased the level of IL- 10 in the spleen, which could be attenuated by wortmannin, a specific PI3K inhibitor. Moreover, the A2BR agonist did not affect the level of IL-10 in the myocardium (Figure 4). Confocal imaging results showed that most of the IL-10 level was elevated in the spleen and was co-localized with CD-11b+ cells (Figure 5).
Figure 3. Western blotting results of p-Akt levels in BAY 60–6583-treated non- injured mice.
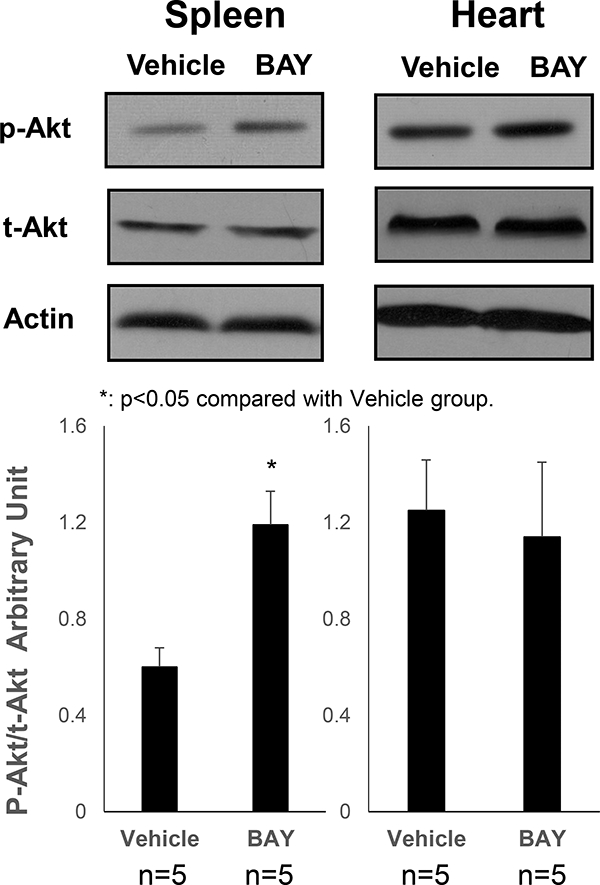
BAY 60–6583 significantly increased the p-Akt levels in the spleen but did not affect the same in the myocardium. *p<0.05 compared to the vehicle group. BAY, BAY 60–6583.
Figure 4. Western blotting results of IL-10 levels in BAY 60–6583-treated non- injured mice.
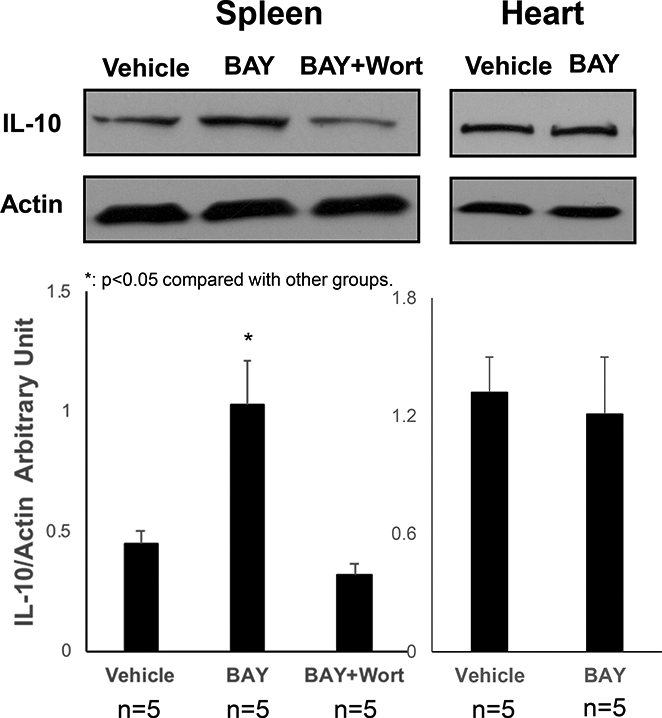
BAY 60–6583 significantly increased the IL-10 levels in spleen, which could be attenuated by wortmannin, a specific PI3K inhibitor, but did not affect IL-10 in the myocardium. *p<0.05 compared to the other groups. BAY, BAY 60–6583; Wort, wortmannin.
Figure 5. Immunofluorescence staining.
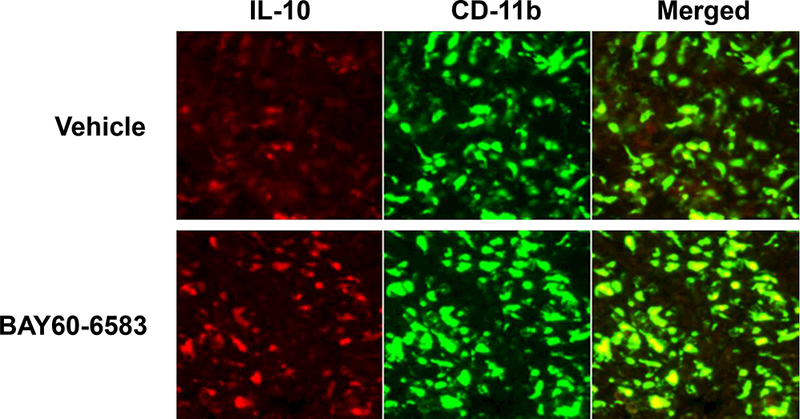
Confocal imaging results showed that majority of IL-10 elevated in the spleen was co-localized with CD-11b+ cells. Red staining presented IL-10, and green staining presented CD11b.
Splenic IL-10 mediated the cardioprotective effects of BAY 60–6583
BAY 60–6583 significantly increased the level of myocardial IL-10 after reperfusion (Figure 6). However, the same was not increased in the reperfused myocardium in splenectomized mice. The cardioprotective effects of BAY 60–6583 on IL-10KO mice were tested. IL-10KO mice presented a higher IS after 40’/60’ IR injury as compared to the WT mice (56.3±2.2% vs 50.2±1%, p<0.05). BAY 60–6583 had no protective effect on IL-10KO mice (55.3±2.1% vs 56.3±2.2, p=NS). Furthermore, BAY 60–6583 failed to reduce the IS in splenectomized mice after IL-10KO splenic leukocyte reconstitution (55.5±2.5 vs 56.4±2.6%, p=NS, Figure 7). The RR among all groups was similar (data not shown).
Figure 6. Western blotting results in the post-ischemic reperfused heart.
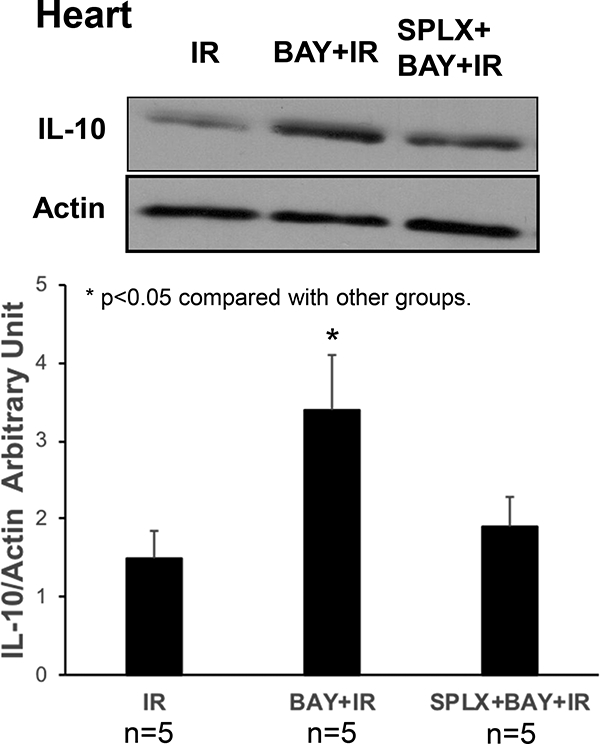
BAY 606583 significantly increased myocardial IL-10 levels after reperfusion. However, the IL- 10 levels were not increased in the reperfused myocardium in splenectomized mice. *p<0.05 compared to the other groups. BAY, BAY 60–6583; SPLX, splenectomy.
Figure 7. Myocardial IS measured using TTC and Phthalo blue staining.
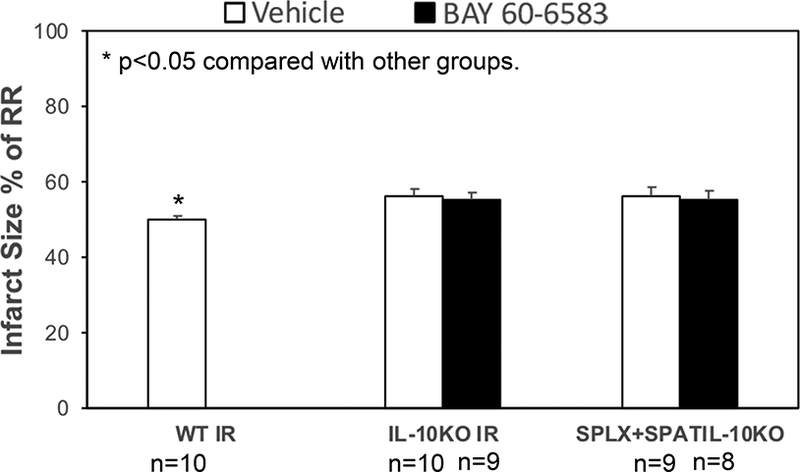
IL-10KO mice had higher IS after IR injury as compared to the WT mice. BAY 60–6583 did not exert a protective effect on IL-10KO mice. Furthermore, BAY 60–6583 failed to reduce the IS in splenectomized mice with IL-10KO splenic-leukocyte reconstitution. *p<0.05 compared to the other groups. IR, myocardial reperfusion injury; SPLX, splenectomy; SPATIL-10KO, IL-10 knockout splenic leukocytes reconstituted mice; WT, wild-type.
DISCUSSION
The present study demonstrated that a systemic administration of A2BAR agonist BAY 60–6583 prior to myocardial ischemia activated a splenic anti-inflammatory pathway, which in turn, limited the inflammatory responses during myocardial reperfusion and reduced the myocardial IS. BAY 60–6583 significantly increased the levels of splenic p-Akt and IL-10 that could be attenuated by pretreatment with the PI3K inhibitor. Moreover, the cardioprotective effects of BAY60–6583 disappeared in the IL- 10KO or splenectomized WT mice with reconstituted IL-10KO splenic leukocytes.
Inflammatory responses play critical roles during myocardial reperfusion injury (5, 17-19). Notably, A2BAR activation before ischemia exerts a cardioprotective effect against myocardial reperfusion injury (11, 20, 21) and A2BAR has anti-inflammatory effects (22-26). However, the mechanism underlying the activated A2BAR-mediated protection of the heart is yet to be elucidated. A2BAR is known to have the lowest affinity to adenosine and is widely expressed in multiple tissues and cell types (1). Accumulating evidence has indicated that the infarct-sparing effect of A2BAR activation is attributed to its activity on immune cells (11, 27-29). Koeppen et al. (28)demonstrated that the A2BAR on bone marrow-derived cells represented an endogenous cardioprotective mechanism during IR injury. The study found that BAY 60–6583, a potent selective A2BAR agonist, did not provide additional cardioprotection in polymorphonuclear leukocytes (PMN)- depleted mice. The authors concluded that the A2BARs on PMNs contributed the protective effects of BAY 60–6583. Recently, the same study (29) showed that adoptive transfer of A2BAR−/− PMN into PMN-depleted mice caused a significantly higher myocardial injury as compared to transfer into the WT PMN cells. Our previous results showed that the pretreatment of BAY 60–6583 promoted the phenotypic switch of myocardial macrophages to an anti-inflammatory M2 subset and attenuated the myocardial reperfusion injury (11). Nonetheless, none of these studies could elucidate the precise mechanisms underlying these anti-inflammatory and infarct-sparing effects of A2BAR activation.
Furthermore, several studies reported a critical role of the cardiosplenic axis in the myocardial reperfusion injury (11-15). Swirski et al. (12) demonstrated that spleen released the monocytes after myocardial infarction, and these participated in the ventricular remodeling process. The study also found that splenic monocytes significantly limited the ventricular remodeling and improved the cardiac function. Moreover, Ismahil et al. (13) reported that the splenic mononuclear phagocyte network was involved in chronic heart failure after myocardial infarction. Sharir et al. (30) demonstrated that splenocyte-derived regulatory T-cells (Tregs) attenuated the left ventricular remodeling. These results showed that splenic leukocytes (monocytes, mononuclear phagocytes, or Tregs) in cardiosplenic axis played different roles. Furthermore, the specific roles of leukocytes might relate to specific pathological processes (31, 32). In addition, previous studies found that activation of splenic leukocytes contributed to myocardial infarct exacerbation during reperfusion after prolonged ischemia in cardiosplenic axis, and thus, limited the splenic inflammatory responses that could protect the heart against myocardial reperfusion injury (14). Interestingly, the splenic leukocytes mediated the myocardial infarct-exacerbating effects of acute hyperglycemia (15). In agreement with previous studies, our results showed that splenectomy significantly reduced the myocardial IS after IRI injury. The cardioprotective effect of A2BAR agonist, BAY 60–6583, was lost in splenectomized mice, which could be restored by reconstituting the splenic leukocytes from WT mice but not A2BRKO mice. These results strongly indicated that the administration of BAY 60–6583 prior to ischemia attenuated the myocardial IS in a splenic leukocyte-dependent manner.
The PI3K/Akt pathway plays a critical role in cardioprotection by A2BAR activation. The pretreatment of PI3K-specific inhibitor significantly attenuated the infarct-sparing effect of A2BAR agonist (11). The PI3K/Akt pathway has also been reported to mediate the anti-inflammatory effects by elevating the IL-10 levels in macrophages (33-35). Interestingly, the activation of A2BAR also augmented the levels of IL-10 in monocytes/macrophages (36-38). Thus, BAY 60–6583 was hypothesized to activate the splenic PI3K/Akt pathway and augment the IL-10 levels, in turn modulating the antiinflammatory effects during myocardial reperfusion. As expected, the results of the present study showed that BAY 60–6583 significantly increased the splenic p-Akt levels; however, no effect was detected on myocardial p-Akt levels in the non-injured mice. IL- 10 has been widely shown to possess anti-inflammatory and cardioprotective effects. Also, our previous study demonstrated that endogenous IL-10 inhibits the production of tumor necrosis factor-alpha (TNF-a) and NO and protects the ischemic and reperfused myocardium by suppressing the recruitment of neutrophils (39). The administration of exogenous IL-10 has been shown to reduce myocardial reperfusion injury (40). Accordingly, the current results showed that the splenic IL-10 level was elevated in BAY 60–6583-treated group, which could be abolished by wortmannin, a selective PI3K inhibitor. However, BAY 60–6583 failed to increase the level of IL-10 in the non-injured myocardium. Moreover, BAY 60–6583 failed to protect the heart in splenectomized WT mice with reconstituted IL-10KO splenic leukocytes. Taken together, a splenic PI3K/Akt/IL-10 pathway is required for the infarct-sparing effect of the A2BAR agonist (Figure 8).
Figure 8. Schematic representation of A2BAR activation-mediated cardioprotection.
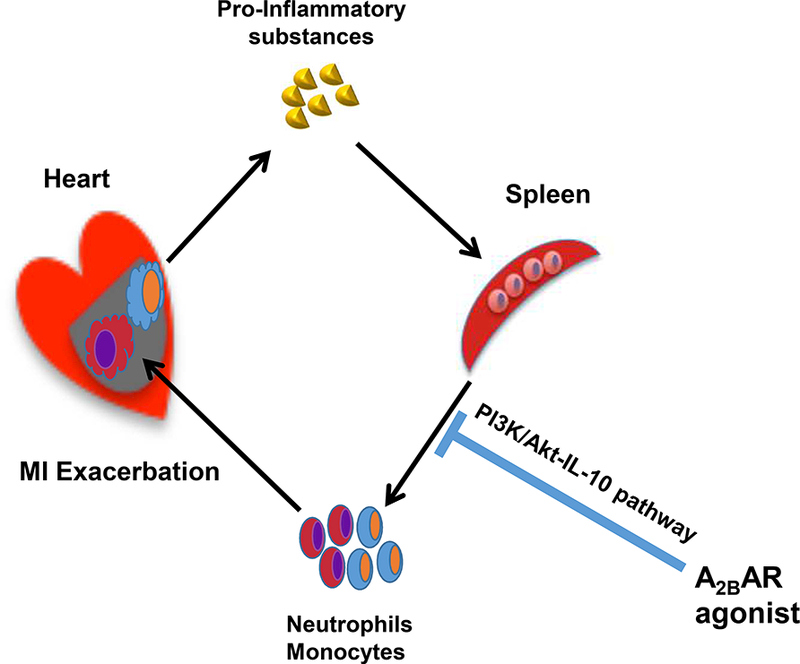
Although all types of adenosine receptors have been reported to induce anti-inflammatory effects (8, 41-43). Accumulating evidence has shown that A2BAR mediates monocyte/macrophage phenotypic switch (11, 25, 37, 44). Also, IL-10 stimulates the M2 macrophage polarization (45-48). As mentioned earlier, the activation of A2BAR augments the levels of IL-10 in monocytes/macrophages (36-38, 49). Interestingly, the confocal imaging results showed that the majority of elevated IL-10 was co-localized with CD11b+ cells, continually representing the monocytes or macrophages, although neutrophils and dendritic cells also expressed low levels of CD11b (50). In addition, our previous study demonstrated that BAY 60–6583 promoted the phenotypic switch of macrophages from pro-inflammatory M1 to anti-inflammatory M2 subset via the PI3K/Akt pathway (11). Nevertheless, further studies are warranted to investigate whether BAY 60–6583 protects the heart against reperfusion injury by modulating the phenotype switching of splenic macrophages subsets.
Study Limitations
The present study did not investigate the precise roles of different subsets of splenic leukocytes, although maximally elevated IL-10 was observed in the CD11b+ cells after A2BAR activation. However, further studies are warranted to investigate the cross-talk among different splenic leukocytes during myocardial reperfusion injury. Moreover, this study was performed in an in vivo mouse model, which was markedly different from a patient with ACS. However, A2BAR has been implicated in human and murine myocardial ischemia (1). Furthermore, several studies have shown that adenosine, in addition to cardioplegic solutions applied in cardiac operations, could improve the myocardial protection (27). Hence, A2BAR may contribute towards myocardial protection in humans.
Conclusions
In summary, the present study demonstrated that A2BAR activation prior to ischemia stimulated the IL-10 production in splenic leukocytes via the PI3K/Akt pathway, which exerted anti-inflammatory effects that reduced myocardial reperfusion injury.
Acknowledgments
Funding sources
This study was funded in part by a National Natural Science Foundation of China Grant (81400213), a key program from Selected Overseas Chinese Scholar Foundation of Tianjin to YT, a Tianjin Medical University General Hospital incubation foundation (ZYYFY2016038) to YN and NIH R01 HL130082 to ZY
Footnotes
Disclosures
None
YT and ZY designed the study and wrote the manuscript. YN and DL did the western blot and immune staining. DL did the animal experiments. Data were collected and analyzed by YN and DL. ILK and BAF help to revise the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gile J, Eckle T ADORA2b Signaling in Cardioprotection. Journal of nature and science 2016:2. [PMC free article] [PubMed] [Google Scholar]
- 2.Takano H, Bolli R, Black RG Jr, Kodani E, Tang XL, et al. A(1) or A(3) adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circulation research 2001:88:520–528. [DOI] [PubMed] [Google Scholar]
- 3.Yang Z, Cerniway RJ, Byford AM, Berr SS, French BA, et al. Cardiac overexpression of A1-adenosine receptor protects intact mice against myocardial infarction. American journal of physiology. Heart and circulatory physiology 2002:282:H949–955. [DOI] [PubMed] [Google Scholar]
- 4.Nayeem MA, Matherne GP, Mustafa SJ Ischemic and pharmacological preconditioning induces further delayed protection in transgenic mouse cardiac myocytes over-expressing adenosine A1 receptors (A1AR): role of A1AR, iNOS and K(ATP) channels. Naunyn-Schmiedeberg’s archives of pharmacology 2003:367:219–226. [DOI] [PubMed] [Google Scholar]
- 5.Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, et al. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation 2006:114:2056–2064. [DOI] [PubMed] [Google Scholar]
- 6.Ge ZD, Peart JN, Kreckler LM, Wan TC, Jacobson MA, et al. Cl-IB- MECA [2-chloro-N6-(3-iodobenzyl)adenosine-5’-N-methylcarboxamide] reduces ischemia/reperfusion injury in mice by activating the A3 adenosine receptor. The Journal of pharmacology and experimental therapeutics 2006:319:1200–1210. [DOI] [PubMed] [Google Scholar]
- 7.Ge ZD, van der Hoeven D, Maas JE, Wan TC, Auchampach JA A(3) adenosine receptor activation during reperfusion reduces infarct size through actions on bone marrow-derived cells. Journal of molecular and cellular cardiology 2010:49:280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian Y, Marshall M, French BA, Linden J, Yang Z The infarct-sparing effect of IB-MECA against myocardial ischemia/reperfusion injury in mice is mediated by sequential activation of adenosine A3 and A 2A receptors. Basic Res Cardiol 2015:110:16. [DOI] [PubMed] [Google Scholar]
- 9.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, et al. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 2008:111:2024–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auchampach JA, Jin X, Wan TC, Caughey GH, Linden J Canine mast cell adenosine receptors: cloning and expression of the A3 receptor and evidence that degranulation is mediated by the A2B receptor. Molecular pharmacology 1997:52:846–860. [DOI] [PubMed] [Google Scholar]
- 11.Tian Y, Piras BA, Kron IL, French BA, Yang Z Adenosine 2B Receptor Activation Reduces Myocardial Reperfusion Injury by Promoting AntiInflammatory Macrophages Differentiation via PI3K/Akt Pathway. Oxidative medicine and cellular longevity 2015:2015:585297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009:325:612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, et al. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circulation research 2014:114:266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian Y, Pan D, Chordia MD, French BA, Kron IL, et al. The spleen contributes importantly to myocardial infarct exacerbation during post-ischemic reperfusion in mice via signaling between cardiac HMGB1 and splenic RAGE. Basic Res Cardiol 2016:111:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian Y, French BA, Kron IL, Yang Z Splenic leukocytes mediate the hyperglycemic exacerbation of myocardial infarct size in mice. Basic Res Cardiol 2015:110:39. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Linden J, Berr SS, Kron IL, Beller GA, et al. Timing of adenosine 2A receptor stimulation relative to reperfusion has differential effects on infarct size and cardiac function as assessed in mice by MRI. American journal of physiology. Heart and circulatory physiology 2008:295:H2328–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z, Day YJ, Toufektsian MC, Ramos SI, Marshall M, et al. Infarctsparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation 2005:111:2190–2197. [DOI] [PubMed] [Google Scholar]
- 18.Han J, Wang D, Yu B, Wang Y, Ren H, et al. Cardioprotection against ischemia/reperfusion by licochalcone B in isolated rat hearts. Oxidative medicine and cellular longevity 2014:2014:134862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Xie C, Zhuang J, Li H, Yao Y, et al. Resveratrol attenuates inflammation in the rat heart subjected to ischemia-reperfusion: Role of the TLR4/NF-kappaB signaling pathway. Molecular medicine reports 2015:11:1120–1126. [DOI] [PubMed] [Google Scholar]
- 20.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, et al. Cardioprotection by ecto-5’-nucleotidase (CD73) and A2B adenosine receptors. Circulation 2007: 115:1581–1590. [DOI] [PubMed] [Google Scholar]
- 21.Maas JE, Wan TC, Figler RA, Gross GJ, Auchampach JA Evidence that the acute phase of ischemic preconditioning does not require signaling by the A 2B adenosine receptor. Journal of molecular and cellular cardiology 2010:49:886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. The Journal of clinical investigation 2006:116:1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konrad FM, Witte E, Vollmer I, Stark S, Reutershan J Adenosine receptor A2b on hematopoietic cells mediates LPS-induced migration of PMNs into the lung interstitium. American journal of physiology. Lung cellular and molecular physiology 2012:303:L425–438. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Yang D, Carroll SH, Eltzschig HK, Ravid K Activation of the macrophage A2b adenosine receptor regulates tumor necrosis factor-alpha levels following vascular injury. Experimental hematology 2009:37:533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Csoka B, Koscso B, Toro G, Kokai E, Virag L, et al. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes 2014:63:850–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csoka B, Nemeth ZH, Rosenberger P, Eltzschig HK, Spolarics Z, et al. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. Journal of immunology (Baltimore, Md. : 1950) 2010:185:542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boros D, Thompson J, Larson DF Adenosine regulation of the immune response initiated by ischemia reperfusion injury. Perfusion 2016:31:103–110. [DOI] [PubMed] [Google Scholar]
- 28.Koeppen M, Harter PN, Bonney S, Bonney M, Reithel S, et al. Adora2b signaling on bone marrow derived cells dampens myocardial ischemia-reperfusion injury. Anesthesiology 2012:116:1245–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo SW, Koeppen M, Bonney S, Gobel M, Thayer M, et al. Differential Tissue-Specific Function of Adora2b in Cardioprotection. Journal of immunology (Baltimore, Md. : 1950) 2015:195:1732–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharir R, Semo J, Shimoni S, Ben-Mordechai T, Landa-Rouben N, et al. Experimental myocardial infarction induces altered regulatory T cell hemostasis, and adoptive transfer attenuates subsequent remodeling. PloS one 2014:9:e113653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofmann U, Frantz S Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circulation research 2015:116:354–367. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann U, Frantz S Role of T-cells in myocardial infarction. European heart journal 2016:37:873–879. [DOI] [PubMed] [Google Scholar]
- 33.Linton MF, Babaev VR, Huang J, Linton EF, Tao H, et al. Macrophage Apoptosis and Efferocytosis in the Pathogenesis of Atherosclerosis. Circulation journal : official journal of the Japanese Circulation Society 2016:80:2259–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W, Xu W, Xiong S Macrophage differentiation and polarization via phosphatidylinositol 3-kinase/Akt-ERK signaling pathway conferred by serum amyloid P component. Journal of immunology (Baltimore, Md. : 1950) 2011:187:1764–1777. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Lu Y, Bian H, Guo L, Zhu H Activation of the alpha7 nicotinic receptor promotes lipopolysaccharide-induced conversion of M1 microglia to M2. American journal of translational research 2017:9:971–985. [PMC free article] [PubMed] [Google Scholar]
- 36.Nemeth ZH, Lutz CS, Csoka B, Deitch EA, Leibovich SJ, et al. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. Journal of immunology (Baltimore, Md. : 1950) 2005:175:8260–8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sciaraffia E, Riccomi A, Lindstedt R, Gesa V, Cirelli E, et al. Human monocytes respond to extracellular cAMP through A2A and A2B adenosine receptors. Journal of leukocyte biology 2014:96:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vijayamahantesh Amit A, Kumar S, Dikhit MR, Jha PK, et al. Up regulation of A2B adenosine receptor on monocytes are crucially required for immune pathogenicity in Indian patients exposed to Leishmania donovani. Cytokine 2016:79:38–44. [DOI] [PubMed] [Google Scholar]
- 39.Yang Z, Zingarelli B, Szabo C Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation 2000:101:1019–1026. [DOI] [PubMed] [Google Scholar]
- 40.Manukyan MC, Alvernaz CH, Poynter JA, Wang Y, Brewster BD, et al. Interleukin-10 protects the ischemic heart from reperfusion injury via the STAT3 pathway. Surgery 2011:150:231–239. [DOI] [PubMed] [Google Scholar]
- 41.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, et al. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. Journal of immunology (Baltimore, Md. : 1950) 1996:157:4634–4640. [PubMed] [Google Scholar]
- 42.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, et al. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor- dependent and independent mechanisms. FASEB J 2000:14:2065–2074. [DOI] [PubMed] [Google Scholar]
- 43.Cheng P, Zuo X, Ren Y, Bai S, Tang W, et al. Adenosine A1-Receptors Modulate mTOR Signaling to Regulate White Matter Inflammatory Lesions Induced by Chronic Cerebral Hypoperfusion. Neurochem Res 2016:41:3272–3277. [DOI] [PubMed] [Google Scholar]
- 44.Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J 2012:26:376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koscso B, Csoka B, Kokai E, Nemeth ZH, Pacher P, et al. Adenosine augments IL-10-induced STAT3 signaling in M2c macrophages. Journal of leukocyte biology 2013:94:1309–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lurier EB, Dalton D, Dampier W, Raman P, Nassiri S, et al. Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing. Immunobiology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, et al. IL- 10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. 2017:112:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopes RL, Borges TJ, Zanin RF, Bonorino C IL-10 is required for polarization of macrophages to M2-like phenotype by mycobacterial DnaK (heat shock protein 70). Cytokine 2016:85:123–129. [DOI] [PubMed] [Google Scholar]
- 49.Koscso B, Csoka B, Selmeczy Z, Himer L, Pacher P, et al. Adenosine augments IL-10 production by microglial cells through an A2B adenosine receptor-mediated process. Journal of immunology (Baltimore, Md. : 1950) 2012:188:445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandor N, Lukacsi S, Ungai-Salanki R, Orgovan N, Szabo B, et al. CD11c/CD18 Dominates Adhesion of Human Monocytes, Macrophages and Dendritic Cells over CD11b/CD18. PloS one 2016:11:e0163120. [DOI] [PMC free article] [PubMed] [Google Scholar]


