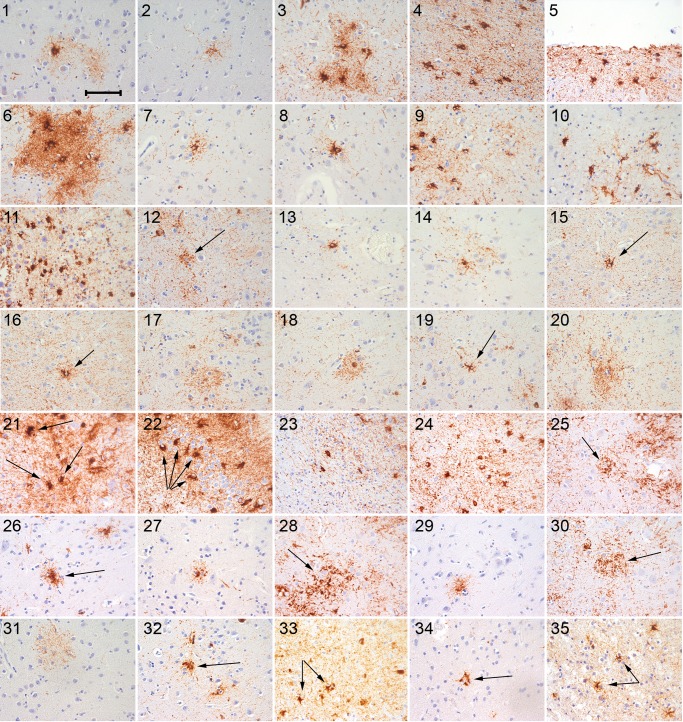
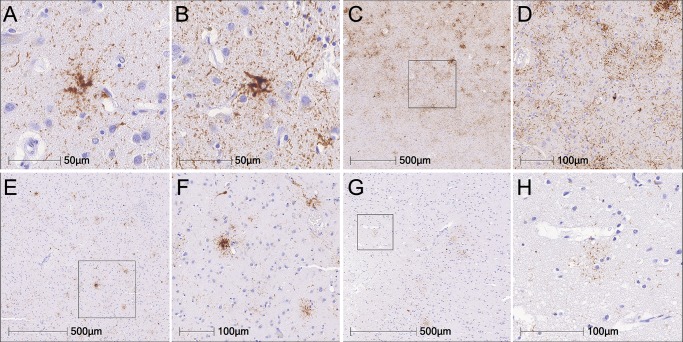
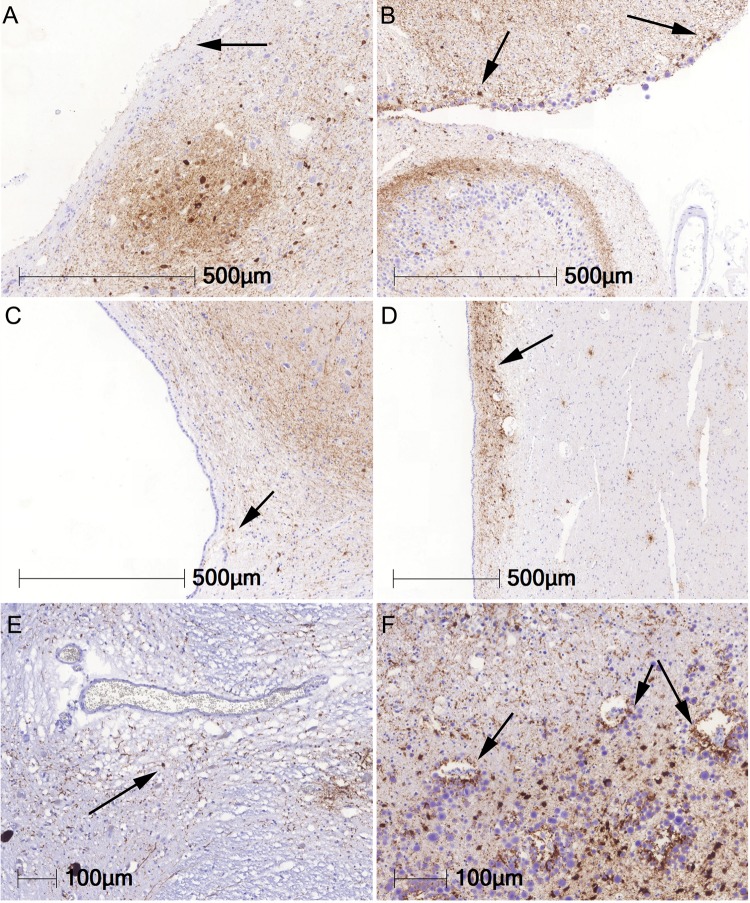
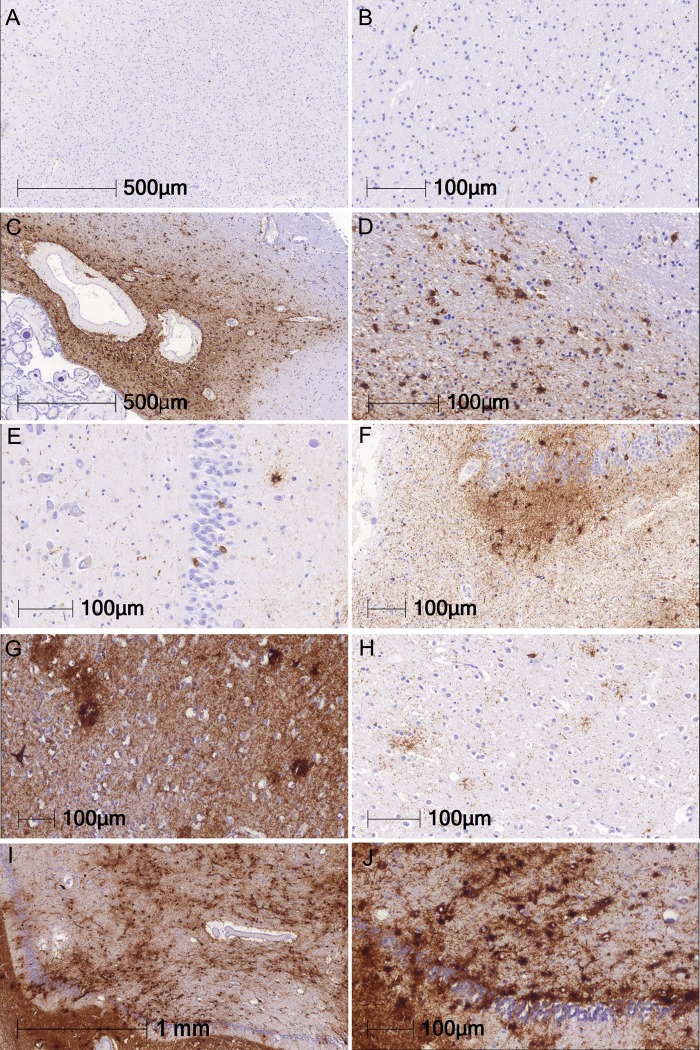
Gabor G Kovacs
Gabor G Kovacs, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,✉,
Sharon X Xie
Sharon X Xie, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Edward B Lee
Edward B Lee, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
John L Robinson
John L Robinson, BA
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Carrie Caswell
Carrie Caswell, MS
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
David J Irwin
David J Irwin, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Jon B Toledo
Jon B Toledo, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Victoria E Johnson
Victoria E Johnson, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Douglas H Smith
Douglas H Smith, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Irina Alafuzoff
Irina Alafuzoff, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Johannes Attems
Johannes Attems, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Janos Bencze
Janos Bencze, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Kevin F Bieniek
Kevin F Bieniek, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Eileen H Bigio
Eileen H Bigio, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Istvan Bodi
Istvan Bodi, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Herbert Budka
Herbert Budka, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Dennis W Dickson
Dennis W Dickson, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Brittany N Dugger
Brittany N Dugger, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Charles Duyckaerts
Charles Duyckaerts, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Isidro Ferrer
Isidro Ferrer, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Shelley L Forrest
Shelley L Forrest, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Ellen Gelpi
Ellen Gelpi, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Stephen M Gentleman
Stephen M Gentleman, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Giorgio Giaccone
Giorgio Giaccone, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Lea T Grinberg
Lea T Grinberg, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Glenda M Halliday
Glenda M Halliday, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Kimmo J Hatanpaa
Kimmo J Hatanpaa, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Patrick R Hof
Patrick R Hof, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Monika Hofer
Monika Hofer, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Tibor Hortobágyi
Tibor Hortobágyi, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
James W Ironside
James W Ironside, FRCPath
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Andrew King
Andrew King, FRCPath
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Julia Kofler
Julia Kofler, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Enikö Kövari
Enikö Kövari, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Jillian J Kril
Jillian J Kril, PhD, FFSc
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Seth Love
Seth Love, FRCPath
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Ian R Mackenzie
Ian R Mackenzie, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Qinwen Mao
Qinwen Mao, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Radoslav Matej
Radoslav Matej, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Catriona McLean
Catriona McLean, MBBS, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
David G Munoz
David G Munoz, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Melissa E Murray
Melissa E Murray, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Janna Neltner
Janna Neltner, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Peter T Nelson
Peter T Nelson, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Diane Ritchie
Diane Ritchie, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Roberta D Rodriguez
Roberta D Rodriguez, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Zdenek Rohan
Zdenek Rohan, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Annemieke Rozemuller
Annemieke Rozemuller, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Kenji Sakai
Kenji Sakai, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Christian Schultz
Christian Schultz, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Danielle Seilhean
Danielle Seilhean, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Vanessa Smith
Vanessa Smith, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Pawel Tacik
Pawel Tacik, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Hitoshi Takahashi
Hitoshi Takahashi, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Masaki Takao
Masaki Takao, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Dietmar Rudolf Thal
Dietmar Rudolf Thal, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Serge Weis
Serge Weis, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Stephen B Wharton
Stephen B Wharton, FRCPath
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Charles L White III
Charles L White III, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
John M Woulfe
John M Woulfe, MD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
Masahito Yamada
Masahito Yamada, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1,
John Q Trojanowski
John Q Trojanowski, MD, PhD
1From the Institute of Neurology, Medical University of Vienna (GGK, HB), Vienna, Austria; Center for Neurodegenerative Disease Research, Institute on Aging and Department of Pathology and Laboratory Medicine of the Perelman School of Medicine at the University of Pennsylvania (GGK, EBL, JLR, DJI, JBT, JQT); and Department of Biostatistics and Epidemiology (SXX, CC); and Department of Neurosurgery, Center for Brain Injury and Repair, University of Pennsylvania (VEJ, DHS), Philadelphia, Pennsylvania; Department of Immunology, Genetics and Pathology, Uppsala University (IA), Uppsala, Sweden; Institute of Neuroscience, Newcastle University (JA), Newcastle upon Tyne, UK; Department of Neuropathology, Institute of Pathology, Faculty of Medicine, University of Debrecen (JB, TH), Debrecen, Hungary; Department of Neuroscience, Mayo Clinic (KFB, DWD, MEM, PT), Jacksonville, Florida; Northwestern University Feinberg School of Medicine, Northwestern ADC Neuropathology Core (EHB, QM), Chicago, Illinois; Clinical Neuropathology, King’s College Hospital and London Neurodegenerative Brain Bank (IB, AK), London, UK; Institute of Neuropathology, University Hospital Zurich (HB), Zurich, Switzerland; University of California San Francisco, Institute for Neurodegenerative Diseases (BND), San Francisco, California; Neuropathology Department, Hôpital de La Salpetrière, AP-HP, UPMC-Sorbonne-University (CD, DS), Paris, France; Institute of Neuropathology, Bellvitge University Hospital, University of Barcelona (IF), CIBERNED, Hospitalet de Llobregat, Barcelona, Spain; Discipline of Pathology, Sydney Medical School, The University of Sydney (SLF, JJK), Sydney, Australia; Neurological Tissue Bank of the Biobank-Hospital Clinic-IDIBAPS, Institut d’Investigacions Biomediques Pi i (EG), Barcelona, Spain; Department of Medicine, Imperial College London (SMG), London, UK; IRCCS Foundation “Carlo Besta” Neurological Institute (GG), Milan, Italy; Memory and Aging Center, Department of Neurology, University of California (LTG), San Francisco, California; Department of Pathology, University of Sao Paulo Medical School (LTG), LIM, São Paulo, Brazil; Brain & Mind Centre, Sydney Medical School, The University of Sydney, and UNSW Medicine & NeuRA (GMH), Sydney, Australia; Department of Pathology, University of Texas Southwestern Medical Center (KJH, CLW), Dallas, Texas; Fishberg Department of Neuroscience, Friedman Brain Institute, and Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai (PRH), New York, New York; Department of Neuropathology, John Radcliffe Hospital (MH), Oxford, UK; Centre for Clinical Brain Sciences, University of Edinburgh (JWI, DR), Edinburgh, UK; Department of Pathology, University of Pittsburgh (JK), Pittsburgh, Pennsylvania; Department of Mental Health and Psychiatry, University Hospitals and University of Geneva School of Medicine (EK), Geneva, Switzerland; Institute of Clinical Neurosciences, University of Bristol, Learning & Research Level 2, Southmead Hospital (SL), Bristol, UK; Department of Pathology and Laboratory Medicine, University of British Columbia (IRM), Vancouver, Canada; Department of Pathology and Molecular Medicine, Thomayer Hospital (RM, ZR), Prague, Czech Republic; Department of Pathology, First Medical Faculty, Charles University (RM, ZR), Prague, Czech Republic; Department of Anatomical Pathology, Alfred Hospital (CM), Prahran, Victoria, Australia; Division of Pathology, St. Michael’s Hospital (DGM), Toronto, Ontario, Canada; Department of Pathology and Sanders-Brown Center on Aging, University of Kentucky (JN, PTN, VS), Lexington, Kentucky; Physiopathology in Aging Lab/Brazilian Aging Brain Study Group-LIM22, University of Sao Paulo Medical School (RDR), Sao Paulo, Brazil; Behavioral and Cognitive Neurology Unit, Department of Neurology, University of São Paulo (RDR), São Paulo, Brazil; Netherlands Brainbank, Amsterdam and Department of Pathology, VU University Medical Center (AR), Amsterdam, The Netherlands; Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Sciences (KS, MY), Kanazawa, Japan; Institute of Neuroanatomy, Centre for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Heidelberg University (CS), Heidelberg, Germany; Department of Neurodegenerative Diseases and Gerontopsychiatry at the University of Bonn Medical Center (PT), Bonn, Germany; Department of Pathology, Brain Research Institute, Niigata University (HT), Niigata, Japan; Department of Neurology, Saitama Medical University International Medical Center (MT), Saitama, Japan; Department of Neuroscience, Katholieke Universiteit-Leuven (DRT); and Department of Pathology, Universitaire Ziekenhuizen-Leuven (DRT), Leuven, Belgium; Laboratory of Neuropathology, Department of Pathology and Neuropathology, Kepler University Hospital, Medical School, Johannes Kepler University (SW), Linz, Austria; Sheffield Institute for Translational Neuroscience, University of Sheffield (SBW), Sheffield, UK; and Department of Pathology and Laboratory Medicine, Centre for Cancer Therapeutics, Ottawa Hospital Research Institute, University of Ottawa (JMW), Ontario, Canada.
1