Abstract
Study Objectives:
To better understand the development of sleep, we characterized the development of circadian rhythms in sleep and wakefulness in the artificially-reared, isolated rat pup using an experimental design that minimized the effects of maternal separation.
Methods:
Neonatal rats were reared in constant conditions (dim red light) while electroencephalographic and electromyographic signals were continuously recorded for up to 3 weeks. This time period spanned the preweaned and weaned ages. The distribution of sleep–wake states was analyzed to estimate the emergence of circadian rhythms.
Results:
Overt ~24-hour rhythms in time spent awake and asleep appear by postnatal day (P)17. A marked bi-modal sleep–wake pattern was also observed, evidenced by the appearance of a pronounced ~12-hour component in the periodogram over the subsequent 3 days (P17–P21). This suggested the presence of two ~24-hour components consistent with the dual-oscillator concept. During this 3-day time window, waking bouts became longer resulting in a repartition of the duration of intervals without non-rapid-eye movement (NREM) sleep into short (<30 minutes) and longer inter-NREM sleep episodes. These longer waking bouts did not immediately result in an increase in NREM sleep delta (0.5–4.0 Hz) power, which is an index of sleep homeostasis in adult mammals. The sleep homeostatic response did not fully mature until P25.
Conclusions:
These results demonstrate that the maturation of circadian organization of sleep–wake behavior precedes the expression of mature sleep homeostasis.
Keywords: ontogenesis, perinatal, maturation, rhythms, neonatal
Statement of Significance
This study provides new insights into the development of sleep regulatory mechanisms in a widely used animal model.
INTRODUCTION
Mammalian sleep is regulated by circadian and homeostatic mechanisms that control the timing and intensity of sleep across the 24-hour day. These regulatory mechanisms undergo dramatic changes during the course of perinatal development.1 The homeostatic response to sleep deprivation, for example, is strikingly different in rodents depending on their age.2–5 In adult rats, sleep deprivation reliably increases non-rapid-eye movement (NREM) sleep electroencephalograph (EEG) delta power, a widely used index of homeostatic sleep propensity. However, in rats prior to approximately the fourth postnatal week, sleep deprivation fails to increase EEG delta power; infant rats instead show only compensatory changes in sleep time, sleep continuity, or phasic motor activity.2,4 Compensatory rebounds in EEG defined rapid eye movement (REM) sleep time following total sleep deprivation or selective REM sleep deprivation are also absent until approximately the fourth postnatal week.2,6 These findings indicate that forms of sleep homeostasis may be present during early development, but coupling to neural mechanisms governing adult, compensatory changes in sleep architecture occurs only after a certain stage of maturation.
Circadian organization of sleep and wakefulness follows a similar developmental plan. In fetal rodents, circadian rhythms in metabolism, enzyme activity, and immediate early gene expression are present in the suprachiasmatic nucleus (SCN).1 However, the coupling of SCN rhythms to sleep and wake neural circuitry is a comparatively late postnatal event.7 In human infants, for example, faint 24-hour periodicities in activity are detectable in newborns, but are not prominent until after the third postnatal month.8 The precise timing of this coupling has been difficult to ascertain because detecting endogenous rhythms requires eliminating entraining and masking signals from the mother and the environment. This is particularly difficult to do in neonatal rodents, which depend upon their dam for warmth, feeding, and grooming (necessary for proper micturition and defecation). In addition, extended periods of maternal separation without controls for temperature, feeding, and grooming elicit a stress response that alters sleep and wake architecture in infant rats.9
In a series of earlier studies, we developed and used an artificial rearing system that minimizes the effects of maternal separation on sleep patterns to chronically measure EEG and electromyographically (EMG) defined REM and NREM sleep in neonatal rats.2,10,11 In the presence of a 12:12 light dark cycle, diurnal/nocturnal patterns of sleep and wakefulness emerged between P16 and P20, and a declining trend in EEG delta power across the rest phase was detectable at P24.10 These findings suggest that circadian regulation of sleep and wakefulness emerges in the third postnatal week and may precede the maturation of adult forms of sleep homeostasis. To more adequately address these issues, we used our artificial rearing system to continuously and quantitatively measure sleep and wakefulness in rat pups born and individually raised under constant (dim red light) conditions from the preweaning (P12) to post-weaning periods (P20+).
MATERIALS AND METHODS
Experimental Design
Time-plugged Long–Evans dams (obtained from Simonson Laboratories, Gilroy, CA) were placed in light-tight ventilated chambers and maintained in constant conditions of dim red light (5–8 lux) and temperature (22°C) with food and water available ad libitum on gestational (G) day 4 (ie, G4). The boxes were kept in a light-tight room also maintained under similar constant conditions. Dams were left undisturbed until parturition (approximately G20) with the exception of cage cleaning and food and water replenishment that occurred once every 3–5 days. At approximately G18, the dams were inspected every 16 hours for new litters. The cleaning schedule and litter inspection times were selected to prevent maternal circadian entrainment to external cues.
Following parturition, the litters were culled to 10 pups. At postnatal day (P) 9 or 10, male pups (n = 12) were removed from the dams and surgically prepared for polysomnographic recording according to previously described methods.11 Briefly, rat pups were anesthetized with methoxyflurane gas. An incision was made over the skull which was cleaned with a solution of hydrogen peroxide followed by application of copalite. EEG electrodes were placed between bregma and lambda. EMG electrodes were sutured into the nuchal muscles and the entire assembly was affixed to the skull using a combination of cyanoacrylate glue and dental acrylic. The wound was then closed with four to five sutures and the animals were treated with the antibiotic gentamicin subcutaneously. All surgical procedures were conducted in the same room and under the same constant conditions as those used for housing the dams and for recording sleep in the pups. At no time were any of the dams or pups exposed to a change in lighting conditions. All animal procedures were in accordance with IACUC guidelines at Stanford University.
Artificial Rearing Techniques
We used a modified version of our previously described system to artificially rear isolated rat pups beginning at P12 for up to 2 weeks.2,10–12 Briefly, neonatal rat pups were fitted with an indwelling, stainless steel cheek cannula that was attached to a flexible feeding tube. The tube was then attached to a timed pump that infused a high-fat milk formula every 35 minutes across the 24-hour day until weaning at P18. The pups were separately housed (under the same constant conditions) in acrylic boxes with bedding material that were enclosed within a water-bath that maintained ambient temperatures at age-appropriate thermo-neutral ranges. Preweaned pups were also inspected and groomed with a moistened cotton ball every 16 hours to induce micturition and defecation and to reduce the effects of maternal separation. Body mass was recorded every 32 hours. Cage cleaning and any necessary changes in milk supply were done at these times as well. Following weaning, the tubes were removed and the pups were provided rat chow and water ad libitum. From this time onward, cage cleaning, body mass measurements, and all other entrances to the recording room only occurred at multiples of 16 hours (eg, 32 and 48 hours etc.). These schedules were used to avoid entrainment cues to developing circadian rhythms.
Polygraphic Recording, Animal Selection, and State Annotation
EEG and EMG signals were routed from the animal via an electrical, counter-balanced tether/commutator to a Grass 7 polygraph as described previously.11 Signals were processed with a high pass filter of 0.3 Hz and a low pass filter of 35 Hz, digitized at 100 Hz, and collected in 10-second epochs on a computer. EMG signals were full-wave rectified, integrated, and stored as one value per epoch. The EEG was also Fourier transformed in each 10-second epoch.11 Polygraphic data were collected continuously from the beginning of each experiment (P12 or P13) until its termination. Only pups that showed sustained body mass gains and good polysomnographic signals (P12–P20) were used for analyses. Mean body masses at P12 and P20 were 27.4 (±0.6) and 38.9 (±1.6), respectively, which represents an average gain in body mass of 42 %. This gain was less that observed in control pups left with their dams in 12:12 light/dark cycles (67 %),11 but within ranges reported for developing rats.13
Some of the recordings had to be terminated after only a few days because the feeding tubes became disconnected. In other cases, the EEG and EMG signals became unusable due to movement of the electrodes as the animal grew. However, we were able to successfully record EEG and EMG signals from most of the animals into the fourth postnatal week (Figure 1). Five animals with the longest records (Figure 2) were selected for circadian analyses. These longer records allowed the detection of stable significant circadian rhythmicity lasting a minimum of three cycles. Analyses concerning sleep–wake distribution and architecture were based on 11 animals. The vigilance states of REM and NREM sleep and wakefulness (W) were determined using an algorithm previously shown to agree with manual scoring (>90%) in neonatal rats.10
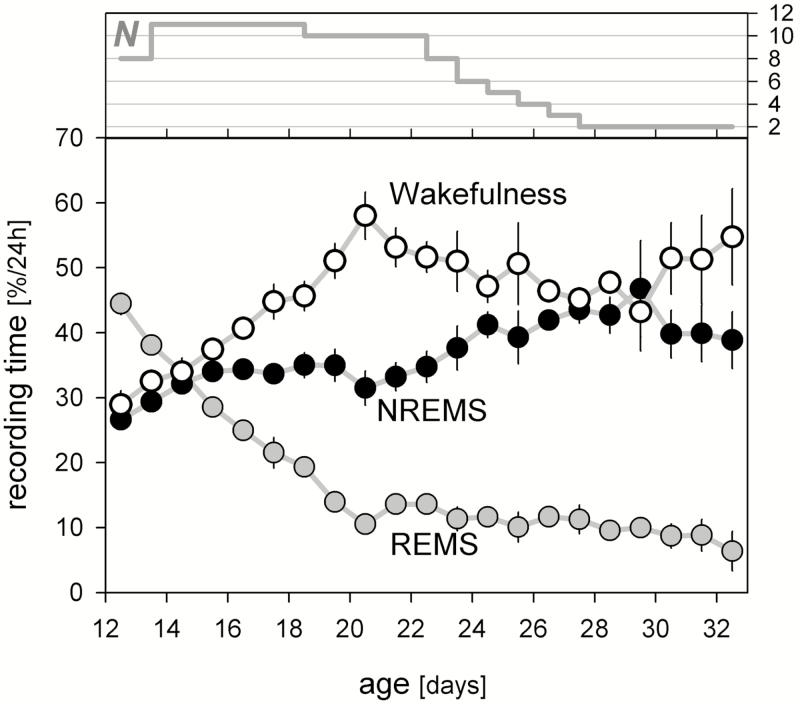
Figure 1.
Developmental changes in time-spent-awake (open), in NREMS (black), and in REMS (grey symbols). Values represent mean daily (ie, 24 hours) % artifact-free recording time (± 1 SEM). Recordings started at midnight after the 11th or 12th postnatal day (n = 8 and 3, respectively). Number of rats (N) contributing to the mean decreased over the course of the experiment (upper curve). NREMS = non-rapid-eye movement sleep; REMS = rapid-eye movement sleep; SEM = standard error of the mean.
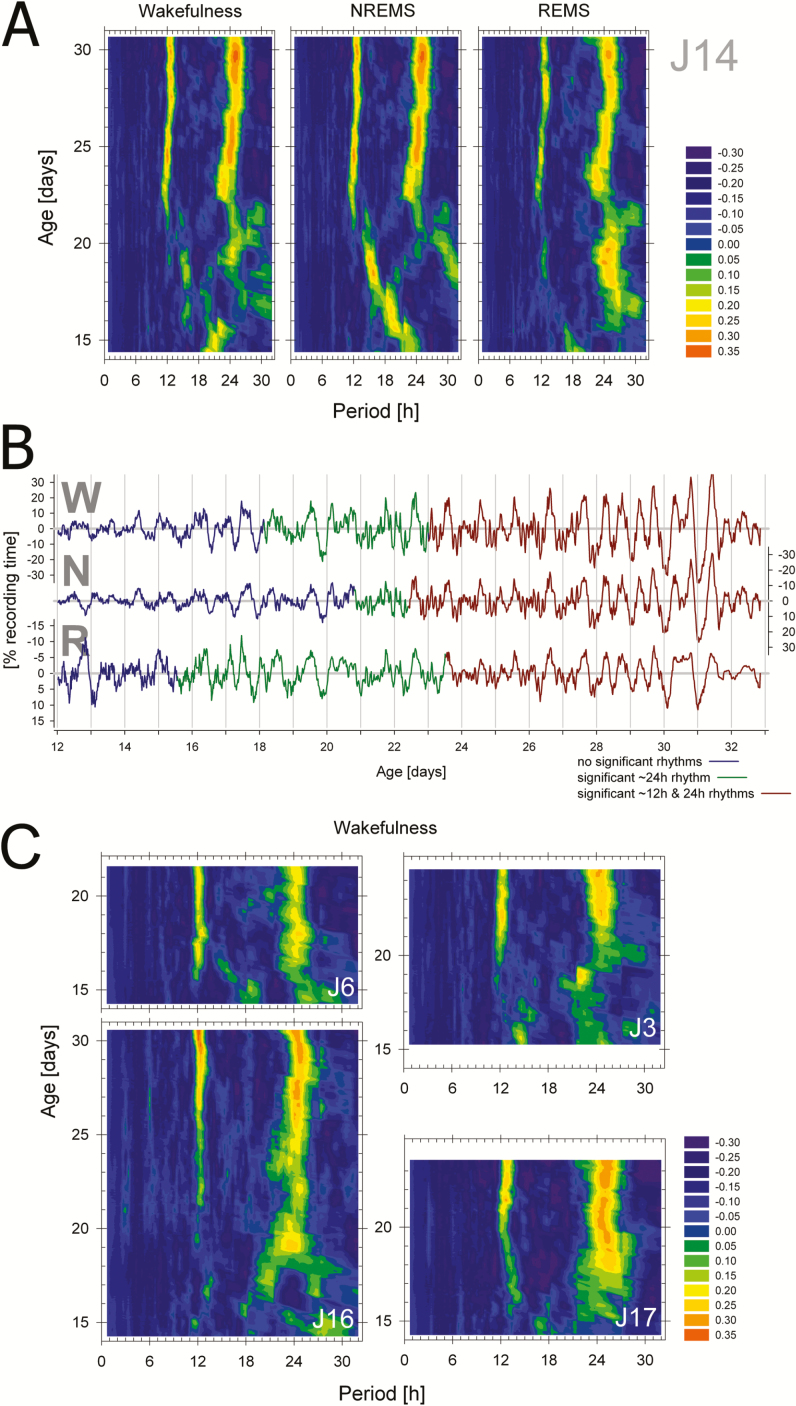
Figure 2.
Appearance of ~12- and 24-hour rhythmicity in the distribution of wakefulness, NREMS, and REMS. (A) Heat maps of periodograms obtained at 20-minute increments for consecutive 4.5-day windows illustrated for one individual rat (J14). Color coded values represent relative Q-values of the periodogram calculated as the difference from the level of Q at p = .01 (see Supplementary Figure 1). Thus green through orange hues represent test periods of significant signal. Note the significant activity in rhythmicity in the 12- and 24-hour ranges starting around postnatal day (P)22 and between P15 and P21, respectively, varying according to behavioral state. Note that the first periodogram that could be calculated was at P14.25; that is, the midpoint of the first 4.5-day window (P12–P16.5). (B) Illustration of the precise onset times of significant 24- (blue-to-green) and 12-hour (green-to-brown) rhythmicity in the original de-trended recordings for wakefulness (W), NREMS (N), and REMS (R) for rat J14. Note that scaling for sleep is inverted to match the wakefulness signal. Note also the presence of a distinct 12-hour component in the sleep–wake distribution especially clear between P27 and P30. Finally, onset times are plotted at midpoint of the 4.5-day sliding window and thus rhythmicity can already be observed in the 2 days prior to onset although a statistical evaluation requires more than two cycles. (C) Periodogram heat maps for the distribution of wakefulness for the four other rats (J3, J6, J16, and J17) with sufficiently long recordings for the detection of stable (>3 days) and significant rhythmicity. Subsequent analyses on appearance of rhythmicity were based on these five animals (see Figure 3 and Table 1). NREMS = non-rapid-eye movement sleep; REMS = rapid-eye movement sleep.
Circadian Analysis of Sleep–Wake States
Because there is no commonly accepted method of analysis for such data, multiple methods were used to ensure that data analyses were unbiased by the methodology. Before the data were analyzed for circadian periodicity, they were “de-trended” to remove the general developmental changes in sleep–wake state amounts on which the circadian changes were superimposed (Figure 1 and Supplementary Figure 1). De-trending was accomplished by subtracting state amount in a given 20-minute interval; that is, the time resolution at which the sleep–wake distribution was analyzed, from the mean value for state amount reached in the 12 hour prior and following this time point (that is, 24 hour; see Supplementary Figure 1).
The presence of significant periodicity in the circadian range (ie, 20–28 hours) was determined by four methods of time-series analysis; that is, the Dörrscheidt and Beck14 and the X2 periodogram analysis algorithms,15 which are both widely used in circadian rhythm studies and best suited to analyze rhythmic data with a stable waveform, the Lomb–Scargle periodogram algorithm (“Peanuts” free-ware16), which was designed to handle non-uniform data, and the Fourier analysis. Only the first three algorithms provide measures of statistical significance for circadian periods. Although each method is best suited for particular applications and has inherent limitations, all three methods produced nearly identical results (see Supplementary Figure 1 for a comparison of the first three methods). Because the Dörrscheidt and Beck method was more conservative in calling a given test period significant, all results reported here are based on this algorithm (see also8).
The periodogram analysis was used to determine the age at which the distribution of each sleep–wake state revealed a significant circadian oscillation. Time spent in each state was calculated for consecutive 20-minute time bins. This bin size accommodated both the need to reduce bin-to-bin variability (smoothing the 10-second scores) and the need for a temporal resolution appropriate for circadian studies. Periodogram analysis was applied to the first 4.5 days of data of each vigilance state and then advanced at 20-minute increments to the end of the data set (illustrated in Supplementary Figure 1). The choice of the 4.5-day window was a compromise between the need for a window that still could provide reasonable time resolution to study circadian ontogeny, and the need to accommodate a minimal number of cycles (ca. 4) that still allowed for statistical evaluation of rhythmicity. A last argument in favor of using shorter rather than longer time windows is that during development rhythms are, by definition, not stationary. Because of the surprising 12-hour component we discovered, we marked the emergence of both 12- and 24-hour “rhythms”. We used the midpoint of the “significant” window as the age at which significant rhythmicity first emerged although one could equally well argue to take window onset as after this time point significant circadian rhythmicity was present. Our estimate might thus be biased towards a 2.25-day delay. Periods were deemed significant at a p = .01.
Sleep–Wake Architecture and Sleep Homeostasis
The time course of the development of the consolidation of waking bouts was analyzed by assessing the relative distribution of wake-bout duration on each day for eight categories each doubling in duration starting with the shortest episode; that is, 10 seconds, and ending with episodes > 10 minutes. Both the number of episodes (expressed per hour of wakefulness), as well as the % with which each of the eight categories contributes to total time spent awake during each day were quantified.
Similarly, the developmental time course of the duration of time between NREM sleep episodes (inter NREM sleep episodes) was determined. This analysis was intended to gain insight into the timing of the maturation of the sleep homeostatic process as measured by NREM sleep delta power. NREM delta power is a widely-used index of sleep homeostasis in adult mammals that appears at a specific time in development.2 It is generally accepted that sleep propensity decreases in the presence of NREM sleep and to accumulate in its absence; that is, during inter NREM sleep episodes comprising both wakefulness and REM sleep.17 In simulation models in rodents it has been assumed that sleep propensity increases at the same rate during both wakefulness and REM sleep18,19 although this assumption has not been experimentally addressed in developing rodents. We determined a distribution of inter-NREM sleep episode durations for seven categories at 10-minute increments starting with episodes < 10 minutes and ending with episodes > 60 minutes. The number of episodes was expressed as a percentage of all episodes present at each developmental age category.
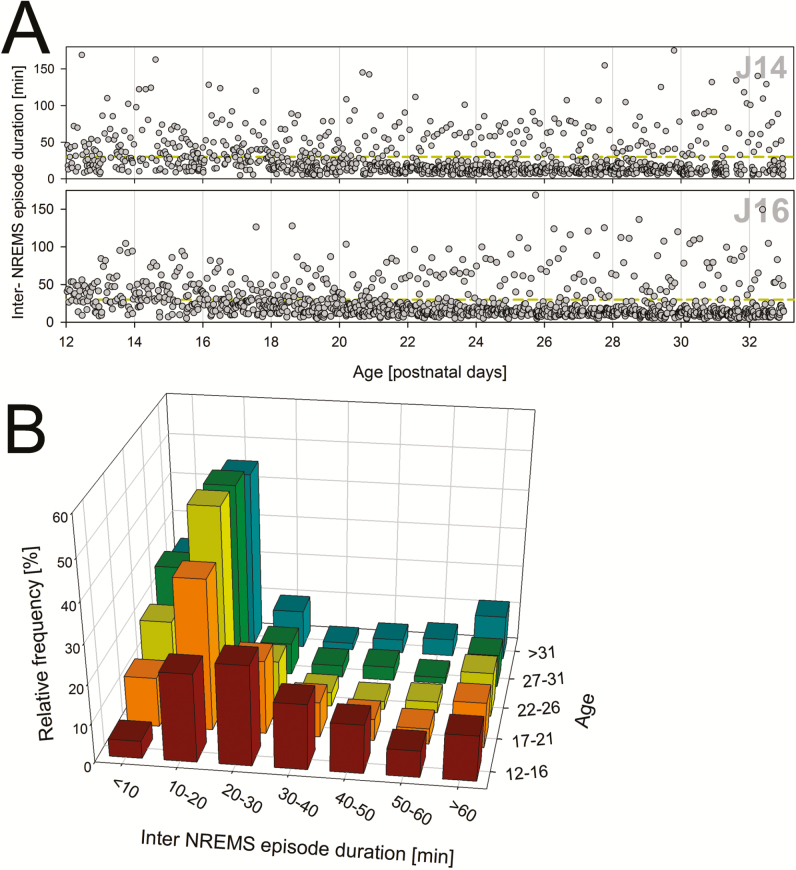
The latter analyses revealed that with developmental age a clear partition between inter NREM sleep episodes shorter and longer than 30 minutes occurred. The effect of time without NREM sleep on subsequent EEG delta power was assessed by correlating each inter-NREM sleep episode duration with the level of EEG delta power reached in the following NREM sleep bout. Only inter-NREM sleep episodes > 30 minutes and delta power values for sustained NREM sleep bouts (>3.5 minutes) were included in the analyses.
Statistical Analyses
Assessing presence of significant rhythmic components (in the 12- and 24-hour ranges) for the developmental time series of the 3 sleep–wake states was done according to the Dörrscheidt and Beck method (see above) with a significance thresholds set at p = .01. Other statistical analyses were performed using SAS (SAS Institute Inc, Cary, NC). Significant effects of state and rhythmic component (12- vs. 24-hours) and their interactions were assessed using analysis-of-variance (ANOVA) and decomposed using post-hoc paired t tests. Simple contrasts were assessed using t tests. Statistical significance was set to p = .05 and results are reported as mean ± standard error of the mean (SEM). SigmaPlot 11 (Systat Software Inc., Chicago, IL) was used for graphs including linear and non-linear regression analyses.
RESULTS
Development of Vigilance State Amounts
The daily time-spent-awake and asleep changed over the course of development, as reported previously.11,20 Daily amounts of wakefulness peaked at P21 and declined steadily until P28 when daily amounts remained constant until the end of the study (Figure 1). Daily amounts of REM sleep decreased steadily until P21 and remained constant thereafter. By contrast, daily amounts of NREM sleep increased gradually until P29. The pattern of vigilance state development in the present study was similar to that observed for pups raised in an light–dark cycle and separated from their dams on alternate days, or acutely recorded for 36 hours.10,11 Therefore, long-term maternal separation did not affect sleep state development.
Development of Circadian Rhythms of Sleep–Wake States
Initially, besides occasional signs in some rats of a 16-hour rhythmicity related to the grooming schedule (eg, Figure 2A and Supplementary Figure 1), no consistent rhythmicity of any period could be discerned across sleep–wake states and individuals. Circadian periodicity in sleep–wake distribution was significant by approximately P17 (Figure 2). The appearance could be pinpointed with reasonable precision within each individual and sleep–wake state allowing statistical assessment of onset times (Figures 2 and 3, Table 1). This was true for wake, NREM sleep, and REM sleep and no difference in onset time was observed among the three sleep–wake states. A rhythm of approximately 12 hours was also detectable, but appeared, on average, 3 days later; that is, at approximately P20 (Table 2). This 12-hour component was not only present in the periodogram but could be directly observed in the sleep–wake distribution, especially clear towards the end of the registration (P27–P30; Figure 2B). At P21.5 (the age when circadian rhythms were present in all states and in all rats), the period of the circadian rhythm was significantly greater than 24 hours (Table 2) consistent with the longer than 24-hour circadian rhythms reported for the adult rat.21,22
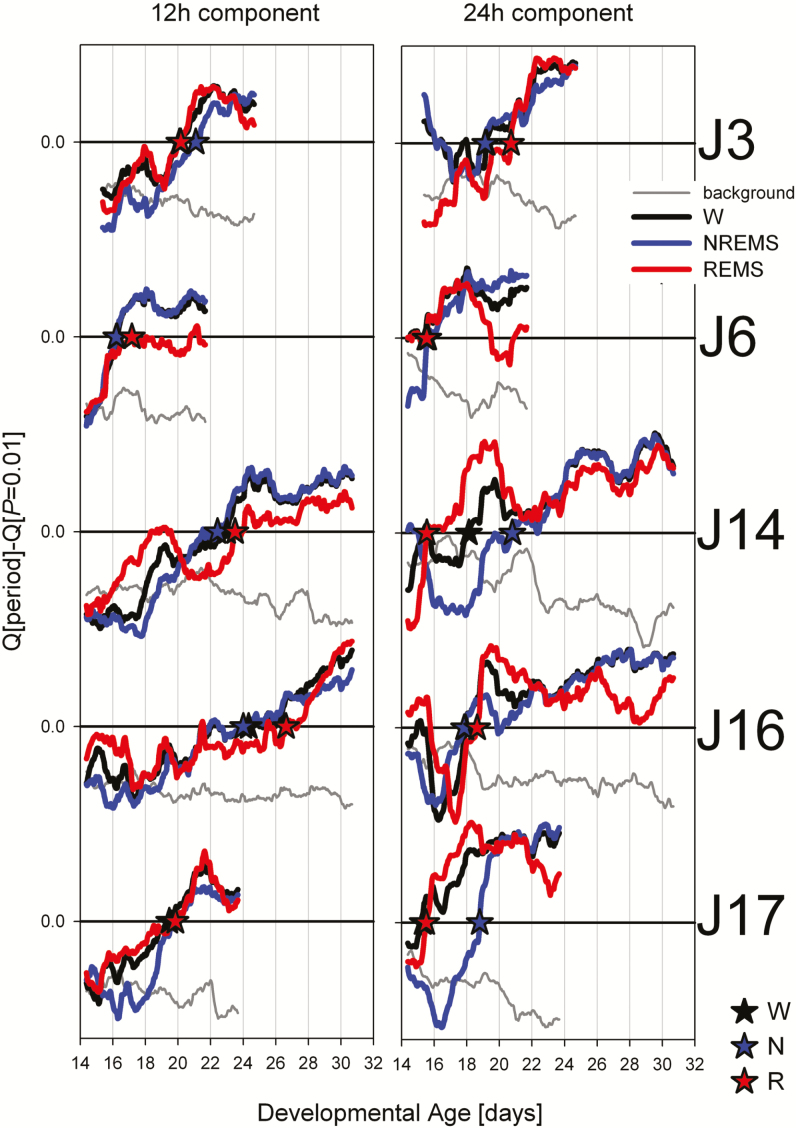
Figure 3.
Postnatal day at which the ~12- (left) and 24-hour (right panels) components of the sleep–wake distribution reached and remained (>3 days) significant for the five rats selected (see Figure 2). Mean Q-values for the two test-period ranges of interest (ie, 11.3–12.7 hours and 23.0–25.3 hours, respectively) were expressed relative to the Q-value reached at p = .01 for these two period ranges (horizontal line at 0 in each panel). Developmental time courses of relative Q-values are indicated for wakefulness (W, black), NREMS (N, blue), and REMS (R, red lines). Asterisks indicate the time point from which the rhythm was deemed significant for each behavioral state and rat. See Table 1 for further statistical evaluation of these onset times. Grey line reflects the Q-values for periods neighboring the regions of interest (labelled “background”); that is, 9.3–11.3 hours/12.7–14.7 hours and 21.0–23.0 hours/25.3–27.3 hours. These background values were averaged for the two test-periods ranges across behavioral states and then analyzed in the same way as the signal. Background activity decreased while signal strength increased in all cases. NREMS = non-rapid-eye movement sleep; REMS = rapid-eye movement sleep.
Table 1.
Onset of Circadian Rhythmicity in the Distribution of the Three Sleep–Wake States.
| ≈12 h | ≈24 h | |
|---|---|---|
| W | P20.6 ± 1.4 | P17.2 ± 0.8 |
| NREMS | P20.7 ± 1.3 | P18.4 ± 0.8 |
| REMS | P21.5 ± 1.6 | P17.2 ± 1.1 |
rANOVA = repeated measures analysis of variance; NREMS = non-rapid-eye movement sleep; REMS = rapid-eye movement sleep; W = Wakefulness.
Mean onset (± SEM; n = 5) in days after birth (P) of significant rhythmicity (p < .01) in the 12-h (≈12 h) and 24-h (≈24 h) ranges. Periodicity onset did not significantly differ among the three behavioral states but started ca. 3.3 days later in the 12-h range (2-way rANOVA: factor “state”: p = .45; “period”: p = .045; interaction: p = .25).
Table 2.
Period of Circadian Rhythms for Wakefulness at P21.5.
| Period (h) | |
|---|---|
| ≈12 h | 12.17 ± 0.10 |
| ≈24 h | 24.44 ± 0.12 |
| 24/12 | 2.01 ± 0.02 |
Circadian period length (± SEM; n = 5) at P21.5 significantly deviated from 24.0 h (t test, p = .024). The period length of the 24-h component was twice that of the 12-h component (24/12) and the 24/12-ratio did not significantly deviate from 2.0 (paired t test, p = .64). P21.5 was chosen because by then significant periodicity was established for all states and rats.
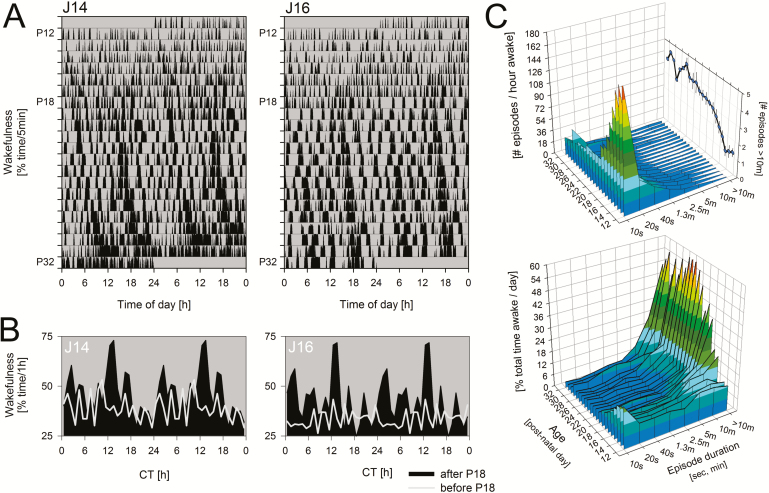
We also examined two additional and related measures reflective of circadian organization; the inter-NREM sleep bout duration and wake-bout duration. Although related, these two measures differ, especially at the earlier ages, when long periods of REM sleep interrupt NREM sleep. Prior to P18, there appeared to be little evidence of consolidated waking periods (Figure 4A). After P18, however, there was a clear shift in the distribution of wake bout lengths towards longer bouts such that by P19, long bouts (>10 minutes) comprised 40% of all time spent awake (Figure 4C). Similarly, at approximately P17–P21, there was a change in the distribution of inter-NREM interval lengths from very short intervals to those longer than 30 minutes (Figure 5). Both changes paralleled the appearance of circadian rhythmicity based on total time spent in each state (Table 1).
Figure 4.
Appearance of consolidated waking bouts during development. (A) Rasterplot illustrating the change of waking bout duration for the two individuals with longest recording (J14 and J16). Vertical black bars represent % time spent awake for consecutive 5-minute intervals. Data are double plotted such that consecutive days are plotted next to and underneath each other for better visualization of circadian rhythms. Note the complete lack of wake consolidation prior to P18. (B) Mean circadian waveform of wakefulness for the two individuals in A over the days prior to (light grey lines) and following (black areas) the day significant 24-hour rhythmicity were observed (ie, P18). Data were folded at the circadian period of each animal to obtain average waking values for consecutive circadian hours. Data are double plotted. (C) Group means for all 11 rats of the distribution of wake-bout duration for consecutive developmental days calculated as the number of episodes per hour awake (upper graph) or as % of total time awake (lower graph) for bout durations ranging from 10 seconds to >10 minutes. Note that 10-second waking episodes dominate up until P18 while the number of >10-minute episodes (expanded on the back panel of the upper graph) gradually increase four-fold after P15. These longest waking bouts (>10 minutes) comprise >40 % of all time-spent-awake after P19 (lower graph).
Figure 5.
Developmental changes in the distribution of inter-NREMS episodes. (A) Each dot represents an interval without NREMS at the age at which it occurred illustrated for two individuals (J14 and J16). Note that in the first 4 days of the recording (aged P12–P16), inter-episode duration are more-or-less evenly distributed while in subsequent days (P17–P21) a marked redistribution occurs with inter-episode durations that are either shorter or longer than 30 minutes (dashed yellow line within each panel). This repartition coincides with the appearance of a circadian component to sleep–wake distribution (see Table 1). (B) This developmental partition into long and short inter-NREMS episode durations is summarized for all 11 rats in a relative frequency histogram. NREMS = non-rapid-eye movement sleep.
Development of Sleep Homeostasis
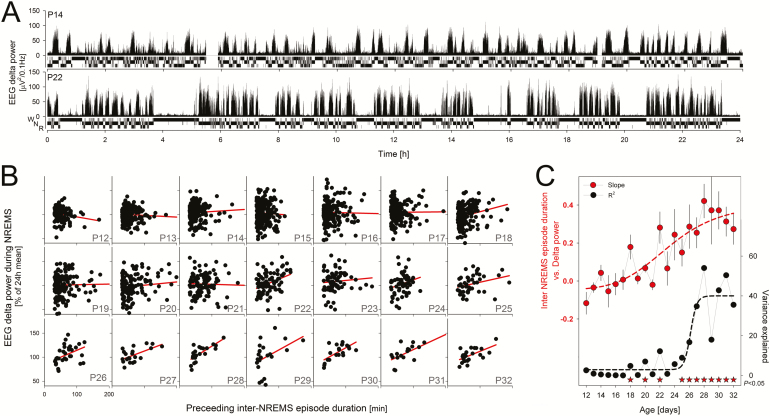
We also examined the relationship between inter-NREM sleep interval length and subsequent levels of NREM sleep delta power (Figures 5 and 6). This is because sleep propensity (as measured by NREM sleep delta power) in adult mammals is thought to discharge only during NREM sleep (and to accumulate during its absence).17 Prior to P24, no clear relationship was present between inter-NREM sleep intervals and subsequent NREM sleep delta power. However, by P24, a positive linear relationship could be discerned (Figure 6B). In addition, by P25 the portion of the variance in NREM sleep delta power explained by the length of the preceding inter-NREM sleep length sharply increased (Figure 6C). This indicated that a mature sleep homeostatic response, under constant conditions, emerged between P24 and P25—which agrees well with results in a 12:12 light–dark schedule.2,10
Figure 6.
Relationship between the duration of inter-NREMS episode duration and EEG delta power (0.5–4.0 Hz) in subsequent NREMS. (A). Illustration (rat J14) of two 24-hour recordings of “raw” sleep–wake data (10-second scores as a hypnogram with waking [w, top], NREM sleep [N, middle], and REM sleep [R, bottom bar]) and matching EEG delta power values during P14 and P22; that is, 4 days before and after P18 when waking bouts (and inter-NREM sleep episodes) consolidated. Note that whereas sleep is organized in discrete bouts separated by waking episodes at P22, no such structuring was observed at P14. Also note that at P22, longer waking bouts tend to be followed by NREM sleep with higher EEG delta power. (B) Linear regression analysis between inter-NREMS bout durations and subsequent EEG delta power was performed for each day (P12–P32). Values for all 11 rats were pooled. Note that number of data points decreases as less rats contribute (see Figure 1). Resulting regression lines in red. Tick marks in lower left panel apply to all other panels. (C) Summary of developmental changes in the correlation between time-spent-without-NREMS and subsequent EEG delta power. The slope of the regression line (95% CI; red symbols) gradually increased and remained significantly positive (CI do not overlap 0) from P24 onwards, linear correlations reach and remain significant from P25 onwards (asterisks; p < .05), and the variance explained (black symbols, R2) by this relationship greatly increased from P26 onwards. Dashed lines represent non-linear regression analyses (function “Logistic” with four parameters, Sigmaplot) fitted in the same way to both variables. CI = confidence intervals; EEG = electroencephalograph; NREMS = non-rapid-eye movement sleep; REM = rapid-eye movement..
DISCUSSION
There are several critical elements in experimentally identifying a “bonafide” endogenous circadian rhythm. First, the behavior or biological process should be observed over several close to 24-hour cycles under constant conditions. This ensures that what is observed is not an induced pattern that disappears once exogenous time cues are eliminated. Second, the sampling period should be sufficiently fine-grained so as to capture an accurate rendering of the circadian rhythm. Thus, the ideal design includes continuous sampling in the same organism across many days. These criteria are easily met in studies in adult animals. These criteria have, however, not been met in past studies of the development of circadian sleep/wake cycles.
We used an artificial rearing technique to continuously measure EEG/EMG defined sleep and wake states in neonatal rats for many days, over ages that spanned the preweaning and weaned periods. Neonatal rats were born in constant conditions, surgeries were performed in constant conditions, and artificial rearing was conducted under the same constant conditions. All experimental manipulations were performed at 16-hour intervals (or multiples of 16 hours); thus, these were unlikely to serve as entraining cues (ie, zeitgebers) as the mammalian circadian clock cannot entrain to periods of 16 (or multiples thereof) hours. Moreover, we did not observe consistent 16-hour induced rhythmicity either, indicating that the interventions were not strong enough to introduce noticeable disturbances in ongoing sleep–wake behavior.
We found that overt changes in sleep–wake organization emerge between the second and third postnatal week. These findings are in good agreement with an earlier investigation in blinded rats which reported 24-hour activity rhythms by the third postnatal week.23 Our results also agree with data from animals reared under 12:12 light–dark schedules, where diurnal/nocturnal differences in sleep–wake amounts, durations or EEG activity appeared between P16 and P28, depending on the strain of rat.10,12,24 However in these latter investigations the presence of a light–dark cycle may have masked or altered the occurrence of endogenous rhythmicity. Here we show that rhythms spontaneously appear and persist in constant conditions indicating that the coupling of the SCN to EEG/EMG measured vigilance states occurs in the second and third postnatal week.
Our results, however, differ in some respects from recent studies reporting day/night differences in sleep/wake organization in isolated P2 Sprague-Dawley rats. As reported by Gall et al.,25 nuchal motor recordings showed differences in sleep cycle number depending on whether the recordings were made in the day or night. However, no day/night differences in wake amounts or bout durations were observed at P2. In addition to differences in rat strains (Sprague-Dawley vs. Long–Evans), there are a number of methodological differences between that study and ours. In contrast to our study, a cross-sectional approach was used, recordings were not made in constant conditions and only two, 2-hour recordings in a 24-hour period were made per animal. Therefore it is not clear if the reported differences in day versus night before the second postnatal week reflect an endogenous 24-hour rhythm or a response to light. Interestingly, however, at older ages when EEG defined states are present, these investigators reported an increase in waking amounts and bout durations in the dark phase by P15 and P21, respectively, which corresponds well with the present results.25 Similar results were reported in Norway rats using a similar experimental design (cross-sectional measures in the presence of a light–dark cycle); no significant difference in wake amounts or bout duration was reported until P15.26
Dual Circadian Rhythms?
We noted the emergence of a marked bi-modal sleep–wake pattern evidenced by the appearance of a pronounced ~12-hour component in the waking periodogram over a 3-day period immediately following the emergence of the ~24-hour components. The period of the 12-hour rhythm did not significantly differ from half the 24-hour circadian component (Table 2), suggesting the two are linked. As is the case in our sleep–wake data, the distribution of overt behaviors over the circadian day are often bi-modal. A typical example of such bi-modality are the two surges in activity related to dawn and dusk that can be observed in many species.27 It has been suggested that these two components are driven by two separate, but phase locked 24-hour oscillators; the evening and morning oscillator (reviewed in27). This coupling gives rise to a 12-hour component in the periodogram, the magnitude of which depends on the phase difference between the two 24-hour components (Supplementary Figure 2A). With the assumption of two 24-hour rhythms each independently affecting sleep and waking but phase coupled at ca. 132°, the observed bi-modality in the time course of wakefulness and the resulting two-peak periodogram could be accurately reproduced (Supplementary Figure 2B). Consistent with the dual-oscillator concept these results might thus suggest the presence of two ~24-hour components, which seem to develop even in the absence of transitions between lighting conditions (ie, dawn and dusk) to which they are phase locked under entrained conditions.
Development of Sleep Homeostasis and Circadian Regulation: Which Emerges First?
The present results support the idea that circadian and mature homeostatic sleep regulatory mechanisms develop at slightly different rates.10 Studies in isolated P12–P20 Long–Evans rats conducted in light–dark cycles, show that sleep deprivation produces compensatory changes in sleep time and continuity; however, adult like changes in NREM sleep delta power are not observed until the end of the fourth postnatal week.2 However, 24-hour organization in sleep and wake amounts and wake bout durations are detectable by P15–P17 and largely mature by P20; several days before the appearance of mature sleep homeostasis, as measured by changes in NREM sleep delta power.10 Slightly different results have been reported in Spraque-Dawley rats, where increases in NREM sleep delta power were observed at P22, but adult-like diurnal/nocturnal organization was not fully present until P29–P30.28 Although strain differences may account for these different results, an additional factor may be masking effects of the light–dark cycle. Here we find under constant conditions that endogenous 24-hour rhythms in sleep and wake amounts are present by P17 in Long–Evans rats, and possibly as early as P14 and P15, depending on when the onset of rhythmicity is assigned (see Materials and Methods section). However, positive linear relationships between inter-NREM sleep intervals and NREM delta power are not observed until P24. Therefore, within the Long–Evans rat strain, it would appear that circadian regulation precedes the appearance of mature forms of sleep homeostasis. While speculative, this raises the interesting possibility that the emergence of longer, more consolidated waking bouts then triggers a mature homeostatic sleep response.
SUPPLEMENTARY MATERIAL
Supplementary data are available at SLEEPJ online.
FUNDING
This research was supported by NICHD 1P50 HD29732-01.
DISCLOSURE STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Jason Ma for his technical assistance in conducting the experiments. Work on this manuscript was performed at Department of Biology, Stanford University, Stanford, CA.
REFERENCES
- 1. Davis FC, Frank MG, Heller HC. Ontogeny of sleep and circadian rhythms. In: Zee PC, Turek FW, eds. Regulation of Sleep and Circadian Rhythms. New York, NY: Marcel Dekker, Inc; 1999: 19–80. [Google Scholar]
- 2. Frank MG, Morrissette R, Heller HC. Effects of sleep deprivation in neonatal rats. Am J Physiol. 1998; 275(1, pt 2): R148–R157. [DOI] [PubMed] [Google Scholar]
- 3. Alföldi P, Tobler I, Borbély AA. Sleep regulation in rats during early development. Am J Physiol. 1990; 258(3, pt 2): R634–R644. [DOI] [PubMed] [Google Scholar]
- 4. Todd WD, Gibson JL, Shaw CS, Blumberg MS. Brainstem and hypothalamic regulation of sleep pressure and rebound in newborn rats. Behav Neurosci. 2010; 124(1): 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nelson AB, Faraguna U, Zoltan JT, Tononi G, Cirelli C. Sleep patterns and homeostatic mechanisms in adolescent mice. Brain Sci. 2013; 3(1): 318–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feng P, Ma Y, Vogel GW. Ontogeny of REM rebound in postnatal rats. Sleep. 2001; 24(6): 645–653. [DOI] [PubMed] [Google Scholar]
- 7. Mirmiran M, Maas YG, Ariagno RL. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med Rev. 2003; 7(4): 321–334. [DOI] [PubMed] [Google Scholar]
- 8. Jenni OG, Deboer T, Achermann P. Development of the 24-h rest- activity pattern in human infants. Infant Behav Dev. 2006; 29(2): 143–152. [DOI] [PubMed] [Google Scholar]
- 9. Hofer MA, Shair H. Control of sleep-wake states in the infant rat by features of the mother-infant relationship. Dev Psychobiol. 1982; 15(3): 229–243. [DOI] [PubMed] [Google Scholar]
- 10. Frank MG, Heller HC. Development of diurnal organization of EEG slow-wave activity and slow-wave sleep in the rat. Am J Physiol. 1997; 273(2, pt 2): R472–R478. [DOI] [PubMed] [Google Scholar]
- 11. Frank MG, Heller HC. Development of REM and slow wave sleep in the rat. Am J Physiol. 1997; 272(6, pt 2): R1792–R1799. [DOI] [PubMed] [Google Scholar]
- 12. Frank MG, Srere H, Ledezma C, O’Hara B, Heller HC. Prenatal nicotine alters vigilance states and AchR gene expression in the neonatal rat: implications for SIDS. Am J Physiol Regul Integr Comp Physiol. 2001; 280(4): R1134–R1140. [DOI] [PubMed] [Google Scholar]
- 13. Götz AA, Wolf M, Stefanski V. Psychosocial maternal stress during pregnancy: effects on reproduction for F0 and F1 generation laboratory rats. Physiol Behav. 2008; 93(4–5): 1055–1060. [DOI] [PubMed] [Google Scholar]
- 14. Dörrscheidt GJ, Beck L. Advanced methods for evaluating characteristic parameters of circadian rhythms. J Math Biol. 1975; 2(2): 107–121. [Google Scholar]
- 15. Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol. 1978; 72(1): 131–160. [DOI] [PubMed] [Google Scholar]
- 16. Ruf T. The Lomb-Scargle periodogram in biological research: analysis of incomplete and unequally spaced time-series. Biol Rhythm Res. 1999;30(2): 178–201. [DOI] [PubMed] [Google Scholar]
- 17. Daan S, Beersma DG, Borbély AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984; 246(2, pt 2): R161–R183. [DOI] [PubMed] [Google Scholar]
- 18. Franken P, Tobler I, Borbély AA. Sleep homeostasis in the rat: simulation of the time course of EEG slow-wave activity. Neurosci Lett. 1991; 130(2): 141–144. [DOI] [PubMed] [Google Scholar]
- 19. Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001; 21(8): 2610–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol. 1970; 2(4): 216–239. [DOI] [PubMed] [Google Scholar]
- 21. Stephan FK. Circadian rhythms in the rat: constant darkness, entrainment to T cycles and to skeleton photoperiods. Physiol Behav. 1983; 30(3): 451–462. [DOI] [PubMed] [Google Scholar]
- 22. Wurts SW, Edgar DM. Circadian and homeostatic control of rapid eye movement (REM) sleep: promotion of REM tendency by the suprachiasmatic nucleus. J Neurosci. 2000; 20(11): 4300–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Honma S, Homna K. Interaction between circadian and ultradian rhythms of spontaneous locomotor activity in rats during the early development. In: Schulz H, Lavie P, eds. Ultradian Rhythms in Physiology and Behavior. Berlin, Germany: Springer Verlag: 1985: 95–109. [Google Scholar]
- 24. Ibuka N. Ontogenesis of circadian sleep-wakefulness rhythms and developmental changes of sleep in the altricial rat and in the precocial guinea pig. Behav Brain Res. 1984; 11(3): 185–196. [DOI] [PubMed] [Google Scholar]
- 25. Gall AJ, Todd WD, Ray B, Coleman CM, Blumberg MS. The development of day-night differences in sleep and wakefulness in norway rats and the effect of bilateral enucleation. J Biol Rhythms. 2008; 23(3): 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Todd WD, Gall AJ, Weiner JA, Blumberg MS. Distinct retinohypothalamic innervation patterns predict the developmental emergence of species-typical circadian phase preference in nocturnal Norway rats and diurnal nile grass rats. J Comp Neurol. 2012; 520(14): 3277–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aschoff C. Circadian activity pattern with two peaks. Ecology. 1966;47(4):657–662. [Google Scholar]
- 28. Gvilia I, Suntsova N, Angara B, McGinty D, Szymusiak R. Maturation of sleep homeostasis in developing rats: a role for preoptic area neurons. Am J Physiol Regul Integr Comp Physiol. 2011; 300(4): R885–R894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.