Abstract
Many of the deadliest bacterial diseases that plague humanity in the modern age are caused by bacterial biofilms that produce chronic infections. However, most of our knowledge of the host immune response comes from the study of planktonic pathogens. While there are similarities in the host response to planktonic and biofilm bacteria, specific immune responses toward biofilms have not been well studied; the only apparent difference is the inability to clear the bacteria allowing the biofilm infection to become chronic. In some cases, the biofilms skew T-cell response toward a balance that allows a stalemate between the host and the pathogen, in which the infection can become persistent. In this minireview, we will summarize well-known examples of this phenomena as well as some emerging studies that may indicate that this situation is much more common than initially thought.
Keywords: biofilm, chronic infection, immune response, Th1/Th2
Biofilm-related infections can skew the immune response toward Th1 or Th2 to favor persistence.
INTRODUCTION
Devastating plagues have shaped the course of human history, resulting in massive morbidity and mortality. Many of these pandemic infections have been attributed to acute bacterial infections, perhaps the most notorious being the plague caused by Yersinia pestis. Other examples include cholera, typhoid fever, tuberculosis and anthrax; all caused by planktonic (free living; single) bacteria. Accordingly, most of the immune response studies have been focused toward this type of bacterial growth. This research has led to a great wealth of information describing the immune response to bacterial pathogens, the data on which goes back to the beginning of the 20th century. Many of the basic mechanisms by which a host responds to different invading microorganism have been described in great detail.
Constant exposure to bacteria has been an unavoidable reality for humans and our ancestral species. While often beneficial, these interactions also pose the risk of encountering pathogens that must be addressed by the host without disrupting commensal homeostasis. The evolutionary pressure associated with this development helped to create two branches of the immune system: innate immunity (II) and acquired immunity (AI). Germline-encoded immunity emerged in sponges 700 million years ago (Yatim and Lakkis 2015). These phylogenetically ancient processes include antimicrobial peptides (AMPs), complement systems, phagocytes and pattern recognition receptors (PRRs). Though lacking fine molecular specificity and immunogenic memory, the genetic requirements for II are passed generationally (Wilmanski, Petnicki-Ocwieja and Kobayashi 2008; Yatim and Lakkis 2015). AI was found first in jawed fish 450 million years ago and is restricted to vertebrates (Akira and Takeda 2004; Yatim and Lakkis 2015). AI permits identification and memory of a range of molecular signatures far more expansive than germline genetics permits, and includes lymphocytes, the major histocompatibility complex (MHC), immunoglobulin (Ig) molecules and recombinase activating genes (Yatim and Lakkis 2015).
The short timeline between evolution of II and AI highlights the importance of synergy between the systems. While AI has a wide range of capabilities, the slow response cannot defend against the initial stages of infection (Akira and Takeda 2004). Classical understanding of II describes that it responds to infection immediately but simply contains infection until AI responds (Medzhitov and Janeway 2000). However, it is now understood that II has a critical role in activation and regulation of subsequent AI. Here, we will discuss the cross-talk that occurs between these two systems—specifically with regard to chronic infections.
CANNONICAL IMMUNE RESPONSE
The first defenses encountered by pathogens are physical barriers at epithelial tissues. These barriers produce conditions that damage bacteria or physically exclude their interaction. AMPs, which are rich in positively charged domains, selectively target negatively charged phospholipid head groups at the outermost leaflet of bacterial membranes (Zasloff 2002). The exact mechanism for AMP function is unclear though several are proposed. However, AMPs are clearly stimulated by LPS, IL-1β and TNF, all of which are upregulated by inflammation from bacterial challenge and are important for downstream immune modulation (Zasloff 2002). Additionally, the concentration of electrolytes on the luminal side is regulated by ion pumps; this activity maintains optimal conditions for AMP function (Zasloff 2002).
Complement killing is another innate response that elicits inflammation and interacts with AI. Complement may proceed through one of three pathways (classical, lectin or alternative pathway). The classical pathway is activated by the C1 antigen–antibody complex and thus, while innate, is related to AI. In contrast, the lectin and alternative pathways are dependent on pathogen-associated molecular patterns (PAMPs, discussed below). The lectin pathway is activated by the PRR mannose-binding lectin binding to mannose residues on bacterial carbohydrates and the alternative pathway is dependent on C3b opsonization at microbial surfaces. The three pathways coalesce at C3 cleavage and activation of the lytic pathway. The lytic pathway produces C5a, a chemoattractant for macrophages and neutrophils (Killick et al.2017). Many intermediate proteins in the complement cascade are signals for activation and differentiation of lymphocytes important for AI (reviewed by Killick et al.). Complement opsonization improves antigen uptake by dendritic cells (DCs), generates local production of C3a/C5a at the APC-T cell interface and regulates the activation of the lymphocytes. Additionally, C3d-opsonized antigens activate B cells via co-engagement of BCR and CD21 at concentrations 25%–50% of concentrations required for activation by antigen alone (Killick et al.2017). Therefore, C3d is an adjuvant for B cells.
PRRs are important for II and for immunologic memory. Although PRRs bind specific PAMPs, genetic limitations restrict the number of ligands recognized. PAMPs are structures produced only by microbial life forms, are essential to pathogenicity and are shared across classes of pathogens (Medzhitov and Janeway 2000). Common PAMPs include LPS, peptidoglycan, lipoteichoic acids, mannans, bacterial DNA, dsRNA and glucans. Upon ligand binding, PRRs activate pathways for the production of inflammatory cytokines. Cytokine release, if further amplified through complement opsonization, recruits lymphocytes and amplifies the response (Medzhitov and Janeway 2000; Keestra-Gounder, Tsolis and Bäumler 2015).
It is clear that II functions are also integral to AI. Activation of T cells is dependent on DCs, which package antigenic peptides onto MHC class I or MHC class II while also upregulating costimulatory molecules, that together lead to full activation of APC function (Yatim and Lakkis 2015). The process is controlled by the presence or absence of costimulatory signals. While APCs may express self-antigens on MHC structures, they do not produce proper costimulation signals. The costimulatory signals CD80 and CD86 are only expressed by APCs when presenting non-self-antigens. Expression of CD80 and CD86 is dependent on TLR signals produced upon PRR/PAMP binding (Medzhitov and Janeway 2000). T cells recognizing the MHC-bound antigen in the absence of these molecules are targeted for senescence or apoptosis (Medzhitov and Janeway 2000; Killick et al.2017). Therefore, the longevity and function of activated T cells is dependent on signaling events that result only from activated II.
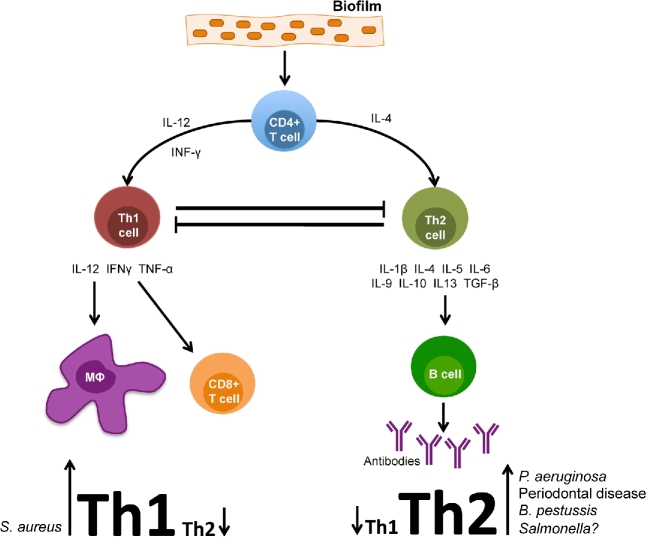
Upon activation, CD4+ helper T cells (Th) demonstrate a regulatory role in AI and activate cytotoxic T cells, B cells and macrophages (Medzhitov and Janeway 2000) (Fig. 1). Th exist in two polarized forms; type 1 Th produce interferon-gamma (IFN-γ), IL-2 and TNF-α. These signals produce phagocyte-dependent inflammation and affect opsonization and complement-fixing antibodies from B cells, macrophage activation, cell cytotoxicity and induce cell-mediated immunity (Romagnani 2000). Alternatively, type 2 Th produce IL-4, IL-5, IL-6, IL-9, IL-10 and IL-13 and regulate immunoglobulin production. Additionally, type 2 Th activate eosinophil differentiation and are inhibitory toward phagocytes (Romagnani 2000). These effects ultimately dampen a proinflammatory environment. About a decade ago a new subset of Th cells was discovered. Th17 cells produce IL-17A, IL-17F, IL-21 and IL-22. Th17 cells play a role in the defense against extracellular pathogens by mediating the recruitment of neutrophils and macrophages to infected tissues and are involved in B cell recruitment through CXCL13 chemokine signaling (Korn et al.2009).
Figure 1.
Schematic representation of the immune response to a bacterial biofilm. Different organisms appear to skew T-cell response toward a balance more favorable for chronic infection (Th1 or Th2). The case for Salmonella is still speculative and thus depicted with a question mark (?).
The regulation of II and AI is a hallmark of the immune response to bacteria. Measured and appropriate responses are integral to health as protection from pathogens is maintained without disrupting beneficial commensals. Both systems work in conjunction to maintain this balance. Although activation is often dependent on two or more signals, redundancies permit detection and response to a variety of infections. This feature adds intrinsic safety by preventing inappropriate activation while also providing a fine-tuned response to the molecular signature encountered.
In the modern age, after the discovery of vaccines and antibiotics, infections caused by bacterial biofilms have gained great importance. In fact, the US National Institutes of Health has estimated that biofilms are responsible for more than 80% of microbial infections (Davies 2003) and cause a significant amount of nosocomial diseases (Wenzel 2007). The recent revelation of the importance of biofilms in human infections is probably due to their involvement in chronic infection, which was not elucidated until the 1980s/1990s. Biofilms are generally more resistant to environmental stress than planktonic cells, including resistance to physical stress, UV radiation, desiccation, antibiotics and the host immune system (Hall-Stoodley, Costerton and Stoodley 2004). The consensus in the field indicates that the AI responds to biofilm infections in the same manner as it responds to that same pathogen in planktonic form. As a specific immune response toward biofilms has not been found, the fundamental difference appears to be the inability to clear the biofilm (Jensen et al.2010; Hänsch 2012; Moser et al.2017). Biofilms evade the immune response by various mechanisms including (i) acting as physical barriers, helping bacteria to avoid detection and phagocytosis (ii) genetically by activating response regulators, genetic switches or suppressors that affect immune cell activity (Leid 2009). One of the key characteristics that allow biofilms to persist is that they can keep the bacteria ‘under the radar’ of the immune system. This is usually attributed to the action of the extracellular polymeric substance (EPS). Perhaps the best-studied EPS is Pseudomonas aeruginosa alginate, which has been shown to reduce chemotaxis, inhibit activation of complement, scavenge hypochlorite and inhibit phagocytosis by macrophages and neutrophils (Jensen et al.2010; Moser et al.2017). The opportunistic pathogens belonging to the Burkholderia cenocepacia complex (BCC) have an EPS with a different chemical composition from alginate but can also inhibit important components of the II response by inhibiting neutrophil chemotactic migration and scavenging of reactive oxygen species (Bylund et al.2006). Established biofilms have been shown to induce necrosis in neutrophil-like dHL60 cell lines, act as a barrier to migration, mask the bacteria from detection and reduce IL-8 production. Interestingly, biofilm production by BCC was induced by growth in the presence of dHL60 cells (Murphy and Caraher 2015). There are many excellent reviews available that cover in great detail the ways in which bacterial biofilms evade, dampen or actively counterattack the host immune response (Jensen et al.2010; Moser and Jensen 2010; Hänsch 2012; Roilides et al.2015; Watters et al.2016; Gunn, Bakaletz and Wozniak 2016; Mulcahy, Isabella and Lewis 2014) and are beyond the scope of this review.
The above-mentioned characteristics allow biofilm infections to become chronic; some examples include chronic bacterial prostatitis, chronic lung infections of cystic fibrosis, periodontitis, typhoid carriers and infections on medical devices like prosthetic heart valves and catheters (Donlan and Costerton 2002). It has been demonstrated that the host and bacterial biofilm reach a stalemate in which the host physically contains the pathogen without complete elimination. The pathogen persists in a state of decreased activity and invasiveness, but survives within the host for a long period of time that has been hypothesized to be in the best interest of the bacteria (Parsek and Singh 2003).
We will discuss how the type of microbial infection that has occurred alters the immune system. As mentioned above, there is a delicate equilibrium between II and IA, each one with its individual responsibilities, strengths and weaknesses, but when working together can efficiently clear invading pathogens and rapidly respond to returning microbial attacks. During chronic infections, the situation differs, as there appears to be a miscoordination between the two arms of the immune system, neither being able to clear the infection and, in some instances, causing tissue damage that further aggravates the infection. There are situations in which biofilms appear to activate the immune response to their benefit. Depending on the pathogen, benefit can vary from either activation of a strong proinflammatory response or premature activation of an anti-inflammatory response.
SKEWING THE BALANCE
Most bacterial infections induce inflammation by activating the II response. The cells of the II system play a key role in the ensuing activation of the AI response, which usually has a delay before taking full effect (Watters et al.2016). Ideally, there is maintenance of the delicate balance between pro- and anti-inflammatory responses. As discussed earlier, T lymphocytes can differentiate into Th1 cells, which tend to be proinflammatory. These cells activate macrophages and the cellular immune response, generating responses active against intracellular pathogens. T lymphocytes can also differentiate into Th2 cells that stimulate a more anti-inflammatory environment that results in the induction of the humoral immune response, mast cells and defense against extracellular bacteria, metazoan parasites such as helminthes and toxins (Romagnani 2000; Moser and Jensen 2010). In many chronic biofilm infections, this system seems to be skewed in a direction that does not lead to clearance (Fig. 1).
Biofilms are bacterial populations that adhere to each other and/or to surfaces and interfaces and are enclosed by a matrix (Costerton et al.1995). The matrix or EPS is composed of various polymers like polysaccharides, proteins and nucleic acid that are secreted by cells. Aggregation as a result of this matrix gives bacteria protection from various forms of environmental stress such as dehydration, UV exposure, salinity, metal toxicity, antimicrobials and phagocytosis (Hall-Stoodley, Costerton and Stoodley 2004).
Pseudomonas aeruginosa and cystic fibrosis
For many biofilm-related phenomena, the best-studied examples involve P. aeruginosa. This opportunistic pathogen forms biofilms in the cyctic fibrosis (CF) lung and skews the balance between Th1 and Th2 responses in this environment (Moser and Jensen 2010; Moser et al.2017; Mauch et al.2017). Typically, CF patients suffering from chronic lung infection display an abundance of polymorphonuclar neutrophils (PMN) and a very robust antibody response, but a feeble cellular response (Moser et al.2017; Mauch et al.2017). In a study of chronically vs. non-chronically infected CF patients (n = 14), the levels of the Th2 marker interleukin 4 (IL-4) were significantly higher in persistently infected patients, while the levels of the IFNγ in non-chronic patients were elevated, indicating that chronic patients have a predominantly Th2 type immune response (Moser et al.2000). Similar findings were observed in animal models: histological analysis of rats vaccinated with alginate in depolymerized form (D-ALG) covalently coupled to P. aeruginosa toxin A (D-Alg toxin A) survived challenge and showed a significant reduction in lung inflammation compared to control animals. Histopathologic analysis showed that the immune infiltrate shifted within 1 week from predominantly innate immune cells (PMNs) to adaptive immune cells (mononuclear cells), as opposed to control animals that showed a response dominated by numerous PMNs, typical of CF patients (Johansen et al.1995). Interestingly, this same group found that Th1 responder C3H/HeN mice (biased toward a Th1-type response) cleared P. aeruginosa infection more efficiently and had higher amounts of IFNγ than Th2 responder BALB/c mice (biased toward a Th2-type response), which had higher amounts of IL-4 (Moser et al.1997). Another group showed that freshly isolated blood PMN and CD4+ T cells from CF patients showed higher anti-inflammatory IL-10 gene transcription than controls (Moss, Hsu and Olds 2000). Furthermore, high levels of B-cells and IgA have been found in P. aeruginosa-infected CF patients (Yonker et al.2015; Mauch et al.2017), again indicating a Th2 response.
Human DCs can also produce different cytokines depending on their surface markers and on environment factors, all of which can induce different Th responses. DCs are divided into myeloid (mDCs) or lymphoid (pDCs) types. mDCs (DCi cells) induce Th1 responses, produce IL-12 and are dependent on granulocyte-macrophage-colony-stimulating factor (GM-CSF). pDCs depend on IL-3, are induced by granulocyte-colony-stimulating factor (G-CSF), induce a Th2 response and are designated DC2 cells. Based on the hypothesis that G-CSF in CF patients should recruit PMNs and induce a DC2 and Th2 response, Moser et al. (2005) found a positive correlation between the GM-CSF/G-CSF ratio, IFNγ and lung responses in CF patients with chronic infection, and an inverse correlation between IL-3 and IFNγ. Furthermore, in an in vivo experiment, these researchers found that treating infected mice with GM-CSF from day 3 to day 6 changed the response from Th2- to Th1-dominated.
Staphylococcus aureus
An opposite phenomenon regarding immune skewing was observed in chronic infections caused by the Gram-positive bacterium, Staphylococcus aureus. C57BL/6J mice were shown to be prone to chronic biofilm infections demonstrating an upregulation of the Th1-linked cytokines TNFα, IL-1β, IL-2, IL-12p70 and the Th17-linked cytokines IL-6 and IL-17. Furthermore, the amount of Th1-type antibodies IgG2a and IgG2b was increased during early infection while the levels of Th2-type Ab IgG1 and suppressive Tregs were not upregulated until late in infection. The authors concluded that an early Th1 and Th17 proinflammatory response, coupled with a downregulated Th2 response, occurred during the early phases of biofilm infection and may cause tissue damage that allowed S. aureus to attach and grow as a biofilm (Prabhakara et al.2011a). In a follow-up experiment, this same group compared the course of infection in Th1-biased C57BL/6J vs. Th2-biased BALB/c mice and found that all C57BL/6J developed chronic infection while about 75% of BALB/c mice spontaneously cleared the bacteria. Additionally, BALB/c mice had higher levels of IL-4 and IL-10 and Treg cell populations than the C57BL/6J, indicating that the anti-inflammatory Th2 response plays a protective role in S. aureus clearance (Prabhakara et al.2011b). Nippe et al. (2011) found similar results using a subcutaneous footpad infection model, C57BL/6 mice were more susceptible than BALB/c and DBA/2. (Th2 biased). C57BL/6 mice showed a lower influx of PMNs, were associated with a Th1 response and displayed a higher secretion of the chemokine CXCL-2. BALB/c and DBA/2 were associated with Th2 response. Interestingly, the lower influx of PMN in susceptible mice was not correlated to a difference in the chemoattractant CXCL-1. Staphylococcus aureus has also been shown to skew the macrophage response from an M1 to an M2 phenotype that is not optimal for bacterial clearance and could favor persistence (Thurlow et al.2011; Hanke, Angle and Kielian 2012).
Periodontal disease
Periodontal disease is another well-known example of a chronic infection that results in skewed T-cell-mediated responses. Although there is conflicting evidence, there seems to be an innate susceptibility in some individuals or the presence of an environmental factor, which switches the response from a T-cell-focused lesion to a B-cell-focused one in periodontal lesions. This subject has been extensively reviewed elsewhere (Berglundh and Donati 2005; Kinane and Mark Bartold 2007; Ohlrich, Cullinan and Seymour 2009). In summary, gingivitis lesions are primarily the result of a Th1 response, while chronic periodontitis is caused by a Th2 response. During gingivitis, there is a strong innate immune response where macrophages and PMNs produce high levels of IL-12 that in turn leads to Th1 cell secretion of IL2 and IFNγ along with suppression of B cells and plasma cells. On the other hand, it has been observed that 65%–70% of periodontitis lesions are composed of plasma or B cells (Berglundh and Donati 2005), leading to the belief that a modest innate immune response primes polyclonal B-cell activation and a Th2 response, followed by progressive periodontal lesions and finally to chronic periodontitis (Ohlrich, Cullinan and Seymour 2009). Th-biased animal models have been used and although conflicting evidence exists, CBA and C57BL/6J (Th1-biased) are more resistant (Baker, Dixon and Roopenian 2000) and have a stronger Th1 response and higher levels of IgG2a (Th1) and lower levels of IL4 (Th2) than BALB/c and DBA/2 (Th2-biased) during periodontal disease.
Emerging stories of chronic infection immune skewing
Bordetella pertussis is the causal agent of whooping cough. It is traditionally known as an acute disease and, although vaccination against the disease was very successful, it has recently resurged in vaccinated adolescents and adults that became silent carriers (Cattelan et al.2015). It has been proposed that this persistent colonization is due to the adoption of a biofilm lifestyle. Safety concerns in the past have led to the change from whole cell vaccines (wPV) to acellular (aPV) vaccines. It has been shown that wPV induce a strong Th1 response that bestows better protection against the pathogen (Barnard et al.1996; Ross et al.2013) than aPV, which induces a Th2 response. This difference has been hypothesized as a possible reason for the failure of the newer vaccine to protect vaccinated individuals, as the Th2 response allows biofilms to develop and persist (Cattelan et al.2015).
The commensal organism Enterococcus faecalis can cause opportunistic infections, especially when biofilms are associated to medical implants. Dislodged biofilms have been shown to invoke reduced levels of the proinflammatory cytokines TNF-α and IL6 in macrophages. Furthermore, biofilms increased the expression of CD80 and CD86, which induce Th1 and Th2 respectively, suggesting a possible skew in T-cell response (Slavik, Hutchcroft and Bierer 1999; Daw et al.2012).
Salmonellae encompass a group of enteric bacteria causing a wide range of diseases that can be divided into two broad groups: those that cause gastroenteritis (e.g. S. enterica serovar Typhimurium) and those that cause systemic infections (e.g. serovar Typhi). Most studies have focused on the planktonic phase of infections where, during initial intestinal invasion, the bacteria induce a potent inflammatory response characterized by a massive influx of neutrophils. More in line with this review is the Salmonella carriage displayed by chronically infected patients. These carriers are usually asymptomatic and are the only known reservoir of the human-restricted S. Typhi. The gallbladder (GB) is the primary site of carriage and there is a direct correlation between presence of gallstones (GSs) and carriage (Schiøler et al.1983). Salmonella biofilms exist on the surface of patient GSs and this condition can be replicated in a mouse GS model of typhoid carriage (Crawford et al.2010). These biofilms are believed to bestow upon Salmonella the recalcitrance to bactericidal bile in the GB environment and to treatment by antibiotics. The GB becomes inflamed during initial infection but we have observed a return to a more normal GB architecture at approximately 21 days post-infection, even though the bacteria are not cleared. Our group is interested in the dynamics that allow this stalemate between the host and the bacteria. Recent transcriptomic analysis using our GS mouse model indicates a shift from a Th1 response during early infection to a Th2 response at 21 DPI (unpublished results). It is yet to be determined whether the host promotes this stalemate in order to protect the GB from the damaging inflammatory response or if Salmonella induces this shift in order to establish a chronic infection. In support of these findings, the balance between Th1 and Th2 has been shown to play a role in Salmonella chronic infection. This pathogen can persist in macrophages in the mesenteric lymph nodes of some mouse models; these chronically infected mice can remain disease-free for a year, but treatment with anti-IFNγ reactivated acute systemic infection symptoms (Monack, Bouley and Falkow 2004). Finally, soluble Salmonella flagellin has been shown to induce a Th2 response (Cunningham et al.2004).
POSSIBLE TREATMENT ALTERNATIVES
Tipping the Th balance toward a more beneficial state has been proposed to improve the course of the chronic bacterial infections. For example, research has been performed to attempt to tip the P. aeruginosa lung infection to a Th1-dominated response. In one study, IFNγ treatment decreased the inflammatory response to chronic P. aeruginosa pneumonia in a rat model (Johansen et al.1996). Additionally, if susceptible BALB/c mice were re-inoculated with P. aeruginosa, the mice became resistant and had a more favorable Th1-type response, similar to that in resistant C3H/HeN mice (Moser et al.2002). Different mechanisms have been proposed as the cause of observed benefit of a Th1 response in CF including (i) an increased stimulation of alveolar macrophages that remove apoptotic and necrotic neutrophils thereby decreasing inflammation, (ii) reduced production of IL-8 that reduces chemoattraction of neutrophils and (iii) downregulation of Th2 responses that reduce B-cell stimulation, antibody response and formation of immune complexes, thereby decreasing tissue damage (Jensen et al.2010). Similar hypotheses have been tested in the case of S. aureus chronic biofilm infections. As mentioned above, M1 macrophages improve the outcome of S. aureus biofilm infections. Treatment with exogenous M1 macrophages inhibited S. aureus biofilms and improved proinflammatory cytokine production in a catheter model (Hanke et al.2013).
CONCLUSIONS
Do bacteria in a biofilm skew the immune response in order to induce a chronic infection? New technologies that allow the analysis of distinct immune cells populations like single-cell RNA sequencing (Papalexi and Satija 2017) and new flow cytometry methods (Perfetto, Chattopadhyay and Roederer 2004) could lead to a better understanding of how the host responds to biofilms and which factors determine the path that immune response might take. Additionally, methods like dual RNA-seq (Westermann et al.2016) could unveil host–pathogen interactions during infection. These might include the release of bacterial effectors by the pathogen or the activation of genetic switches by both organisms. Whether bacterial biofilms are taking advantage of vulnerabilities in our defenses or if they are actively inducing a skewed immune response remains to be elucidated. Given the well-established examples and the emerging studies it is clear that it is a widespread phenomenon in biofilm-related chronic illnesses. A better understanding of the mechanisms and details could lead to possible generalized treatment options or those tailored toward a particular pathogen.
Acknowledgements
We would like to thank Dr Purnima Dubey for proofreading the manuscript and for providing insightful suggestions.
FUNDING
The work presented here was funded by grants AI109002 and AI116917 from the National Institutes of Health to JSG.
Conflict of interest. None declared.
REFERENCES
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 2004;4:499–511. [DOI] [PubMed] [Google Scholar]
- Baker PJ, Dixon M, Roopenian DC. Genetic control of susceptibility to Porphyromonas gingivalis-induced alveolar bone loss in mice. Infect Immun 2000;68:5864–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard A, Mahon BP, Watkins J et al. Th1/Th2 cell dichotomy in acquired immunity to Bordetella pertussis: variables in the in vivo priming and in vitro cytokine detection techniques affect the classification of T-cell subsets as Th1, Th2 or Th0: variables in the in vivo priming and in vitro cytokine detection techniques affect the classification of T-cell subsets as Th1, Th2 or Th0. Immunology 1996;87:372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglundh T, Donati M. Aspects of adaptive host response in periodontitis. J Clin Periodontol 2005;32:87–107. [DOI] [PubMed] [Google Scholar]
- Bylund J, Burgess L-A, Cescutti P et al. Exopolysaccharides from Burkholderia cenocepacia inhibit neutrophil chemotaxis and scavenge reactive oxygen species. J Biol Chem 2006;281:2526–32. [DOI] [PubMed] [Google Scholar]
- Cattelan N, Dubey P, Arnal L et al. Bordetella biofilms: a lifestyle leading to persistent infections. Pathog Dis 2016;74:ftv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Lewandowski Z, Caldwell DE et al. Microbial biofilms. Annu Rev Microbiol 1995;49:711–45. [DOI] [PubMed] [Google Scholar]
- Crawford RW, Rosales-Reyes R, Ramírez-Aguilar Mde LL et al. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. P Natl Acad Sci USA 2010;107:4353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AF, Khan M, Ball J et al. Responses to the soluble flagellar protein FliC are Th2, while those to FliC on Salmonella are Th1. Eur J Immunol 2004;34:2986–95. [DOI] [PubMed] [Google Scholar]
- Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2003;2:114–22. [DOI] [PubMed] [Google Scholar]
- Daw K, Baghdayan AS, Awasthi S et al. Biofilm and planktonic Enterococcus faecalis elicit different responses from host phagocytes in vitro. FEMS Immunol Med Mic 2012;65:270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002;15:167–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn JS, Bakaletz LO, Wozniak DJ. What's on the outside matters: the role of the extracellular polymeric substance of gram-negative biofilms in evading host immunity and as a target for therapeutic intervention. J Biol Chem 2016;291:12538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2004;2:95–108. [DOI] [PubMed] [Google Scholar]
- Hanke ML, Angle A, Kielian T. MyD88-dependent signaling influences fibrosis and alternative macrophage activation during Staphylococcus aureus biofilm infection. PLoS One 2012;7:e42476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke ML, Heim CE, Angle A et al. Targeting macrophage activation for the prevention and treatment of Staphylococcus aureus biofilm infections. J Immunol 2013;190:2159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänsch GM. Host defence against bacterial biofilms: “Mission Impossible?” ISRN Immunol 2012;2012:1–17. [Google Scholar]
- Jensen PØ, Givskov M, Bjarnsholt T et al. The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol Med Mic 2010;59:292–305. [DOI] [PubMed] [Google Scholar]
- Johansen HK, Hougen HP, Cryz SJ Jr et al. Vaccination promotes TH1-like inflammation and survival in chronic Pseudomonas aeruginosa pneumonia in rats. Am J Resp Crit Care 1995;152:1337–46. [DOI] [PubMed] [Google Scholar]
- Johansen HK, Hougen HP, Rygaard J et al. Interferon-gamma (IFN-gamma) treatment decreases the inflammatory response in chronic Pseudomonas aeruginosa pneumonia in rats. Clin Exp Immunol 1996;103:212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keestra-Gounder AM, Tsolis RM, Bäumler AJ. Now you see me, now you don’t: the interaction of Salmonella with innate immune receptors. Nat Rev Microbiol 2015;13:206–16. [DOI] [PubMed] [Google Scholar]
- Killick J, Morisse G, Sieger D et al. Complement as a regulator of adaptive immunity. Semin Immunopathol 2017, DOI: 10.1007/s00281-017-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane DF, Mark Bartold P. Clinical relevance of the host responses of periodontitis. Periodontol 2000 2007;43:278–93. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M et al. IL-17 and Th17 cells. Annu Rev Immunol 2009;27:485–517. [DOI] [PubMed] [Google Scholar]
- Leid JG. Bacterial biofilms resist key host defenses. Microbe 2009;4(2):66–70. [Google Scholar]
- Mauch RM, Jensen PØ, Moser C et al. IgG avidity to Pseudomonas aeruginosa over the course of chronic lung biofilm infection in cystic fibrosis. J Cyst Fibros 2017, DOI: 10.1016/j.jcf.2017.08.012. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C. Innate immunity. N Engl J Med 2000;343:338–44. [DOI] [PubMed] [Google Scholar]
- Monack DM, Bouley DM, Falkow S. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNgamma neutralization. J Exp Med 2004;199:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser C, Jensen PØ. Adaptive Immune Responses and Biofilm Infections. New York, NY: Springer, 2010, 201–14. [Google Scholar]
- Moser C, Jensen PØ, Kobayashi O et al. Improved outcome of chronic Pseudomonas aeruginosa lung infection is associated with induction of a Th1-dominated cytokine response. Clin Exp Immunol 2002;127:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser C, Jensen PØ, Pressler T et al. Serum concentrations of GM-CSF and G-CSF correlate with the Th1/Th2 cytokine response in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. APMIS 2005;113:400–9. [DOI] [PubMed] [Google Scholar]
- Moser C, Johansen HK, Song Z et al. Chronic Pseudomonas aeruginosa lung infection is more severe in Th2 responding BALB/c mice compared to Th1 responding C3H/HeN mice. APMIS 1997;105:838–42. [PubMed] [Google Scholar]
- Moser C, Kjaergaard S, Pressler T et al. The immune response to chronic Pseudomonas aeruginosa lung infection in cystic fibrosis patients is predominantly of the Th2 typeNote. APMIS 2000;108:329–35. [DOI] [PubMed] [Google Scholar]
- Moser C, Pedersen HT, Lerche CJ et al. Biofilms and host response - helpful or harmful. APMIS 2017;125:320–38. [DOI] [PubMed] [Google Scholar]
- Moss RB, Hsu YP, Olds L. Cytokine dysregulation in activated cystic fibrosis (CF) peripheral lymphocytes. Clin Exp Immunol 2000;120:518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy LR, Isabella VM, Lewis K. Pseudomonas aeruginosa biofilms in disease. Microb Ecol 2014;68:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Caraher E. Residence in biofilms allows Burkholderia cepaciacomplex (Bcc) bacteria to evade the anti-microbial activities of neutrophil-like dHL60 cells. Pathog Dis 2015;73:ftv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nippe N, Varga G, Holzinger D et al. Subcutaneous infection with S. aureus in mice reveals association of resistance with influx of neutrophils and Th2 response. J Invest Dermatol 2011;131:125–32. [DOI] [PubMed] [Google Scholar]
- Ohlrich EJ, Cullinan MP, Seymour GJ. The immunopathogenesis of periodontal disease. Aust Dent J 2009;54(Suppl 1):S2–10. [DOI] [PubMed] [Google Scholar]
- Papalexi E, Satija R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat Rev Immunol 2017, DOI: 10.1038/nri.2017.76. [DOI] [PubMed] [Google Scholar]
- Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 2003;57:677–701. [DOI] [PubMed] [Google Scholar]
- Perfetto SP, Chattopadhyay PK, Roederer M. Innovation: seventeen-colour flow cytometry: unravelling the immune system. Nat Rev Immunol 2004;4:648–55. [DOI] [PubMed] [Google Scholar]
- Prabhakara R, Harro JM, Leid JG et al. Murine immune response to a chronic Staphylococcus aureus biofilm infection. Infect Immun 2011a;79:1789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakara R, Harro JM, Leid JG et al. Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infect Immun 2011b;79:5010–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roilides E, Simitsopoulou M, Katragkou A et al. How biofilms evade host defenses. Microbiol Spectr 2015;3, DOI: 10.1128/microbiolspec.MB-0012-2014. [DOI] [PubMed] [Google Scholar]
- Romagnani S. T-cell subsets (Th1 versus Th2). Ann Allergy Asthma Immunol 2000;85:9–21. [DOI] [PubMed] [Google Scholar]
- Ross PJ, Sutton CE, Higgins S et al. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog 2013;9:e1003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiøler H, Christiansen ED, Høybye G et al. Biliary calculi in chronic Salmonella carriers and healthy controls: a controlled study. Scand J Infect Dis 1983;15:17–9. [DOI] [PubMed] [Google Scholar]
- Slavik JM, Hutchcroft JE, Bierer BE. CD80 and CD86 are not equivalent in their ability to induce the tyrosine phosphorylation of CD28. J Biol Chem 1999;274:3116–24. [DOI] [PubMed] [Google Scholar]
- Thurlow LR, Hanke ML, Fritz T et al. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 2011;186:6585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters C, Fleming D, Bishop D et al. Host responses to biofilm. Prog Mol Biol Transl 2016;142:193–239. [DOI] [PubMed] [Google Scholar]
- Wenzel RP. Health care-associated infections: major issues in the early years of the 21st century. Clin Infect Dis 2007;45:S85–8. [DOI] [PubMed] [Google Scholar]
- Westermann AJ, Förstner KU, Amman F et al. Dual RNA-seq unveils noncoding RNA functions in host-pathogen interactions. Nature 2016;529:496–501. [DOI] [PubMed] [Google Scholar]
- Wilmanski JM, Petnicki-Ocwieja T, Kobayashi KS. NLR proteins: integral members of innate immunity and mediators of inflammatory diseases. J Leukocyte Biol 2008;83:13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatim KM, Lakkis FG. A brief journey through the immune system. Clin J Am Soc Nephro 2015;10:1274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonker LM, Cigana C, Hurley BP et al. Host-pathogen interplay in the respiratory environment of cystic fibrosis. J Cyst Fibros 2015;14:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature 2002;415:389–95. [DOI] [PubMed] [Google Scholar]