Abstract
Background
A high fructose intake has been shown to be associated with increased serum urate concentration, whereas ascorbate (vitamin C) may lower serum urate by competing with urate for renal reabsorption.
Objective
We assessed the combined association, as the fructose:vitamin C intake ratio, and the separate associations of dietary fructose and vitamin C intakes on prevalent hyperuricemia.
Methods
We conducted cross-sectional analyses of dietary intakes of fructose and vitamin C and serum urate concentrations among Jackson Heart Study participants, a cohort of African Americans in Jackson, Mississippi, aged 21–91 y. In the analytic sample (n = 4576), multivariable logistic regression was used to examine the separate associations of dietary intakes of fructose and vitamin C and the fructose:vitamin C intake ratio with prevalent hyperuricemia (serum urate ≥7 mg/dL), after adjusting for age, sex, smoking, waist circumference, systolic blood pressure, estimated glomerular filtration rate, diuretic medication use, vitamin C supplement use, total energy intake, alcohol consumption, and dietary intake of animal protein. Analyses for individual dietary factors (vitamin C, fructose) were adjusted for the other dietary factor.
Results
In the fully adjusted model, there were 17% greater odds of hyperuricemia associated with a doubling of the fructose:vitamin C intake ratio (OR: 1.17; 95% CI: 1.08, 1.28), 20% greater odds associated with a doubling of fructose intake (OR: 1.20; 95% CI: 1.08, 1.34), and 13% lower odds associated with a doubling of vitamin C intake (OR: 0.87; 95% CI: 0.78, 0.97). Dietary fructose and the fructose:vitamin C intake ratio were more strongly associated with hyperuricemia among men than women (P-interaction ≤ 0.04).
Conclusion
Dietary intakes of fructose and vitamin C are associated with prevalent hyperuricemia in a community-based population of African Americans.
Keywords: urate; fructose; vitamin C; dietary intake, hyperuricemia
Introduction
Elevated serum uric acid or urate has been found to be associated with an increased risk of developing multiple chronic diseases, including hypertension (1–5), cardiovascular disease (6–9), and chronic kidney disease (10–13), all of which are particularly prevalent among African Americans. Hyperuricemia is a direct cause of gout through urate crystallization in joint fluid (14, 15). Excessive serum urate appears to increase renin production and cause microvascular and glomerular lesions, which contributes to the development of glomerular hypertension and kidney injury (10). A randomized clinical trial also reported that reducing serum urate concentrations slowed chronic kidney disease progression (16). A recent study that used data from the NHANES found that the population average serum urate concentration increased by 0.15 mg/dL and the prevalence of hyperuricemia increased by 3.2% from 1988–1994 to 2007–2008 (17). Gout incidence was higher among American Americans than among whites and the magnitude of the prevalence increase over time was higher among African Americans (17, 18).
Dietary factors may influence serum urate concentrations (19). Fructose and vitamin C, which are common in the diet, have been found to have opposing actions on serum concentrations of urate. Both observational and experimental studies have shown that a higher dietary intake of fructose is associated with higher serum urate concentrations (20, 21). The metabolism of fructose produces xanthine and hypoxanthine, which are then used to synthesize uric acid, resulting in elevated serum urate (22). In contrast, vitamin C (ascorbic acid) competes with serum urate for renal reabsorption, thereby reducing serum urate concentrations, especially among people with hyperuricemia (23). Evidence has shown that a coregulation process exists between urate and ascorbate in antioxidant and oxidant activities (24).
The dietary intake of fructose has steadily increased in the United States (25). In a study incorporating data from NHANES and the National Food Consumption Survey, the estimated average dietary intake of fructose increased from 37 g/d to 49 g/d from 1977 to 2005 (26), approaching fructose intake amounts that are associated with adverse health outcomes (27–29). The predominant sources of fructose in the diet are nonalcoholic beverages, contributing 46% of total fructose intake (26). Vitamin C intakes have improved in the United States, especially among African Americans. The prevalence of vitamin C deficiency (defined as a serum concentration <11.4 μmol/L) in non-Hispanic blacks decreased from 21.9% in 1988–1994 to 6.7% in 2003–2004 (30). In 1999–2000, ∼12% of US adults were taking vitamin C supplements; however, the usage was significantly lower among African Americans (5.1%) than among whites (14.7%) (31). Given the increasing trend of both fructose and vitamin C intakes and their opposite direction of association with serum urate concentrations, we examined the association of dietary fructose and vitamin C, as a ratio and separately, with serum urate concentration and prevalent hyperuricemia in a community-based African-American population.
Methods
Study design
The Jackson Heart Study (JHS) is a prospective cohort of African-American men and women that was designed to investigate risk factors for cardiovascular disease and related cardiometabolic disorders. The details on the design of this study have been previously published (32). Briefly, between 2000 and 2004, 5306 participants were recruited and enrolled from 3 counties in the Jackson, Mississippi metropolitan statistical area (Hinds, Madison, and Rankin). The JHS consists of 22% participants randomly selected from the Jackson, Mississippi study site of the Atherosclerosis Risk in Communities Study (ARIC; 1989) Jackson site, 17% from a randomly selected community sample, 30% from a structured volunteer sample, and the remaining 31% enrolled as family members of JHS participants (33). The original cohort had an eligible age range of 35–84 y of age. Allowance for flexibility in age of family members resulted in a broader age range of enrolled JHS participants (21–91 y).
In the present study, we conducted cross-sectional analyses of baseline dietary and serum urate data. Home interviews and clinical medical examinations were conducted, and blood and urine specimens were collected during the baseline visit. The study protocol was approved by the institutional review boards at the University of Mississippi Medical Center and Johns Hopkins University, and the research was conducted in compliance with the Helsinki Declaration.
Study population
This analysis targeted the entire JHS cohort. We excluded those who had missing data for dietary intake of fructose, dietary intake of vitamin C, or serum urate (n = 252); those who were taking medication for gout (n = 61); and those who had missing information on covariates (n = 417). After applying these exclusion criteria, the analytic sample size was 4576 participants.
Assessment of dietary intake
Dietary intake was assessed with the use of an FFQ modified from the Delta Nutrition Intervention Research Initiative (NIRI) FFQ (34). This FFQ was administered by trained interviewers to JHS participants during the baseline study visit. Participants reported their usual frequency of consumption and portion sizes for 158 food items over the past 6 mo. The Delta NIRI FFQ was developed by the Lower Mississippi NIRI Food Frequency Working Group to assess intakes of regional foods (35). Dietary intakes of fructose and vitamin C were estimated on the basis of the frequency of consumption and portion size of each food item and its nutritional content.
The main exposures were fructose (grams per day), vitamin C (milligrams per day), and the fructose:vitamin C intake ratio (fructose:vitamin C intake ratio = fructose/vitamin C). Fructose, vitamin C, and the fructose:vitamin C intake ratio were log2-transformed to allow for the interpretation of each unit increase on the log2 scale as a doubling. Given that the log2-transformed ratio is equivalent to the difference in the individual log2-transformed dietary factors [log2(fructose:vitamin C intake ratio) = log2(fructose/vitamin C) = log2(fructose) − log2(vitamin C)], this quantity can be interpreted as the relative intake difference between fructose and vitamin C. These dietary exposures were then divided into quartiles for the analysis. We compared levels of dietary intake of fructose and vitamin C in our study population with established levels. Fructose intake was dichotomized as normal (≤50 g/d) or high (>50 g/d) (29). Vitamin C intake was dichotomized as meeting the Institute of Medicine recommendation (>90 mg/d for men and >75 mg/d for women) or not meeting the Institute of Medicine recommendation (≤90 mg/d for men and ≤75 mg/d for women) (36). Other dietary variables included in the analysis were total energy intake (kilocalories per day), alcohol intake (grams per day), and animal protein intake (grams per day).
Outcome ascertainment
The primary outcome of interest was serum urate concentration. In addition to analyzing serum urate concentration as a continuous variable, we defined prevalent hyperuricemia as serum urate ≥7 mg/dL, which has been used in previous studies (17, 37). Fasting blood samples were collected from study participants during the baseline study visit. Serum specimens were prepared and stored at −80°C within 2 h after collection. Serum urate concentrations were measured in the University of Mississippi Medical Center laboratory with the use of the uricase method (CV: 1–2%) (38).
Measurement of other covariates
During the baseline study visit, information was obtained on demographic characteristics (date of birth, sex) and health behaviors (smoking status) with the use of a structured questionnaire. We categorized smoking status as current smoker, former smoker, or never smoker.
During the study visit, blood pressure and waist circumference were measured. Sitting blood pressure was obtained with the use of a Hawksley random zero sphygmomanometer (Hawksley and Sons Ltd.) with the proper cuff size. The average of 2 blood pressure measurements was used in the analysis. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or taking antihypertensive medication. Waist circumference was measured to the nearest centimeter. Serum creatinine was measured with the use of the enzymatic method with the Vitros Ortho-Clinical Diagnostics Analyzer and standardized with the use of the isotope dilution MS traceable method (39). We calculated estimated glomerular filtration rate (eGFR) with the use of the 2009 Chronic Kidney Disease Epidemiology Collaboration equation based on serum creatinine (40). Participants were asked to bring their current medications (used within the past 2 wk) to the study visit, including prescription and over-the-counter medications, vitamin and mineral supplements, and herbal or home remedies.
Statistical analysis
Baseline characteristics of the study population and dietary factors were reported with the use of descriptive statistics (means, SDs, proportions) for the overall analytic population, according to quartiles of fructose:vitamin C intake ratio and by sex. We used linear regression models to examine the association between each of the 3 dietary exposures separately (fructose, vitamin C, and fructose:vitamin C intake ratio) and serum urate concentration. We used logistic regression models to calculate ORs for the association between the 3 dietary exposures and prevalent hyperuricemia. We tested for trend with the use of the median within each quartile to evaluate the ordered relation across quartiles of the 3 dietary exposures for both the continuous (serum urate concentration) and binary (hyperuricemia) outcomes. We graphically displayed the association between fructose:vitamin C intake ratio and serum urate concentration with the use of a restricted cubic spline with knots at the 25th, 50th , and 75th percentiles. In addition, we graphically displayed the association between eGFR and serum urate concentration with the use of a scatterplot and a locally weighted scatterplot smoothing curve.
We constructed multivariable regression models adjusted for different sets of covariates: model 1 was adjusted for age, sex, smoking status, waist circumference, systolic blood pressure, eGFR, and medication use (diuretic medication, vitamin C supplement); model 2 was adjusted for age, sex, smoking status, waist circumference, systolic blood pressure, eGFR, and dietary factors (total energy, alcohol, animal protein); and model 3 was adjusted for the covariates in models 1 and 2 (age, sex, smoking status, waist circumference, systolic blood pressure, eGFR, medication use, dietary factors). In model 4, vitamin C and fructose were mutually adjusted for each other (i.e., when fructose was the main exposure, we adjusted for vitamin C; when vitamin C was the main exposure, we adjusted for fructose).
To examine whether an extremely high intake of vitamin C would influence our results, we conducted 2 sensitivity analyses: 1) we excluded individuals taking vitamin C supplements and 2) we excluded individuals with high dietary intakes of vitamin C (>500 mg/d). To assess a priori–hypothesized sex differences in the association between the dietary exposures and serum urate concentration and prevalent hyperuricemia, we conducted stratified analyses by sex and tested for interaction with the use of a likelihood ratio test.
We performed statistical analyses with Stata statistical software, version 13.0 (StataCorp). Tests were 2-sided and significance was assessed at an α level of 0.05.
Results
Baseline characteristics
Among the 4576 JHS participants in the analytic sample, the mean age at baseline was 56 y and 35% were men (Table 1). Approximately one-third of participants were taking diuretic medications and 9% were taking vitamin C supplements. Those participants in the lowest quartile (quartile 1) of fructose:vitamin C intake ratio were older, had a lower baseline eGFR, were more likely to have hypertension and diabetes, and were more likely to report taking diuretic medications and vitamin C supplements at baseline than the higher quartiles (quartiles 2–4). We did not observe substantial variations in BMI, waist circumference, or systolic blood pressure across quartiles. At lower quartiles of fructose:vitamin C intake ratios, total energy intake as well as the consumption of alcohol, animal protein, and sugar-sweetened beverages were lower relative to higher quartiles of fructose:vitamin C intake ratio.
TABLE 1.
Baseline demographic and health characteristics and dietary factors by quartile of the dietary fructose:vitamin C intake ratio in African-American adults1
| Quartile of fructose:vitamin C intake ratio | |||||
|---|---|---|---|---|---|
| Overall study population | Quartile 1: 0.0–0.1 | Quartile 2: 0.1–0.2 | Quartile 3: 0.2–0.3 | Quartile 4: 0.3–4.6 | |
| (n = 4576) | (n = 1144) | (n = 1144) | (n = 1144) | (n = 1144) | |
| Age, y | 55.5 ± 12.6 | 58.3 ± 12.0 | 55.3 ± 12.7 | 54.3 ± 13.0 | 54.2 ± 12.5 |
| Male sex, % | 35.4 | 31.8 | 35.7 | 38.5 | 35.7 |
| BMI, kg/m2 | 31.8 ± 7.2 | 31.6 ± 7.1 | 31.8 ± 6.8 | 31.6 ± 7.3 | 32.1 ± 7.7 |
| Waist circumference, cm | 100.6 ± 16.1 | 100.6 ± 16.3 | 100.5 ± 15.3 | 100.2 ± 16.6 | 101.0 ± 16.0 |
| Systolic blood pressure, mm Hg | 126.9 ± 18.2 | 127.1 ± 17.6 | 126.0 ± 17.5 | 127.5 ± 18.9 | 127.1 ± 18.9 |
| eGFR, mL/(min × 1.73 m2) | 94.0 ± 21.7 | 91.8 ± 21.3 | 93.9 ± 21.6 | 94.9 ± 22.4 | 95.6 ± 21.2 |
| Smoking status, % | |||||
| Current smoker | 12.3 | 10.1 | 10.5 | 11.5 | 17.1 |
| Former smoker | 18.7 | 21.7 | 17.0 | 16.3 | 19.8 |
| Never smoker | 69.0 | 68.3 | 72.6 | 72.2 | 63.1 |
| Hypertension (yes), % | 60.1 | 64.5 | 60.8 | 58.7 | 56.6 |
| Diabetes (yes), % | 21.7 | 28.5 | 25.1 | 18.0 | 15.2 |
| Diuretic medication use, % | 32.6 | 38.4 | 33.7 | 28.8 | 29.7 |
| Vitamin C supplement use, % | 9.0 | 30.2 | 3.0 | 1.9 | 1.0 |
| Fructose:vitamin C intake ratio, g/mg | 0.20 (0.13–0.30) | 0.08 (0.04–0.11) | 0.16 (0.14–0.18) | 0.24 (0.22–0.27) | 0.44 (0.36–0.62) |
| Intakes of | |||||
| Fructose, g/d | 24.2 (14.9–39.2) | 15.3 (10.0–24.4) | 20.0 (12.7–28.6) | 28.2 (19.0–39.7) | 43.0 (28.2–65.5) |
| Vitamin C, mg/d | 120 (78–191) | 216 (116–606) | 123 (82–176) | 116 (80–163) | 84 (58–123) |
| Energy, kcal/d | 2235 ± 911 | 2056 ± 891 | 2152 ± 875 | 2339 ± 913 | 2393 ± 923 |
| Alcohol, g/d | 3.8 ± 11.9 | 3.5 ± 10.9 | 3.4 ± 9.5 | 4.3 ± 12.6 | 4.1 ± 14.0 |
| Animal protein, g/d | 49.7 ± 28.9 | 47.1 ± 29.7 | 48.7 ± 28.2 | 51.7 ± 28.2 | 51.2 ± 29.2 |
| SSBs, mL/d | 561 ± 792 | 232 ± 395 | 307 ± 450 | 572 ± 677 | 1130 ± 1096 |
Values are means ± SDs, medians (IQRs), or percentages. eGFR, estimated glomerular filtration rate; SSB, sugar-sweetened beverage.
Levels of dietary intake of fructose and vitamin C
For the overall study population, the median intake of fructose was 24.2 g/d (IQR: 14.9–39.2 g/d), the median intake of vitamin C was 120 mg/d (IQR: 78–191 mg/d), and the median fructose:vitamin C intake ratio was 0.20 g · mg−1 · d−1 (IQR: 0.13–0.30 g · mg−1 · d−1) (Table 1). A total of 709 (15.5%) participants consumed a high amount of fructose (>50 g/d) and 1209 (26.4%) participants consumed amounts of vitamin C below the recommended level of dietary intake (Supplemental Table 1).
Association between dietary factors and serum urate
After adjusting for age, sex, smoking, waist circumference, systolic blood pressure, eGFR, diuretic medication use, vitamin C supplement use, total energy intake, alcohol consumption, and dietary intake of animal protein and vitamin C (model 4), a doubling of fructose intake was associated with an increase in serum urate concentration of 0.11 mg/dL (95% CI: 0.06, 0.15 mg/dL; Table 2). Relative to the lowest quartile, participants in the highest quartile of fructose intake had a 0.30-mg/dL higher serum urate concentration (95% CI: 0.17, 0.42 mg/dL; model 4).
TABLE 2.
Associations between dietary intakes of fructose and vitamin C or the fructose:vitamin C intake ratio and serum urate concentrations in African-American adults1
| Quartile of 3 dietary exposures3 | ||||||
|---|---|---|---|---|---|---|
| Continuous exposures2 | Quartile 1 (n = 1114) | Quartile 2 (n = 1114) | Quartile 3 (n = 1114) | Quartile 4 (n = 1114) | P-trend | |
| Fructose, g/d | 1.0–15.2 | 15.2–25.0 | 25.0–41.5 | 41.5–339.5 | ||
| Model 1 | 0.06 (0.02, 0.09) | Reference | 0.05 (−0.06, 0.15) | 0.10 (0.00, 0.20) | 0.18 (0.07, 0.28) | 0.001 |
| Model 2 | 0.08 (0.04, 0.13) | Reference | 0.07 (−0.04, 0.18) | 0.15 (0.03, 0.26) | 0.26 (0.13, 0.38) | <0.001 |
| Model 3 | 0.09 (0.05, 0.13) | Reference | 0.07 (−0.03, 0.18) | 0.16 (0.05, 0.27) | 0.26 (0.14, 0.38) | <0.001 |
| Model 4 | 0.11 (0.06, 0.15) | Reference | 0.09 (−0.02, 0.19) | 0.18 (0.07, 0.29) | 0.30 (0.17, 0.42) | 0.53 |
| Vitamin C, mg/d | 16.8–78.8 | 78.9–123.6 | 123.6–199.3 | 199.8–1037.6 | ||
| Model 1 | −0.02 (−0.06, 0.02) | Reference | −0.03 (−0.13, 0.07) | 0.00 (−0.10, 0.11) | −0.06 (−0.17, 0.06) | 0.43 |
| Model 2 | −0.06 (−0.09, −0.02) | Reference | −0.02 (−0.12, 0.09) | 0.00 (−0.11, 0.11) | −0.13 (−0.25, −0.02) | 0.02 |
| Model 3 | −0.02 (−0.06, 0.02) | Reference | −0.02 (−0.13, 0.08) | 0.01 (−0.10, 0.11) | −0.05 (−0.17, 0.08) | 0.58 |
| Model 4 | −0.05 (−0.09, −0.01) | Reference | −0.06 (−0.16, 0.05) | −0.05 (−0.16, 0.06) | −0.13 (−0.26, 0.00) | 0.30 |
| Fructose:vitamin C | 0.0–0.1 | 0.1–0.2 | 0.2–0.3 | 0.3–4.6 | ||
| intake ratio, g/mg | ||||||
| Model 1 | 0.07 (0.04, 0.11) | Reference | 0.02 (−0.09, 0.13) | 0.05 (−0.06, 0.16) | 0.15 (0.04, 0.26) | 0.008 |
| Model 2 | 0.08 (0.05, 0.12) | Reference | 0.09 (−0.02, 0.19) | 0.09 (−0.01, 0.2) | 0.20 (0.09, 0.31) | <0.001 |
| Model 3 | 0.08 (0.04, 0.11) | Reference | 0.03 (−0.08, 0.14) | 0.06 (−0.06, 0.17) | 0.17 (0.05, 0.28) | 0.004 |
1Values are β-coefficients (95% CIs); n = 4576. Model 1 adjusted for age, sex, smoking status, waist circumference, systolic blood pressure, estimated glomerular filtration rate, and medication use (diuretic medication, vitamin C supplement); model 2 adjusted for age, sex, smoking status, waist circumference, systolic blood pressure, estimated glomerular filtration rate, and dietary factors (total energy intake, alcohol, animal protein); model 3 adjusted as for model 1 plus dietary factors; and model 4 adjusted as for model 3 plus vitamin C (for the models with fructose as the main exposure) or fructose (for the models with vitamin C as the main exposure).
2The results (β-coefficients) from the continuous analysis can be interpreted as the increase (if positive) or decrease (if negative) in serum urate concentration per doubling of the dietary factor.
3The results (β-coefficients) from the quartile analysis can be interpreted as the increase (if positive) or decrease (if negative) in serum urate concentration associated with a higher quartile (quartile 2, 3, or 4) of dietary intakes of fructose and vitamin C and the fructose:vitamin C intake ratio relative to the lowest quartile (quartile 1).
A doubling of vitamin C intake was associated with a decrease in serum urate (−0.05 mg/dL; 95% CI: −0.09, −0.01 mg/dL) in the fully adjusted model (model 4). Participants in the highest quartile of vitamin C intake had a 0.13-mg/dL lower serum urate concentration (95% CI: −0.26, 0.00 mg/dL) relative to those in the lowest quartile of vitamin C intake (model 4).
When these 2 dietary factors were expressed together as a ratio, there was a 0.08-mg/dL (95% CI: 0.04, 0.11 mg/dL) increase in serum urate per doubling of the fructose:vitamin C intake ratio in the fully adjusted model (model 4; Table 2). Participants in the highest quartile of fructose:vitamin C intake ratio had 0.17-mg/dL (95% CI: 0.05-, 0.28-mg/dL) higher serum urate concentrations than did participants in the lowest quartile of the fructose: vitamin C intake ratio (P-trend = 0.004). Results for the association between fructose:vitamin C intake ratio and serum urate were similar across the 3 models, with a slight attenuation after adjusting for use of diuretic medications and use of vitamin C supplements (model 1 and model 3 compared with model 2). There was a significant inverse association between eGFR and the serum urate concentration (Pearson's r = −0.41, P < 0.001; Figure 1).
FIGURE 1.
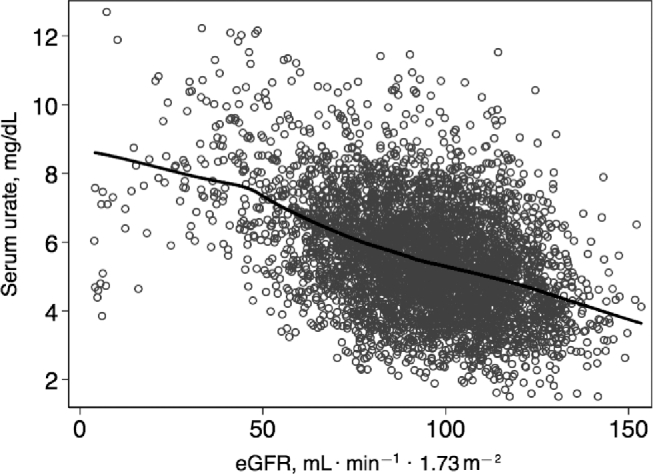
Scatterplot of kidney function (eGFR) and serum urate concentrations in African-American adults (n = 4576). The solid line represents the lowess curve for eGFR and serum urate concentration. eGFR, estimated glomerular filtration rate; lowess, locally weighted scatterplot smoothing.
Association between dietary factors and hyperuricemia
Similar patterns were observed for the dichotomized outcome of hyperuricemia defined as serum urate ≥7 mg/dL (Table 3) as for the continuous outcome of serum urate. A doubling of fructose intake was associated with 20% (OR: 1.20; 95% CI: 1.08, 1.34) greater odds of hyperuricemia in the fully adjusted model (model 4). Participants in the highest quartile of fructose intake had 1.63 times (OR: 1.63; 95% CI: 1.20, 2.21) greater odds of hyperuricemia compared with participants in the lowest quartile of fructose intake. Higher dietary intakes of vitamin C were significantly associated with lower odds of hyperuricemia in the fully adjusted model (model 4).
TABLE 3.
Association between dietary intakes of fructose and vitamin C and fructose:vitamin C intake ratio and odds of hyperuricemia (≥7 mg/dL) in African-American adults1
| Quartile of 3 dietary exposures3 | ||||||
|---|---|---|---|---|---|---|
| Continuous exposures2 | Quartile 1 (n = 1114) | Quartile 2 (n = 1114) | Quartile 3 (n = 1114) | Quartile 4 (n = 1114) | P-trend | |
| Fructose, g/d | 1.0–15.2 | 15.2–25.0 | 25.0–41.5 | 41.5–339.5 | ||
| Model 1 | 1.08 (0.99, 1.18) | Reference | 0.99 (0.77, 1.27) | 1.07 (0.83, 1.38) | 1.25 (0.97, 1.62) | 0.11 |
| Model 2 | 1.13 (1.02, 1.25) | Reference | 1.02 (0.80, 1.31) | 1.16 (0.90, 1.51) | 1.43 (1.07, 1.90) | 0.02 |
| Model 3 | 1.15 (1.04, 1.28) | Reference | 1.05 (0.81, 1.36) | 1.19 (0.91, 1.56) | 1.48 (1.10, 1.99) | 0.01 |
| Model 4 | 1.20 (1.08, 1.34) | Reference | 1.10 (0.85, 1.43) | 1.28 (0.98, 1.69) | 1.63 (1.20, 2.21) | 0.64 |
| Vitamin C, mg/d | 16.8–78.8 | 78.9–123.6 | 123.6–199.3 | 199.8–1037.6 | ||
| Model 1 | 0.91 (0.83, 1.00) | Reference | 0.89 (0.70, 1.14) | 0.87 (0.68, 1.11) | 0.76 (0.57, 1.00) | 0.08 |
| Model 2 | 0.89 (0.81, 0.97) | Reference | 0.90 (0.71, 1.14) | 0.88 (0.69, 1.14) | 0.70 (0.54, 0.92) | 0.02 |
| Model 3 | 0.92 (0.83, 1.02) | Reference | 0.90 (0.70, 1.15) | 0.88 (0.68, 1.14) | 0.77 (0.57, 1.04) | 0.16 |
| Model 4 | 0.87 (0.78, 0.97) | Reference | 0.85 (0.66, 1.09) | 0.80 (0.61, 1.04) | 0.66 (0.48, 0.91) | 0.90 |
| Fructose:vitamin C | 0.0–0.1 | 0.1–0.2 | 0.2–0.3 | 0.3–4.6 | ||
| intake ratio, g/mg | ||||||
| Model 1 | 1.16 (1.07, 1.26) | Reference | 0.96 (0.74, 1.26) | 1.01 (0.77, 1.33) | 1.48 (1.14, 1.92) | 0.003 |
| Model 2 | 1.17 (1.08, 1.26) | Reference | 1.06 (0.82, 1.36) | 1.07 (0.83, 1.37) | 1.56 (1.22, 1.99) | <0.001 |
| Model 3 | 1.17 (1.08, 1.28) | Reference | 0.98 (0.75, 1.28) | 1.03 (0.79, 1.35) | 1.54 (1.18, 2.01) | 0.001 |
1Values are ORs (95% CIs); n = 4576. Model 1 adjusted for age, sex, smoking status, waist circumference, systolic blood pressure, estimated glomerular filtration rate, and medication use (diuretic medication, vitamin C supplement); model 2 adjusted for age, sex, smoking status, waist circumference, systolic blood pressure, estimated glomerular filtration rate, and dietary factors (total energy intake, alcohol, animal protein); model 3 adjusted as for model 1 plus dietary factors; and model 4 adjusted as for model 3 plus vitamin C (for the models with fructose as the main exposure) or fructose (for the models with vitamin C as the main exposure).
2ORs for hyperuricemia per doubling of the dietary factor.
3The results (ORs) from the quartile analysis can be interpreted as greater (if >1) or lower (if <1) odds of hyperuricemia associated with a higher quartile (quartile 2, 3, or 4) of dietary intakes of fructose and vitamin C or the fructose:vitamin C intake ratio relative to the lowest quartile (quartile 1).
When these 2 dietary factors were combined as the fructose:vitamin C intake ratio, the odds of hyperuricemia were 1.54 times greater for participants in the highest compared with the lowest quartile of fructose:vitamin C intake ratio (model 3 OR: 1.54; 95% CI: 1.18, 2.01; P-trend = 0.001). The odds of hyperuricemia were 17% greater per doubling of fructose:vitamin C intake ratio in the fully adjusted model (model 3 OR: 1.17; 95% CI: 1.08, 1.28).
Sensitivity analyses of vitamin C intakes
After excluding participants who were using vitamin C supplements (n = 413), the results for the remaining 4163 participants who were not using vitamin C supplements were similar to the main findings for serum urate (Supplemental Table 2) and hyperuricemia (Supplemental Table 3). Likewise, among the 4047 participants with vitamin C intakes ≤500 mg/d, results were similar to those from the main analysis for the outcome of serum urate concentration (Supplemental Table 4) as well as for the outcome of hyperuricemia (Supplemental Table 5).
Evaluation of sex differences
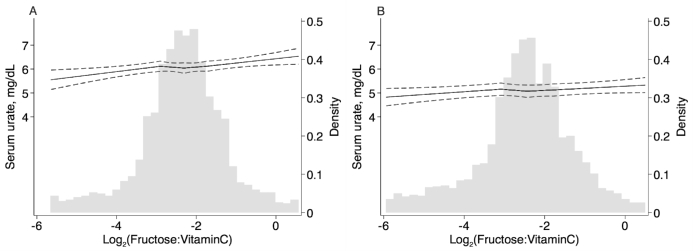
Dietary intakes of fructose and vitamin C and serum urate concentrations were higher among men than among women (Supplemental Table 6). In sex-stratified analyses, the magnitude of the results was stronger for men than for women for the association between the 3 dietary exposures and serum urate concentration (Supplemental Table 7) as well as for the association between the 3 dietary exposures and hyperuricemia (Supplemental Table 8). For example, per doubling of the fructose:vitamin C intake ratio, serum urate concentration was higher by 0.05 mg/dL among women (95% CI: 0.01, 0.09 mg/dL; model 3) and by 0.13 mg/dL among men (95% CI: 0.06, 0.19 mg/dL; model 3) (Supplemental Table 7). The increase in serum urate concentrations as the fructose:vitamin C intake ratio increased was more pronounced among men (Figure 2A) than among women (Figure 2B). There was a significant interaction by sex on the association between fructose (both P ≤ 0.04) and the fructose:vitamin C intake ratio (both P-interaction = 0.01) and both outcomes (i.e., serum urate and hyperuricemia) but not for vitamin C as a separate exposure (both P-interaction > 0.40).
FIGURE 2.
Restricted cubic spline of serum urate concentration by fructose:vitamin C intake ratio among men (A) (n = 1620) and women (B) (n = 2956). The solid lines represent the restricted cubic spline of serum urate concentration by fructose:vitamin C intake ratio, with knots at the 25th, 50th, and 75th percentiles. Fructose:vitamin C intake ratios were truncated at the 1st and 99th percentiles. Dashed lines represent 95% CIs. The gray-shaded histogram represents the distribution of the fructose:vitamin C intake ratio. Regression models were adjusted for age, smoking status, waist circumference, systolic blood pressure, estimated glomerular filtration rate, medication use (diuretic medication, vitamin C supplement), and dietary factors (total energy, alcohol, and animal protein intakes).
Discussion
In this study in 4576 community-based African Americans, we found that a dietary intake of fructose that is relatively higher than the intake of vitamin C (i.e., the fructose:vitamin C intake ratio) was significantly associated with higher serum urate concentration and greater odds of hyperuricemia. The magnitude of the association between the fructose:vitamin C intake ratio and both serum urate and hyperuricemia were stronger among men than women.
Several previous studies have shown that certain dietary factors influence serum urate concentration. Alcohol, animal sources of protein (e.g., red meat and seafood), and fructose-enriched food items (e.g., high-fructose corn syrup and sugar-sweetened beverages) have been identified as modifiable risk factors for elevated serum urate and gout, whereas vegetable sources of protein (e.g., soy), low-fat dairy products, coffee, and vitamin C have been associated with lower serum urate concentrations (23, 41–44). A clinical trial found that there was a significant dose-response effect of different consumption amounts of high-fructose corn syrup–sweetened beverages (providing 0%, 10%, 17.5%, and 25% of energy requirements) on 24-h mean serum urate concentration (45). In another randomized clinical trial, Huang et al. (46) found that the use of a vitamin C supplement (500 mg/d) for 2 mo compared with placebo significantly reduced the serum urate concentration among nonsmokers.
To the best of our knowledge, the present study is the first to examine the combined influence of dietary intakes of fructose and vitamin C on serum urate concentration with the use of the fructose:vitamin C intake ratio as a measure of relative difference in these 2 dietary factors. In an analysis of 3 prospective cohorts (Nurses’ Health Study I, Nurses’ Health Study II, and Health Professionals Follow-Up Study), dietary intakes of fructose and vitamin C were not independently associated with incident hypertension after adjusting for the other dietary factor (analyses of fructose intake were adjusted for vitamin C and vice versa) (47). These investigators suggested a mediating role of serum urate on the association between fructose, vitamin C, and incident hypertension, but the results for serum urate as a mediator were not reported.
In our analyses, we found that the dietary intake of fructose as a separate exposure, and when combined as a ratio with vitamin C, had significant independent associations with serum urate concentrations and hyperuricemia after controlling for other covariates. If reducing the intake of fructose is difficult to achieve, then increasing the intake of foods that are rich in vitamin C and low in fructose (e.g., fruit and vegetables) is a potentially easier approach to maintaining or reducing serum urate.
Our a priori–specified sex-stratified analyses showed that the association between the dietary intake of fructose and the fructose:vitamin C intake ratio and serum urate concentration was stronger among men than in women. This observation is supported by findings from previous research. For example, in NHANES 2001–2002, it was reported that men with a high dietary intake of added sugars and sugar-sweetened beverages had higher plasma uric acid concentrations, and there was no significant association among women (P-interaction < 0.01) (48). In a mouse study, there were differences in the types and locations of transport proteins expressed in female compared with male mice in response to a high-fructose diet, which suggests that sex is a determinant of metabolic adaptation to fructose intake (49). Furthermore, there are differences by sex on the association between genetic variants of the putative fructose transporter solute carrier family 2, facilitated glucose transporter member 9 (SLC2A9) and serum uric acid concentrations (50).
The underlying mechanism of the opposing actions of fructose and vitamin C on serum urate has been documented in previous studies. The phosphorylation of fructose increases the degradation from ATP to AMP, which, as a result, turns on the purine degradation pathway and generates serum urate (51). In contrast, vitamin C lowers serum urate concentration through a uricosuric effect by competing with serum urate on the urate transporter 1 (also known as solute carrier family 22, member 12, or SLC22A12) for re-absorption in the kidney proximal tubule (52, 53). Thus, a higher dietary intake of vitamin C could stimulate renal excretion of urate synthesized during the fructose metabolic process, thereby balancing the serum urate concentration.
Our study has several limitations to acknowledge. First, dietary intakes of fructose and vitamin C were estimated with the use of self-report of food consumption assessed via FFQ, which may be influenced by measurement error and recall bias. Second, the cross-sectional study design limited our ability to establish temporality between dietary intake and serum urate concentration. However, because the FFQ estimated the average dietary intake during the past 6 mo, we can be confident that these estimates of dietary intakes of fructose and vitamin C preceded the measurement of serum urate concentration. Measurements of serum urate were not available at follow-up study visits. Reverse causality was less likely to be a concern in our study, especially after we excluded individuals who were taking gout medication at baseline. Third, there may be residual confounding due to incomplete adjustment for dietary factors. In conclusion, the dietary fructose:vitamin C intake ratio was significantly associated with the serum urate concentration and prevalent hyperuricemia among African Americans.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—JLH and CMR: designed the research study; AC and BAY: provided the essential materials; ZZ and CMR: performed the statistical analysis, wrote the manuscript, and had primary responsibility for final content; JC, AK, and MAM-D: provided content-related expertise; RK: provided methodologic expertise; and all authors: read and approved the final manuscript.
Notes
The Jackson Heart Study is supported by contracts from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities (HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, and HHSN268201300050C). CMR is supported by a Mentored Research Scientist Development Award from the National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK107782). BAY is supported in part by funds from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK102134) and the Veterans Affairs Puget Sound Health Care System.
Supplemental Tables 1–8 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at http://academic.oup.com/jn/.
Abbreviations used: eGFR, estimated glomerular filtration rate; JHS, Jackson Heart Study; NIRI, Nutrition Intervention Research Initiative.
References
- 1. Alper AB, Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension 2005;45:34–8. [DOI] [PubMed] [Google Scholar]
- 2. Mellen PB, Bleyer AJ, Erlinger TP, Evans GW, Nieto FJ, Wagenknecht LE, Wofford MR, Herrington DM. Serum uric acid predicts incident hypertension in a biethnic cohort: the Atherosclerosis Risk in Communities Study. Hypertension 2006;48:1037–42. [DOI] [PubMed] [Google Scholar]
- 3. Krishnan E, Kwoh CK, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension 2007;49:298–303. [DOI] [PubMed] [Google Scholar]
- 4. Nagahama K, Inoue T, Kohagura K, Kinjo K, Ohya Y. Associations between serum uric acid levels and the incidence of hypertension and metabolic syndrome: a 4-year follow-up study of a large screened cohort in Okinawa, Japan. Hypertens Res 2015;38:213–8. [DOI] [PubMed] [Google Scholar]
- 5. Anand N, Padma V, Prasad A, Alam KC, Javid MSM. Serum uric acid in new and recent onset primary hypertension. J Pharm Bioallied Sci 2015;7:S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tuttle KR, Short RA, Johnson RJ. Sex differences in uric acid and risk factors for coronary artery disease. Am J Cardiol 2001;87:1411–4. [DOI] [PubMed] [Google Scholar]
- 7. Niskanen LK, Laaksonen DE, Nyyssönen K, Alfthan G, Lakka H-M, Lakka TA, Salonen JT. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med 2004;164:1546–51. [DOI] [PubMed] [Google Scholar]
- 8. Kuo C-F, Yu K-H, Luo S-F, Ko Y-S, Wen M-S, Lin Y-S, Hung K-C, Chen C-C, Lin C-M, Hwang J-S. Role of uric acid in the link between arterial stiffness and cardiac hypertrophy: a cross-sectional study. Rheumatology 2010:keq095. [DOI] [PubMed] [Google Scholar]
- 9. Mehta T, Nuccio E, McFann K, Madero M, Sarnak MJ, Jalal D. Association of uric acid with vascular stiffness in the Framingham Heart Study. Am J Hypertens 2015;28:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kang D-H, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ. A role for uric acid in the progression of renal disease. J Am Soc Nephrol 2002;13:2888–97. [DOI] [PubMed] [Google Scholar]
- 11. Borghi C, Rosei EA, Bardin T, Dawson J, Dominiczak A, Kielstein JT, Manolis AJ, Perez-Ruiz F, Mancia G. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens 2015;33:1729–41. [DOI] [PubMed] [Google Scholar]
- 12. De Cosmo S Viazzi F, Pacilli A, Giorda C, Ceriello A, Gentile S, Russo G, Rossi MC, Nicolucci A, Guida P. Serum uric acid and risk of CKD in type 2 diabetes. Clin J Am Soc Nephrol 2015;10:1921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma L, Wei L, Chen H, Zhang Z, Yu Q, Ji Z, Jiang L. Influence of urate-lowering therapies on renal handling of uric acid. Clin Rheumatol 2016;35:133–41. [DOI] [PubMed] [Google Scholar]
- 14. Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 1977;20:895–900. [DOI] [PubMed] [Google Scholar]
- 15. Pittman JR, Bross MH. Diagnosis and management of gout. Am Fam Physician 1999;59:1799–806. [PubMed] [Google Scholar]
- 16. Goicoechea M, de Vinuesa SG, Verdalles U, Verde E, Macias N, Santos A, de Jose AP, Cedeño S, Linares T, Luño J. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis 2015;65:543–9. [DOI] [PubMed] [Google Scholar]
- 17. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 2011;63:3136–41. [DOI] [PubMed] [Google Scholar]
- 18. Maynard JW, McAdams-DeMarco MA, Law A, Kao L, Gelber AC, Coresh J, Baer AN. Racial differences in gout incidence in a population-based cohort: Atherosclerosis Risk in Communities Study. Am J Epidemiol 2014;179:576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol 2010;22:165–72. [DOI] [PubMed] [Google Scholar]
- 20. Stanhope KL, Medici V, Bremer AA, Lee V, Lam HD, Nunez MV, Chen GX, Keim NL, Havel PJ. A dose-response study of consuming high-fructose corn syrup–sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr 2015;101:1144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, Bremer AA, Berglund L, McGahan JP, Keim NL. Consumption of fructose-but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr Metabol 2012;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DeBosch BJ, Chen Z, Finck BN, Chi M, Moley KH. Glucose transporter-8 (GLUT8) mediates glucose intolerance and dyslipidemia in high-fructose diet-fed male mice. Mol Endocrinol 2013;27:1887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao X, Curhan G, Forman JP, Ascherio A, Choi HK. Vitamin C intake and serum uric acid concentration in men. J Rheumatol 2008;35:1853–8. [PMC free article] [PubMed] [Google Scholar]
- 24. Medow MS, Bamji N, Clarke D, Ocon AJ, Stewart JM. Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. J Appl Physiol 2011;111:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- 26. Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 2009;139(Suppl):1228S–35S. [DOI] [PubMed] [Google Scholar]
- 27. Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang D-H, Gersch MS, Benner S, Sánchez-Lozada LG. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 2007;86:899–906. [DOI] [PubMed] [Google Scholar]
- 28. Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, Shafiu M, Segal M, Glassock RJ, Shimada M. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev 2009;30:96–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Livesey G, Taylor R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr 2008;88:1419–37. [DOI] [PubMed] [Google Scholar]
- 30. Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr 2009;90:1252–63. [DOI] [PubMed] [Google Scholar]
- 31. Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol 2004;160:339–49. [DOI] [PubMed] [Google Scholar]
- 32. Taylor HA Jr., Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis 2005;15:S6–4. [PubMed] [Google Scholar]
- 33. The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 34. Carithers TC, Talegawkar SA, Rowser ML, Henry OR, Dubbert PM, Bogle ML, Taylor HA, Tucker KL. Validity and calibration of food frequency questionnaires used with African-American adults in the Jackson Heart Study. J Am Diet Assoc 2009;109:1184–93, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tucker KL, Maras J, Champagne C, Connell C, Goolsby S, Weber J, Zaghloul S, Carithers T, Bogle ML. A regional food-frequency questionnaire for the US Mississippi Delta. Public Health Nutr 2005;8:87–96. [PubMed] [Google Scholar]
- 36. Monsen ER. Dietary Reference Intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc 2000;100:637–40. [DOI] [PubMed] [Google Scholar]
- 37. Bardin T, Richette P. Definition of hyperuricemia and gouty conditions. Curr Opin Rheumatol 2014;26:186–91. [DOI] [PubMed] [Google Scholar]
- 38. Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci 2004;328:131–44. [DOI] [PubMed] [Google Scholar]
- 39. Wang W, Young BA, Fulop T, de Boer IH, Boulware LE, Katz R, Correa A, Griswold ME. Effects of serum creatinine calibration on estimated renal function in African Americans: the Jackson Heart Study. Am J Med Sci 2015;349:379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ 2008;336:309–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Villegas R, Xiang Y-B, Elasy T, Xu W-H, Cai H, Cai Q, Linton M, Fazio S, Zheng W, Shu X-O. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: the Shanghai Men's Health Study. Nutr Metabol Cardiovasc Dis 2012;22:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Teng GG, Pan A, Yuan JM, Koh WP. Food sources of protein and risk of incident gout in the Singapore Chinese Health Study. Arthritis Rheumatol 2015;67:1933–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park KY, Kim HJ, Ahn HS, Kim SH, Park EJ, Yim SY, Jun JB. Effects of coffee consumption on serum uric acid: systematic review and meta-analysis. Semin Arthritis Rheum 2016;45:580–6. [DOI] [PubMed] [Google Scholar]
- 45. Stanhope KL, Medici V, Bremer AA, Lee V, Lam HD, Nunez MV, Chen GX, Keim NL, Havel PJ. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr 2015;101:1144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang HY, Appel LJ, Choi MJ, Gelber AC, Charleston J, Norkus EP, Miller ER. The effects of vitamin C supplementation on serum concentrations of uric acid: results of a randomized controlled trial. Arthritis Rheum 2005;52:1843–7. [DOI] [PubMed] [Google Scholar]
- 47. Forman JP, Choi H, Curhan GC. Fructose and vitamin C intake do not influence risk for developing hypertension. J Am Soc Nephrol 2009;20:863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gao X, Qi L, Qiao N, Choi HK, Curhan G, Tucker KL, Ascherio A. Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension 2007;50:306–12. [DOI] [PubMed] [Google Scholar]
- 49. Sharma N, Li L, Ecelbarger CM. Sex differences in renal and metabolic responses to a high-fructose diet in mice. Am J Physiol Renal Physiol 2015;308:F400–F10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brandstätter A, Kiechl S, Kollerits B, Hunt SC, Heid IM, Coassin S, Willeit J, Adams TD, Illig T, Hopkins PN. Sex-specific association of the putative fructose transporter SLC2A9 variants with uric acid levels is modified by BMI. Diabetes Care 2008;31:1662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med 2005;143:499–516. [DOI] [PubMed] [Google Scholar]
- 52. Mitch WE, Johnson MW, Kirshenbaum JM, Lopez RE. Effect of large oral doses of ascorbic acid on uric acid excretion by normal subjects. Clin Pharmacol Ther 1981;29:318–21. [DOI] [PubMed] [Google Scholar]
- 53. Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T. Molecular identification of a renal urate–anion exchanger that regulates blood urate levels. Nature 2002;417:447–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.