Abstract
Objectives
The present longitudinal, multi-method, and multi-informant study examined biological, neuropsychological, and social predictors of medical adherence and responsibility among early adolescents with spina bifida (SB).
Methods
Youth with SB (M age = 11.40 at Time 1) and their parents and teachers completed surveys, and families and peers completed observational assessments, at two biennial data collection time points (n = 112 for both time points). Multinomial logistic regressions tested predictors of group membership (adherent vs. nonadherent and child responsible vs. not responsible with SB medical tasks).
Results
Consistent with the bio-neuropsychosocial model, several risk factors emerged for SB management. Impaired gross motor classification and low IQ were barriers to obtaining medical responsibility, and high family stress and executive dysfunction were barriers to adherence and responsibility.
Conclusions
This study offered intervention targets to promote self-management and adherence for youth with SB and their families, including parent stress-management and family problem-solving.
Keywords: adherence, adolescents, health behavior, spina bifida
Medical nonadherence poses significant risks for patients, families, and the larger healthcare system. For adolescents with chronic health conditions, medical nonadherence is a primary cause of mortality, treatment failure, preventable secondary conditions, higher health care utilization, increased health spending, and reduced quality of life (Fredericks, Magee, Opipari-Arrigan, Shieck, Well, & Lopez, 2008; Caterino etal., 2006; Kennard etal., 2004). The successful transfer of medical responsibilities from parent to adolescent is intricately connected to adherence outcomes. Evidence-based models of pediatric medical management also underscore the influence of modifiable and nonmodifiable individual, family, and social characteristics associated with medical self-management and adherence (Grey, Schulman-Green, Knafl, & Reynolds, 2015; Modi etal., 2012; Schwartz, Tuchman, Hobbie, & Ginsberg, 2011). Research, however, has yet to establish enduring predictor variables that relate to medical adherence and responsibility for certain vulnerable pediatric populations, such as spina bifida (SB). To address this limitation, the current study tested the utility of a bio-neuropsychosocial model of adjustment (Holmbeck & Devine, 2010) through the evaluation of biological, neuropsychological, and social predictors of catheterization and bowel program adherence and responsibility during early adolescence in youth with SB. According to this model, medical management may be influenced by biological (e.g., SB severity), neuropsychological (e.g., executive functioning), and social (e.g., family and peer functioning) variables, particularly during critical stages of development such as adolescence.
During adolescence, many youth with chronic health conditions gain increased responsibility for their medical regimen (Anderson, Ho, Brackett, Finkelstein, & Laffel, 1997; Quittner, Modi, Lemanek, Ievers-Landis, & Rapoff, 2008). By the time youth with SB are 12–13 years old, most have obtained responsibility for catheterization and many have obtained responsibility for bowel programs (Stepansky, Roache, Holmbeck, & Schultz, 2010). Unfortunately, rates of adherence among adolescents are generally much lower than adherence rates in younger children and adults (i.e., 50% adherence rate among adolescents; La Greca & Mackey, 2009). This drop in adherence is thought to reflect a transitional period in which the adolescent assumes increased responsibility for his or her medical care, while parents become less involved (Miller & Harris, 2011), as well as other salient developmental issues of adolescence that may negatively impact medical self-management (e.g., individuation and separation from the family and greater affiliation with peers; Rapoff, 2011). While ongoing parental involvement has been associated with more optimal SB self-management during adolescence (Psihogios & Holmbeck, 2013; Sawin, Bellin, Roux, Buran, & Brei, 2009), the transfer of medical responsibilities from parent to adolescent is considered a necessary process for transitioning from pediatric to adult health care (Reed-Knight, Blount, & Gilleland, 2014).
Adolescents with SB are considered an understudied and underserved population who tend to achieve lower overall levels of autonomy during adolescence compared with typically developing children (Davis, Shurtleff, Walker, Seidel, & Duguay, 2006; Friedman, Holmbeck, DeLucia, Jandasek, & Zebracki, 2009). Adolescents with SB complete several daily medical tasks, including urinary catheterization, bowel management programs, medications, dietary modifications (e.g., high fiber), and skin checks to prevent pressure ulcers. Nonadherence to these tasks is associated with numerous preventable secondary complications, such as urinary tract infections from poor adherence to catheterization (Caterino etal., 2006) and constipation and incontinence from nonadherence to bowel programs (Dicianno etal., 2008). While preliminary investigations suggest that up to 50% of children and adolescents with SB are nonadherent to specific aspects of their disease regimen (Psihogios, Kolbuck, & Holmbeck, 2015), existing research has not adequately described the individual and contextual factors that impact adherence to SB treatments.
Individuals with SB may struggle with adherence and the transfer of medical responsibilities for several reasons. First, many youth with SB typically demonstrate low average cognitive capabilities (Riddle, Morton, Sampson, Vachha, & Adams, 2005; Wills, 1993) and struggle with aspects of executive functioning, such planning, problem-solving, focused attention, and working memory (e.g., Dennis, Landry, Barnes, & Fletcher, 2006). In a cross-sectional study, O’Hara and Holmbeck (2013) found that lower executive functions were associated with poorer medical adherence and lower medical responsibility. Family functioning variables also have been linked to SB medical adherence, with higher levels of family conflict associated with lower levels of adherence, and higher levels of family cohesion predictive of more optimal adherence (Psihogios & Holmbeck, 2013; Stepansky etal., 2010). However, research on youth with SB has not evaluated medical adherence and responsibility during adolescence in relation to other relevant predictors, such as condition severity and social functioning with peers, factors that are included in Holmbeck and Devine’s (2010) bio-neuropsychosocial model of adjustment for individuals with SB.
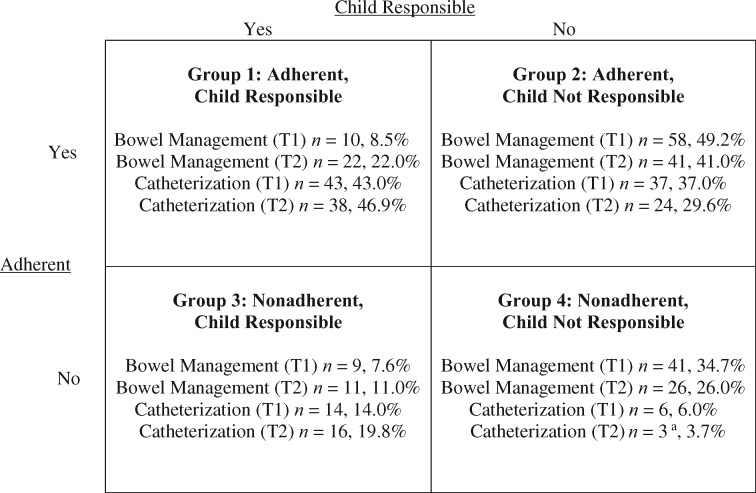
The purpose of the study was to evaluate the predictive utility of four different domains of functioning on catheterization and bowel program responsibility and adherence among young adolescents with SB: (1) biological, (2) neuropsychological, (3) family, and (4) peer. We focused on adherence and responsibility with catheterization and bowel programs only, as these tasks are prominent components of SB medical care, and nonadherence to these tasks is associated with common but preventable secondary complications. As seen in Figure 1, adherence and responsibility were evaluated in an integrative manner, where participants were classified as high versus low on both variables, thus yielding a 2 × 2 grid. We expected that youth with less severe SB (greater gross motor functioning, fewer shunt revisions, and lower lesion levels), fewer neuropsychological challenges (higher IQ and less executive dysfunction), more adaptive family functioning (higher family cohesion, and lower family conflict and stress), and more adaptive peer functioning (higher friendship quality and emotional support from a peer, and lower peer conflict) would most likely be a member of the “Adherent, Child Responsible” category for their catheterization and bowel programs. Salient predictors of less optimal outcomes (i.e., limited child responsibility and/or nonadherence) may clarify intervention targets for successfully transferring SB responsibilities to adolescents, without the characteristic decrease in medical adherence.
Figure 1.
Adherence and responsibility groups for catheterization and bowel program.
Note. Total n = 118 for bowel management at Time 1 (T1); Total n = 100 for bowel management at Time 2 (T2); Total n = 100 for catheterization at Time 1 (T1); Total n = 81 for catheterization at Time 2 (T2); the McNemar–Bowker Test showed that Time 1 (T1) and Time 2 (T2) group membership did not significantly differ (p > .05).
aNot tested for statistical significance owing to Group 4 n < 5.
Method
Participants
Participants were part of a larger, longitudinal study examining family, psychosocial, and neurocognitive functioning among youth with SB. This report utilized data from Time 1 and, approximately 2 years later, Time 2. Families of youth with SB were recruited from four hospitals and a statewide SB association in the Midwest. Inclusion criteria consisted of (1) diagnosis of SB; (2) age 8–15 years at Time 1; (3) ability to speak or read English or Spanish; (4) involvement of at least one primary caregiver; and (5) residence within 300 miles of lab to allow for home-based data collections. Of the original 246 families who met eligibility criteria, 163 families agreed to participate but 21 of those families could not be contacted or later declined, and two families eventually did not meet inclusion criteria. The final sample included 140 families of children and adolescents with SB (see demographics in Table I). Children of families who declined participation did not differ from those who participated with respect to type of SB (e.g., myelomeningocele or other), χ2 (1) = 0.0002, p > .05, shunt status, χ2 (1) = 0.003, p > .05, or occurrence/nonoccurrence of shunt infections, χ2 (1) = 1.08, p > .05. Of the original 140 participants, 112 completed Time 2 (i.e., 80% of the sample, M age = 13.41, SD = 2.41). Participants at Time 2 did not differ from youth who did not participate with respect to gender, χ2 = 0.28, p >.05, socioeconomic status (SES), t (128) = 1.86, p >.05, type of SB (myelomeningocele or other), χ2 (1) = 1.19, p >.05, lesion level (thoracic or other), χ2 (1) = 0.72, p >.05, or shunt status, χ2 (1) = 2.73, p >.05. However, youth who did not participate at Time 2 were significantly older at Time 1, t (138) = 3.02, p = .003, than those who did participate.
Table I.
Child Demographic and Spina Bifida Information at Time 1
| Variable | M (SD) or % (N = 140) |
|---|---|
| Age: | 11.4 (2.5) |
| Gender | |
| Male | 45.7% |
| Female | 54.3% |
| Ethnicity | |
| White | 52.1% |
| Hispanic | 26.4% |
| African American | 12.1% |
| Other | 5.8% |
| Hollingshead SES | 39.4 (15.9) |
| Spina bifida type | |
| Myelomeningocele | 86.4% |
| Lipomeningocele | 6.4% |
| Other | 5.8% |
| Unknown/not reported | 1.4% |
| Lesion level | |
| Thoracic | 16.4% |
| Lumbar | 48.6% |
| Sacral | 29.3% |
| Unknown/not reported | 1.4% |
| Shunt present | 77.9% |
| Ambulation | |
| Braces | 81.1% |
| Wheelchair | 61.4% |
Note. The percentages of children who use braces or wheelchairs do not add up to 100% because many children use both methods of ambulation.
Each child with SB was asked to invite a peer to participate. Inclusion criteria for peers were as follows: (1) within 2 years of the target child’s age, and (2) ability to speak and read English or Spanish. Of the original 140 families, 121 recruited a peer that met these criteria. While families were strongly encouraged to invite friends who were not related to the target child, 15 peers were related to the child with SB and were removed from relevant analyses. Thus, 106 youth with SB (76%) and their friends were included in the peer-related analyses at Time 1 (M peer age = 10.98 years, SD = 2.75, 55.7% female, 64.2% Caucasian, 17.9% Hispanic, 8.5% African American, and 6.6% other racial background).
Procedure
Trained undergraduate and graduate student research assistants collected data during scheduled home visits. Families received monetary compensation ($150 for families, $50 for peers) and gifts (e.g., t-shirts and pens) for participation. Informed consent from parents and assent from children and their peers were obtained. Parents were asked to complete release of information forms to allow for additional data collection from teachers, health professionals, and medical charts. Youth with SB and their parents independently completed questionnaires, in separate rooms, and together participated in videotaped semi-structured family interaction tasks. Research assistants read questionnaires out loud to participants when requested or when reading difficulties were observed or described by youth or parents. Additionally, neuropsychological testing of the child was completed. Youth with SB and a peer individually completed questionnaires about general friendship characteristics and the specific friendship of the participating child with SB and peer. Youth with SB and peers also engaged in videotaped semi-structured interaction tasks.
Measures
Medical Responsibility (Time 1 and Time 2)
Bowel and catheterization subscales of Sharing of Spina Bifida Management Responsibilities (SOSBMR) were used to examine who takes primary responsibility for these two tasks. The SOSBMR was adapted from the Diabetes Family Responsibility Questionnaire (Anderson, Auslander, Jung, Miller, & Santiago, 1990) and consists of 34 items that describe SB or general health-related tasks relevant to adolescents with SB. Parents independently rated who was primarily responsible for each task on a 3-point scale (i.e., Parent, Child, Equal, or Not Applicable). These items are grouped into several subscales: health care appointments, communication about SB, medications, general needs and self-care, ambulation, skin care, catheterization, bowel management, exercise, and diet. Mother and father reports were significantly correlated at Time 1 (r = .76) and Time 2 (r = .75) and were averaged together to form one mean parent score at each time point. The combined parent-report of medical responsibility was found to have excellent alphas at Time 1 (bowel subscale α = .97; catheterization subscale α = .94) and Time 2 (bowel subscale α = .95; catheterization subscale α = .97). To create categorical variables (“Child Responsible” vs. “Child Not Responsible”; Psihogios, Kolbuck, & Holmbeck. 2015), subscale mean scores (ranging from 1 to 3) were calculated for catheterization and bowel items. Means ≥2.1 (i.e., slightly above “shared responsibility”) were re-labeled “Child Responsible,” whereas means below 2.1 (i.e., scores ranging from “shared responsibility” to “parent responsibility”) were re-labeled “Child Not Responsible.”
Medical Adherence (Time 1 and Time 2)
The Spina Bifida Self-Management Profile (SBSMP) measured adherence to SB medical treatments (Wysocki & Gavin, 2006). The SBSMP is a 14-item questionnaire that focuses on seven dimensions of SB medical regimen (catheterization, bowel program, skin/wound care, exercise, appointments, medications, dealing with urinary tract infections), with higher scores indicating higher levels of SB medical adherence. Mother and father responses were averaged together to form a mean score for each time point as they were significantly correlated at Time 1 (r = .45) and 2 (r = .35). Scale reliability could not be computed for this sample owing to the low number of participants who completed every item (i.e., parents can endorse “not applicable” for certain items). Categorical variables from two items assessing adherence to catheterization and two items assessing adherence to bowel programs were created based on clinically meaningful cut points described in a previous manuscript (Psihogios, Kolbuck, & Holmbeck, 2015). As an example for catheterization, a score of “1” (adherent) was given to families who indicate that they miss catheterizing four to five times per week or less (i.e., less than once per day). A score of “0” (nonadherent) was given to families who indicate that they miss catheterizing one time or more per day (i.e., at least seven times per week or more). As an example for bowel management, a score of “1” (adherent) was assigned to families who indicated that their child takes bowel medications 50%–79% of the time or greater. A score of “0” (nonadherent) was assigned to families who indicated that their child takes bowel medications <50% of the time.
Biological Variables
Medical records were used to determine lesion level and number of shunt revisions. We analyzed lesion level as a continuous variable by assigning participants a score that ranged from 1 to 30, with lower numbers representing lower-level lesions. We used a modified version of the Gross Motor Function Classification System to assign the target child a gross motor classification. Information from mother-report was used to assign the target a gross motor classification scale level ranging from Level I: No braces, crutches, walker, or wheelchair (i.e., 100% unassisted walking) to Level IV: Uses wheelchair at school, long outings (i.e., <50% walking) (Rosenbaum, Palisano, Bartlett, Galuppi, & Russell, 2008).
Neuropsychological Variables
The Wechsler Abbreviated Scale of Intelligence was used as a proxy for general intellectual functioning (Wechsler, 1999). The Vocabulary and Matrix Reasoning subtests were administered to youth with SB to obtain an estimate of IQ. The Behavior Rating Inventory of Executive Function (BRIEF) was used as a parent- and teacher-report measure of child executive dysfunction (Gioia, Isquith, Guy, & Kenworthy, 2000). Higher scores on the BRIEF represent higher levels of executive dysfunction. As mother, father, and teacher reports were moderately correlated (r = .30 to .57), we created an aggregate score from item-level means across reporters, which had adequate internal consistency (α = .98). Similar to other studies of youth with SB (O’Hara & Holmbeck, 2013; Lennon etal., 2015), we opted to use the item-level mean across reporters, rather than T-scores, as there is typically more variability at the item level. Further, normative data differs for parent and teacher report and we wished to aggregate scores across reporters to reduce the number of analyses. Using neuropsychological performance data, we created an executive functioning performance composite score based on the mean age scaled scores. Scales in this composite score included the Delis-Kaplan Executive Function System (D-KEFS) Verbal Fluency subtests (i.e., Letter Fluency, Category Fluency, and Category Switching) and the Planned Connections subtest from the Cognitive Assessment System (Delis, Kaplan, & Kramer, 2001; Naglieri & Das, 1997). Internal consistency across these scales was adequate (α = .89).
Family Variables: Questionnaires
Parents separately completed the Parent-Adolescent Conflict scale, a brief version of the Issues Checklist (Robin & Foster, 1989). We did not compute alpha coefficients for this measure, as family members only answer items they have personally discussed and rarely answer every item. Mother and father reports of family conflict were correlated (r = .48) and combined to create a mean parent report of family conflict. We used the Family Environment Scale, Form R to measure parents’ perceptions of family cohesion on a Likert scale (Moos & Moos, 1994). Owing to a significant correlation (r = .46), mother and father reports were averaged together to form a mean cohesion score (α = .62). The Family Stress Scale was used to measure the intensity of common stressors in families with a child with a chronic health condition (Quittner, Glueckauf, & Jackson, 1990). Mother- and father-reported family stress were significantly correlated (r = .40) and thus averaged together to form a combined parent-reported stress score (α = .92).
Family Variables: Observational Methods
Family conflict and cohesion were investigated by evaluating observations across four family interaction tasks. The family interactions consisted of four, counterbalanced, structured tasks: (1) interactive game, (2) discussion of two age-appropriate vignettes about social situations, (3) discussion of transferring disease-specific responsibilities to the child, and (4) discussion of family conflict issues that were frequently endorsed on questionnaires by family members. Trained research assistants coded each family interaction task using the Family Interaction Macro-coding System (Holmbeck, Zebracki, Johnson, Belvedere, & Hommeyer, 2007; Kaugars etal., 2010). Research assistants received approximately 10 hr of training before coding the videotapes and were required to reach a reliability of 90% agreement with an expert coder before coding. Family cohesion (seven items) and family conflict (two items) subscales were examined. Excellent internal consistency for the cohesion dimension (α = .90) and acceptable internal consistency for the conflict dimension (α = .66) were found. Interrater reliability between two coders was adequate (ICC = 0.50 for the conflict dimension; ICC = 0.70 for the cohesion dimension).
Peer Variables: Questionnaires
In the Friendship Activity Questionnaire, youth with SB rated their best friend across five scales of friendship qualities (Bukowski, Hoza, & Boivin, 1994). A total score was used, which showed adequate internal consistency (α = .88). For the Emotional Support Questionnaire, the participant’s mean score from the friend category across all seven dimensions was used to assess closeness and support with one nominated close friend (α = .88).
Peer Variables: Observational Methods
Peer conflict was investigated by coding peer interaction tasks. Counterbalanced tasks included (1) toy ranking (i.e., ranking a set of toys based on how much the children enjoyed playing with them), (2) develop a commercial advertising an ambiguous object for 5 min, (3) plan an adventure, and (4) discuss previous peer conflicts and brainstorm problem-solving ideas that could have been used to resolve conflict. The Peer Interaction Macro-Coding Scale is an adaptation of several previous coding systems (Holmbeck, Belvedere, Gorey-Ferguson, & Schneider, 1995; Johnson & Holmbeck, 1999; Smetana, Yau, Restrepo, & Braeges, 1991). Research assistants received approximately 10 hr of training before coding the videotapes and were required to reach a reliability of 90% agreement with an expert coder before coding. Two trained undergraduate or graduate research assistants coded each peer interaction task. Only the dyadic conflict scale was used (five items). Inter-rater reliability for the conflict scale was adequate; child with SB: ICC = 0.75; peer: ICC = 0.77 (Holbein etal., 2014), as was the internal consistency (target α = .86; peer α = .89).
Data Analytic Plan
Multinomial logistic regressions were used to explore the main study hypotheses. For all analyses, we ran Time 1 predictors (biological, neuropsychological, family, or peer) predicting Time 1 or Time 2 catheterization or bowel adherence/responsibility groups (i.e., four groups for catheterization, four groups for bowel management, see Figure 1). Group 1, “Adherent, Child Responsible,” with bowel management or catheterization was the reference category for all analyses (i.e., Group 1 compared with Group 2, 3, or 4). Power was computed based on the fewest number of participants who had catheterization or bowel program management data at Time 2 (i.e., n = 81 for catheterization at Time 2). Assuming a power of .80, an alpha of .05, and an estimated R2 of .15 (a medium effect size), a sample of 91 was required for the analyses with up to five independent variables (Cohen, 1992). Therefore, for most analyses, the current study had enough power to detect medium to large effects. Owing to limited power, we did not control for Time 1 medical adherence and responsibility variables or relevant covariates (child age and family SES) when conducting Time 2 logistical regression analyses. Furthermore, we did not evaluate Group 4 at Time 2 (i.e., “Not Adherent, Child Not Responsible”) for catheterization management owing to the low n in that group (i.e., less than five participants; see Figure 1).
Owing to the limitations of the categorical approach (e.g., limited power to control for Time 1 medical variables and relevant covariates because of lower n when catheterization/bowel management tasks were “not applicable”), we conducted exploratory hierarchical regression analyses to determine whether evaluating overall medical adherence or responsibility as outcomes (i.e., continuous variables, across all medical domains including catheterization, bowel management, skin/wound care, exercise) and controlling for relevant confounds (child age, SES) would support main study findings. Further, this allowed for testing the bio-neuropsychosocial model by simultaneously considering all relevant predictors. Covariates were entered in the first block (child age, SES, and for prospective analyses, medical responsibility or adherence at Time 1), followed by neuropsychological, family, biological, and peer variables, respectively. Outcome variables were Time 1 or Time 2 overall medical responsibility or medical adherence (across all medical domains; four analyses total).
Results
Means, standard deviations, and scale ranges for variables used in the analyses are presented in Table II. Descriptive analyses of the four groups determined frequencies in each medical adherence and responsibility group (i.e., four categorical groups) for bowel and catheterization management at Time 1 and Time 2 (see Figure 1). Multinomial logistic regressions were conducted to determine whether the groups differed based on age or SES, using Group 1 (“Adherent, Child Responsible”) as the reference group. As expected, for bowel management at Time 1, participants who fell in Group 2 (χ2 (1) = −0.53, p = .01) and Group 4 (χ2 (1) = −0.55, p =. 01; i.e., “Child Not Responsible” groups) were younger. Similarly, at Time 2, participants in Group 2 (χ2 (1) = −0.29, p = .04) and Group 4 (χ2 (1) = −0.36, p = .02) were younger. Additionally, at Time 2, participants who fell in Group 2 (“Adherent, Not Responsible” for bowel management had lower SES (χ2 (1) = −0.04, p = .03). Regarding catheterization at Time 1, participants who fell in Group 2 (χ2 (1) = −0.22, p = .03) were younger and had lower SES (χ2 (1) = −0.03, p = .04). Similarly, at Time 2, participants in Group 2 were younger (χ2 (1) = −0.30, p = .02).
Table II.
Biological, Neuropsychological, Family, Peer, and Continuous Medical Variables (Medical Adherence and Responsibility)
| Variable | N | M | SD | Actual range | Possible range |
|---|---|---|---|---|---|
| Biological variables (Time 1) | |||||
| Lesion level | 121 | 7.36 | 3.41 | 1.00–16.00 | 1.00–30.00 |
| Number of shunt revisions | 94 | 2.95 | 3.61 | 0.00–16.00 | a |
| Gross motor functioning | 133 | 2.89 | 1.07 | 1.00–4.00 | 1.00-4.00 |
| Neuropsychological variables (Time 1) | |||||
| Parent/teacher report (Item Mean, BRIEF) | 138 | 1.69 | 0.32 | 1.01–2.68 | 1.00–3.00 |
| Executive function performance test (scaled score) | 128 | 6.88 | 3.13 | 1.00–13.75 | 1.00–19.00 |
| Intellectual function test data (WASI; standard score) | 134 | 85.85 | 19.70 | 55.00–137.00 | 40.00–160.00 |
| Family variables (Time 1) | |||||
| Family conflict (observational) | 139 | 2.03 | 0.45 | 1.00–3.35 | 1.00–5.00 |
| Family cohesion (observational) | 139 | 3.36 | 0.40 | 2.24–4.19 | 1.00–5.00 |
| Parent-report family conflict (PAC) | 133 | 1.70 | 0.51 | 1.00–3.91 | 1.00–4.00 |
| Parent-report family cohesion (FES) | 127 | 3.11 | 0.10 | 2.22–3.89 | 1.00–4.00 |
| Parent-report family stress (FSS) | 127 | 1.99 | 0.85 | 1.00–4.21 | 1.00–5.00 |
| Peer variables (Time 1) | |||||
| Peer conflict (observational) | 123 | 1.93 | 0.42 | 1.15–3.55 | 1.00–5.00 |
| Emotional peer support (ESQ) | 119 | 3.08 | 0.58 | 1.00–4.00 | 1.00–4.00 |
| Friendship quality (FAQ) | 126 | 3.62 | 0.54 | 1.87–4.64 | 1.00–5.00 |
| Medical variables (parent report; across all medical tasks) | |||||
| Medical adherence (Time 1; Z-Score) | 125 | −0.01 | 0.45 | −1.86–1.15 | a |
| Medical adherence (Time 2; Z-Score) | 106 | 0.02 | 0.44 | −1.72–0.81 | a |
| Medical responsibility (Time 1) | 124 | 1.73 | 0.38 | 1.00–3.00 | 1.00–3.00 |
| Medical responsibility (Time 2) | 106 | 1.91 | 0.42 | 1.09–3.00 | 1.00–3.00 |
Note. BRIEF = Behavioral Rating Inventory of Executive Function; WASI = Wechsler Abbreviated Scale of Intelligence; PAC (Parent-Adolescent Conflict Scale); FES = Family Environmental Scale; FSS = Family Stress Scale; ESQ = Emotional Support Questionnaire; FAQ = Friendship Activity Questionnaire.
Possible range not defined.
Biological Factors Related to Adherence/Responsibility
Bowel Management
Cross-sectional analyses indicated that measures of lesion level, number of shunt revisions, and gross motor classification did not significantly predict group membership (p’s > .05) at Time 1. Longitudinal analyses found that gross motor functioning level significantly predicted whether a child was “Adherent, Child Not Responsible” (M = 2.97) versus “Adherent, Child Responsible” (M = 2.28) to bowel programs at Time 2, B = .74, Wald χ2 (1) = 4.07, p = .04. The odds ratio indicated that as gross motor functioning level increased by one unit (higher scores indicate greater gross motor impairment), the odds of being “Adherent, Child Not Responsible” (rather than “Adherent, Child Responsible”) increased by 2.09 units (see Table III).
Table III.
Significant Multinomial Logistic Regression Analyses
| 95% CI for odds ratio |
||||
|---|---|---|---|---|
| B (SE) | Lower | Odds ratio | Upper | |
| Time 1 Bowel Management | ||||
| Group 1 vs. Group 3 | ||||
| Family cohesion 3.60 (1.78)* 1.13 36.54 1185.29 (Parent report) | ||||
| Group 1 vs. Group 4 | ||||
| BRIEF | 4.99 (1.66)** | 5.75 | 147.58 | 3788.80 |
| Family stress | 1.59 (0.81)* | 1.01 | 4.90 | 23.81 |
| Time 1 Catheterization | ||||
| Group 1 vs. Group 2 | ||||
| Gross motor functioning classification | 0.67 (0.34)* | 1.01 | 1.96 | 3.80 |
| Time 2 Bowel Management | ||||
| Group 1 vs. Group 2 | ||||
| Gross motor functioning classification | 0.74 (0.36)* | 1.02 | 2.09 | 4.25 |
| IQ | −0.06 (0.02)* | 0.90 | 0.94 | 0.99 |
| Peer conflict | 1.80 (0.85)* | 1.14 | 6.02 | 31.68 |
| Group 1 vs. Group 3 | ||||
| Emotional support 1.63 (0.81)* 1.04 5.10 25.11 from peers | ||||
| Group 1 vs. Group 4 | ||||
| BRIEF | 3.14 (1.26)* | 1.97 | 23.20 | 273.11 |
| Family stress | 1.88 (0.87)* | 1.18 | 6.53 | 36.10 |
| Time 2 Catheterization | ||||
| Group 1 vs. Group 2 | ||||
| Gross motor functioning classification | 0.75 (0.36)* | 1.05 | 2.11 | 4.24 |
| Group 1 vs. Group 3 | ||||
| Family cohesion (observed) | 3.04 (1.47)* | 1.18 | 20.82 | 367.13 |
Note. *p < .05; **p < .01; Group 1 (“Adherent, Child Responsible”) is the reference group; Group 2 = “Adherent, Child Not Responsible”; Group 3 = ”Nonadherent, Child Responsible”); Group 4 = “Nonadherent, Child Not Responsible.”
Catheterization
Gross motor functioning level concurrently related to whether a child was “Adherent, Child Not Responsible” (M = 3.51) versus “Adherent, Child Responsible” (M = 2.62) to catheterization at Time 1, B = 0.67, Wald χ2 (1) = 3.91, p = .04. The odds ratio showed that as gross motor functioning level increased by one unit (higher scores indicate greater gross motor impairment), the odds of being “Adherent, Child Not Responsible” increased by 1.96 units (see Table III). Similarly, gross motor functioning level significantly predicted whether a child was “Adherent, Child Not Responsible” (M = 3.24) versus “Adherent, Child Responsible” (M = 2.51) to catheterization at Time 2, B = 0.75, Wald χ2 (1) = 4.41, p = .04. The odds ratio indicated that as the gross motor classification increased by one unit, the odds of being “Adherent, Child Not Responsible” increased by 2.11 units.
Neuropsychological Factors Related to Adherence/Responsibility
Bowel Management
Executive dysfunction was concurrently related to whether a child was “Nonadherent, Child Not Responsible” (M = 1.83) versus “Adherent, Child Responsible” (M = 1.51) to bowel programs, B = 4.99, Wald χ2 (1) = 9.10, p = .003. The odds ratio indicated that as problem scores on the BRIEF increased by one unit (with higher scores representing higher executive dysfunction), the odds of being “Nonadherent, Child Not Responsible” increased by 147.58 units (see Table III). BRIEF scores at Time 1 also significantly predicted whether a child was “Nonadherent, Child Not Responsible” (M = 1.81) versus “Adherent, Child Responsible” (M = 1.51) with bowel management at Time 2, B = 3.14, Wald χ2 (1) = 6.25, p = .01. The odds ratio showed that as total number of problems on the BRIEF increased by one unit, the odds of being “Not Adherent, Child Not Responsible” increased by 23.20 units. Further, IQ scores predicted whether a child was “Adherent, Child Not Responsible” (M = 83.89) versus “Adherent, Child Responsible” (M = 99.70) to bowel programs at Time 2, B = −.06, Wald χ2 (1) = 6.64, p = .01. The odds ratio showed that as IQ increased by one unit, the odds of being “Adherent, Child Not Responsible” decreased by 0.94 units.
Catheterization
There were no significant findings for neuropsychological variables (p’s > .05) predicting catheterization group membership.
Family Functioning Factors Related to Adherence/Responsibility
Bowel Management
Family cohesion significantly predicted whether a child was “Nonadherent, Child Responsible” (M = 3.23) versus “Adherent, Child Responsible” (M = 3.01) to bowel programs, B = 3.60, Wald χ2 (1) = 4.11, p = .04 at Time 1. Counterintuitively, the odds ratio indicated that as family cohesion increased by one unit, the odds of being “Nonadherent, Child Responsible” increased by 36.54 units (see Table III). Family stress significantly predicted whether a child was “Nonadherent, Child Not Responsible” (M = 2.27) versus “Adherent, Child Responsible” (M = 1.75) to bowel programs at Time 1, B = 1.59, Wald χ2 (1) = 3.89, p = .04. The odds ratio indicated that as family stress increased by one unit, the odds of being “Nonadherent, Child Not Responsible” increased by 4.90 units. Similarly, prospective analyses showed that family stress significantly predicted whether a child was “Nonadherent, Child Not Responsible” (M = 2.03) versus “Adherent, Child Responsible” (M = 1.72) to bowel programs at Time 2, B = 1.88, Wald χ2 (1) = 4.63, p = .03. The odds ratio indicated that as family stress increased by one unit, the odds of being “Nonadherent, Child Not Responsible” increased by 6.53 units.
Catheterization
There were no significant cross-sectional associations between measures of family functioning and group membership at Time 1 (p’s > .05). Prospective analyses indicated that observed family cohesion predicted whether a child was “Nonadherent, Child Responsible” (M = 3.62) versus “Adherent, Child Responsible” (M = 3.39) to catheterization at Time 2, B = 3.04, Wald χ2 (1) = 4.30, p = .04. The odds ratio showed that as observed family cohesion increased by one unit, the odds of being “Nonadherent, Child Responsible” increased by 20.83 units (see Table III).
Peer Functioning Factors Related to Adherence/Responsibility
Bowel Management
Friendship quality, peer conflict, and emotional support from peers did not relate to group membership at Time 1 (p’s > .05). Observed peer conflict significantly predicted whether a child was “Adherent, Child Not Responsible” (M = 2.02) versus “Adherent, Child Responsible” (M = 1.73), B = 1.80, Wald χ2 (1) = 4.43, p = .03. The odds ratio showed that as observed peer conflict increased by one unit, the odds of being “Adherent, Child Not Responsible” increased by 6.02 units (see Table III). Emotional support from peers significantly predicted whether a child was “Nonadherent, Child Responsible” (M = 3.47) versus “Adherent, Child Responsible” (M = 3.05), B = 1.63, Wald χ2 (1) = 4.02, p = .04. The odds ratio showed that as emotional support from peers increased by one unit, the odds of being “Nonadherent, Child Responsible” increased by 5.10 units (see Table II).
Catheterization
There were no significant findings for peer functioning variables (p’s > .05) predicting catheterization group membership.
Exploratory Analyses
For Time 1 adherence, lower SES (B = −.01 β = −.36, t (63) = −2.50, p = .02) and more executive dysfunction (measured by the BRIEF; B = −.57, β = −.40, t (63) = −2.72, p =. 01) related to poorer adherence. Lower medical adherence (B = .32, β = .38, t (48) = 2.61, p =. 01) and higher observed family cohesion at Time 1 (B = −.34, β = −.35, t (48) = −2.36, p = .03) predicted poorer adherence at Time 2. For Time 1 medical responsibility, older child age (B = .08, β = .56, t (63) = 3.92, p = .0003) and lower lesion level (B = −.05 β = −.48, t (63) = −3.30, p = .002) related to more child responsibility. For Time 2, higher observed family conflict at Time 1 (B = .26 β = .27, t (48) = 2.83, p = .01) related to more child responsibility at Time 2.
Discussion
The purpose of this multisource, multimethod study was to examine biological, neuropsychological, and social predictors of medical adherence and responsibility in an early adolescent SB sample at two, biennial study time points. This study extended the current literature by testing the utility of a bio-neuropsychosocial model of adjustment (Holmbeck & Devine, 2010) to evaluate the concurrent and prospective utility of four different domains of functioning for the development of medical responsibility and adherence in youth with SB. Strengths of this investigation included use of mother-, father-, child-, and teacher-reported data, observational measures of family and peer dynamics, and the longitudinal nature of the study.
In main study analyses, limitations in gross motor functioning were associated with concurrent and longitudinal predictors of lower medical responsibility. These findings, coupled with previous data on high rates of parent-facilitated adherence to medical recommendations (Psihogios & Holmbeck, 2013), indicate that parents appear to manage more severe SB quite well. Notably, parents of youth with more severe SB demonstrated resilience in their ability to manage more severe disease factors and subsequent medical demands. Nevertheless, adolescents who struggle to become autonomous with their medical care may be the same individuals who will struggle to meet other medical and nonmedical independence goals, such as successfully transitioning to adult-centered care (Sawyer & Macnee, 2010) and obtaining employment (Zukerman, Devine, & Holmbeck, 2011). Youth with SB who have more severe disease markers may need additional, ongoing health care interventions and supports (e.g., access to patient advocates and vocational rehabilitation) to promote independence skills in medical and nonmedical domains.
Neuropsychological functioning emerged as one of the strongest predictors of medical adherence and responsibility, particularly for bowel management. Similar to past research (e.g., Tarazi, Andrew Zabel, & Mahone, 2008; O’Hara and Holmbeck, 2013; Friedman etal., 2009), we discovered that the neuropsychological impairments associated with SB (e.g., executive dysfunction and intellectual difficulties) negatively impacted a child’s ability to obtain independence. Executive dysfunction also emerged as a barrier to medical adherence. Although some parents were successful in managing medical responsibilities, other parents struggled to adhere to medical recommendations while caring for their neuro-cognitively complex child. Potentially, a child’s symptoms of executive dysfunction may undermine a parent’s ability to manage their child’s care; a child with poor inhibition and emotional control may be oppositional to their bowel program. Another explanation is that caring for the developmental needs of a child with more profound neuropsychological deficits may cause significant stress for parents, which we identified as another important risk factor for nonadherence.
In the family domain, we discovered complex relationships between medical management and family dynamics. Contrary to past studies that have shown a positive relationship between family cohesion and medical adherence (e.g., Stepansky etal., 2010), we found higher levels of concurrent family cohesion in families of youth who were responsible for, but also nonadherent to, bowel treatments. Past research shows that when parents of youth with SB balance emotional support, affection, and approval with age-appropriate expectations and consequences, adherence was maximized (O’Hara & Holmbeck, 2013). It is possible that families of youth with SB who show high levels of family cohesion and child responsibility, but nonadherence to treatments, may struggle with setting age-appropriate expectations with the medical regimen. We also found that parents who reported high levels of family stress were more likely to report concurrent and longitudinal parental nonadherence to their child’s bowel program. Similar to other pediatric populations (Fredericks, Lopez, Magee, Shieck, & Opipari-Arrigan, 2007), our study found that family stress was a significant barrier to parents’ medical adherence, as well as limited child engagement in their bowel regimen. These complex family findings demonstrate the fine balance that parents must strike between supporting their young adolescent’s developing autonomy, managing their own stress levels, setting age-appropriate limits, and engaging in problem-solving discussions with their child about medical responsibilities.
Within the peer domain, we found that youth with high levels of peer conflict were adherent, but not responsible for their bowel program. Unfortunately, youth who do not obtain responsibility for their medical management in adolescence may be at risk for further difficulties with peers (e.g., peer rejection) owing to an inability to keep pace with peers’ growing independence. We also found a significant association between child-reported emotional support from peers and child nonadherence to bowel treatments. Youth who have a strong affiliation with peers may be more prone to forgetting medical management responsibilities owing to spending time with friends, or disregard medical responsibilities in favor of being more like their typically developing peers.
While examining medical adherence and child medical responsibility simultaneously is valuable for designing interventions aimed at the successful transfer of medical responsibilities to youth, our categorical approach of limited our statistical power and ability to test the bio-neuropsychosocial model by simultaneously considering all relevant predictors. To augment main study findings, we conducted exploratory hierarchical regression analyses, which included all predictors and accounted for relevant covariates (child age, SES, and for prospective analyses, medical responsibility or adherence at Time 1). We found that higher lesion level (which relates to greater SB severity) correlated with less child responsibility with all medical tasks at Time 1. This finding is similar to our main study results, which showed that parents maintain their involvement in the disease regimen for youth with higher SB severity. Similar to our categorical analyses, more executive dysfunction (measured by the BRIEF) related to poorer concurrent adherence. Regarding family functioning, we again found support that higher levels of observed family cohesion at Time 1 were associated with poorer adherence at Time 2.
Different from main study findings, we showed that higher family conflict at Time 1 related to more child responsibility with the medical regimen 2 years later. Higher family conflict at Time 1 may reflect disagreements between family members during the initial steps of the transfer process. Importantly, a few main study findings were not supported by exploratory analyses, such as higher family stress being associated with poorer adherence and medical responsibility. Differences in findings may reflect consideration of covariates, such as age and SES, which also have pervasive impacts on a broad range of pediatric health-care outcomes (Mullins etal., 2011; Miller & Harris, 2011). Future research should consider how these nonmodifiable demographic variables affect modifiable factors (such as family dynamics), and in turn, directly and indirectly influence medical self-management.
There are several limitations of the current study that should be addressed in future work. First, the sample size was small, particularly when evaluating the categorical catheterization groups. This limitation in statistical power also precluded our ability to control for baseline medical adherence and responsibility in the longitudinal categorical analyses as well as relevant confounds (e.g., child age and family SES). Further, wide confidence intervals were found in some cases; thus, odds ratios should be interpreted with caution. Third, this sample included a large age range of youth (ages 8–15 years at Time 1 and youth ages 10–18 years at Time 2), who were at diverse stages of development. To address this limitation, we examined whether age differed by group and controlled for age in the exploratory hierarchical linear regression analyses. As expected, participants in the “Not Responsible” groups (i.e., Groups 2 and 4) were younger than participants in the reference group (Group 1). Thus, findings related to the “Not Responsible” group may have been influenced by age. There were also limitations regarding the parent-report questionnaire measure of medical adherence. While self-report measures possess key advantages, including being low-cost, minimally burdensome to families, and easy to administer, they may inflate adherence rates owing to social desirability (Stirratt etal., 2015). Further, main study findings focused on the two SB medical tasks that are salient for most individuals with SB, but did not take into account other relevant medical tasks (e.g., conducting routine skin checks) or the young adolescent’s individualized prescribed regimen. This was partially addressed by evaluating overall adherence and responsibility (across all medical domains) in the exploratory analyses.
Consistent with the bio-neuropsychosocial model of adjustment for pediatric SB, biological (e.g., lesion level, gross motor functioning), neuropsychological (e.g., executive functioning), and psychosocial (e.g., family cohesion and stress) risk factors emerged as important targets for provision of medical management resources and interventions. This study represents one of the first to consider medical adherence and child responsibility simultaneously as outcomes, which is an important first step toward developing effective self-management interventions for youth with SB. This study offers potential targets for medical self-management interventions for young adolescents with poorly managed SB, including parent stress-management and collaborative family-based, problem-solving to navigate the characteristic neuropsychological challenges associated with medical management. For example, parent problem-solving skills training has been shown to be an efficacious intervention for improving distress among mothers of children with cancer (Sahler etal., 2013), and may be generalized to reducing stress among parents of children with SB, while also promoting adaptive problem-solving with medical tasks. Mobile health (mHealth) interventions via smart phone applications are also a promising platform for delivering medical self-management interventions to individuals with SB (Dicianno etal., 2016) and, based on findings in the present study, mobile messages may require tailoring based on the individual’s unique disease and neuropsychological, and social characteristics.
According to the bio-neuropsychosocial model, risk and resilience factors likely influence each other, with each factor evolving and changing over time (Holmbeck & Devine, 2010). Thus, future work should replicate findings with a larger sample size, while considering the interactions among these variables across time (e.g., the transactional relationship between child neuropsychological factors and family dynamics) in relation to SB medical adherence and the allocation of treatment responsibilities. Finally, evaluating the development of medical self-management skills into emerging adulthood, when young adults with SB seek a successful transition to adult health care, will be an important focus of future research.
Acknowledgment
This study is part of an ongoing, longitudinal study. The authors express their gratitude to participating children, families, physicians, nurses, and teachers.
Funding
This research was supported in part by grants from the National Institute of Child Health and Human Development (R01 HD048629) and the March of Dimes (12-FY13-271).
Conflict of interest: None declared.
References
- Anderson B. J., Auslander W. F., Jung K. C., Miller J. P., Santiago J. V. (1990). Assessing family sharing of diabetes responsibilities. Journal of Pediatric Psychology, 15, 477–492. [DOI] [PubMed] [Google Scholar]
- Anderson B. J., Ho J., Brackett J., Finkelstein D., Laffel L. (1997). Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. Journal of Pediatrics, 130, 257–265. [DOI] [PubMed] [Google Scholar]
- Bukowski W. M., Hoza B., Boivin M. (1994). Measuring friendship quality during pre-and early adolescence: The development and psychometric properties of the Friendship Qualities Scale. Journal of Social and Personal Relationships, 11, 471–484. [Google Scholar]
- Caterino J. M., Scheatzle M. D., D’antonio J. A. (2006). Descriptive analysis of 258 emergency department visits by spina bifida patients. The Journal of Emergency Medicine, 31, 17–22. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1992). A power primer. Psychological Bulletin, 112, 155.. [DOI] [PubMed] [Google Scholar]
- Davis B. E., Shurtleff D. B., Walker W. O., Seidel K. D., Duguay S. (2006). Acquisition of autonomy skills in adolescents with myelomeningocele. Developmental Medicine & Child Neurology, 48, 253–258. [DOI] [PubMed] [Google Scholar]
- Delis D. C., Kaplan E., Kramer J. H. (2001). Delis-Kaplan Executive Function System (D-KEFS): Psychological Corporation. [Google Scholar]
- Dennis M., Landry S. H., Barnes M., Fletcher J. M. (2006). A model of neurocognitive function in spina bifida over the life span. Journal of the International Neuropsychological Society, 12, 285–296. [DOI] [PubMed] [Google Scholar]
- Dicianno B. E., Fairman A. D., McCue M., Parmanto B., Yih E., McCoy A., Pramana G., Yu D. X., McClelland J., Collins D. M., Brienza D. M. (2016). Feasibility of using mobile health to promote self-management in Spina Bifida. American Journal of Physical Medicine & Rehabilitation, 95, 425–437. [DOI] [PubMed] [Google Scholar]
- Dicianno B. E., Kurowski B. G., Yang J. M. J., Chancellor M. B., Bejjani G. K., Fairman A. D., Lewis N., Sotirake J. (2008). Rehabilitation and medical management of the adult with spina bifida. American Journal of Physical Medicine & Rehabilitation, 87, 1027–1050. [DOI] [PubMed] [Google Scholar]
- Fredericks E. M., Magee J. C., Opipari-Arrigan L., Shieck V., Well A., Lopez M. J. (2008). Adherence and health-related quality of life in adolescent liver transplant recipients. Pediatric Transplantation, 123, 289–299. [DOI] [PubMed] [Google Scholar]
- Fredericks E., Lopez M., Magee J., Shieck V., Opipari-Arrigan L. (2007). Psychological functioning, nonadherence and health outcomes after pediatric liver transplantation. American Journal of Transplantation, 7, 1974–1983. [DOI] [PubMed] [Google Scholar]
- Friedman D., Holmbeck G. N., DeLucia C., Jandasek B., Zebracki K. (2009). Trajectories of autonomy development across the adolescent transition in children with spina bifida. Rehabilitation Psychology, 54, 16.. [DOI] [PubMed] [Google Scholar]
- Gioia G. A., Isquith P. K., Guy S. C., Kenworthy L. (2000). Test review behavior rating inventory of executive function. Child Neuropsychology, 6, 235–238. [DOI] [PubMed] [Google Scholar]
- Grey M., Schulman-Green D., Knafl K., Reynolds N. R. (2015). A revised self-and family management framework. Nursing Outlook, 63, 162–170. [DOI] [PubMed] [Google Scholar]
- Holbein C. E., Lennon J. M., Kolbuck V. D., Zebracki K., Roache C. R., Holmbeck G. N. (2014). Observed differences in social behaviors exhibited in peer interactions between youth with spina bifida and their peers: Neuropsychological correlates. Journal of Pediatric Psychology, 40, 320–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck G. N., Belvedere M., Gorey-Ferguson L., Schneider J. (1995). Manual for family macro-coding. Unpublished manual. Loyola University of Chicago. [Google Scholar]
- Holmbeck G. N., Devine K. A. (2010). Psychosocial and family functioning in spina bifida. Developmental Disabilities Research Reviews, 16, 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck G. N., Zebracki K., Johnson S., Belvedere M., Hommeyer J. (2007). Parent-child interaction macro-coding manual. Unpublished coding system. Chicago: Loyola University Chicago. [Google Scholar]
- Johnson S., Holmbeck G. (1999). Parental overprotectiveness coding manual. Unpublished manuscript. Loyola University Chicago. [Google Scholar]
- Kaugars A. S., Zebracki K., Kichler J. C., Fitzgerald C. J., Greenley R. N., Alemzadeh R., Holmbeck G. N. (2010). Use of an observational coding system with families of adolescents: Psychometric properties among pediatric and healthy populations. Journal of Pediatric Psychology, 36, 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennard B. D., Stewart S. M., Olvera R., Bawdon R. E., Lewis C. P., Winick N. J. (2004). Nonadherence in adolescent oncology patients: Preliminary data on psychological risk factors and relationships to outcome. Journal of Clinical Psychology in Medical Settings, 11, 31–39. [Google Scholar]
- La Greca A. M., Mackey E. R. (2009). Type 1 diabetes mellitus In Behavioral approaches to chronic disease in adolescence (pp. 85–100): New York, NY: Springer. [Google Scholar]
- Lennon J. M., Klages K. L., Amaro C. M., Murray C. B., Holmbeck G. N. (2015). Longitudinal study of neuropsychological functioning and internalizing symptoms in youth with Spina Bifida: Social competence as a mediator. Journal of Pediatric Psychology, 40, 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. A., Harris D. (2011). Measuring children's decision-making involvement regarding chronic illness management. Journal of Pediatric Psychology, 37, 292–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A. C., Pai A. L., Hommel K. A., Hood K. K., Cortina S., Hilliard M. E., Guilfoyle SM, Gray W. N., Drotar D. (2012). Pediatric self-management: A framework for research, practice, and policy. Pediatrics, 129, 473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos R., Moos B. (1994). Family environment scale manual: Development, applications, research (3rd edn). Palo Alto, CA: Consulting Psychologist Press. [Google Scholar]
- Mullins L. L., Wolfe-Christensen C., Chaney J. M., Elkin T. D., Wiener L., Hullmann S. E., Fedele D. A., Junghans A. (2011). The relationship between single-parent status and parenting capacities in mothers of youth with chronic health conditions: The mediating role of income. Journal of Pediatric Psychology, 36, 249–257. [DOI] [PubMed] [Google Scholar]
- Naglieri J. A., Das J. P. (1997). Cognitive assessment system administration and scoring manual. Itasca, IL: Riverside Publishing. [Google Scholar]
- O'Hara L. K., Holmbeck G. N. (2013). Executive functions and parenting behaviors in association with medical adherence and autonomy among youth with spina bifida. Journal of Pediatric Psychology, 38, 675–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psihogios A. M., Holmbeck G. N. (2013). Discrepancies in mother and child perceptions of spina bifida medical responsibilities during the transition to adolescence: Associations with family conflict and medical adherence. Journal of Pediatric Psychology, 38, 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psihogios A. M., Kolbuck V., Holmbeck G. N. (2015). Condition self-management in pediatric spina bifida: A longitudinal investigation of medical adherence, responsibility-sharing, and independence skills. Journal of Pediatric Psychology, 40, 790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quittner A. L., Glueckauf R. L., Jackson D. N. (1990). Chronic parenting stress: Moderating versus mediating effects of social support. Journal of Personality and Social Psychology, 59, 1266.. [DOI] [PubMed] [Google Scholar]
- Quittner A. L., Modi A. C., Lemanek K. L., Ievers-Landis C. E., Rapoff M. A. (2008). Evidence-based assessment of adherence to medical treatments in pediatric psychology. Journal of Pediatric Psychology, 33, 916–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoff M. A. (2011). Adherence to pediatric medical regimens Second Edition. New York, NY: Springer Science & Business Media [Google Scholar]
- Reed-Knight B., Blount R. L., Gilleland J. (2014). The transition of health care responsibility from parents to youth diagnosed with chronic illness: A developmental systems perspective. Family, Systems, & Health, 32, 219–234. [DOI] [PubMed] [Google Scholar]
- Riddle R., Morton A., Sampson J., Vachha B., Adams R. (2005). Performance on the NEPSY among children with spina bifida. Archives of Clinical Neuropsychology, 20, 243–248. [DOI] [PubMed] [Google Scholar]
- Robin A. L., Foster S. L. (1989). Negotiating parent-adolescent conflict: A behavioral-family systems approach. New York, NY: Guilford Press. [Google Scholar]
- Rosenbaum P. L., Palisano R. J., Bartlett D. J., Galuppi B. E., Russell D. J. (2008). Development of the gross motor function classification system for cerebral palsy. Developmental Medicine & Child Neurology, 50, 249–253. [DOI] [PubMed] [Google Scholar]
- Sahler O. J. Z., Dolgin M. J., Phipps S., Fairclough D. L., Askins M. A., Katz E. R., Noll R. B., Butler R. W. (2013). Specificity of problem-solving skills training in mothers of children newly diagnosed with cancer: Results of a multisite randomized clinical trial. Journal of Clinical Oncology, 31, 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin K. J., Bellin M. H., Roux G., Buran C. F., Brei T. J. (2009). The experience of self‐management in adolescent women with spina bifida. Rehabilitation Nursing, 34, 26–38. [DOI] [PubMed] [Google Scholar]
- Sawyer S. M., Macnee S. (2010). Transition to adult health care for adolescents with spina bifida: Research issues. Developmental Disabilities Research Reviews, 16, 60–65. [DOI] [PubMed] [Google Scholar]
- Schwartz L., Tuchman L., Hobbie W., Ginsberg J. (2011). A social‐ecological model of readiness for transition to adult‐oriented care for adolescents and young adults with chronic health conditions. Child Care, Health and Development, 37, 883–895. [DOI] [PubMed] [Google Scholar]
- Smetana J. G., Yau J., Restrepo A., Braeges J. L. (1991). Adolescent-parent conflict in married and divorced families. Developmental Psychology, 27, 1000. [Google Scholar]
- Stepansky M. A., Roache C. R., Holmbeck G. N., Schultz K. (2010). Medical adherence in young adolescents with spina bifida: Longitudinal associations with family functioning. Journal of Pediatric Psychology, 35, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirratt M. J., Dunbar-Jacob J., Crane H. M., Simoni J. M., Czajkowski S., Hilliard M. E., Aikens J. E., Hunter C. M., Velligan D. I., Huntley K., Ogedegbe G., Rand C. S., Schron E., Huntley K. (2015). Self-report measures of medication adherence behavior: Recommendations on optimal use. Translational Behavioral Medicine, 5, 470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi R. A., Andrew Zabel T., Mahone M. E. (2008). Age-related differences in executive function among children with spina bifida/hydrocephalus based on parent behavior ratings. The Clinical Neuropsychologist, 22, 585–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (1999). WASI: Wechsler abbreviated scale of intelligence manual. San Antonio, TX: Harcourt Assessment, Inc. [Google Scholar]
- Wills K. W. (1993). Neuropsychological functioning in children with spina bifida and/or hydrocephalus. Journal of Clinical Child Psychology, 22, 247–265. [Google Scholar]
- Wysocki T., Gavin L. (2006). Paternal involvement in the management of pediatric chronic diseases: Associations with adherence, quality of life, and health status. Journal of Pediatric Psychology, 31, 501–511. [DOI] [PubMed] [Google Scholar]
- Zukerman J. M., Devine K. A., Holmbeck G. N. (2011). Adolescent predictors of emerging adulthood milestones in youth with spina bifida. Journal of Pediatric Psychology, 36, 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]