Abstract
Study Objectives
Sleep bruxism (SB) is considered as a possible etiological factor for temporomandibular disorder (TMD) pain. However, polysomnographic (PSG) studies, which are current “gold standard” diagnostic approach to SB, failed to prove an association between SB and TMD. A possible explanation could be that PSG studies have considered only limited characteristics of SB activity: the number of SB events per hour and, sometimes, the total duration of SB per night. According to the sports sciences literature, lack of adequate rest time between muscle activities leads to muscle overloading and pain. Therefore, the aim of this study was to determine whether the intervals between bruxism events differ between patients with and without TMD pain.
Methods
Two groups of female volunteers were recruited: myofascial TMD pain group (n=124) and non-TMD control group (n=46). From these groups, we selected 86 (69%) case participants and 37 (80%) controls who had at least two SB episodes per night based on PSG recordings. A linear mixed model was used to compare case and control groups over the repeated observations of interepisode intervals.
Results
The duration of interepisode intervals was statistically similar in the case (mean [standard deviation {SD}] 1137.7 [1975.8] seconds)] and control (mean [SD] 1192.0 [1972.0] seconds) groups. There were also a similar number of SB episodes per hour and a total duration of SB episodes in both groups.
Conclusions
The current data fail to support the idea that TMD pain can be explained by increasing number of SB episodes per hour of sleep or decreasing the time between SB events.
Keywords: facial pain, myalgia, muscle contraction, sleep, polysomnography
Statement of Significance.
The role of sleep bruxism (SB) in the etiology of jaw-muscle pain remains controversial. In this paper, we aimed to explain developing of jaw-muscle pain by taking into account the duration of “rest-time” intervals between SB events. Because no significant difference between case group with jaw-muscle pain and pain-free control group was detected, we conclude that the duration of “rest-time” intervals is not the key factor. Further research is needed to clarify the possible etiological factors for jaw-muscle pain.
INTRODUCTION
Sleep bruxism (SB) is a repetitive jaw-muscle activity characterized by clenching or grinding of the teeth and/or by bracing or thrusting of the mandible during sleep.1 It is a rather common condition. Reported prevalence is up to 41% (range: 3.5%–40.6%) in the general adult population,2 with the highest estimates coming from studies that used self-report to diagnose SB. Currently, polysomnography (PSG) with audio-video recordings is considered the most accurate method to diagnose SB.3,4 Based on PSG studies,5 the reported prevalence of SB in the general population is around 7%. SB has long been considered as a possible etiological factor for temporomandibular disorders (TMDs).6–8 TMD is a collective term embracing a number of clinical problems of the musculoskeletal structures of the masticatory system. The most frequently reported symptom is pain originating from the masticatory muscles, which is often aggravated during function.9–12
To diagnose jaw-muscle pain (myofascial TMD pain) as well as other TMDs, structured self-report and clinical instruments like the Research Diagnostic Criteria for TMD10 (RDC/TMD) and the Diagnostic Criteria for TMD12 (DC/TMD) are recommended. In the dental office, jaw-muscle pain can be found in many patients who are assumed to engage in SB. It can be speculated that patients with developed jaw-muscle pain are presumed to have “overloaded” their muscles, which is not unlikely to occur due to the unconscious nature of SB. Several hypotheses have been proposed to explain the development of overuse-related muscle pain.13 The most important of these are the duration of the muscle activity,14 the type of muscle contraction15 (ie, concentric or eccentric), and the rest intervals between subsequent muscle contractions.16 It is widely accepted that the balance between muscle work and recovery plays a crucial role in preventing overloading.17 A lack of adequate recovery might thus lead to muscle overloading and pain.
Previous studies about the association between SB and jaw-muscle pain, which used PSG and the RDC/TMD as the respective diagnostics approaches, have used only few characteristics of jaw-muscle activity to describe SB, viz, the number of bruxism episodes per hour of sleep and, in some cases, the total duration of bruxism episodes.18,19 These studies yielded contradictory results: some found an association between TMD pain and SB20, whereas others detected either no association21 or even a negative one.22,23
There are no studies so far describing associations between the duration of interepisode intervals (IEI) and jaw-muscle pain. However, this characteristic of jaw-muscle activity could be important in producing muscle overloading that in turn may cause jaw-muscle pain. Therefore, the aim of this study was to determine, based on sleep-laboratory PSG recordings, whether the intervals between SB events (IEI) differ between patients with and without myofascial face pain. We hypothesized shorter IEI in TMD patients than non-TMD control participants.
MATERIAL AND METHODS
The study was approved by The Institutional Review Board at the New York University (NYU) School of Medicine (New York, New York) (study # I07-303).
Participants
Participants were recruited from among patients seeking treatment at the NYU College of Dentistry. Before entering the study, all participants completed a full informed consent process and signed an informed consent form.
For the present study, only female volunteers were recruited, given the higher prevalence of TMD pain in women.24 The participants were allocated in the case group or in the control group based on the presence or absence of myofascial TMD pain, independent of their own beliefs regarding the presence or absence of SB. The control sample was a demographic match to the case participants regarding age, socioeconomic status, and race.
The RDC/TMD was used to establish the diagnosis of myofascial TMD pain. Two raters used RDC/TMD training tapes and materials and were initially calibrated to high levels of diagnostic concordance, with repeat periodic reliability testing throughout the study. One of the two raters was the “main” rater providing the study data, whereas the other rater (who was experienced in using the RDC) served as gold standard.
Potential participants were excluded from either group if they reported a history of trauma to the face, acute dental problems, or recent extensive dental treatment. At least 48 hours should have passed between the latest dental treatment and the RDC/TMD examination. Also, persons were excluded from participation if they were pregnant, habitually smoked after bedtime, habitually slept less than 4 hours per night, had a neuropathic facial pain condition, or had been diagnosed with severe obstructive sleep apnea (ie, an apnea-hypopnea index 30 or more events/hour of sleep) requiring continuous positive airway pressure, which would have interfered with SB measurement (see below). Moreover, studying the IEI between SB events requires the presence of at least two SB episodes per night. Thus, participant who presented less than two SB episodes per night based on PSG registration (see below) were excluded from the final analysis.
In all, 124 women with a diagnosis of myofascial TMD pain and 46 pain-free control participants completed the sleep laboratory studies. From them, 86 case participants (69%) and 37 controls (80%) had at least two SB episodes. Thus, the final analysis of IEI between SB events was based on data from 123 participants.
Polysomnography
The PSG registrations were performed at a sleep laboratory affiliated with the NYU School of Medicine. Participants were studied in the sleep laboratory for two consecutive nights. The first night allowed for adaptation to the sleep laboratory environment. The second night was used for the registration of jaw-muscle activity and sleep architecture. Data from the first night were, however, used for the statistical analysis in 10 instances: three cases failed to return for the second night and six cases and one control were missing data during the second night due to technical problems.
The onset and offset times of the nocturnal PSG recordings were determined from each participant’s habitual sleep times, with the recordings running approximately from 10:30 pm to 07:00 am. The PSG record consisted of a six-channel electroencephalogram, a bilateral electrooculogram, a bilateral submental (chin) and anterior tibialis electromyogram (EMG), a right and left masseter and temporalis EMG, an electrocardiogram, chest and abdominal motion (by means of belts with piezoelectric sensors), body position, airflow by nasal pressure transducer and nasal-oral thermistor, and oximetry.
Outcome Measures
PSG data were exported to Stellate Harmonie software (Natus, San Carlos, California) for analysis. Two raters independently scored sleep stages, arousals, apneas, and periodic limb movements. Inter-rater reliability for identification of SB episodes by the two scorers was excellent (κ = 0.89). For reasons unrelated to this study, one of the sleep scorers was not blinded to the participants’ case-control status.
Jaw-muscle activity was analyzed using the Research Diagnostic Criteria for SB (RDC/SB) for the right masseter.3 Masseter activity that exceeded twice the amplitude of the relaxed waking EMG level before sleep was used as the threshold. Against this reference, phasic episodes were defined by three or more brief (> 0.25 seconds and < 2.0 seconds) EMG bursts. Tonic episodes were scored if the burst duration was longer than 2 seconds. A mixed episode corresponds to phasic and tonic bursts, separated by an interval lasting less than 3 seconds. EMG episodes that were separated by an IEI of at least 3 seconds were scored as different episodes.
Data Analysis
As a first step, the frequency of SB episodes per hour of sleep was calculated and participants who had two or more SB episodes per night were selected. Then, the frequency of SB episodes per hour of sleep, the duration of SB episodes, and the duration of the intervals between SB episodes were calculated. The time between sleep onset and the first SB episode, as well as the time between the last SB episode and the end of sleep, were excluded from the last calculation.
Independent samples t-tests were used to compare age and educational levels between the two groups, and a chi-square test was used to compare groups on race, ethnicity, marital, and work status. A median test was used to compare groups on the average number and average duration of the SB episodes. A linear mixed model was used to compare case and control groups over the repeated observations of IEI, following rank transformation of those durations to correct skew in their distribution. The choice of rank transformation, instead of commonly used logarithm or square root one, was based on our experience that suggests that rank transformation deals better with outliers.25 All tests employed a significance level of five percent. Statistical analysis was performed using IBM SPSS Statistics 23 software (IBM Corp., Armonk, New York, USA).
RESULTS
The control and case groups did not differ in terms of measured demographic characteristics. Controls and cases were, respectively, of similar age (mean [standard deviation {SD}] = 34.7 [12.9] vs. 37.8 [13.4] years; p = .23), educational level (mean [SD] = 15.6 [2.1] vs. 15.9 [2.2] years; p = .57), race (61.1 vs. 70% white; p = .23), Hispanic ethnicity (26.7 vs. 17.3%; p = .77), currently employed for pay (69.4 vs. 59.5%; p = 0.31), and never married (66.7 vs. 65.8%; χ2 = 1.5, p = .92). Cases reported a mean (SD) characteristic pain intensity of 5.1 (1.75) and median pain chronicity of 84 months.
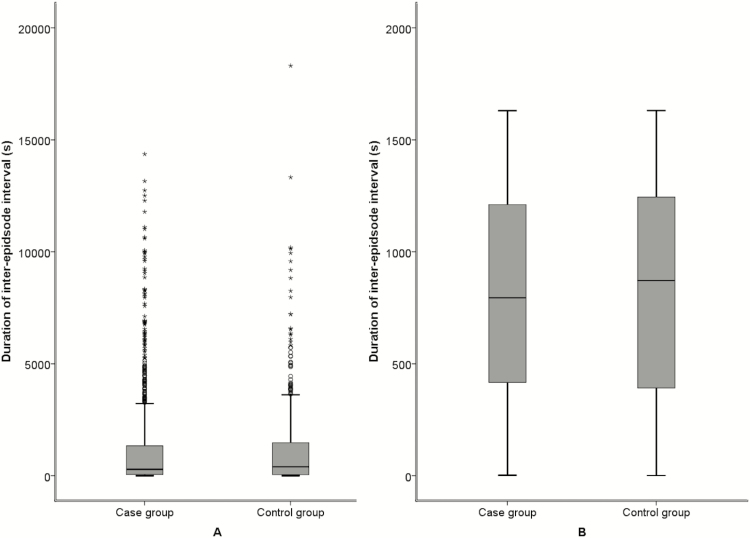
Polysomnography. The number of SB episodes per hour of sleep ranged widely in this sample, from 0 to 11, with a median of 1.4 episodes. There were a similar number of SB episodes per hour in control and case groups (mean [SD] = 2.2 [1.9] and 2.1 [2.0]), respectively (p = .77). The duration of SB episodes per night also varied widely, with durations ranging from 0.9 to 402.6 seconds and a median duration of 45 seconds. Groups had a similar total duration of SB episodes (mean [SD] = 72.2 [71.4] seconds for the control group and 66.7 [74.3] seconds for the case group; p = .3). Figure 1 shows the distribution of IEI before and after rank transformation was performed. As shown, there was considerable skew in the crude distribution (A), so that while median intervals were between 5 and 6 minutes (281 seconds in cases and 393 seconds in controls), those intervals could range above 4 hours. Rank transformation of these data greatly improved the symmetry of these distributions (B), and those data were used in further analysis. Although mean rank was lower among cases than controls, consistent with the hypothesized shorter latency to next event, the linear mixed model analysis indicated statistically similar levels in the two groups. PSG results are summarized in Table 1.
Figure 1.
Box plot showing the distribution of the duration of the intervals between sleep bruxism (SB) episodes in control and case participants before (A) and after (B) rank transformation.
Table 1.
Polysomnograpy (PSG): Comparison of Sleep Bruxism (SB) Measures in Myofascial Temporomandibular Pain Disorder (TMD) Cases and Controls.
| PSG Measure | Controls (n=37) | Cases (n=86) | p value | ||
|---|---|---|---|---|---|
| Mean (SD) | Median | Mean (SD) | Median | ||
| RMMA episodes (per hour) | 2.2 (1.9) | 1.4 | 2.1 (2.0) | 1.4 | .77 |
| Duration (all episodes, seconds) | 72.2 (71.4) | 48.9 | 66.7 (74.3) | 41.2 | .3 |
| Duration of interepisode intervals (seconds) | 1192.0 (1972.0) | 393 | 1137.7 (1975.8) | 281 | .51 |
| Duration of interepisode intervals (rank) | 827.4 (482.4) | 864 | 806.7 (463.5) | 793 | .44 |
SD = standard deviation.
DISCUSSION
The aim of this study was to test, using sleep-laboratory PSG recordings, whether the IEI between SB events differ between patients with and without myofascial pain. According to the sports sciences literature, lack of adequate rest-time between muscle activities leads to muscle overloading and pain.26 However, the recommended RDC/SB mainly focus on two characteristics of jaw-muscle contraction: the number of bruxism episodes and bursts per hour of sleep. In this paper, we hypothesized that the same SB activity (ie, with the same number of episodes and bursts) can have a different effect on the jaw muscles depending on its distribution over the night. For example, patients with jaw-muscle pain can have their SB episodes distributed over the night in a skewed fashion, with the episodes mainly being present in a short period of time. This can lead to much more load and possible muscle injury than in cases where the SB episodes are more or less equally distributed over the night. To test this hypothesis, we compared the duration of IEI between SB events in individuals with jaw-muscle pain and pain-free controls. The most important finding of this study is that there is no significant difference between the case and control groups in the duration of IEI. The two groups also did not differ in the number of SB episodes per hour of sleep or in the duration of the SB episodes. The last findings echo the results reported by Raphael et al.,21 from whose data set we selected current participants with two or more SB episodes per hour of sleep. It is noteworthy that more control than case participants (80% vs. 69%; p = .045, two-tailed Fisher’s Exact test) were included in the present study from the data set of Raphael et. al.21 This is consistent with the earlier reported observation that TMD-pain patients have fewer SB episodes than non-TMD controls.21–23
The relationship between SB and TMD pain has been the topic of several studies over the last years.27 The exact nature of the relationship between these conditions, however, is still unknown, with two major but conflicting theories aiming to explain the association: the Vicious Cycle Theory and the Pain Adaptation Model.28,29 The Vicious Cycle Theory suggests that an initiating factor, such as SB, results in pain that reflexively leads to muscle spasm. In turn, this spasm leads to further pain and dysfunction, thus completing the loop. However, the evidence that supports the Vicious Cycle Theory is limited.30–32 The Pain Adaptation Model, on the other hand, suggests that muscle pain leads to a reduction in muscle activity, which protects the muscle system from further injury and promotes healing.33,34 This model is commonly considered as the most appropriate explanation for the effects of pain on muscle performance.35 Although neither the Vicious Cycle Theory nor the Pain Adaptation Model considers the temporal delays in “causality” that might exist between pain and muscle function, most of the studies on SB-TMD pain association that used PSG-based diagnostics also support the Pain Adaptation Model, providing evidence of negative or no association between the two. One PSG-based paper that supports the Vicious Cycle Theory21 used a single-night PSG, which is not in line with the RDC/SB that recommends two nights of PSG registrations and reported very high rates of SB episodes in both case and control groups, suggesting a unique sample.
Interestingly, the results of our research are consistent neither with the Vicious Cycle Theory nor with the Pain Adaptation Model. Based on the Pain Adaptation Model, one could expect that patients with jaw-muscle pain would have less SB activity than the control group, whereas based on the Vicious Cycle Theory, a decrease in jaw-muscle activity is to be expected. However, in this research, we did not find any difference in SB activity between groups. This suggests that the association between pain and muscle function is not hardwired and that other factors than pain alone determine the motor outcome.34 Indeed, pain has a multidimensional nature with several characteristics: duration, intensity, location in the specific part of motor unit, and individual response to pain.36–39 Moreover, the jaw-muscle system has a functional and structural heterogeneity.40,41 It is likely that this complex biomechanical system adapts to pain in different ways in order to maintain required functional integrity and to protect itself from further injury. This adaptation may not necessarily lead to muscle performance decrement but rather to a redistribution of function to other uninjured units.42
The above reasoning suggests that other factors than SB may play a role in maintaining jaw-muscle pain. In 2006, a large-scale project entitled Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) started to identify the risk factors for TMD. This prospective cohort study evaluated 202 phenotypic risk factors from six domains: sociodemographic, general health status, pain sensitivity, cardiac autonomic function, and psychological and clinical orofacial characteristics.43 From these, the frequency of somatic symptoms, for example, a running nose, fatigue, and dizziness, was the strongest psychosocial predictor of TMD incidence.44 Smaller contributions were found for measures of psychological stress, anxiety, obsessive-compulsive feelings, pain-coping strategies, and sleep quality. Moreover, genetic associations were found, implicating six single-nucleotide polymorphisms as risk factors for chronic TMD. This emphasizes that TMD is a complex disorder which is caused by interplay of multiple genetic and environmental factors, and that a univariate association between SB and jaw-muscle pain does not represent the actual, far more complex situation.
As suggested by the sports science literature, successful training must involve load but also must include adequate recovery periods.17 As a consequence of load, the athlete may experience acute feelings of fatigue or even pain. Followed by an adequate rest period, the acute fatigue results in a positive adaptation or improvement in performance. This is the basis of effective training programs. However, in case a disruption occurs between appropriate training load and adequate recovery, the athletes may develop a so-called nonfunctional overtraining which will lead to a decrease in performance, depression, and pain that may last for several weeks or months. Several confounding factors have been reported to contribute to nonfunctional overloading, such as inadequate diet, somatic symptoms (eg, upper respiratory tract infections), psychosocial distress (family or work related), and sleep disorders.17 This multifactorial explanatory model of overtraining syndrome seems to have much in common with the results of OPPERA study, which also suggests an interplay between similar factors in the etiology of TMD pain.
The noticeable difference between the overloading in sports and the data we presented in the current study is the time scale at which the loading and recovery occurred. In sports science literature, the training which includes both loading and recovery periods takes days to weeks (if not months). However, when SB IEI were considered in the present research, the time intervals lasted only seconds to minutes. The load and recovery model from sports literature could be applied to SB in case variable activity would be present for days to weeks. This would probably provide a different outcome than reported in the present research. Unfortunately, the current study design, with two nights of SB registration, did not allow to study the “rest” intervals at a longer time scale.
On the other hand, although the sport science literature does not provide evidence regarding the effect of short-lasting (eg, seconds) rest-time intervals on muscle injury, this evidence is available from experimental animal studies. Numerous experimental studies have shown that shorter rest-time interval (eg, seconds to minutes) between muscle contraction indeed lead to more muscle injury and dysfunction.16,45
Most participants evidenced a low rate of SB events. From our sample of 123 participants with two and more SB episodes per night, only 53 case participants (61.2%) and 23 controls (62.2 %) fulfilled minimal criteria for Sleep Bruxism Diagnosis46 but 18 (14.6% of the studied sample) had more than four SB episodes per hour of sleep, fulfilling the criteria for the high SB intensity group (Table 2). As the best test of the muscle overloading theory would be conducted among those with some critically high rate of SB, we compared inter-event intervals between cases and controls within each of the three SB intensity groups. Results were similar in each stratum (analysis not shown), but further work with extreme subjects may be necessary.
Table 2.
Distribution of participants Based on Sleep Bruxism (SB) Diagnostic Cutoff Criteria.
| SB intensity | Controls Count (%) | Cases Count (%) | Total Count (%) |
|---|---|---|---|
| Low (>1 and ≤2 episodes/hour) | 9 (24.3) | 20 (23.3) | 29 (23.6) |
| Moderate (>2 and ≤4 episodes/hour) | 8 (21.6) | 21 (24.4) | 29 (23.6) |
| High (>4 episodes/hour) | 6 (16.2) | 12 (14) | 18 (14.6) |
Although the results of our study suggest that the duration of interepisode SB intervals is not the “key factor” in the explanatory model of TMD pain, the quality of “rest intervals” still may play a role in the etiology of myofascial TMD pain. Previously, the analysis of EMG activity occurring outside of defined SB and other motor events showed that the levels were significantly higher in myofascial TMD patients compared to non-TMD controls.47 These long-lasting periods of elevated EMG activity between SB episodes could play a role in inadequate muscle recovery and eventually lead to persistent jaw-muscle pain. This suggestion is in line with the modified stress-hyperactivity-pain theory proposed by Ohrbach and McCall,48 which focused on chronic low-grade hyperactivity.
The fact that sleep background EMG activity was significantly higher for woman with myofascial TMD pain than for control woman rises a question about the thresholds used to define SB events. The background EMG activity is routinely used as the threshold to identify SB activity. One can speculate that using that threshold, which is significantly higher for pain participants than for controls, could introduce a bias. The higher threshold could lead to inclusion less SB episodes in the case group than in the control group, which may have compromised the outcomes of the study. The alternative for the threshold based on background EMG activity would be the one based on percentages from the maximum voluntary contractions (MVC), as proposed by Lavigne et al.3 However, using this threshold in myofascial TMD-pain participants may also introduce a bias. Participants with pain in their jaw muscles could try to avoid more pain during function and therefore do not express maximum bite force during the MVC recording. Further research is needed to establish the most reliable threshold for EMG activity during sleep, when participants with jaw-muscle pain are investigated.
Given these limits, current data fail to support the idea that TMD pain can be explained by increasing number of SB episodes per hour of sleep or decreasing time between SB events.
DISCLOSURE STATEMENT
Dr. Lobbezoo received funding from the University of Amsterdam/New York University Visiting Guest Faculty Program 2014–2015, for his Visiting Professorship at New York University College of Dentistry. The original study (Raphael et al. 21) was funded in part by grant R01 DE018569 from the National Institutes of Health, Bethesda, MD. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
REFERENCES
- 1. Lobbezoo F, Ahlberg J, Glaros AG et al. Bruxism defined and graded: an international consensus. J Oral Rehabil. 2013; 40(1): 2–4. [DOI] [PubMed] [Google Scholar]
- 2. Manfredini D, Restrepo C, Diaz-Serrano K, Winocur E, Lobbezoo F. Prevalence of sleep bruxism in children: a systematic review of the literature. J Oral Rehabil. 2013; 40(8): 631–642. [DOI] [PubMed] [Google Scholar]
- 3. Lavigne GJ, Rompré PH, Montplaisir JY. Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study. J Dent Res. 1996; 75(1): 546–552. [DOI] [PubMed] [Google Scholar]
- 4. Raphael KG, Santiago V, Lobbezoo F. Is bruxism a disorder or a behaviour? Rethinking the international consensus on defining and grading of bruxism. J Oral Rehabil. 2016; 43(10): 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maluly M, Andersen ML, Dal-Fabbro C et al. Polysomnographic study of the prevalence of sleep bruxism in a population sample. J Dent Res. 2013; 92(7 Suppl): 97S–103S. [DOI] [PubMed] [Google Scholar]
- 6. Glaros AG, Glass EG, McLaughlin L. Knowledge and beliefs of dentists regarding temporomandibular disorders and chronic pain. J Orofac Pain. 1994; 8(2): 216–222. [PubMed] [Google Scholar]
- 7. Velly AM, Philippe P, Gornitsky M. Heterogeneity of temporomandibular disorders: cluster and case-control analyses. J Oral Rehabil. 2002; 29(10): 969–979. [DOI] [PubMed] [Google Scholar]
- 8. Osterberg T, Carlsson GE. Relationship between symptoms of temporomandibular disorders and dental status, general health and psychosomatic factors in two cohorts of 70-year-old subjects. Gerodontology. 2007; 24(3): 129–135. [DOI] [PubMed] [Google Scholar]
- 9. McNeill C, Mohl ND, Rugh JD, Tanaka TT. Temporomandibular disorders: diagnosis, management, education, and research. J Am Dent Assoc. 1990; 120(3): 253, 255, 257 passim. [DOI] [PubMed] [Google Scholar]
- 10. Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992; 6(4): 301–355. [PubMed] [Google Scholar]
- 11. Okeson J. Orofacial pain: guidelines for classification, assessment, and management. 3rd ed Chicago: Quintessence Publishing; 1996 [Google Scholar]
- 12. Schiffman E, Ohrbach R, Truelove E et al. ; International RDC/TMD Consortium Network, International association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 2014; 28(1): 6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kreher JB, Schwartz JB. Overtraining syndrome: a practical guide. Sports Health. 2012; 4(2): 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rashedi E, Nussbaum MA. Cycle time influences the development of muscle fatigue at low to moderate levels of intermittent muscle contraction. J Electromyogr Kinesiol. 2016; 28: 37–45. [DOI] [PubMed] [Google Scholar]
- 15. Lieber RL, Fridén J. Muscle damage is not a function of muscle force but active muscle strain. J Appl Physiol (1985). 1993; 74(2): 520–526. [DOI] [PubMed] [Google Scholar]
- 16. Cutlip RG, Geronilla KB, Baker BA et al. Impact of stretch-shortening cycle rest interval on in vivo muscle performance. Med Sci Sports Exerc. 2005; 37(8): 1345–1355. [DOI] [PubMed] [Google Scholar]
- 17. Meeusen R, Duclos M, Foster C et al. ; European College of Sport Science; American College of Sports Medicine Prevention, diagnosis, and treatment of the overtraining syndrome: joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med Sci Sports Exerc. 2013; 45(1): 186–205. [DOI] [PubMed] [Google Scholar]
- 18. Camparis CM, Formigoni G, Teixeira MJ, Bittencourt LR, Tufik S, de Siqueira JT. Sleep bruxism and temporomandibular disorder: Clinical and polysomnographic evaluation. Arch Oral Biol. 2006; 51(9): 721–728. [DOI] [PubMed] [Google Scholar]
- 19. Smith MT, Wickwire EM, Grace EG et al. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009; 32(6): 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rossetti LM, Pereira de Araujo Cdos R, Rossetti PH, Conti PC. Association between rhythmic masticatory muscle activity during sleep and masticatory myofascial pain: a polysomnographic study. J Orofac Pain. 2008; 22(3): 190–200. [PubMed] [Google Scholar]
- 21. Raphael KG, Sirois DA, Janal MN et al. Sleep bruxism and myofascial temporomandibular disorders: a laboratory-based polysomnographic investigation. J Am Dent Assoc. 2012; 143(11): 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lavigne GJ, Rompré PH, Montplaisir JY, Lobbezoo F. Motor activity in sleep bruxism with concomitant jaw muscle pain. A retrospective pilot study. Eur J Oral Sci. 1997; 105(1): 92–95. [DOI] [PubMed] [Google Scholar]
- 23. Rompré PH, Daigle-Landry D, Guitard F, Montplaisir JY, Lavigne GJ. Identification of a sleep bruxism subgroup with a higher risk of pain. J Dent Res. 2007; 86(9): 837–842. [DOI] [PubMed] [Google Scholar]
- 24. LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997; 8(3): 291–305. [DOI] [PubMed] [Google Scholar]
- 25. Conover WJ, Iman RL. Rank transformations as a bridge between parametric and nonparametric statistic. Am Stat. 1981;35(3)124–129. [Google Scholar]
- 26. Meeusen R, Duclos M, Gleeson M, Rietjens G, Steinacker J, Urhausen A. Prevention, diagnosis and treatment of the overtraining syndrome—ECSS position statement “task force”. Eur J Sport Sci. 2006;6(1):1–14. [DOI] [PubMed] [Google Scholar]
- 27. Manfredini D, Lobbezoo F. Relationship between bruxism and temporomandibular disorders: a systematic review of literature from 1998 to 2008. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109(6): e26–e50. [DOI] [PubMed] [Google Scholar]
- 28. Travell JG, Rinzler S, Herman M. Pain and disability of the shoulder and arm. Treatment by intramuscular infiltration with procaine hydrochloride. J Am Med Assoc. 1942;120:417–422. [Google Scholar]
- 29. Lund JP, Donga R, Widmer CG, Stohler CS. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991; 69(5): 683–694. [DOI] [PubMed] [Google Scholar]
- 30. Stohler CS, Zhang X, Lund JP. The effect of experimental jaw muscle pain on postural muscle activity. Pain. 1996; 66(2-3): 215–221. [DOI] [PubMed] [Google Scholar]
- 31. Matre DA, Sinkjaer T, Svensson P, Arendt-Nielsen L. Experimental muscle pain increases the human stretch reflex. Pain. 1998; 75(2-3): 331–339. [DOI] [PubMed] [Google Scholar]
- 32. Svensson P, De Laat A, Graven-Nielsen T, Arendt-Nielsen L. Experimental jaw-muscle pain does not change heteronymous H-reflexes in the human temporalis muscle. Exp Brain Res. 1998; 121(3): 311–318. [DOI] [PubMed] [Google Scholar]
- 33. Lund JP, Lavigne G, Dubner R, Sessle B.. Orofacial Pain: From Basic Science to Clinical Management. Chicago: Quintessence; 2001. [Google Scholar]
- 34. Janal MN, Raphael KG, Klausner J, Teaford M. The role of tooth-grinding in the maintenance of myofascial face pain: a test of alternate models. Pain Med. 2007; 8(6): 486–496. [DOI] [PubMed] [Google Scholar]
- 35. Murray GM, Peck CC. Orofacial pain and jaw muscle activity: a new model. J Orofac Pain. 2007; 21(4): 263–78; discussion 279. [PubMed] [Google Scholar]
- 36. Svensson P, Macaluso GM, De Laat A, Wang K. Effects of local and remote muscle pain on human jaw reflexes evoked by fast stretches at different clenching levels. Exp Brain Res. 2001; 139(4): 495–502. [DOI] [PubMed] [Google Scholar]
- 37. Jensen MP, Nielson WR, Kerns RD. Toward the development of a motivational model of pain self-management. J Pain. 2003; 4(9): 477–492. [DOI] [PubMed] [Google Scholar]
- 38. Viane I, Crombez G, Eccleston C, Devulder J, De Corte W. Acceptance of the unpleasant reality of chronic pain: effects upon attention to pain and engagement with daily activities. Pain. 2004; 112(3): 282–288. [DOI] [PubMed] [Google Scholar]
- 39. Bodéré C, Téa SH, Giroux-Metges MA, Woda A. Activity of masticatory muscles in subjects with different orofacial pain conditions. Pain. 2005; 116(1-2): 33–41. [DOI] [PubMed] [Google Scholar]
- 40. Hannam AG, McMillan AS. Internal organization in the human jaw muscles. Crit Rev Oral Biol Med. 1994; 5(1): 55–89. [DOI] [PubMed] [Google Scholar]
- 41. van Eijden TM, Turkawski SJ. Morphology and physiology of masticatory muscle motor units. Crit Rev Oral Biol Med. 2001; 12(1): 76–91. [DOI] [PubMed] [Google Scholar]
- 42. Westgaard RH, de Luca CJ. Motor unit substitution in long-duration contractions of the human trapezius muscle. J Neurophysiol. 1999; 82(1): 501–504. [DOI] [PubMed] [Google Scholar]
- 43. Slade GD, Ohrbach R, Greenspan JD et al. Painful temporomandibular disorder: decade of discovery from OPPERA studies. J Dent Res. 2016; 95(10): 1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fillingim RB, Ohrbach R, Greenspan JD et al. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J Pain. 2013;14(12 Suppl):75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stauber WT, Willems ME. Prevention of histopathologic changes from 30 repeated stretches of active rat skeletal muscles by long inter-stretch rest times. Eur J Appl Physiol. 2002; 88(1-2): 94–99. [DOI] [PubMed] [Google Scholar]
- 46. Rompré PH, Daigle-Landry D, Guitard F, Montplaisir JY, Lavigne GJ. Identification of a sleep bruxism subgroup with a higher risk of pain. J Dent Res. 2007; 86(9): 837–842. [DOI] [PubMed] [Google Scholar]
- 47. Raphael KG, Janal MN, Sirois DA et al. Masticatory muscle sleep background electromyographic activity is elevated in myofascial temporomandibular disorder patients. J Oral Rehabil. 2013; 40(12): 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ohrbach R, McCall WDJr. The stress-hyperactivity-pain theory of myogenic pain. Proposal for a revised theory. Pain Forum. 1996;5:51–66. [Google Scholar]