Abstract
Objective
To characterize the clinical correlates and outcome of inflammatory ocular disease (IOD) among patients with ANCA-associated vasculitides (AAV).
Methods
Medical records of potential cases of AAV seen at Mayo Clinic from 2003 to 2013, inclusive, were reviewed to identify confirmed cases meeting the diagnosis of AAV using the Chapel Hill Consensus Conference 2012 descriptors. Records of confirmed cases of AAV were then further reviewed for IOD, and clinical characteristics, treatment and outcomes abstracted.
Results
A total of 1171 confirmed cases of AAV were identified of which 183 patients (mean age 49.0 years; 51% female; 95% Caucasian) had IOD. The most common manifestation of IOD was injection of the eye (57%) followed by eye pain (46%) and visual acuity loss (18%). Scleritis was the most common type of IOD (22%) followed by episcleritis (21%), orbital inflammation (18%), lacrimal duct stenosis (10%) and uveitis (9%). Oral glucocorticoids were used to treat IOD in the majority of patients (96%). CYC and rituximab were the most frequently used immunosuppressive agents (54 and 36%, respectively). Of those with orbital inflammation, 52% underwent therapeutic surgical intervention. Clinical remission of IOD was achieved in 91% of patients but relapses were seen in 23%. Significant visual acuity loss was observed in only six patients.
Conclusion
IOD is a common manifestation of AAV and seen in about 16% of patients with AAV. Scleritis, episcleritis and orbital inflammation are the most common subtypes. Most patients respond well to glucocorticoids and immunosuppression, but relapse of IOD is common.
Keywords: ANCA associated vasculitis, inflammatory eye disease, orbital inflammation, cohort study
Rheumatology key messages
Inflammatory ocular disease occurred in about one-fifth of patients with ANCA-associated vasculitides.
Scleritis, episcleritis and orbital inflammation were the most common subtypes of inflammatory ocular disease.
Subtype of inflammatory ocular disease was predictive for response to treatment.
Introduction
ANCA-associated vasculitides (AAV) are a group of systemic vasculitides that primarily affect small and medium-sized vessels. ANCA consists of two subtypes, namely perinuclear ANCA (p-ANCA), usually directed against MPO-ANCA, and cytoplasmic ANCA (c-ANCA), almost always directed against PR3-ANCA. PR3-ANCA is detected in the majority of patients with granulomatosis with polyangiitis (GPA) while MPO-ANCA is detected in the majority of patients with microscopic polyangiitis (MPA) and approximately half of patients with eosinophilic GPA (EGPA) [1]. ANCA specificity is predictive for response to treatment. Rituximab is more effective than CYC as induction therapy in patients with AAV who have PR3-ANCA while both treatments are equally effective in those with MPO-ANCA. Similarly, ANCA specificity is predictive for long-term prognosis as patients with positive PR3-ANCA are at higher risk of relapse than those with positive MPO-ANCA [2].
AAV have a variable clinical expression with multi-system involvement. The most commonly affected organs include lungs, kidneys, skin, paranasal sinuses, joints and peripheral nerves. Involvement of the lung and kidney is associated with a less favourable prognosis with a higher morbidity and mortality [3].
Inflammatory ocular disease (IOD) is a frequent manifestation of AAV. All parts of the eye and orbit can be affected. However, data on epidemiology, clinical presentation, relationship with ANCA status and outcome of AAV patients with IOD are limited as previously reported studies generally had a small number of subjects [4–8]. The current study was conducted with the aim of describing clinical characteristics of IOD in AAV in a cohort of patients who were seen at the Mayo Clinic, MN, USA.
Methods
Data source and study population
Medical records of potential cases of patients with AAV seen at Mayo Clinic from 2003 to 2013 were identified from the institutional database using diagnostic codes related to ANCA-associated vasculitis, GPA, Wegener’s granulomatosis, MPA, EGPA and Churg-Strauss syndrome. Those medical records were individually reviewed to confirm the diagnosis of AAV based on physician diagnosis, compatible clinical presentation and pertinent laboratory investigation such as ANCA and pathology that fulfilled the Chapel Hill Consensus Conference 2012 descriptors [9]. Medical records of the confirmed cases of AAV were then further reviewed for IOD. Data on demographics, clinical diagnosis, systemic and ophthalmological manifestations, laboratory investigations, medical treatments, surgical interventions, outcomes and complications were abstracted. BVAS for granulomatosis with polyangiitis (BVAS/GPA) at IOD diagnosis was calculated from the extent of organ involvement [10].Approval for this study was obtained from the Mayo Clinic institutional review board and the need for informed consent was waived.
Statistical analysis
Descriptive statistics (means, percentages, etc.) were used to summarize the data. Chi-squared and rank sum tests were used for comparisons between groups. A P < 0.05 was considered statistically significant for all analyses. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
A total of 1955 medical records with diagnostic codes related to AAV were identified from the institutional database. After individual medical review, diagnosis of AAV was confirmed in 1171 patients. Most of the excluded patients were patients who were initially evaluated for AAV but were ultimately found to have other diagnoses. Our search algorithm picks up any patient who ever had the diagnostic codes of AAV attached to the medical record. The high sensitivity but low specificity of diagnostic codes of AAV has been observed in other databases as well [11]. Of the 1171 included patients with confirmed AAV, 183 patients (mean age at IOD diagnosis 49.0 years; 51% female; 95% Caucasian) had IOD. The median follow-up after IOD diagnosis was 6.6 years. At diagnosis, PR3-ANCA was positive in 117 patients (64%) and MPO-ANCA was positive in 39 patients (21%). ANCA serology was negative in 27 patients (15%), and none of these became positive during follow-up. GPA was the most common clinical diagnosis (152 patients, 83%), followed by MPA (23 patients, 13%) and EGPA (8 patients, 4%). Tables 1 and 2 summarize the demographics, clinical characteristics and outcome of patients with IOD in this cohort by ANCA subtype and clinical diagnosis, respectively.
Table 1.
Demographics and clinical characteristics of inflammatory ocular disease in patients with ANCA-associated vasculitis by ANCA subtype
| Treatment | Negative ANCA | p-ANCA/ MPO-ANCA | c-ANCA/ PR3-ANCA | Total | P-value |
|---|---|---|---|---|---|
| (n = 27) | (n = 39) | (n = 117) | (n = 183) | ||
| Demographics | |||||
| Age at diagnosis of IOD, mean (s.d.), years | 51.0 (20.6) | 53.9 (17.9) | 46.9 (16.3) | 49.0 (17.5) | 0.053 |
| Female, n (%) | 16 (59) | 20 (51) | 57 (49) | 93 (51) | 0.613 |
| Ethnicity, n (%) | 0.131 | ||||
| Caucasian | 26 (96) | 36 (92) | 112 (96) | 174 (95) | |
| African-American | 0 (0) | 1 (3) | 0 (0) | 1 (1) | |
| Native Hawaiian/other Pacific islander | 1 (4) | 0 (0) | 0 (0) | 1 (1) | |
| Asian | 0 (0) | 1 (3) | 3 (3) | 4 (2) | |
| Native American | 0 (0) | 1 (3) | 0 (0) | 1 (1) | |
| Other | 0 (0) | 0 (0) | 2 (2) | 2 (1) | |
| Duration of follow-upa, median (IQR), years | 6.0 (3.3–9.7) | 5.5 (2.1–9.9) | 7.0 (3.9–10.9) | 6.6 (3.4–10.7) | 0.318 |
| Clinical characteristics | |||||
| IOD onset prior or at diagnosis of AAV, n (%) | 18 (67) | 27 (69) | 52 (44) | 97 (53) | 0.008 |
| IOD onset after diagnosis of AAV, n (%) | 9 (33) | 12 (31) | 65 (56) | 86 (47) | |
| Time from IOD to AAV diagnosis for those with IOD onset prior to/at diagnosis of AAV, mean (s.d.), months | 11.1 (11.3) | 6.0 (13.8) | 5.9 (11.7) | 6.9 (12.3) | 0.035 |
| Time from AAV diagnosis to IOD for those who had IOD onset after AAV, mean (s.d.), months | 92.2 (89.5) | 25.9 (22.4) | 64.4 (64.9) | 61.9 (65.4) | 0.084 |
| BVAS/GPA at IOD onset, mean (s.d.) | 2.7 (2.6) | 5.7 (4.2) | 5.3 (3.9) | 5.0 (3.9) | 0.002 |
| Presenting symptoms, n (%) | |||||
| Ocular injection | 4 (15) | 24 (62) | 77 (66) | 105 (57) | <0.001 |
| Eye pain | 14 (52) | 14 (36) | 56 (48) | 84 (46) | 0.343 |
| Gradual visual loss | 6 (22) | 2 (5) | 14 (12) | 22 (12) | 0.110 |
| Sudden visual loss | 2 (7) | 3 (8) | 6 (5) | 11 (6) | 0.799 |
| Diplopia | 12 (44) | 8 (21) | 10 (9) | 30 (16) | <0.001 |
| Epiphora | 5 (19) | 1 (3) | 19 (16) | 25 (14) | 0.072 |
| Photophobia | 1 (4) | 5 (13) | 15 (13) | 21 (11) | 0.390 |
| Headache | 3 (11) | 6 (15) | 9 (8) | 18 (10) | 0.366 |
| Proptosis | 5 (19) | 4 (10) | 7 (6) | 16 (9) | 0.107 |
| Floaters | 1 (4) | 1 (3) | 2 (2) | 4 (2) | 0.802 |
| Dry eye | 1 (4) | 2 (5) | 0 (0) | 3 (2) | 0.061 |
| Type of eye disease, n (%) | |||||
| Scleritis | 1 (4) | 5 (13) | 34 (29) | 40 (22) | 0.005 |
| Episcleritis | 1 (4) | 10 (26) | 28 (24) | 39 (21) | 0.052 |
| Orbital inflammation | 12 (44) | 6 (15) | 15 (13) | 33 (18) | 0.001 |
| Lacrimal duct stenosis | 3 (11) | 0 (0) | 16 (14) | 19 (10) | 0.053 |
| Uveitis | 1 (4) | 1 (3) | 14 (12) | 16 (9) | 0.120 |
| Conjunctivitis | 1 (4) | 5 (13) | 6 (5) | 12 (7) | 0.197 |
| Cranial nerve II, IV or VI palsy | 3 (11) | 6 (15) | 2 (2) | 11 (6) | 0.004 |
| Peripheral ulcerative keratitis | 0 (0) | 4 (10) | 3 (3) | 7 (4) | 0.051 |
| Dacryoadenitis | 3 (11) | 2 (5) | 3 (3) | 8 (4) | 0.142 |
| Optic neuritis | 1 (4) | 3 (8) | 3 (3) | 7 (4) | 0.351 |
| Amaurosis fugax | 2 (8) | 2 (5) | 3 (3) | 7 (4) | 0.443 |
| Retinal vasculitis | 1 (4) | 1 (3) | 1 (1) | 3 (2) | 0.505 |
| Laterality of IOD, n (%) | 0.927 | ||||
| Unilateral | 17 (63) | 23 (59) | 69 (59) | 109 (60) | |
| Bilateral | 10 (37) | 16 (41) | 48 (41) | 74 (40) | |
| Complications | |||||
| Posterior synechiae, n (%) | 0 (0) | 1 (3) | 0 (0) | 1 (1) | 0.156 |
| Cataract, n (%) | 5 (19) | 9 (23) | 22 (19) | 36 (20) | 0.833 |
| Glaucoma, n (%) | 1 (4) | 3 (8) | 11 (9) | 15 (8) | 0.618 |
| Optic nerve atrophy, n (%) | 1 (4) | 2 (5) | 6 (5) | 9 (5) | 0.951 |
| Relapse | |||||
| At least one relapse, n (%) | 4 (15) | 10 (26) | 28 (24) | 42 (23) | 0.540 |
First occurrence of eye disease to last follow-up. AAV: ANCA-associated-vasculitis; c-ANCA: cytoplasmic ANCA; p-ANCA: peri-ANCA; BVAS/GPA: BVAS for granulomatosis with polyangiitis; IOD: inflammatory ocular disease.
Table 2.
Demographics and clinical characteristics of inflammatory ocular disease in patients with ANCA-associated vasculitis by clinical diagnosis
| Treatment | EGPA | GPA | MPA | Total | P-value |
|---|---|---|---|---|---|
| (n = 8) | (n = 152) | (n = 23) | (n = 183) | ||
| Demographics | |||||
| Age at diagnosis of IOD, mean (s.d.), years | 59.6 (8.2) | 48.0 (17.5) | 51.4 (18.8) | 49.0 (17.5) | 0.111 |
| Female, n (%) | 1 (13) | 79 (52) | 13 (57) | 93 (51) | 0.079 |
| Ethnicity, n (%) | 0.578 | ||||
| Caucasian | 8 (100) | 145 (95) | 21 (91) | 174 (95) | |
| African-American | 0 (0) | 0 (0) | 1 (4) | 1 (1) | |
| Native Hawaiian/other Pacific islander | 0 (0) | 1 (1) | 0 (0) | 1 (1) | |
| Asian | 0 (0) | 3 (2) | 1 (4) | 4 (2) | |
| Native American | 0 (0) | 1 (1) | 0 (0) | 1 (1) | |
| Other | 0 (0) | 2 (1) | 0 (0) | 2 (1) | |
| Duration of follow-up,a median (IQR) years | 8.4 (4.9–10.3) | 6.7 (3.7–10.7) | 5.0 (1.9–9.0) | 6.6 (3.4–10.7) | 0.283 |
| Clinical characteristics | |||||
| IOD onset prior or at diagnosis of AAV, n (%) | 3 (38) | 80 (53) | 14 (61) | 97 (53) | 0.509 |
| IOD onset after diagnosis of AAV, n (%) | 5 (63) | 72 (47) | 9 (39) | 86 (47) | |
| Time from IOD to AAV diagnosis for those with IOD onset prior to/at diagnosis of AAV, mean (s.d.), months | 0.8 (0.8) | 7.1 (12.9) | 7.1 (9.8) | 6.9 (12.3) | 0.557 |
| Time from AAV diagnosis to IOD for those who had IOD onset after AAV, mean (s.d.), months | 48.9 (51.6) | 67.8 (68.3) | 22.3 (22.5) | 61.9 (65.4) | 0.076 |
| BVAS/GPA at IOD onset, mean (s.d.) | 5.6 (3.8) | 4.9 (3.9) | 5.5 (4.2) | 5.0 (3.9) | 0.624 |
| Presenting symptoms, n (%) | |||||
| Ocular injection | 2 (25) | 89 (59) | 14 (61) | 105 (57) | 0.163 |
| Eye pain | 0 (0) | 74 (49) | 10 (43) | 84 (46) | 0.026 |
| Gradual visual loss | 0 (0) | 21 (14) | 1 (4) | 22 (12) | 0.242 |
| Sudden visual loss | 3 (38) | 8 (5) | 0 (0) | 11 (6) | <0.001 |
| Diplopia | 3 (38) | 22 (14) | 5 (22) | 30 (16) | 0.175 |
| Epiphora | 0 (0) | 23 (15) | 2 (9) | 25 (14) | 0.363 |
| Photophobia | 0 (0) | 17 (11) | 4 (17) | 21 (11) | 0.398 |
| Headache | 1 (13) | 14 (9) | 3 (13) | 18 (10) | 0.820 |
| Proptosis | 0 (0) | 16 (11) | 0 (0) | 16 (9) | 0.167 |
| Floaters | 0 (0) | 3 (2) | 1 (4) | 4 (2) | 0.700 |
| Dry eye | 0 (0) | 3 (2) | 0 (0) | 3 (2) | 0.733 |
| Type of eye disease, n (%) | |||||
| Scleritis | 0 (0) | 36 (24) | 4 (17) | 40 (22) | 0.246 |
| Episcleritis | 2 (25) | 32 (21) | 5 (22) | 39 (21) | 0.964 |
| Orbital inflammation | 0 (0) | 32 (21) | 1 (4) | 33 (18) | 0.060 |
| Lacrimal duct stenosis | 0 (0) | 19 (13) | 0 (0) | 19 (10) | 0.115 |
| Uveitis | 0 (0) | 15 (10) | 1 (4) | 16 (9) | 0.457 |
| Conjunctivitis | 0 (0) | 9 (6) | 3 (13) | 12 (7) | 0.326 |
| Cranial nerve II, IV or VI palsy | 3 (38) | 4 (3) | 4 (17) | 11 (6) | <0.001 |
| Peripheral ulcerative keratitis | 0 (0) | 5 (3) | 2 (9) | 7 (4) | 0.383 |
| Dacryoadenitis | 0 (0) | 8 (5) | 0 (0) | 8 (4) | 0.426 |
| Optic neuritis | 1 (13) | 5 (3) | 1 (4) | 7 (4) | 0.412 |
| Amaurosis fugax | 2 (25) | 5 (3) | 0 (0) | 7 (4) | 0.005 |
| Retinal vasculitis | 0 (0) | 2 (1) | 1 (4) | 3 (2) | 0.528 |
| Laterality of IOD, n (%) | 0.243 | ||||
| Unilateral | 5 (63) | 94 (62) | 10 (43) | 109 (59) | |
| Bilateral | 3 (38) | 58 (38) | 13 (57) | 74 (41) | |
| Complications | |||||
| Posterior synechiae, n (%) | 0 (0) | 0 (0) | 1 (4) | 1 (1) | 0.030 |
| Cataract, n (%) | 0 (0) | 31 (20) | 5 (22) | 36 (20) | 0.355 |
| Glaucoma, n (%) | 0 (0) | 13 (9) | 2 (9) | 15 (8) | 0.688 |
| Optic nerve atrophy, n (%) | 0 (0) | 8 (5) | 1 (4) | 9 (5) | 0.791 |
| Relapse | |||||
| At least one relapse, n (%) | 0 (0) | 38 (25) | 4 (17) | 42 (23) | 0.207 |
First occurrence of eye disease to last follow-up. AAV: ANCA-associated vasculitis; BVAS/GPA: BVAS for granulomatosis with polyangiitis; EGPA: eosinophilic granulomatosis with polyangiitis; GPA: granulomatosis with polyangiitis; IOD: inflammatory ocular disease; MPA: microscopic polyangiitis.
Overall, among the 183 patients with IOD, IOD was one of the initial manifestations that led to diagnosis of AAV in about half of them (53%) with median time from IOD to diagnosis of AAV of 1.8 months. IOD was more likely to be the initial manifestation of MPO-ANCA-associated disease (69%) and negative-ANCA disease (67%) than PR3-ANCA-associated disease (45%; P = 0.008). However, this difference was not observed with the analysis by clinical diagnosis. IOD occurred after the diagnosis of AAV in 47% of patients with median time from diagnosis of AAV to the first occurrence of IOD of 40.2 months. Mean (s.d.) BVAS/GPA during active IOD was 5.0 (3.9). Mean BVAS/GPA was significantly lower among those with negative ANCA [2.7 (2.6)] compared with those with MPO-ANCA-associated disease [5.7 (4.2)] and PR3-ANCA-associated disease [5.3 (3.9); P = 0.002]. This difference was not observed with the analysis by clinical diagnosis.
The most common manifestation of IOD was ocular injection (105 patients, 57%) followed by eye pain (84 patients, 46%), visual acuity (VA) loss (33 patients, 18%; gradual loss 22 patients, 12% and sudden loss 11 patients, 6%), diplopia (30 patients, 16%), epiphora (25 patients, 14%) and photophobia (21 patients, 11%). IOD was bilateral in 74 patients (40%). Percentages of bilateral involvement for specific type of IOD are as follows: 75% for conjunctivitis, 73% for cranial nerve palsy, 67% for retinal vasculitis, 53% for episcleritis, 50% for optic neuritis, 33% for orbital inflammation, 29% for peripheral ulcerative keratitis, 29% for amaurosis fugax, 28% for scleritis and 25% for uveitis. The majority of those who had bilateral IOD had the disease in both eyes at the same time (60 patients, 81%); eyes’ involvement was sequential in 14 patients (19%).
Scleritis was the most common subtype of IOD (40 patients, 22%) followed by episcleritis (39 patients, 21%), orbital inflammation (inflammatory orbital pseudotumour/orbital myositis) (33 patients, 18%), lacrimal duct stenosis (19 patients, 10%), uveitis (16 patients, 9%), conjunctivitis (12 patients, 7%), cranial nerve III, IV or VI palsy (11 patients, 6%), dacryoadenitis (8 patients, 4%), optic neuritis (7 patients, 4%), amaurosis fugax (7 patients, 4%), peripheral ulcerative keratitis (7 patients, 4%) and retinal vasculitis (3 patients, 2%).
In the analysis by serology (Table 1), the order of frequency of subtype of IOD among patients with PR3-ANCA-associated disease was similar to the order of frequency of subtype of IOD for the entire cohort as they were the majority of patients in this cohort. Among those with MPO-ANCA-associated disease, episcleritis, orbital inflammation and cranial nerve III, IV or VI palsy were more common than scleritis. On the other hand, orbital inflammation was by far the most common subtype of IOD (44%) among those with negative ANCA. A total of five patients had positive non-specific ANCA (i.e. positive c-ANCA or p-ANCA without positive MPO-ANCA and PR3-ANCA). One had orbital pseudotumour and optic neuropathy, one had episcleritis, one had scleritis and two had amaurosis fugax. The presenting symptoms include eye pain (two patients), sudden visual loss (one patient), gradual vision loss (one patient), dryness (one patient), floater (one patient) and ocular injection (two patients).
Similarly, in the analysis by clinical diagnosis (Table 2), the order of frequency of subtype of IOD among patients with GPA was similar to the order of frequency of subtype of IOD for the entire cohort as they were the majority of patients in this cohort. Scleritis, episcleritis and conjunctivitis were also common among patients with MPA. However, orbital pseudotumour/myositis was very uncommon; it was found in only one case. IOD was an uncommon manifestation of EGPA with only eight cases observed in this cohort. Cranial nerve III, IV or VI palsy, amaurosis fugax, optic neuritis and episcleritis were the subtypes of IOD seen in EGPA.
Detailed ophthalmological examination was available in 36 of 40 patients with scleritis. All of them had anterior scleritis. Data on subtype of anterior scleritis were available in 30 cases. Diffuse anterior scleritis was the most common subtype (16 cases) followed by nodular anterior scleritis (9 cases), scleromalacia perforans (3 cases) and necrotizing anterior scleritis (2 cases).
Orbital inflammation/pseudotumour was observed in 33 patients. ANCA was negative in about one-third of them (36%), while PR3-ANCA was positive in 45% and MPO-ANCA was positive in 19%. All but one patient had clinical diagnosis of GPA. Imaging of the orbit was obtained in all patients and contiguous sinus involvement was noted in the majority of patients (28 of 33 patients). Most of these patients with orbital inflammation (88%) underwent orbital biopsy which revealed necrotizing vasculitis (52%), small vessel vasculitis without necrosis (14%), chronic non-specific inflammation (21%) and granulomatous inflammation (14%). Orbital inflammation was the only manifestation at diagnosis in 10 patients (30% of all patients with orbital inflammation). Among those 10 patients, ANCA was negative in 3, MPO-ANCA was positive in 3 and PR3-ANCA was positive in 4. Uveitis was observed in 16 patients; 15 had anterior uveitis and 1 had panuveitis. The majority of them (14 patients, 88%) had PR3-ANCA. Similarly, the majority of them (15 patients, 94%) had clinical diagnosis of GPA.
Oral glucocorticoids were used to treat IOD in the majority of patients (175 patients, 96%) while topical glucocorticoids were used in 28% (52 patients). CYC was the most frequently used immunosuppressive agent (99 patients, 54%) followed by rituximab (65 patients, 36%), MTX (54 patients, 30%), AZA (50 patients, 27%) and MMF (27 patients, 15%). These immunosuppressive agents were often used in combination (most commonly, MTX and rituximab). Treatment decisions were made based on the patient’s overall disease presentation and only in the minority of patients based only on the eye disease.
Of 33 patients with orbital inflammation/pseudotumour, 15 (45%) underwent debulking surgery and 2 (6%) underwent orbital decompression surgery. Over half of these patients (17 patients, 52%) also received intra-orbital glucocorticoid injections. Twenty-eight patients (85%) with orbital inflammation/pseudotumour achieved remission with therapy. Of 19 patients who had lacrimal duct stenosis from AAV, 15 (79%) underwent dacryocystorhinostomy. Medications and surgical treatments for eye diseases by ANCA subtype and by clinical diagnosis are provided in Table 3.
Table 3.
Treatment of inflammatory ocular disease in patients with ANCA-associated vasculitis by ANCA subtype and by clinical diagnosis
| Treatment | Total | Negative ANCA | p-ANCA/ MPO | c-ANCA/ PR3 | P-value | EGPA | GPA | MPA | P-value |
|---|---|---|---|---|---|---|---|---|---|
| (n = 183) | (n = 27) | (n = 39) | (n = 117) | (n = 8) | (n = 152) | (n = 23) | |||
| Medications | |||||||||
| Topical glucocorticoids | 52 (28) | 2 (7) | 12 (31) | 38 (32) | 0.032 | 0 (0) | 42 (28) | 10 (43) | 0.055 |
| Oral glucocorticoids | 176 (96) | 23 (85) | 37 (95) | 115 (98) | 0.011 | 7 (88) | 147 (97) | 21 (91) | 0.257 |
| Intra-orbital glucocorticoids injection | 17 (9) | 9 (33) | 1 (3) | 7 (6) | <0.001 | 0 (0) | 17 (11) | 0 (0) | 0.148 |
| CYC | 99 (54) | 9 (33) | 18 (46) | 72 (62) | 0.016 | 3 (38) | 86 (57) | 10 (43) | 0.315 |
| Rituximab | 65 (36) | 7 (26) | 11 (28) | 47 (40) | 0.212 | 2 (25) | 60 (39) | 3 (13) | 0.039 |
| Anti-TNF-α therapy | 2 (1) | 2 (7) | 0 (0) | 0 (0) | 0.003 | 0 (0) | 2 (1) | 0 (0) | 0.814 |
| MTX | 54 (30) | 9 (33) | 7 (18) | 38 (32) | 0.203 | 0 (0) | 50 (33) | 4 (17) | 0.055 |
| MMF | 27 (15) | 2 (7) | 6 (15) | 19 (16) | 0.503 | 0 (0) | 22 (14) | 5 (22) | 0.319 |
| AZA | 50 (27) | 5 (19) | 10 (26) | 35 (30) | 0.471 | 2 (25) | 42 (28) | 6 (26) | 0.977 |
| HCQ | 1 (1) | 0 (0) | 1 (3) | 0 (0) | 0.156 | 0 (0) | 0 (0) | 1(4) | 0.030 |
| Oral NSAIDs | 3 (2) | 0 (0) | 0 (0) | 3 (3) | 0.423 | 0 (0) | 2 (1) | 1 (4) | 0.528 |
| Surgical interventions | |||||||||
| Dacryocystorhinostomy | 15 (8) | 3 (11) | 0 (0) | 12 (10) | 0.108 | 0 (0) | 15 (10) | 0 (0) | 0.189 |
| Orbital decompression surgery | 2 (1) | 1 (4) | 0 (0) | 1 (1) | 0.334 | 0 (0) | 2 (1) | 0 (0) | 0.814 |
| Orbital debulking surgery | 16 (9) | 6 (22) | 3 (8) | 7 (6) | 0.026 | 0 (0) | 16 (11) | 0 (0) | 0.167 |
All values are given as n (%). c-ANCA: cytoplasmic ANCA; p-ANCA: peri-nuclear ANCA; EGPA: eosinophilic granulomatosis with polyangiitis; GPA: granulomatosis with polyangiitis; MPA; micropolyangiitis.
Clinical remission of eye inflammation was achieved in 167 patients (91%). Analysis by clinical diagnosis revealed that 100% of patients with EGPA and MPA achieved clinical remission while 89% of patients with GPA achieved this (P = 0.19). Analysis by serology revealed a similar percentage of remission between PR3-ANCA-associated disease and MPO-ANCA-associated disease (92 and 97%, respectively) but the percentage was significantly lower among those with negative ANCA (78%, P = 0.011).
Relapse of IOD was common. A total 42 patients (23%) had at least one relapse during follow-up which included 13 patients with scleritis (33% of all patients with scleritis), 13 episcleritis (33% of all patients with episcleritis), 5 orbital inflammation/pseudotumour (15% of all patients with orbital inflammation/pseudotumour), 3 uveitis (19% of all patients with uveitis), 2 lacrimal duct stenosis (11% of all patients with lacrimal duct stenosis), 1 lacrimal gland disease (13% of all patients with lacrimal gland disease), 1 conjunctivitis (8% of all patients with conjunctivitis), 1 amaurosis fugax (14% of all patients with amaurosis fugax) and 1 eyelid skin lesion (which is the only patient with eyelid skin lesion). In all but five patients, the ocular involvement in relapse was of the same type as the initial IOD. Relapse of IOD occurred in isolation in 22 patients and in association with flare of other organs in 20 patients. The median number of relapses was 1. Clinical diagnosis was not predictive of relapse (25% in GPA, 17% in MPA and 0% in EGPA; P = 0.21), nor of serological status (24% in PR3-ANCA-associated disease, 26% in MPO-ANCA-associated disease and 15% in negative ANCA disease; P = 0.54). During follow-up, 36 patients (20%) developed cataract, 15 patients (8%) developed glaucoma and 9 patients (5%) developed optic nerve atrophy.
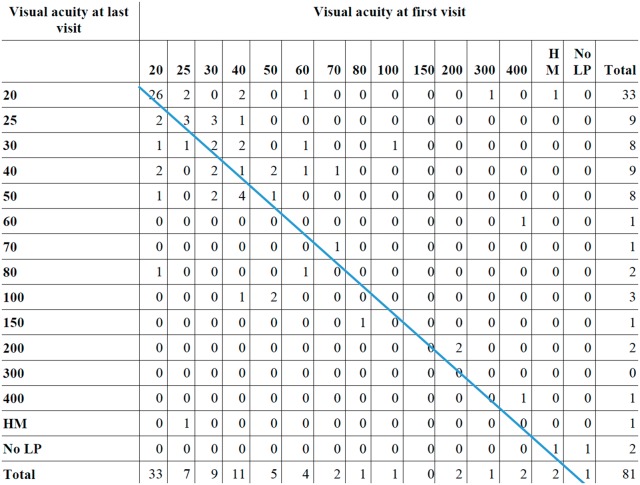
VA of the worst eye of 81 patients who had at least two ophthalmology visits at Mayo Clinic is summarized in Fig. 1. A total of 33 patients (41%) had VA of 20/20 at the initial visit and only 7 of them had worsening of VA at the last visit. Among the 48 patients who had VA worse than 20/20 at the first visit, 20 (42%) had improved VA at the last visit, 12 (25%) had the same VA and 16 (33%) had worsened VA. However, only six patients (7%) lost more than three lines of VA. Of those six patients, three had orbital inflammation, one uveitis, one scleritis and one had conjunctivitis. Cataracts developing during follow-up were responsible for reduced VA in two patients with scleritis and conjunctivitis. A total of four patients (5%) had VA of < 20/200 at last visit.
Fig. 1.
Visual acuity of worst eye at first and last visit of 81 inflammatory ocular disease patients
Numbers to the left of the line represent worse vision during follow-up while numbers to the right of the line represent improved vision. HM: hand motion; LP: light perception.
Discussion
This is a large retrospective cohort study with a long period of follow-up that investigates the clinical characteristics and outcomes of IOD in patients with AAV. Eye involvement was frequent, with 16% of patients developing IOD.
Frequency of eye involvement varied considerably among previous studies, ranging from 6 to 70% [7, 12–15] depending on studied populations and methods used to identify the cohort and IOD. In the current study, among those 16% of patients with AAV who had IOD, IOD was one of the initial manifestations leading to diagnosis of AAV in about half of them. Thus, clinicians must have a high degree of suspicion for AAV on evaluating patients with ocular inflammation. ANCA serology could be considered as a screening tool; ANCA were positive in the majority of AAV patients with IOD.
Scleritis and episcleritis were the most common types of IOD in this cohort, particularly for those with PR3-ANCA-associated disease, which is in line with previous studies [6, 12, 16]. Ocular injection and eye pain, the usual manifestations of scleritis and episcleritis, were the most frequent ophthalmological manifestations in this cohort. Necrotizing variant of anterior scleritis has been reported in a variable number of patients in the literature, with one study reporting necrotizing scleritis in about two-third of patients with scleritis [5, 17]. However, it was observed in only two patients from the total of 40 patients with scleritis in the current study.
A total of 33 patients had orbital inflammation/pseudotumour, which was the third most common subtype of IOD. Of these, the majority had orbital pain as one of the presenting symptoms (76%). Other common presenting symptoms included diplopia (45%) and injection of the eye (27%). The majority of these patients had positive biopsy for either granuloma or vasculitis (83%). This observation is consistent with previous studies that reported biopsy findings of either granuloma or vasculitis in 75–85% of patients with orbital inflammation [6, 16, 18, 19]. Interestingly, 30% of those with orbital inflammation did not have other organ involvement at the time of AAV diagnosis and one-third of these patients had negative ANCA. Contiguous sinus involvement was noted in the majority of patients with orbital inflammation/pseudotumour. A non-contiguous orbital infiltrate or inflammatory dacryoadenitis without any sinus disease was less common but underscores the notion that AAV should be included in the differential diagnosis of any patient who presents with orbital inflammation regardless of associated symptoms and/or ANCA status.
Oral glucocorticoids were the mainstay of IOD treatment in this cohort. CYC and rituximab were the most frequently used immunosuppressive agents. Over half of patients with orbital inflammation underwent surgical intervention (debulking surgery or decompression surgery) in addition to immunosuppressive therapy. Similarly, the majority of patients with lacrimal duct stenosis from AAV underwent dacryocystorhinostomy. Response to treatment is generally good as over 90% of patients achieved remission. Only six patients lost more than three lines of VA during follow-up and only four patients had VA of the worse eye less than 20/200 at the last visit. The outcome of this cohort is more favourable compared with older studies that observed significant visual loss in up to 15% of patients [7, 18, 20]. The improvement may reflect a better understanding of the disease and increased availability of treatment options in more recent years. However, relapse remained common. Interestingly, about half of the time, relapse of IOD occurred in isolation without flare of other organs.
In this cohort, neither categorization of patients per serological status or clinical phenotype was predictive of clinical outcome. Subtype of IOD was more useful to predict relapse/response to treatment. For example, the percentage of patients who did not achieve remission in the entire cohort was only 9% but it was as high as 15% among those with orbital inflammation. Similarly, about one-third of patients with scleritis/episcleritis experienced relapse while only 13% of patients with lacrimal gland disease and 8% of patients with conjunctivitis experienced relapse. It should be noted that in the analysis by serology, the percentage of patients who did not achieve remission was higher among those with negative ANCA compared with those with either PR3-ANCA-associated disease or MPO-ANCA-associated disease. However, the difference was primarily driven by the fact that about half of patients with IOD who had negative ANCA had orbital inflammation/pseudotumour.
The major strengths of this study are that it is based upon a large cohort with long duration of follow-up. The database allows capture of IOD that occurred after the initial diagnosis of AAV. Data on treatment and outcome were also available. Medical records of all cases were individually reviewed. Therefore, the accuracy of the diagnosis was high. The major limitations are those inherent in the retrospective study design, as the data on clinical manifestations and laboratory investigation were not systematically collected in a prospective fashion and, therefore, all the pertinent information might not be recorded. Not all of the AAV patients in the cohort came to ophthalmological attention, so that subclinical eye involvement may not have been detected in an unknown number of cases. Some patients who had a milder type of IOD, such as episcleritis and conjunctivitis, had only one ophthalmology visit. Therefore, data on VA during follow-up visit were only available in about half of the cohort. It is also possible that the patients in this cohort may not represent the true spectrum of the disease in the general population as it consists of AAV patients who were seen at a single tertiary care centre.
Conclusion
IOD occurred in about one-fifth of patients with AAV. Scleritis, episcleritis and orbital inflammation were the most common subtypes of IOD. Response to treatment was generally satisfactory with over 90% achieving clinical remission. Significant reduction of VA was infrequent.
Funding: This study was funded by the John M. Nasseff, Sr Clinician Career Development Award in Rheumatology to Ashima Makol, MD and supported by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). The content of this manuscript is solely the responsibility of the authors and the sponsors of these grants did not have any involvement with the study design, data collection, analysis, interpretation, in the writing of the manuscript, or the decision to submit the manuscript for publication.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Weiner M, Segelmark M.. The clinical presentation and therapy of diseases related to anti-neutrophil cytoplasmic antibodies (ANCA). Autoimmun Rev 2016;15:978–82. [DOI] [PubMed] [Google Scholar]
- 2. Cornec D, Cornec-Le Gall E, Fervenza FC, Specks U.. ANCA-associated vasculitis – clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol 2016;12:570–9. [DOI] [PubMed] [Google Scholar]
- 3. Lee T, Gasim A, Derebail VK. et al. Predictors of treatment outcomes in ANCA-associated vasculitis with severe kidney failure. Clin J Am Soc Nephrol 2014;9:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caster JC, Shetlar DJ, Pappolla MW, Yee RW.. Microscopic polyangiitis with ocular involvement. Arch Opthalmol 1996;114:346–8. [DOI] [PubMed] [Google Scholar]
- 5. Harper SL, Letko E, Samson CM. et al. Wegener’s granulomatosis: the relationship between ocular and systemic disease. J Rheumatol 2001;28:1025–32. [PubMed] [Google Scholar]
- 6. Pakrou N, Selva D, Leibovitch I.. Wegener’s granulomatosis: Ophthalmic manifestations and management. Semin Arthitis Rheum 2006;35:284–92. [DOI] [PubMed] [Google Scholar]
- 7. Hoffman GS, Kerr GS, Leavitt RY. et al. Wegener’s granulomatosis: an analysis of 158 patients. Ann Intern Med 1992;116:488–98. [DOI] [PubMed] [Google Scholar]
- 8. Haynes BF, Fishman ML, Fauci AS, Wolff SM.. The ocular manifestations of Wegener’s granulomatosis. Fifteen years experience and review of literature. Am J Med 1977;63:789–99. [DOI] [PubMed] [Google Scholar]
- 9. Jennette JC, Falk RJ, Bacon PA. et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 10. Stone JH, Hoffman GS, Merkel PA. et al. A disease-specific activity index for Wegener’s granulomatosis. Modification of the Birmingham vasculitis activity score. Arthritis Rheum 2001;44:912–20. [DOI] [PubMed] [Google Scholar]
- 11. Boyd M, Specks U, Finkielman JD.. Accuracy of the ICD-9 code for identification of patients with Wegener’s granulomatosis. J Rheumatol 2010;37:474. [DOI] [PubMed] [Google Scholar]
- 12. Fauci AS, Haynes BJ, Katz P, Wolff SM.. Wegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med 1983;98:76–85. [DOI] [PubMed] [Google Scholar]
- 13. Straatsma BR. Ocular manifestations of Wegener’s granulomatosis. Am J Med 1957;63:789–99. [DOI] [PubMed] [Google Scholar]
- 14. Englund M, Merkel PA, Tomasson G, Segelmark M, Mohammad AJ.. Comorbidities in patients with antineutrophil cytoplasmic antibody-associated vasculitis versus the general population. J Rheumatol 2016;43:1553–8. [DOI] [PubMed] [Google Scholar]
- 15. Reinhold-Keller E, Beuge N, Latza U. et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis. Long-term outcome in 155 patients. Arthritis Rheum 2000;43:1021–32. [DOI] [PubMed] [Google Scholar]
- 16. Perry SR, Rootman J, White VA.. The clinical and pathologic constellation of Wegener granulomatosis of the orbit. Ophthalmology 1997;104:683–94. [DOI] [PubMed] [Google Scholar]
- 17. Gu J, Zhou S, Ding R. et al. Necrotizing scleritis and peripheral ulcerative keratitis associated with Wegener’s granulomatosis. Ophthalmol Ther 2013;2:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sadiq SA, Jenning CR, Jones NS, Downes RN.. Wegener’s granulomatosis: the ocular manifestation revisited. Orbit 2000;19:253–61. [DOI] [PubMed] [Google Scholar]
- 19. Fechner FP, Faquin WC, Pilch BZ.. Wegener’s granulomatosis of the orbit: a clinicopathological study of 15 patients. Laryngoscope 2002;112:1945–50. [DOI] [PubMed] [Google Scholar]
- 20. Bullen CL, Liesegang TJ, McDonald TJ, DeREmee RA.. Ocular complication of Wegener’s granulomatosis. Q J Med 1993;90:279–90. [DOI] [PubMed] [Google Scholar]