Abstract
Background: Inadequate protein intake and hypoalbuminemia, indicators of protein-energy wasting, are among the strongest mortality predictors in hemodialysis patients. Hemodialysis patients are frequently counseled on dietary phosphorus restriction, which may inadvertently lead to decreased protein intake. We hypothesized that, in hypoalbuminemic hemodialysis patients, provision of high-protein meals during hemodialysis combined with a potent phosphorus binder increases serum albumin without raising phosphorus levels.
Methods: We conducted a randomized controlled trial in 110 adults undergoing thrice-weekly hemodialysis with serum albumin <4.0 g/dL recruited between July 2010 and October 2011 from eight Southern California dialysis units. Patients were randomly assigned to receive high-protein (50–55 g) meals during dialysis, providing 400–500 mg phosphorus, combined with lanthanum carbonate versus low-protein (<1 g) meals during dialysis, providing <20 mg phosphorus. Prescribed nonlanthanum phosphorus binders were continued over an 8-week period. The primary composite outcome was a rise in serum albumin of ≥0.2 g/dL while maintaining phosphorus between 3.5–<5.5 mg/dL. Secondary outcomes included achievement of the primary outcome's individual endpoints and changes in mineral and bone disease and inflammatory markers.
Results: Among 106 participants who satisfied the trial entrance criteria, 27% (n = 15) and 12% (n = 6) of patients in the high-protein versus low-protein hemodialysis meal groups, respectively, achieved the primary outcome (intention-to-treat P-value = 0.045). A lower proportion of patients in the high-protein versus low-protein intake groups experienced a meaningful rise in interleukin-6 levels: 9% versus 31%, respectively (P = 0.009). No serious adverse events were observed.
Conclusion: In hypoalbuminemic hemodialysis patients, high-protein meals during dialysis combined with lanthanum carbonate are safe and increase serum albumin while controlling phosphorus.
Keywords: hemodialysis, high-protein, hyperphosphatemia, hypoalbuminemia, protein-energy wasting
INTRODUCTION
Protein-energy wasting is a common condition and a major risk factor for adverse outcomes including higher death risk in chronic kidney disease (CKD) patients undergoing maintenance hemodialysis [1]. Biochemical markers of protein-energy wasting such as hypoalbuminemia (defined as a serum albumin <4.0 g/dL) are among the most potent predictors of death risk in dialysis patients [2–4]. Given that heightened catabolism, low-protein intake and dialytic amino acid and protein losses may predispose hemodialysis patients to hypoalbuminemia and subsequent protein-energy wasting, the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) Clinical Practice Guidelines recommend a higher protein intake of 1.2 g/kg body weight/day in this population [2]. Despite these recommendations, epidemiologic data show that >50% of hemodialysis patients have inadequate dietary protein intake (<1.0 g/kg/day) as estimated by their calculated urea kinetic–based normalized protein catabolic rates (nPCRs), also known as normalized protein nitrogen appearance (nPNA) [5].
Multiple barriers may hinder the achievement of these nutritional targets in hemodialysis patients. For example, many high-protein foods are rich in phosphorus, leading to hyperphosphatemia, which has been associated with renal bone disease, cardiovascular disease including vascular calcification and higher mortality risk [6–11]. As such, hemodialysis patients are frequently counseled on dietary phosphorus restriction, which may inadvertently lead to a reduction in protein intake [8, 12, 13]. Second, thrice-weekly dialysis sessions may coincide with core meal times, leading to inadequate food intake on dialysis treatment days [14]. This issue may be compounded by the fact that many outpatient dialysis units in the USA refrain from administering meals and prohibit outside food or beverage consumption during dialysis, given concerns about postprandial hypotension, aspiration, infection risk, staff burden and financial constraints [13, 15]. Third, the importance of nutritional status, as well as the benefits of nutritional supportive measures, may remain under-recognized and underprioritized in the hemodialysis population.
It has been suggested that dietary liberalization in conjunction with greater use of phosphorus binders may be a more effective strategy in addressing the inadequate protein intake of hemodialysis patients [12, 13, 16]. To better inform the field, we designed the randomized controlled trial Fosrenol (lanthanum carbonate) for Enhancing Dietary Protein Intake in Hypoalbuminemic Dialysis Patients (FrEDI) in order to test the hypothesis that the provision of high-protein meals during hemodialysis treatment sessions in the dialysis clinic combined with a potent phosphorus binder in hypoalbuminemic hemodialysis patients would increase serum albumin levels without adversely impacting their phosphorus levels.
MATERIALS AND METHODS
Study design and population
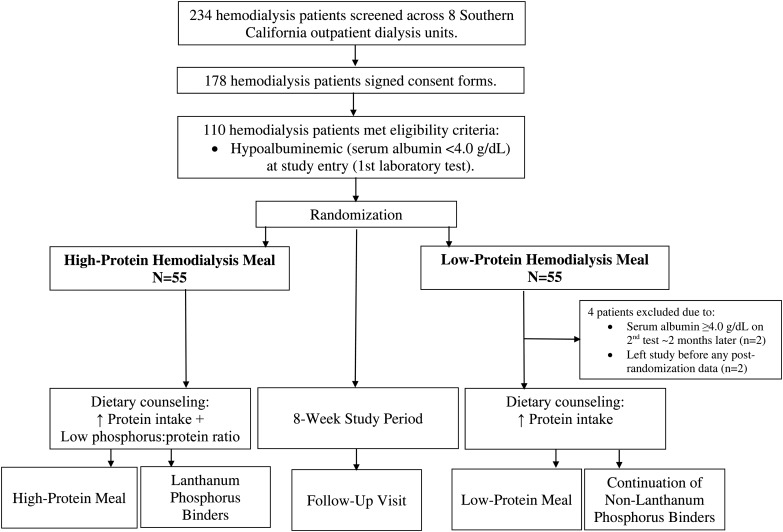
We designed the FrEDI randomized controlled trial to compare the effect of high-protein meals administered during dialysis treatments combined with lanthanum carbonate as a potent binder versus low-protein meals during dialysis in 110 prevalent hemodialysis patients. Study coordinators recruited patients from eight outpatient dialysis clinics in Southern California from 1 July 2010 through 31 October 2011 (see ClinicalTrials.gov; NCT0111694). Inclusion criteria were the following: (i) ages 18–85 years; (ii) thrice-weekly hemodialysis for ≥3 months; (iii) hypoalbuminemia, defined as a baseline bromocresol green serum albumin level <4.0 g/dL on two measurements separated by ∼2 months; (iv) capability of safe and independent oral food intake during hemodialysis treatments and (v) informed (written) consent to participate in the study. Patients were excluded if they (i) had received lanthanum carbonate phosphate binder(s) within 2 weeks of study entry, (ii) had a medical condition that may limit increasing dietary protein intake (e.g. inability to eat or maintain ingested food) or (iii) were unwilling to sign the consent form. All patients were eligible to receive vitamin D pharmacotherapies, including calcitriol, cholecalciferol, doxercalciferol, ergocalciferol and paricalcitol, as well as cinacalcet, at the discretion of the treating nephrologist. The sponsor, investigators and patients were unaware of the treatment assignments until the first meal box was opened by the patient. The study was approved by the Institutional Review Board of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center and was conducted in accordance with the ethical principles of the Declaration of Helsinki, the Good Clinical Practice guidelines of the International Conference of Harmonisation and local regulatory requirements.
Study intervention
Eligible patients underwent a washout period of 2 weeks (i.e. in the event they previously received lanthanum carbonate >2 weeks before study entry), attended a dietary counseling session with the study dietitians and underwent pre-study examination in the Outpatient General Clinical Research Center of Harbor-UCLA. They subsequently underwent 1:1 randomization to the high-protein versus low-protein hemodialysis meal groups using computer-generated sequences by the senior database manager, who was blinded to patient assignment (Figure 1). Allocation was recorded using sealed envelopes. Patients assigned to the high-protein hemodialysis meal group received high-protein meals in the form of prepared food in meal boxes (see Supplementary data) during the first 60 min of each thrice-weekly hemodialysis treatment over a period of 8 weeks (a total of 24 meals during 24 hemodialysis treatment sessions), which were administered as prepared boxes containing meals with 50–55 g of protein, 850 kcal and a low phosphorus:protein ratio of <10 mg/g, yielding 400–450 mg of natural phosphorus. Patients in the high-protein hemodialysis meal group also received 0.5–1.5 g of lanthanum carbonate with each meal box that was titrated every 2 weeks as necessary. Lanthanum carbonate was specifically selected given its high phosphate-binding capacity and low pill burden compared with other phosphorus binders [17]. Patients assigned to the low-protein hemodialysis meal group received prepared boxes containing meals with minimal protein (<1 g), <20 mg phosphorus and low caloric (<50 kcal) content, such as salads, during the first 60 min of each hemodialysis treatment over the study period (see Supplementary data). They were also allowed to continue their preexisting nonlanthanum carbonate phosphate binders. Meal boxes for both study groups were prepared at the Los Angeles Biomedical Research Institute/Harbor UCLA General Clinical Research Center Bio-Nutrition Department (see Supplementary data for detailed methods). Both treatment groups received routine dietary counseling on consuming high-protein intake at home (i.e. dietary protein intake to achieve or maintain an nPCR of 1.0 g/kg/day). The high-protein hemodialysis meal group received additional counseling on consuming a low phosphorus:protein ratio diet in which they were advised to avoid processed foods with high phosphorus additives [18].
FIGURE 1.
Study population.
Study outcomes
The primary composite outcome was an increase in serum albumin of ≥0.2 g/dL while maintaining a target serum phosphorus range of 3.5–<5.5 mg/dL, based on NKF-KDOQI Clinical Practice Guidelines [2], following the 8-week intervention period. This serum albumin threshold was selected according to prior data demonstrating associations with lower mortality, hospitalization risk and treatment costs in hemodialysis patients [19–21]. Secondary outcomes included (i) achievement of the individual endpoints of the primary composite outcome, (ii) change in chronic kidney disease–mineral and bone disease biochemical parameters [serum calcium, parathyroid hormone (PTH), alkaline phosphatase], (iii) change in nPCR, (iv) change in serum electrolytes that correlate with protein intake (serum bicarbonate, potassium), (v) change in dialysis adequacy parameters [single-pool Kt/V (spKt/V), urea reduction ratio], (vi) change in inflammatory parameters [tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), C-reactive protein (CRP)], (vii) change in hematologic parameters (hemoglobin, white blood cell count, platelet count), (viii) change in lipid levels [low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides] and the adipokine leptin and (ix) change in postdialysis weight following the 8-week study intervention.
Biochemical measurements
Baseline serum albumin and phosphorus levels were measured within 5 days prior to randomization and intervention, then every month thereafter. Most other secondary outcomes of interest were also concurrently measured on a monthly basis, including calcium, PTH, alkaline phosphatase, nPCR, bicarbonate, potassium, spKt/V, urea reduction ratio, hemoglobin, white blood cell count and platelet count. PTH was measured using a first-generation immunoradiometric PTH assay (Nichols, San Juan Capistrano, CA, USA [22]). Non-routine blood tests including TNF-α, IL-6, CRP and leptin were measured prior to the trial and then after the trial ended in those who completed the entire 8-week study.
Statistical analysis
Intention-to-treat analyses were performed to include all those who participated in the study with serum albumin levels <4.0 g/dL and who had at least one serum albumin measurement after the trial started [23]. Comparison of baseline characteristics of the high-protein versus low-protein intake groups were conducted using t-test, Wilcoxon rank sum, or χ2 tests depending upon data type. For the primary outcome, a χ2 test was used to compare the proportion of patients in the two treatment groups who achieved a combined rise in serum albumin of ≥0.2 mg/dL while maintaining serum phosphorus levels of 3.5 – <5.5 mg/dL. Based on intention-to-treat power analyses (α = 0.05, 1 − β = 0.80), 110 participants (55 in each of the 2 groups) were targeted for an anticipated increase in serum albumin of ≥0.2 mg/dL. For the secondary outcomes examining changes in laboratory and weight parameters, we compared the (i) postintervention values, (ii) change in values (defined as the preintervention value minus the postintervention value) using Wilcoxon rank sum tests and (iii) the proportion of patients achieving a clinically relevant change in levels using χ2 tests between the two treatment groups. We also compared the preintervention (baseline) and postintervention (follow-up) levels within treatment groups using the Wilcoxon signed-rank test. Analyses and figures were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) and Stata version 13.1 (StataCorp, College Station, TX, USA).
RESULTS
Study population
Between July 2010 and October 2011, a total of 234 patients were screened, among whom 178 patients consented to participation. Among these patients, 110 patients met initial eligibility criteria and underwent randomization to the high-protein (n = 55) and low-protein hemodialysis meal (n = 55) groups (Figure 1). In the low-protein hemodialysis meal group, four patients were excluded due to (i) having had a serum albumin level of ≥4.0 g/dL upon second measurement 2 months later (n = 2) and (ii) withdrawing from the study prior to obtaining any postrandomization serum albumin data (n = 2), resulting in 106 patients who had at least one postrandomization data point based on the intention-to-treat principle. The final study population was diverse in terms of sex, race and ethnicity (Table 1), and all sociodemographic, case-mix and baseline laboratory test characteristics were well-balanced across the two treatment groups except for IL-6 levels, which were higher in the high-protein intake group (P = 0.026).
Table 1.
Baseline characteristics among 106 hemodialysis patients randomized to the high-protein (n = 55) versus low-protein (n = 51) hemodialysis meal groups
| High-protein hemodialysis meal | Low-protein hemodialysis meal | P-value* | |
|---|---|---|---|
| % (n) | 52 (55) | 48 (51) | N/A |
| Sociodemographic and case-mix characteristics | |||
| Age (years), mean ± SD | 53 ± 15 | 57 ± 14 | 0.163 |
| Female (%) | 56 | 55 | 0.880 |
| Race (%) | |||
| White | 64 | 41 | 0.167 |
| Black | 27 | 41 | |
| Asian | 7 | 10 | |
| Pacific Islander | 2 | 6 | |
| Missing | 0 | 2 | |
| Hispanic ethnicity (%) | 60 | 41 | 0.053 |
| Married (%) | 40 | 39 | 0.934 |
| Primary insurance (%) | 0.842 | ||
| Medicare/Medicaid | 80 | 78 | |
| Other | 20 | 22 | |
| Diabetes (%) | 62 | 65 | 0.758 |
| Weight (kg), median (IQR) | 74.2 (63.9–86.7) | 73.9 (64.2– 91.4) | 0.830 |
| BMI (kg/m2), median (IQR) | 28.3 (24.4–31.5) | 27.1 (23.6–29.9) | 0.226 |
| Dialysis treatment characteristics | |||
| Access type (%) | 0.880 | ||
| AVF or AVG | 87 | 88 | |
| CVC | 13 | 12 | |
| Dialysis session time (min), median (IQR) | 211 (210–240) | 210 (195–240) | 0.261 |
| Ultrafiltration volume (L), median (IQR) | 2.4 (1.7–2.8) | 2.4 (1.9–2.7) | 0.809 |
| Laboratory test characteristics Median (IQR) | |||
| Serum albumin (g/dL) | 3.6 (3.5–3.8) | 3.7 (3.6–3.8) | 0.163 |
| Phosphorus (mg/dL) | 5.3 (4.4–6.8) | 4.9 (4.2–6.5) | 0.297 |
| Calcium (mg/dL) | 8.8 (8.2–9.2) | 8.8 (8.3–9.1) | 0.857 |
| PTH (pg/mL) | 358 (283–560) | 283 (138–466) | 0.061 |
| Alkaline phosphatase (U/L) | 89 (68–123) | 79 (63–109) | 0.159 |
| nPCR (g/kg/day) | 1.00 (0.84–1.18) | 1.05 (0.84–1.21) | 0.390 |
| Bicarbonate (mEq/L) | 23 (21–26) | 23 (21–25) | 0.358 |
| Potassium (mEq/L) | 5.0 (4.5–5.4) | 4.7 (4.5–5.3) | 0.317 |
| Serum creatinine (mg/dL) | 8.9 (6.7–10.6) | 9.0 (7.1–11.4) | 0.584 |
| Blood urea nitrogen (mg/dL) | 59 (47–68) | 54 (46–64) | 0.386 |
| Serum prealbumin (mg/dL) | 25 (21–31) | 26 (24–32) | 0.375 |
| spKt/V | 1.59 (1.48–1.87) | 1.56 (1.35–1.85) | 0.653 |
| URR | 75 (71–79) | 74 (68–79) | 0.338 |
| Hemoglobin (g/dL) | 11.2 (10.5–11.9) | 11.4 (10.8–12.0) | 0.479 |
| WBC (×109/L) | 6.3 (5.0–8.7) | 6.9 (5.4–8.2) | 0.805 |
| Platelet count (×109/L) | 210 (177–253) | 240 (186–279) | 0.159 |
| LDL (mg/dL) | 60 (43–77) | 67 (48–84) | 0.197 |
| HDL (mg/dL) | 32 (25–43) | 36 (29–48) | 0.277 |
| Triglycerides (mg/dL) | 98 (71–145) | 92 (70–121) | 0.527 |
| TNF-α (pg/mL) | 3.84 (2.80–6.76) | 3.42 (2.55–4.91) | 0.195 |
| IL-6 (pg/mL) | 5.01 (3.13–12.17) | 3.21 (2.26–6.92) | 0.026 |
| Leptin (ng/mL) | 25.1 (6.6–54.2) | 26.3 (7.3–48.2) | 0.786 |
| C-reactive protein (mg/L) | 0.64 (0.19–1.38) | 0.37 (0.18–0.81) | 0.117 |
| Medications | |||
| Nonlanthanum phosphorus binders (%) | 46 | 49 | 0.757 |
| Cinacalcet (%) | 16 | 22 | 0.463 |
BMI, body mass index; AVF, arteriovenous fistula; AVG, arteriovenous graft; CVC, central venous catheter; PTH, parathyroid hormone; nPCR, normalized protein catabolic rate; spKt/V, single-pool Kt/V; URR, urea reduction ratio; WBC, white blood cell count; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6.
*P-value estimated using t-test, Wilcoxon rank sum or χ2 tests.
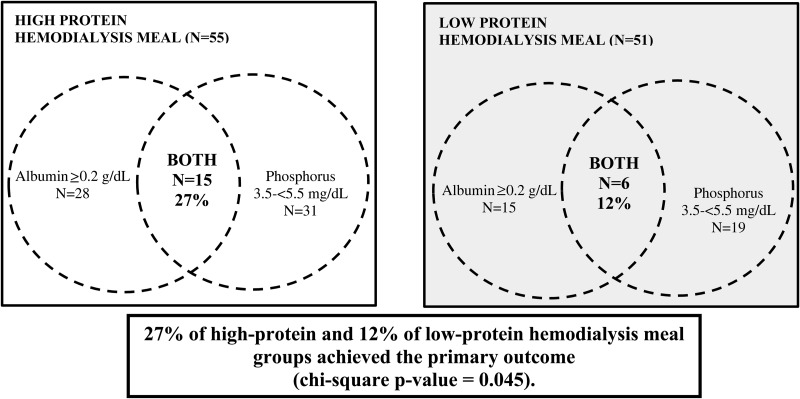
Primary outcome
Following the 8-week intervention period, 27% of patients in the high-protein intake group and 12% of patients in the low-protein intake group achieved the primary composite outcome of a combined increase in serum albumin of ≥0.2 g/dL and a target serum phosphorus range of 3.5–<5.5 mg/dL (P-value = 0.045; Figure 2). In the high-protein intake group, 28, 31 and 15 patients achieved a ≥0.2 g/dL increase in serum albumin (Table 2), serum phosphorus 3.5–<5.5 mg/dL and both endpoints, respectively (Figure 2). In the low-protein group, 15, 19 and 6 patients achieved a ≥0.2 g/dL increase in serum albumin, serum phosphorus 3.5–<5.5 mg/dL and both endpoints, respectively.
FIGURE 2.
Proportion of hemodialysis patients randomized to the high-protein versus low-protein hemodialysis meal groups who achieved the primary outcome (increase in serum albumin ≥0.2 g/dL and serum phosphorus 3.5–<5.5 mg/dL).
Table 2.
Comparison of the proportion of hemodialysis patients randomized to the high-protein versus low-protein hemodialysis meal groups who achieved a clinically significant change [Δ; defined as the median (IQR) difference in the preintervention value minus the postintervention value) in weight and laboratory characteristics postintervention
| High-protein hemodialysis meal, | Low-protein hemodialysis meal, | P-value* | |
|---|---|---|---|
| % (n) | % (n) | ||
| Δ Serum albumin ≥0.2 (g/dL) | 51 (28) | 29 (15) | 0.024 |
| Δ Phosphorus ≥0.5 (mg/dL) | 31 (17) | 47 (24) | 0.088 |
| Δ Calcium ≥0.5 (mg/dL) | 18 (10) | 16 (8) | 0.732 |
| Δ PTH ≥50 (pg/mL) | 56 (14) | 59 (13) | 0.831 |
| Δ Alkaline Phos. ≥25 (U/L) | 18 (10) | 16 (8) | 0.732 |
| Δ nPCR ≥0.2 (g/kg/day) | 27 (15) | 22 (11) | 0.461 |
| Δ Bicarbonate ≥5 (mEq/L) | 8 (4) | 14 (7) | 0.354 |
| Δ Potassium ≥0.5 (mEq/L) | 20 (11) | 33 (17) | 0.120 |
| Δ Serum creatinine ≥1.0 (mg/dL) | 26 (14) | 19 (9) | 0.387 |
| Δ Blood urea nitrogen ≥20 (mg/dL) | 16 (9) | 16 (8) | 0.924 |
| Δ Serum prealbumin ≥0.2 (mg/dL) | 39 (17) | 34 (16) | 0.649 |
| Δ spKt/V ≥0.2 | 23 (10) | 18 (8) | 0.561 |
| Δ URR ≥10 | 9 (5) | 10 (5) | >0.999 |
| Δ Hemoglobin ≥0.5 (g/dL) | 31 (17) | 36 (18) | 0.581 |
| Δ WBC ≥25 (×109/L) | 0 (0) | 0 (0) | N/A |
| Δ Platelet count ≥50 (×109/L) | 15 (8) | 16 (8) | 0.867 |
| Δ LDL ≥25 (mg/dL) | 18 (8) | 13(6) | 0.504 |
| Δ HDL ≥10 (mg/dL) | 18 (8) | 17 (8) | 0.887 |
| Δ Triglycerides ≥25 (mg/dL) | 38 (17) | 27 (13) | 0.270 |
| Δ TNF-α ≥5 (pg/mL) | 20 (9) | 27 (13) | 0.455 |
| Δ IL-6 ≥5 (pg/mL) | 9 (4) | 31 (15) | 0.010 |
| Δ Leptin ≥10 (ng/mL) | 22 (10) | 18 (9) | 0.642 |
| Δ C-reactive protein ≥1.0 (mg/L) | 9 (4) | 13 (6) | 0.741 |
| Δ Weight ≥5 (kg) | 0 (0) | 4 (2) | 0.229 |
| Δ BMI ≥5 (kg/m2) | 0 (0) | 2 (1) | 0.481 |
PTH, parathyroid hormone; Alkaline Phos., alkaline phosphatase; nPCR, normalized protein catabolic rate; spKt/V, single-pool Kt/V; URR, urea reduction ratio; WBC, white blood cell count; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; BMI, body mass index.
*P-value calculated by χ2 or Fisher's exact test.
Secondary outcomes
Comparison of postintervention laboratory values showed that patients in the high-protein intake group had significantly higher PTH values versus those in the low-protein intake group {median 505 [interquartile range (IQR) 239–703] pg/mL versus 330 (192–516) respectively (P = 0.043)}, whereas there did not appear to be significant differences in other postintervention laboratory or weight values (Table 3).
Table 3.
Comparison of postintervention weight and laboratory test characteristics among hemodialysis patients randomized to the high-protein versus low-protein hemodialysis meal groups following study completion
| High-protein hemodialysis meal | Low-protein hemodialysis meal | P-value* | |
|---|---|---|---|
| Laboratory test characteristics Median (IQR) | |||
| Serum albumin (g/dL) | 3.8 (3.6–4.0) | 3.7 (3.5–3.9) | 0.151 |
| Phosphorus (mg/dL) | 5.2 (4.4–6.2) | 5.5 (4.5–6.6) | 0.433 |
| Calcium (mg/dL) | 8.8 (8.2–9.3) | 8.7 (8.1–9.0) | 0.331 |
| PTH (pg/mL) | 505 (239–703) | 330 (192–516) | 0.043 |
| Alkaline phosphatase (U/L) | 98 (73–119) | 77 (61–120) | 0.093 |
| nPCR (g/kg/day) | 1.00 (0.87–1.26) | 1.05 (0.79–1.34) | 0.556 |
| Bicarbonate (mEq/L) | 23 (21–25) | 23 (21–25) | 0.908 |
| Potassium (mEq/L) | 4.8 (4.5–5.2) | 4.9 (4.3–5.5) | 0.937 |
| Serum creatinine (mg/dL) | 9.2 (7.4–11.6) | 8.8 (6.5–11.3) | 0.520 |
| Blood urea nitrogen (mg/dL) | 59 (47–69) | 58 (48–74) | 0.676 |
| Serum prealbumin (mg/dL) | 24 (19–30) | 26 (22–34) | 0.293 |
| spKt/V | 1.69 (1.47–1.88) | 1.55 (1.40–1.86) | 0.214 |
| URR | 76 (72–79) | 74 (70–78) | 0.139 |
| Hemoglobin (g/dL) | 11.2 (10.4–11.9) | 11.3 (10.7–11.8) | 0.707 |
| WBC (×109/L) | 7.0 (5.7–8.7) | 7.4 (5.8–8.3) | 0.887 |
| Platelet count (×109/L) | 217 (175–268) | 222 (179–282) | 0.742 |
| LDL (mg/dL) | 63 (44–86) | 60 (42–79) | 0.671 |
| HDL (mg/dL) | 94 (65–177) | 93 (63–129) | 0.346 |
| Triglycerides (mg/dL) | 94 (65–177) | 93 (63–129) | 0.346 |
| TNF-α (pg/mL) | 5.45 (3.41–8.86) | 5.90 (3.55–10.67) | 0.910 |
| IL-6 (pg/mL) | 5.20 (3.41–9.85) | 6.09 (3.30–14.03) | 0.624 |
| Leptin (ng/mL) | 22.2 (7.2–81.3) | 24.5 (6.7–49.0) | 0.582 |
| C-reactive protein (mg/L) | 0.75 (0.35–1.60) | 0.48 (0.21–1.27) | 0.198 |
| Case-mix characteristics Median (IQR) | |||
| Weight (kg) | 73.4 (64.1–88.0) | 75.2 (64.8–92.3) | 0.602 |
| BMI (kg/m2) | 28.3 (24.2–31.9) | 27.5 (24.5–30.8) | 0.450 |
PTH, parathyroid hormone; nPCR, normalized protein catabolic rate; spKt/V, single-pool Kt/V; URR, urea reduction ratio; WBC, white blood cell count; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; BMI, body mass index.
*P-value calculated by Wilcoxon rank sum tests.
When the change in laboratory and weight values (pre-intervention levels minus postintervention levels) were compared across treatment groups, those in the high-protein intake group experienced a greater increase in serum albumin versus the low protein-intake group [median 0.2 (IQR −0.1–0.3) g/dL versus 0.0 (−0.2, 0.2), respectively (P = 0.007)] (Table 4). In contrast, patients in the low-protein intake group experienced a greater increase in IL-6 levels as compared with the high-protein intake group [median 1.07 (−0.56–6.69) pg/mL versus −0.52 (−3.66–1.70), respectively (P = 0.002)]. When we compared the proportion of patients who achieved a clinically significant change in laboratory and weight values, we similarly observed that a greater proportion of those in the high-protein versus low-protein intake groups achieved a clinically significant increase in serum albumin ≥0.2 g/dL (51% versus 29%, respectively; P = 0.024), whereas a greater proportion of patients in the low-protein intake versus high-protein intake group experienced a clinically significant increase in IL-6 levels ≥5 pg/mL (31% versus 9%, respectively; P = 0.010) (Table 2).
Table 4.
Comparison of the change [Δ; defined as the median (IQR) difference in the preintervention value minus the postintervention value) in postintervention weight and laboratory characteristics among hemodialysis patients randomized to the high-protein versus low-protein hemodialysis meal groups
| High-protein hemodialysis meal | Low-protein hemodialysis meal | P-value* | |
|---|---|---|---|
| Δ Serum albumin (g/dL) | 0.2 (−0.1–0.3) | 0.0 (−0.2–0.2) | 0.007 |
| Δ Phosphorus (mg/dL) | −0.4 (−1.2–1.0) | 0.4 (−0.8–1.1) | 0.133 |
| Δ Calcium (mg/dL) | −0.1 (−0.3–0.3) | −0.1–(−0.5–0.3) | 0.732 |
| Δ PTH (pg/mL) | 104 (−64–306) | 73 (4–196) | 0.941 |
| Δ Alkaline Phosphatase (U/L) | 1 (−7–15) | 0 (−10–13) | 0.554 |
| Δ nPCR (g/kg/day) | −0.01 (−0.14–0.25) | −0.02 (−0.17–0.17) | 0.473 |
| Δ Bicarbonate (mEq/L) | −1 (−3–2) | 1 (−2–2) | 0.117 |
| Δ Potassium (mEq/L) | 0.0 (−0.4–0.4) | 0.1 (−0.3–0.6) | 0.211 |
| Δ Serum creatinine (mg/dL) | 0.2 (−0.4–1.0) | −0.4 (−1.2–0.8) | 0.020 |
| Δ Blood urea nitrogen (mg/dL) | 0 (−12–13) | 0 (−7–14) | 0.932 |
| Δ Serum prealbumin (mg/dL) | 1.7 (−4.9–2.2) | −1.4 (−3.3–3.1) | 0.413 |
| Δ spKt/V | −0.01 (−0.14–0.18) | −0.02 (−0.17–0.14) | 0.700 |
| Δ URR | 0 (−3–3) | 0 (−3–2) | 0.893 |
| Δ Hemoglobin (g/dL) | −0.3 (−0.9–0.8) | −0.2 (−1.3–0.8) | 0.815 |
| Δ WBC (×109/L) | 0.4 (−0.2–1.5) | 0.2 (−1.2–1.3) | 0.359 |
| Δ Platelet count (×109/L) | 8 (−19–29) | −9 (−31–18) | 0.057 |
| Δ LDL (mg/dL) | 7 (−12–18) | −4 (−25–13) | 0.103 |
| Δ HDL (mg/dL) | −2 (−10–5) | −1 (−10–7) | 0.988 |
| Δ Triglycerides (mg/dL) | −4 (−35–35) | 2 (−29–26) | 0.930 |
| Δ TNF-α (pg/mL) | 2.01 (−1.37–3.82) | 2.17 (−0.23–5.39) | 0.346 |
| Δ IL-6 (pg/mL) | −0.52 (−3.66–1.70) | 1.07 (−0.56–6.69) | 0.002 |
| Δ Leptin (ng/mL) | 1.1 (−9.7–9.6) | −1.5 (−9.6–5.7) | 0.198 |
| Δ C-reactive protein (mg/L) | 0.07 (0.17–0.38) | 0.00 (−0.18–0.29) | 0.727 |
| Δ Weight (kg) | 0.4 (−0.4–1.4) | 0.7 (−0.8–2.1) | 0.535 |
| Δ BMI (kg/m2) | 0.1 (−0.2–0.5) | 0.3 (−0.3–0.7) | 0.569 |
PTH, parathyroid hormone; nPCR, normalized protein catabolic rate; spKt/V, single-pool Kt/V; URR, urea reduction ratio; WBC, white blood cell count; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; BMI, body mass index.
*P-value calculated by Wilcoxon rank sum test.
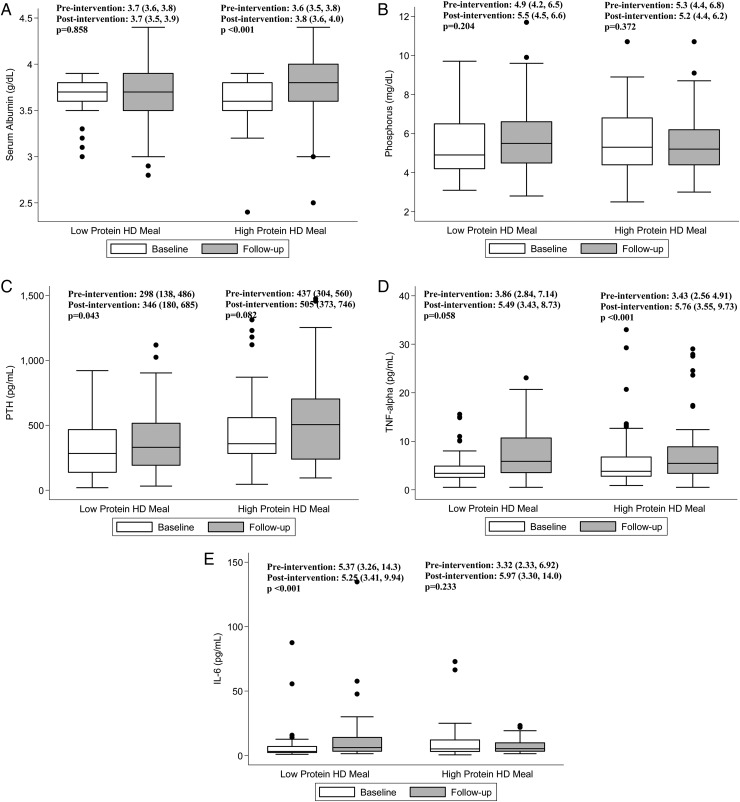
When preintervention (baseline) and postintervention (follow-up) levels within treatment groups were compared (Table 5 and Figure 3), we found that patients in the high-protein intake group experienced a significant increase in serum albumin [median 3.6 (IQR 3.5–3.8) g/dL and 3.8 (3.6–4.0) pre- and postintervention, respectively; P < 0.001], whereas those in the low-protein intake group did not [median 3.7 (IQR 3.6–3.8) g/dL and 3.7 (3.5–3.9) pre- and post-intervention, respectively; P = 0.858]. In contrast, patients in the low-protein intake group experienced a significant increase in PTH [median 298 (IQR 138–486) pg/mL and 346 (180–685) pre- and postintervention, respectively; P = 0.043], whereas those in the high-protein intake group did not [median 437 (IQR 304–560) pg/mL and 505 (373–746) pre- and postintervention, respectively; P = 0.082]. Furthermore, patients in the low-protein intake group experienced a significant increase in TNF-α [median 3.43 (IQR 2.56–4.91) pg/mL and 5.76 (3.55–9.73) pre- and postintervention, respectively; P < 0.001] and IL-6 [median 3.32 (IQR 2.33–6.92) pg/mL and 5.90 (3.30–14.0) pre- and postintervention, respectively; P < 0.001], whereas those in the high-protein intake group did not. Notably, neither treatment group experienced a clinically significant change in serum bicarbonate or potassium levels.
Table 5.
Comparison of weight and laboratory characteristics [median (IQR)] pre- and postintervention among hemodialysis patients randomized to the high-protein versus low-protein hemodialysis meal groups
| High-protein hemodialysis meal |
Low-protein hemodialysis meal |
|||||
|---|---|---|---|---|---|---|
| Preintervention | Postintervention | P-value* | Preintervention | Postintervention | P-value* | |
| Serum albumin (g/dL) | 3.6 (3.5–3.8) | 3.8 (3.6–4.0) | <0.001 | 3.7 (3.6–3.8) | 3.7 (3.5–3.9) | 0.858 |
| Phosphorus (mg/dL) | 5.3 (4.4–6.8) | 5.2 (4.4–6.2) | 0.372 | 4.9 (4.2–6.5) | 5.5 (4.5–6.6) | 0.204 |
| Calcium (mg/dL) | 8.8 (8.2–9.2) | 8.8 (8.2–9.3) | 0.558 | 8.8 (8.3–9.1) | 8.7 (8.1–9.0) | 0.268 |
| PTH (pg/mL) | 437 (304–560) | 505 (373–746) | 0.082 | 298 (138–486) | 346 (180–685) | 0.043 |
| Alkaline phos. (U/L) | 89 (68–123) | 98 (73–119) | 0.275 | 79 (63–109) | 77 (61–120) | 0.853 |
| nPCR (g/kg/day) | 1.00 (0.84–1.18) | 1.00 (0.87–1.26) | 0.388 | 1.05 (0.84–1.21) | 1.05 (0.79–1.34) | 0.853 |
| Bicarbonate (mEq/L) | 23 (21–26) | 23 (21–25) | 0.178 | 23 (21–25) | 23 (21–25) | 0.381 |
| Potassium (mEq/L) | 5.0 (4.5–5.4) | 4.8 (4.5–5.2) | 0.554 | 4.7 (4.5–5.3) | 4.9 (4.3–5.5) | 0.306 |
| Serum creatinine (mg/dL) | 8.86 (6.70–10.64) | 9.17 (7.41–11.64) | 0.042 | 9.03 (7.07–11.45) | 8.91 (6.53–11.33) | 0.182 |
| Blood urea nitrogen (mg/dL) | 54 (46–64) | 59 (47–69) | 0.460 | 59 (47–68) | 58 (48–74) | 0.631 |
| Serum prealbumin (mg/dL) | 26 (21–32) | 24 (20–30) | 0.099 | 26 (23–32) | 26 (22–33) | 0.349 |
| spKt/V | 1.59 (1.48–1.84) | 1.67 (1.45–1.82) | 0.849 | 1.57 (1.35–1.88) | 1.57 (1.42–1.86) | 0.652 |
| URR | 75 (71–79) | 76 (72–79) | 0.580 | 74 (69–79) | 74 (70–78) | 0.859 |
| Hemoglobin (g/dL) | 11.2 (10.5–11.9) | 11.2 (10.4–11.9) | 0.402 | 11.5 (10.8–12.0) | 11.3 (10.7–11.8) | 0.330 |
| WBC (×109/L) | 6.3 (5.0–8.7) | 7.0 (5.7–8.7) | 0.054 | 6.9 (5.4–8.2) | 7.4 (5.8–8.3) | 0.548 |
| Platelet count (×109/L) | 210 (177–253) | 218 (175–268) | 0.077 | 240 (186–279) | 222 (179–282) | 0.409 |
| LDL (mg/dL) | 57 (42–77) | 63 (45–85) | 0.310 | 66 (47–84) | 60 (41–81) | 0.242 |
| HDL (mg/dL) | 32 (23–42) | 31 (22–37) | 0.397 | 36 (29–48) | 36 (26–45) | 0.447 |
| Triglycerides (mg/dL) | 100 (71–147) | 91 (65–157) | 0.798 | 91 (69–120) | 92 (62–127) | 0.944 |
| TNF-α (pg/mL) | 3.86 (2.84–7.14) | 5.49 (3.43–8.73) | 0.058 | 3.43 (2.56–4.91) | 5.76 (3.55–9.73) | <0.001 |
| IL-6 (pg/mL) | 5.37 (3.26–14.28) | 5.25 (3.41–9.94) | 0.233 | 3.32 (2.33–6.92) | 5.97 (3.30–14.03) | <0.001 |
| Leptin (ng/mL) | 29.2 (6.5–48.5) | 22.0 (7.5–68.4) | 0.291 | 26.28 (7.32–48.18) | 21.83 (6.74–47.60) | 0.412 |
| C-reactive protein (mg/L) | 0.75 (0.23–1.51) | 0.76 (0.40–1.82) | 0.276 | 0.40 (0.18–0.83) | 0.50 (0.21–1.27) | 0.552 |
| Weight (kg) | 74.2 (63.9–86.7) | 73.4 (64.1–88.0) | 0.084 | 73.9 (64.2–91.4) | 75.2 (64.8–92.3) | 0.072 |
| BMI (kg/m2) | 28.3 (24.4–31.5) | 28.3 (24.2–31.9) | 0.104 | 27.1 (23.6–29.9) | 27.5 (24.5–30.8) | 0.082 |
PTH, parathyroid hormone; Alkaline phos., alkaline phosphatase; nPCR, normalized protein catabolic rate; spKt/V, single-pool Kt/V; URR, urea reduction ratio; WBC, white blood cell count; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; BMI, body mass index.
*P-value calculated by Wilcoxon signed-rank test.
FIGURE 3.
Comparison of selected laboratory characteristics pre- and postintervention among hemodialysis (HD) patients randomized to the high-protein versus low-protein hemodialysis meal groups.
Clinical practice guideline targets and medication treatment patterns
While a greater proportion of patients in the high-protein intake versus low-protein hemodialysis meal groups achieved the KDOQI Clinical Practice Guidelines serum phosphorus target of 3.5–<5.5 mg/dL (56% versus 38%, respectively; P = 0.049), there were no differences in achievement of the PTH target of 150–300 pg/mL across treatment groups (23% and 31%, respectively; P = 0.464) (Table 6).
Table 6.
Comparison of the proportion of hemodialysis patients randomized to the high-protein versus low-protein hemodialysis meal groups who achieved serum phosphorus and parathyroid hormone National Kidney Foundation–Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) Clinical Practice Guideline targets postintervention
| High-protein hemodialysis meal, % (n) | Low-protein hemodialysis meal, % (n) | P-value | |
|---|---|---|---|
| Phosphorus target 3.5–<5.5 mg/dL |
56 (31) | 37 (19) | 0.049 |
| PTH target 150–300 pg/mL |
23 (8) | 31 (11) | 0.464 |
Prior to the trial there was an even distribution of non-lanthanum phosphorus binder use across treatment arms (46% versus 49% among high-protein versus low-protein hemodialysis meal groups, respectively; P = 0.757; Table 1). During the study, 24% versus 33% of patients continued nonlanthanum binders, 8% versus 15% newly initiated binders, 29% versus 15% ceased binders and 39% versus 38% remained without binders in the high-protein versus low-protein hemodialysis meal groups, respectively (P = 0.371).
Adverse events
Over the course of the study, there were no severe adverse events or nonsevere adverse events reported, including allergic reactions, aspiration while consuming meals during hemodialysis or death. Minor events were reported related to the cold temperature of the meals or dislike of the prepared food in cold meal boxes. Patients in the high-protein hemodialysis meal group also reported higher satisfaction with high-protein meals during hemodialysis.
DISCUSSION
In this pilot randomized controlled trial, a greater proportion of hypoalbuminemic hemodialysis patients who received in-center high-protein hemodialysis meals combined with lanthanum carbonate during dialysis treatment experienced a clinically relevant increase in serum albumin levels while maintaining serum phosphorus levels in the target range versus those who received low-protein hemodialysis meals. We also found that the low-protein hemodialysis meal group experienced a greater increase in IL-6 levels as compared with the high-protein hemodialysis meal group. Upon comparing pre- versus postintervention laboratory values within treatment groups, those in the high-protein hemodialysis meal group experienced a significant increase in serum albumin whereas those in the low-protein hemodialysis meal group experienced a significant increase in PTH, IL-6, and TNF-α levels.
Prior studies have shown that serum albumin levels <4.0 g/dL are predictive of higher death risk in hemodialysis patients, and that even mild increases in serum albumin of ≥0.2 g/dL over time are associated with improved survival, lower hospitalization risk, and reduced treatment costs [19–21, 24]. While the degree to which a decline in serum albumin reflects protein-energy wasting, increased protein catabolism, dialytic protein and amino acid losses and inflammation in these studies remains unclear, large observational studies show that moderate increases in protein intake are also associated with greater survival compared to reductions in protein intake in this population. In a study of >30 000 hemodialysis patients whose trajectory of protein intake ascertained by nPCR and serum phosphorus levels were examined over 6 months, those whose protein intake increased over time had greater survival, whereas those whose protein intake decreased over time, irrespective of phosphorus level, experienced higher death risk [25].
A growing body of observational studies and pilot randomized controlled trials suggest that administration of oral nutritional supplements with a high protein content during hemodialysis treatments is effective in improving nutritional parameters. In a study of eight hemodialysis patients with deranged nutritional status by Pupim et al. [26], provision of oral protein intake during dialysis was shown to improve protein homeostasis and oppose the catabolic effects of hemodialysis based on protein turnover studies conducted before, during and after dialysis. In a study of 85 hemodialysis patients with malnutrition by Caglar et al. [27], following administration of an oral nutritional supplement during dialysis over 6 months, there was a significant increase in both serum albumin and prealbumin levels. More recently, in a 2 × 2 factorial, pilot, feasibility randomized controlled trial by Rattanasompattikul et al. [28] of 84 hypoalbuminemic hemodialysis patients who received an oral nutritional supplement, pentoxifylline, oral nutritional supplement + pentoxifylline or placebo over a 16-week study period, those who received the oral nutritional supplement alone experienced a significant increase in serum albumin.
To our knowledge, ours is the first randomized controlled trial to show that provision of a high-protein meal during hemodialysis treatment in the dialysis center in conjunction with a potent phosphorus binder such as lanthanum carbonate effectively increases serum albumin while maintaining serum phosphorus levels within target ranges recommended by clinical practice guidelines. Lanthanum was selected because studies of phosphate-binding in humans have shown that lanthanum has the highest relative phosphate-binding coefficient (RPBC) in comparison to other phosphorus binders, and hence has a lower pill burden [17]. Although animal studies have shown that lanthanum may accumulate in organs (e.g. liver), no significant adverse effects have yet been reported in dialysis patients [29, 30]. In terms of other mineral and bone disease markers assessed as secondary outcomes, we observed a significant increase in PTH levels in the low-protein hemodialysis meal group but not in the high-protein group. While the postintervention PTH levels were higher in the high-protein versus low-protein hemodialysis meal groups, there were no significant differences in achievement of serum PTH target ranges recommended by the NKF-KDOQI guidelines. Notably, we did not observe significant differences in certain electrolytes that correlate with protein intake (e.g. potassium or serum bicarbonate level) between the two groups [16, 31].
Another novel finding was the greater increase in inflammatory markers (interleukin-6 and TNF-α) observed in the low-protein hemodialysis meal group but not in the high-protein group. In hemodialysis patients with protein-energy wasting, there is a strong link between malnutrition and inflammation in which both may play synergistic roles in the development of wasting [1]. It is plausible that in our patients assigned to the high-protein hemodialysis meal arm, the amelioration of hypoalbuminemia and subsequent protein-energy wasting may have also evaded a worsening inflammatory status over time in these patients. In animal models of starvation, activation of inflammatory cytokines has been observed, such that otherwise normal rats randomized to receive a diet low in protein and energy experienced an increase in acute phase proteins and cytokines, which was not seen in control-fed rats [32].
Our study further corroborates the potential safety and nutritional benefits of administering meals during hemodialysis treatments in the dialysis center. In contrast to the US, meals are routinely provided in outpatient hemodialysis units in certain European and Asian countries [13, 16]. For example, dialysis patients in Germany, where meals are frequently provided, demonstrate better nutritional markers and survival than their US counterparts [33]. As there may be additional advantages beyond nutritional outcomes (e.g. enhanced patient satisfaction, improved health-related quality of life, increased adherence to hemodialysis treatment), further studies are needed to determine the benefits of intradialytic meals in hemodialysis patients [13]. In addition, while higher protein intake may potentially confer a higher uremic toxic burden due to the metabolism of amino acids, we did not observe a clinically or statistically significant difference in the change in serum blood urea nitrogen levels between or within the high-protein versus low-protein hemodialysis meal treatment arms.
The strengths of our study include its multicenter design; inclusion of patients who were diverse with regards to sex, race and ethnicity and eligibility to receive vitamin D pharmacotherapies and cinacalcet, along with phosphorus binders, across both treatment groups. However, several limitations of the study bear mention. First, as our study population was a selected group of hemodialysis patients in Southern California who consented to participate in the study, their case-mix characteristics (i.e. comorbidity burden) and adherence to phosphorus binders may not be reflective of the broader US hemodialysis population. Second, our study design did not allow for the separate evaluation of the effect of lanthanum carbonate and high- versus low-protein hemodialysis meals upon the outcome of interest. Third, while lanthanum carbonate was utilized in the high-protein hemodialysis meal group, given its high phosphorus-binding capacity and lower pill burden compared with other phosphorus binders [12], it remains uncertain if similar achievement of serum phosphate targets would be observed with alternative binders. Fourth, the observed improvements in serum albumin levels in the high-protein hemodialysis meal group may have been partly due to the dietary counseling provided at baseline and/or the possibility that the high-protein group's energy-dense (>2 kcal/g), high-calorie (850 kcal) meals corrected an energy deficit on dialysis treatment days. Notably, the latter is not supported by changes in body weight. Fifth, although not statistically significant, there was an imbalance in racial/ethnic distribution across treatment arms, such that a greater proportion of white patients and a lower proportion of black patients were in the high-protein versus low-protein hemodialysis meal groups. Prior studies have shown that black hemodialysis patients have a tendency toward higher serum albumin levels compared with their white counterparts [34]. Thus, residual confounding on this basis would likely bias the relationship between high- versus low-protein hemodialysis meal intake towards the null, rendering our results even more conservative. Lastly, while the high-protein hemodialysis meal group demonstrated improvement in surrogate endpoints of nutritional status, further study is needed to determine whether this intervention favorably impacts hard outcomes.
In conclusion, in hypoalbuminemic hemodialysis patients, provision of high-protein meals with lanthanum carbonate during dialysis treatment appears to be safe and effectively increases serum albumin while controlling serum phosphorus levels. At this time, further studies are needed to determine if high-protein hemodialysis meals in conjunction with phosphorus binders can improve hard outcomes such as survival in this population.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
We thank DaVita Clinical Research (DCR) for allowing us to conduct this study in their outpatient dialysis clinics and for providing the clinical data for this research study. Portions of these data were presented as an abstract at the 2012 International Congress on Nutrition and Metabolism in Renal Disease in Honolulu, HI (26–30 June 2012) and the 2013 National Kidney Foundation Spring Clinical Meeting in Lake Buena Vista, FL (2–6 April 2013). The study was supported by funding from Shire as an independent investigator-initiated proposal.
CONFLICT OF INTEREST STATEMENT
K.K.-Z. received funding to conduct this investigator-initiated study from Shire, a manufacturer of lanthanum carbonate. K.K.-Z. also received honoraria from Abbott, AbbVie, Alexion, Amgen, AstraZeneca, AVEO Oncology, Chugai, DaVita, Fresenius, Genentech, Haymarket Media, Hospira, Kabi, Keryx, Novartis, Pfizer, Relypsa, Resverlogix, Sandoz, Sanofi, Shire, Vifor, UpToDate and ZS-Pharma. C.P.K. is the recipient of a research grant from Shire, OPKO, Amgen, AbbVie and Bayer, and received honoraria from AstraZeneca, Abbott Nutrition, Relypsa and ZS-Pharma. Other authors have not declared any relevant conflicts of interest. Results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Kovesdy CP, Kalantar-Zadeh K. Accuracy and limitations of the diagnosis of malnutrition in dialysis patients. Semin Dial 2012; 25: 423–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(6 Suppl 2): S1–S140 [DOI] [PubMed] [Google Scholar]
- 3. Beddhu S, Kaysen GA, Yan G. et al. Association of serum albumin and atherosclerosis in chronic hemodialysis patients. Am J Kidney Dis 2002; 40: 721–727 [DOI] [PubMed] [Google Scholar]
- 4. Lacson E Jr, Wang W, Hakim RM. et al. Associates of mortality and hospitalization in hemodialysis: potentially actionable laboratory variables and vascular access. Am J Kidney Dis 2009; 53: 79–90 [DOI] [PubMed] [Google Scholar]
- 5. Shinaberger CS, Kilpatrick RD, Regidor DL. et al. Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am J Kidney Dis 2006; 48: 37–49 [DOI] [PubMed] [Google Scholar]
- 6. Block GA, Klassen PS, Lazarus JM. et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15: 2208–2218 [DOI] [PubMed] [Google Scholar]
- 7. Fouque D, Roth H, Pelletier S. et al. Control of mineral metabolism and bone disease in haemodialysis patients: which optimal targets? Nephrol Dial Transplant 2013; 28: 360–367 [DOI] [PubMed] [Google Scholar]
- 8. Kalantar-Zadeh K, Kuwae N, Regidor DL. et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 2006; 70: 771–780 [DOI] [PubMed] [Google Scholar]
- 9. Ketteler M, Floege J. Calcification and the usual suspect phosphate: still guilty but there are other guys behind the scenes. Nephrol Dial Transplant 2006; 21: 33–35 [DOI] [PubMed] [Google Scholar]
- 10. Ketteler M, Westenfeld R, Schlieper G. et al. Pathogenesis of vascular calcification in dialysis patients. Clin Exp Nephrol 2005; 9: 265–270 [DOI] [PubMed] [Google Scholar]
- 11. Schlieper G, Brandenburg V, Djuric Z. et al. Risk factors for cardiovascular calcifications in non-diabetic Caucasian haemodialysis patients. Kidney Blood Press Res 2009; 32: 161–168 [DOI] [PubMed] [Google Scholar]
- 12. Fouque D, Horne R, Cozzolino M. et al. Balancing nutrition and serum phosphorus in maintenance dialysis. Am J Kidney Dis 2014; 64: 143–150 [DOI] [PubMed] [Google Scholar]
- 13. Kalantar-Zadeh K, Ikizler TA. Let them eat during dialysis: an overlooked opportunity to improve outcomes in maintenance hemodialysis patients. J Ren Nutr 2013; 23: 157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burrowes JD, Larive B, Cockram DB. et al. Effects of dietary intake, appetite, and eating habits on dialysis and non-dialysis treatment days in hemodialysis patients: cross-sectional results from the HEMO study. J Ren Nutr 2003; 13: 191–198 [DOI] [PubMed] [Google Scholar]
- 15. Kalantar-Zadeh K, Cano NJ, Budde K. et al. Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat Rev Nephrol 2011; 7: 369–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalantar-Zadeh K, Tortorici AR, Chen JL. et al. Dietary restrictions in dialysis patients: is there anything left to eat? Semin Dial 2015; 28: 159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daugirdas JT, Finn WF, Emmett M. et al. The phosphate binder equivalent dose. Semin Dial 2011; 24: 41–49 [DOI] [PubMed] [Google Scholar]
- 18. Sullivan C, Sayre SS, Leon JB. et al. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: a randomized controlled trial. JAMA 2009; 301: 629–635 [DOI] [PubMed] [Google Scholar]
- 19. Kalantar-Zadeh K, Kilpatrick RD, Kuwae N. et al. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant 2005; 20: 1880–1888 [DOI] [PubMed] [Google Scholar]
- 20. Kopple JD, Cheung AK, Christiansen JS. et al. OPPORTUNITY: a randomized clinical trial of growth hormone on outcome in hemodialysis patients. Clin J Am Soc Nephrol 2008; 3: 1741–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lacson E Jr, Ikizler TA, Lazarus JM. et al. Potential impact of nutritional intervention on end-stage renal disease hospitalization, death, and treatment costs. J Ren Nutr 2007; 17: 363–371 [DOI] [PubMed] [Google Scholar]
- 22. Nussbaum SR, Zahradnik RJ, Lavigne JR. et al. Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem 1987; 33: 1364–1367 [PubMed] [Google Scholar]
- 23. Stel VS, Zoccali C, Dekker FW. et al. The randomized controlled trial. Nephron Clin Pract 2009; 113: c337–c342 [DOI] [PubMed] [Google Scholar]
- 24. Owen WF Jr, Lew NL, Liu Y. et al. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med 1993; 329: 1001–1006 [DOI] [PubMed] [Google Scholar]
- 25. Shinaberger CS, Greenland S, Kopple JD. et al. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr 2008; 88: 1511–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pupim LB, Majchrzak KM, Flakoll PJ. et al. Intradialytic oral nutrition improves protein homeostasis in chronic hemodialysis patients with deranged nutritional status. J Am Soc Nephrol 2006; 17: 3149–3157 [DOI] [PubMed] [Google Scholar]
- 27. Caglar K, Fedje L, Dimmitt R. et al. Therapeutic effects of oral nutritional supplementation during hemodialysis. Kidney Int 2002; 62: 1054–1059 [DOI] [PubMed] [Google Scholar]
- 28. Rattanasompattikul M, Molnar MZ, Lee ML. et al. Anti-Inflammatory and Anti-Oxidative Nutrition in Hypoalbuminemic Dialysis Patients (AIONID) study: results of the pilot-feasibility, double-blind, randomized, placebo-controlled trial. J Cachexia Sarcopenia Muscle 2013; 4: 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aime S, Canavese C, Stratta P. Advisory about gadolinium calls for caution in the treatment of uremic patients with lanthanum carbonate. Kidney Int 2007; 72: 1162–1163 [DOI] [PubMed] [Google Scholar]
- 30. Slatopolsky E, Liapis H, Finch J. Progressive accumulation of lanthanum in the liver of normal and uremic rats. Kidney Int 2005; 68: 2809–2813 [DOI] [PubMed] [Google Scholar]
- 31. Kovesdy CP, Regidor DL, Mehrotra R. et al. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol 2007; 2: 999–1007 [DOI] [PubMed] [Google Scholar]
- 32. Ling PR, Smith RJ, Kie S. et al. Effects of protein malnutrition on IL-6-mediated signaling in the liver and the systemic acute-phase response in rats. Am J Physiol Regul Integr Comp Physiol 2004; 287: R801–R808 [DOI] [PubMed] [Google Scholar]
- 33. Wizemann V. Regular dialysis treatment in Germany: the role of non-profit organisations. J Nephrol 2000; 13(Suppl 3): S16–S19 [PubMed] [Google Scholar]
- 34. Leavey SF, Strawderman RL, Young EW. et al. Cross-sectional and longitudinal predictors of serum albumin in hemodialysis patients. Kidney Int 2000; 58: 2119–2128 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.