Abstract
Porphyromonas gingivalis, an anaerobic Gram-negative bacterium critically involved in the development of human periodontitis, belongs to the late colonizers of the oral cavity. The success of this pathogen in the host colonization and infection results from the presence of several virulence factors, including extracellular peptidylarginine deiminase (PPAD), an enzyme that converts protein arginine residues to citrullines. A common opportunistic fungal pathogen of humans, Candida albicans, is also frequently identified among microorganisms that reside at subgingival sites. The aim of the current work was to verify if protein citrullination can influence the formation of mixed biofilms by both microorganisms under hypoxic and normoxic conditions. Quantitative estimations of the bacterial adhesion to fungal cells demonstrated the importance of PPAD activity in this process, since the level of binding of P. gingivalis mutant strain deprived of PPAD was significantly lower than that observed for the wild-type strain. These results were consistent with mass spectrometric detection of the citrullination of selected surface-exposed C. albicans proteins. Furthermore, a viability of P. gingivalis cells under normoxia increased in the presence of fungal biofilm compared with the bacteria that formed single-species biofilm. These findings suggest a possible protection of these strict anaerobes under unfavorable aerobic conditions by C. albicans during mixed biofilm formation.
Keywords: periodontitis, citrullination, peptidylarginine deiminase, Candida albicans, mixed biofilms, enolase, macrophage, cytokine
Porphyromonas gingivalis exploits protein citrullination during formation of mixed-species biofilms with Candida albicans.
INTRODUCTION
Porphyromonas gingivalis, a Gram-negative rod-shaped bacterium, is an obligatory anaerobe that contributes to the subgingival plaque biofilm in human individuals suffering from periodontitis, a chronic inflammatory disease associated with the degradation of gingival tissues followed by tooth loss (Socransky et al.1998; Darveau, Hajishengallis and Curtis 2012). Numerous virulence factors produced by this pathogen, including adhesive molecules such as hemagglutinins and fimbriae, hemin-binding proteins, secreted proteases and other hydrolytic enzymes, strongly contribute to bacterial ability to modulate the activity of host immune system and enable the tissue colonization (Lamont and Jenkinson 1998).
Recently, an additional potential P. gingivalis virulence factor has been identified—an extracellular enzyme, peptidylarginine deiminase (PPAD), that converts positively charged arginine residues in protein molecules to neutral citrulline residues in a process known as deimination or citrullination (McGraw et al.1999; Zhao et al.2017). This type of post-translational modification can significantly change the charge of protein molecule, thus potentially affecting its structure, function and ability to interact with other molecules. The citrullination of human proteins and peptides by PPAD can exert an influence on the functionality of many different systems responsible for the maintenance of homeostasis in the host organism and the response to infectious agents, with notable examples of disturbing the complement system activity due to the inactivation of anaphylatoxin C5a (Bielecka et al.2014) or facilitating bacterial invasion on gingival fibroblasts related to the induction of prostaglandin E2 signaling pathway (Gawron et al.2014). Additionally, the citrullination of C-terminal arginine can modulate the function of a proinflammatory and vasoactive peptide, bradykinin, thus regulating the inflow of plasma to the infected gingival pocket (McGraw et al.1999). Deimination of C-terminal arginine by PPAD also accounts for abolishing the biological activity of the epidermal growth factor and contributes to increased tissue destruction (Pyrc et al.2013). It has also been shown that PPAD is able to modify peptides derived from two human proteins, fibrinogen and α-enolase, and the citrullination of these proteins, primarily performed by human peptidylarginine deiminases, plays an important role in the pathogenesis of rheumatoid arthritis (Wegner et al. 2010a,b). To date, P. gingivalis PPAD is the only known bacterial peptidylarginine deiminase (Gabarrini et al.2015).
As has recently been reported, PPAD does not exclusively citrullinate molecules of the colonized host. Several proteins located at the surface of P. gingivalis cells were also identified to contain the modified arginine residues (Stobernack et al.2016). The citrullinated bacterial proteins can significantly contribute to the virulence, e.g. by a participation to the colonization of the subgingival niche, directly associated with the formation of mixed-species biofilms. Taking into consideration this hypothesis, it seems appropriate to investigate the significance of PPAD activity to the interactions of P. gingivalis with both the human host and members of biofilm heterogeneous microbial community. Apart from a great variety of bacterial organisms that form dental biofilms, one of the most important fungal pathogens of humans—Candida albicans—has also often been isolated from different locations in the oral cavity, including root canals and deep periodontal pockets (O’Donnell et al.2015; Gomes et al.2017; Marsh and Zaura 2017).
C andida albicans is primarily a commensal microorganism that can be identified in the mouths of nearly all individuals in human population, and is usually harmless for them as long as the immune system works efficiently (Peters et al.2017). However, when the immunity is compromised owing to various severe diseases, and the delicate balance between the host and the fungus is markedly disturbed, C. albicans demonstrates its pathogenic nature, causing various mycoses with variable severity, ranging from oral lesions and mucosal candidiasis to disseminated, systemic infections, often life threatening (Sardi et al.2013; Lewis and Williams 2017). Interestingly, the role of C. albicans in complex, polymicrobial biofilms formed in the oral cavity is crucial and indispensable, because C. albicans actively contributes to the composition and functions of such biofilms, e.g. reducing oxygen levels and allowing the survival and growth of anaerobic bacteria under aerobic conditions (Fox et al.2014; Janus et al.2017; Lambooij et al.2017). Taking into consideration a possibility that P. gingivalis and C. albicans can be found at the same infection niche and this cohabitation may result in the increase in the potential for pathogenicity and the ability to infect host tissues (Tamai, Sugamata and Kiyoura 2011), this work aimed to investigate whether one of the recently proposed bacterial virulence factors, i.e. PPAD, could influence the interactions between C. albicans and P. gingivalis and affect the formation of dual-species biofilm.
As macrophages constitute a significant part of the cells recovered from gingival tissue of chronic periodontitis patients and seem to play an essential role in the development and progression of periodontal diseases by mediating the host immune response (Zhou et al. 2005), we used these cells as a model to compare the host responses to the single species- or mixed bacterial–fungal infections.
MATERIALS AND METHODS
Strains and growth conditions
Porphyromonas gingivalis wild-type strain W83 (ATCC® BAA-308™) from American Type Culture Collection (Manassas, VA, USA) and the mutant strain deprived of PPAD (Δppad), obtained as described previously (Wegner et al.2010b), were grown on agar plates supplemented with 5% v/v sheep blood and in Schaedler broth (BTL, Lodz, Poland) supplemented with 250 mg L−1 L-cysteine-HCl (Sigma, St. Louis, MO, USA), 0.5 mg L−1 vitamin K (Sigma), 5 mg L−1 hemin (Sigma) and, for the mutant strain, with 5 mg L−1 erythromycin, at 37°C in an anaerobic chamber (90% N2, 5% CO2, 5% H2). Candida albicans strain 3147 (ATCC® 10231™) purchased from American Type Culture Collection was cultured aerobically in the YPD solid or liquid medium (1% yeast extract, 2% soybean peptone and 2% glucose) (Sigma) at 30°C. Cells were counted using optical density measurements after centrifugation of the bacterial (5000 × g, 20 min) or fungal (3000 × g, 5 min) liquid cultures at 4°C, and washing the cell pellet three times with sterile phosphate buffered saline (PBS), pH 7.4 (Biowest, Nuaillé, France).
Biofilm formation in 96-well microtiter plates
Candida albicans cells, 5 × 105 in 100 μL of RPMI 1640 medium (Biowest), were placed into the wells of 96-well high-binding black microplate with clear, flat bottom (Corning Incorporated, Kennenbunk, ME, USA) and incubated aerobically for 16 h at 37°C. Before the use in the biofilm formation assay, 2 × 109P. gingivalis cells in 1 mL of PBS were mixed with 0.4 μL of CellTrace CFSE (5(6)-carboxyfluorescein N-hydroxysuccinimidyl ester) staining solution (Thermo Fisher Scientific, Waltham, MA, USA) and labeled in the dark at 37°C for 40 min. After that, the bacterial cells were harvested by centrifugation (5000 × g, 10 min) and the excess of unbound tracer was removed by the following washing steps with: 1 mL of PBS, 1 mL of Schaedler broth diluted 1:1 with PBS and then 1 mL of PBS. After that, the suspensions of labeled P. gingivalis cells were prepared in RPMI 1640 medium (1 × 107 or 1 × 108 bacterial cells per 50 μL) and added to the wells with previously formed fungal biofilm. Microplates were incubated for 3 or 24 h at 37°C under normoxic or anoxic conditions. The latter were maintained using GENbox jar with GENbox anaer generator (bioMérieux S.A., Marcy l’Etoile, France). According to the manufacturer's instructions, just after 30 min the oxygen concentration in the chamber is close to 0%. In addition, control samples were prepared with only the cells of one species applied to the wells (5 × 105C. albicans cells, or 1 × 107 or 1 × 108P. gingivalis cells per 150 μL of RPMI 1640). After incubation, the supernatant was carefully removed from biofilm and the adsorbed cells were washed three times with 200 μL of sterile PBS. Each sample was prepared in technical triplicate and in at least three biological replicates.
The viability of both cell types was assessed using colony-forming unit (CFU) assay after their mechanical detachment from microplate wells and performing 10-fold serial dilutions. The C. albicans cells were cultured aerobically at 30°C for 24 h on YPD agar plates, whereas P. gingivalis cells were grown anaerobically at 37°C for 7 days on blood agar plates in GENbox jar with GENbox anaer generator. To control experimental repeatability, four replicates were prepared from each sample. After the incubation under conditions suitable for each microorganism, the numbers of colonies were counted.
To examine the level of the adhesion of CFSE-labeled P. gingivalis cells to fungal biofilms, the fluorescence intensity was measured with the excitation and emission wavelengths of 480 and 520 nm, respectively, using Synergy H1 Microplate Reader (BioTek Instruments, Winooski, VT, USA).
Biofilm visualization with confocal scanning laser microscopy
Imaging of single- or dual-species biofilms was performed using a Zeiss LSM 710 confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany) with a ×40 oil immersion objective, as a Z-stack of images. For this purpose, 1 × 106C. albicans cells in 400 μL of RPMI 1640 were applied into the chamber of Eppendorf Cell Imaging Coverglasses of 170 μm thickness (Eppendorf, Hamburg, Germany), coated with 0.05% poly-L-lysine (Sigma) and cultured under aerobic conditions at 37°C for 16 h to form biofilm. Then, the growth medium was removed and 1 × 108 CFSE-labeled P. gingivalis cells in 400 μL of RPMI 1640 were added to the chambers and further incubated at 37°C for 24 h under aerobic or anaerobic conditions, as described above. Similarly, the samples of single-species biofilm were also prepared. After growth, biofilms were rinsed three times with 400 μL of PBS to wash out the unbound cells and, prior to imaging, fungal cells were stained with 400 μL of the 0.1% aqueous solution (v/v) of Calcofluor White Stain (CFW) (Sigma) for 5 min. The excess of stain was removed, sterile PBS was pipetted to the chambers with biofilms, and the samples were viewed using excitation wavelength of 405 nm to visualize fungal cells stained with CFW and 488 nm to detect bacterial cells labeled with CFSE. The thickness of biofilm layers was analyzed using ZEN Lite Microscopy Software (Carl Zeiss).
Identification of citrullinated surface-exposed proteins of C. albicans with mass spectrometry
The identification of fungal cell wall proteins that may have been modified by the bacterial PPAD enzyme during biofilm formation was performed using liquid chromatography-coupled tandem mass spectrometry (LC-MS/MS) with an HCTUltra ETDII ion-trap mass spectrometer equipped with an electrospray ionization ion source (Bruker, Bremen, Germany) and coupled to an ultrahigh-performance liquid chromatograph Dionex Ultimate 3000 system (Carlsbad, CA, USA). Candida albicans cells (5 × 108) and P. gingivalis W83 or Δppad cells (5 × 109) were co-cultured in 5 mL RPMI 1640 medium at 37°C for 24 h with gentle agitation (30 rpm) in an orbital rotary shaker MaxQ 4000 (Thermo Fisher Scientific) under aerobic or anaerobic conditions. Additionally, the monospecies cultures were grown under the same conditions. The cells that formed a biofilm on the bottom of the flasks were detached mechanically and collected, while the culture supernatant was discarded. After harvesting the cells by centrifugation (3000 × g, 5 min) and washing them three times with 1 mL of 25 mM ammonium bicarbonate buffer (NH4HCO3), 100 μL of the same buffer with 5 mM dithiotreitol and 1 μg of sequencing-grade trypsin (Promega, Madison, WI, USA) were added, followed by the incubation for 10 min at 37°C. After this time, peptides from fungal surface-exposed proteins were released by trypsin to the solution and then subjected to further identification. Briefly, the remaining cells were centrifuged and discarded, while the supernatants were collected, filtered through 0.22-μm pore filter units to remove any remaining cells and further incubated overnight at 37°C. The undigested proteins were removed from the samples by addition of trifluoroacetic acid to the final concentration of 0.1% (v/v) and centrifugation (12 000 × g, 12 min) of any precipitates. Samples were dried in a SpeedVac (Martin Christ, Osterode am Harz, Germany), dissolved in 10% acetonitrile with 0.1% formic acid and the trypsin-generated peptides were subjected to separation on a 100 mm × 2.1 mm Accucore C18 column (particle size of 2.6 μm) (Thermo Fisher Scientific), with a gradient of 10%–55% of 0.1% formic acid in 80% acetonitrile for 60 min, with a flow rate of 0.1 mL min−1. The resulting lists of peaks were used to search against the SwissProt protein database with an in-house Mascot server (v.2.3.0; Matrix Science, London, UK) and a taxonomy restriction to Fungi (548 872 sequences for all entries, 31 582 sequences for fungal proteins) with following parameters: peptide mass tolerance ± 0.3 Da, fragment mass tolerance ± 0.5 Da, allowing two missed cleavages. Citrullination of arginine residues and deamidation of asparagine and glutamine residues, both resulting in the mass increase by 1 Da, have been chosen as variable modifications. After automatic search, the MS/MS spectra for peptides indicated as modified were manually reviewed by the verification of sequence-specific fragment ions to confirm the identification of citrullination. The experiments were performed in triplicates and only those peptides that were identified as having particular arginine residue modified to citrulline in all three replicates have been considered in this work.
THP-1 cell culture and macrophage differentiation
The human acute monocytic leukemia cell line (THP-1) was cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 U mL−1 penicillin and 100 mg mL−1 streptomycin (all compounds purchased from Cytogen) at 37°C in a humidified atmosphere of 95% air and 5% CO2. THP-1 cells were seeded in 12-well tissue culture plates at a density of 1 × 106 cells/well in the complete medium, and treated with a final concentration of 10 ng mL−1 phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich) for 48 h to induce maturation toward adherent macrophage-like cells. The number and extent of differentiation were determined using the Scepter Handheld Automated Cell Counter (Millipore). After cell differentiation, the fresh culture medium without antibiotics was added but the PMA content was preserved for the entire duration of experiments. Inoculation with selected microorganisms was performed after 24 h of cell incubation under the above-described conditions.
THP-1 co-culture with mixed microbial cells
In the study of host responses to formation of mixed biofilm, THP-1 macrophages were incubated with microbial cells at a multiplicity of infection (THP-1:yeast:bacteria ratio) of 1:1:100 for 3 or 24 h, at 37°C. After centrifugation of the cell suspensions (200 × g, 5 min), the supernatants were removed and the remaining cell pellets were washed three times with PBS and used for RNA isolation. Each sample was analyzed in triplicate in three independent experiments.
Gene expression analyses
Total RNA was extracted from human cells using TRIzol reagent (Invitrogen) according to the protocol provided by the manufacturer. Total RNA was purified with the GenJET RNA purification kit (Thermo Scientific) and On-Column DNase I Digestion Set (Sigma-Aldrich) in order to remove all genomic DNA before performing reverse transcription reactions with the SuperScript III First-Strand Synthesis System according to the manufacturer's instructions (Invitrogen). Expression of individual genes was analyzed using quantitative real-time PCR (qPCR) with primers listed in Table 1. PCR amplification was performed by the SYBR green-based detection assay and the StepOnePlus real-time PCR system (Applied Biosystems, Waltham, MA) in 10-μL reaction mixture composed of 2 μL of cDNA, 0.2 μL of 10 μM forward (FW) and reverse (RV) primers, and 5 μL of SYBR KAPA master mix (Kapa Biosystems, Wilmington, MA) under the following conditions: initial denaturation at 95°C for 5 min and 40 cycles of denaturation at 95°C for 20 s, primer annealing at 58°C (for IL-8 encoding gene—at 53°C) for 15 s, and extension at 72°C for 20 s. Data analyses were performed with the real-time PCR system Sequence Detection software (version 1.4; Applied Biosystems). The average threshold cycle (CT) value was obtained and normalized to the average CT value of the gene encoding human glyceraldehyde-3-phosphate dehydrogenase. The comparative CT method was used to quantify gene expression, and relative expression was determined by the 2−ΔΔCTmethod (Livak and Schmittgen 2001).
Table 1.
List of primers used in this study.
| target | primer | sequence (5΄ → 3΄) |
|---|---|---|
| IL1β | FW | CTTTGAAGCTGATGGCCCTAAA |
| RV | AGTGGTGGTCGGAGATTCGT | |
| IL6 | FW | GGCACTGGCAGAAAACAACC |
| RV | GGCAAGTCTCCTCATTGAATCC | |
| IL8 | FW | CACCGGAAGGAACCATCTCACT |
| RV | TCAGCCCTCTTCAAAAACTTCTCC | |
| IL10 | FW | TGAGAACCAAGACCCAGACA |
| RV | AAGGCATTCTTCACCTGCTC | |
| MCP-1 | FW | CAGCCAGATGCAATCAATGC |
| RV | TGCTGCTGGTGATTCTTCTATAGCT | |
| TNFα | FW | TCCTCCAGACACCCTCAACC |
| RV | AGGCCCCAGTTTGAATTCTT | |
| GAPDH | FW | GGGAAGCTTGTCATCAATGG |
| RV | CATCGCCCCACTTGATTTTG |
Statistical analysis
Data were presented as means ± SD (standard deviation) and analyzed using GraphPad Prism software (GraphPad, LaJolla, CA, USA) with an one-way ANOVA test. The differences were considered statistically significant at P < 0.05 (unless otherwise stated).
RESULTS
Formation of dual-species biofilms by P. gingivalis and C. albicans under anaerobic and aerobic conditions
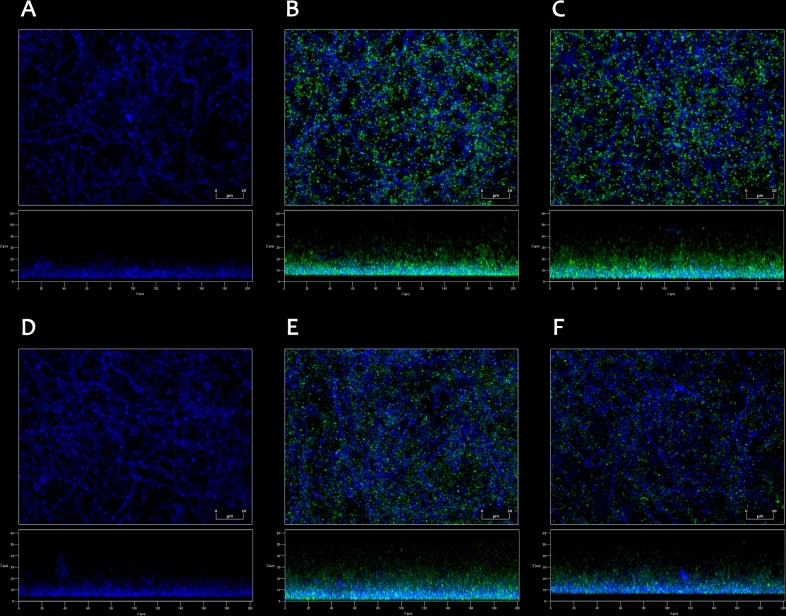
To investigate a possibility that the activity of bacterial PPAD might be induced during the interactions between C. albicans and P. gingivalis upon forming dual-species biofilms, two bacterial strains have been used, the reference wild-type strain P. gingivalis W83 and P. gingivalis W83 knockout strain (Δppad), that does not express the PPAD-encoding gene and demonstrates inability to perform protein citrullination (Wegner et al.2010b). In the current study, a model of co-infection was selected, based on the assumption that a formation of a biofilm by aerobic fungi might affect the colonization of the same infection site by anaerobic bacteria. Under both aerobic and anaerobic conditions, the formation of dual-species biofilms by C. albicans and P. gingivalis strains W83 or Δppad was confirmed by microscopic observations (Fig. 1). Bacterial cells, green fluorescently labeled prior to the addition to pre-formed fungal biofilms, were shown to be located in a close proximity to C. albicans hyphal forms, suggesting mutual interactions between these two microorganisms.
Figure 1.
Formation of mixed biofilms by C. albicans and P. gingivalis wild-type W83 strain (B, E) and P. gingivalis Δppad strain (C, F), observed by confocal laser scanning microscopy. Dual-species biofilms were prepared using 1 × 106C. albicans cells and 1 × 108P. gingivalis cells of each used strain and incubated in RPMI 1640 medium without phenol red for 24 h at 37°C under anaerobic (A–C) or aerobic (D–F) conditions. (A, D) Monospecies fungal biofilms are presented as a reference. Fungal cells (blue) were stained just before imaging with 0.1% Calcofluor White Stain, and bacterial cells (green) were labeled with CFSE prior to addition to the chambers. The images were analyzed with ZEN Lite Microscopy Software.
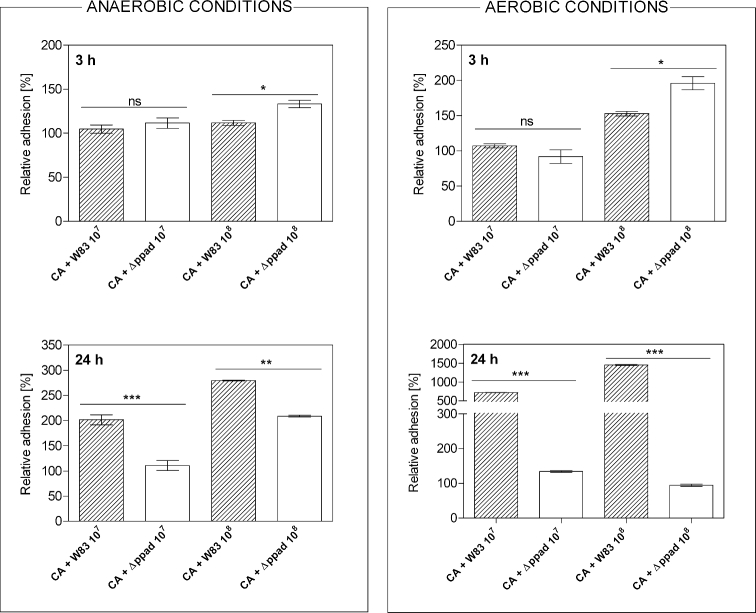
To qualitatively evaluate these interactions in a microplate model, the measurements of the fluorescence intensity representing the number of bacterial cells adhered to fungi were carried out after 3 or 24 h of mutual contact of both microorganisms (Fig. 2). The results were presented as relative adhesion (assuming the level of bacterial adhesion to microplate wells to represent 100%), in order to avoid the effect of general differences in the adhesion ability between tested strains, occurring after a shorter incubation time. In the initial phase of biofilm formation, the differences in the relative adhesion of P. gingivalis W83 strain compared to Δppad strain were rather slight. However, after prolonged time of contact between microorganisms, the relative adhesion was reduced in the case of Δppad strain. This difference, in favor of the wild strain, was up to 100% under anaerobic conditions, while under aerobic conditions the level of adhesion of W83 cells to fungal cells was several fold higher compared to Δppad strain. This observation confirms the important role of PPAD activity for the adherence of bacterial cells to C. albicans. It was also important to consider a dependence of the bacterial cells’ viability during biofilm formation on the presence or absence of already formed fungal biofilm (Fig. 3). Porphyromonas gingivalis CFU assay was performed after co-culture of fungi and bacteria under optimal anoxic growth conditions or in the presence of atmospheric oxygen at a concentration of about 20.9% that is lethal to bacteria after longer incubation. Interestingly, under aerobic conditions in each of the experimental variants (lower vs higher number of bacterial cells used), both bacterial strains exhibited an increased viability in the presence of fungi than when grown alone for 24 h (Fig. 3).
Figure 2.
The relative adhesion of P. gingivalis cells to C. albicans biofilm after 3 or 24 h of co-culture under anaerobic or aerobic conditions. Fungal biofilm was prepared by culturing 5 × 105 cells (CA) in 100 μL RPMI 1640 medium per microplate well for 24 h at 37°C under aerobic conditions. 1 × 107 or 1 × 108 cells of P. gingivalis W83 strain or Δppad strain, both labeled with CFSE, were added to be further incubated for 3 or 24 h. The fluorescence intensity of CFSE-labeled cells was measured with the excitation/emission wavelengths of 480/520 nm. The adhesion of bacteria to the microplate wells was assumed to represent 100%. Bars on the graph represent the mean values of three determinations ± SD. Statistical significance levels were determined with one-way ANOVA test with Tukey's multiple comparison test and marked with **for P < 0.01, ***for P < 0.001 and ‘ns’ for ‘not significant’.
Figure 3.
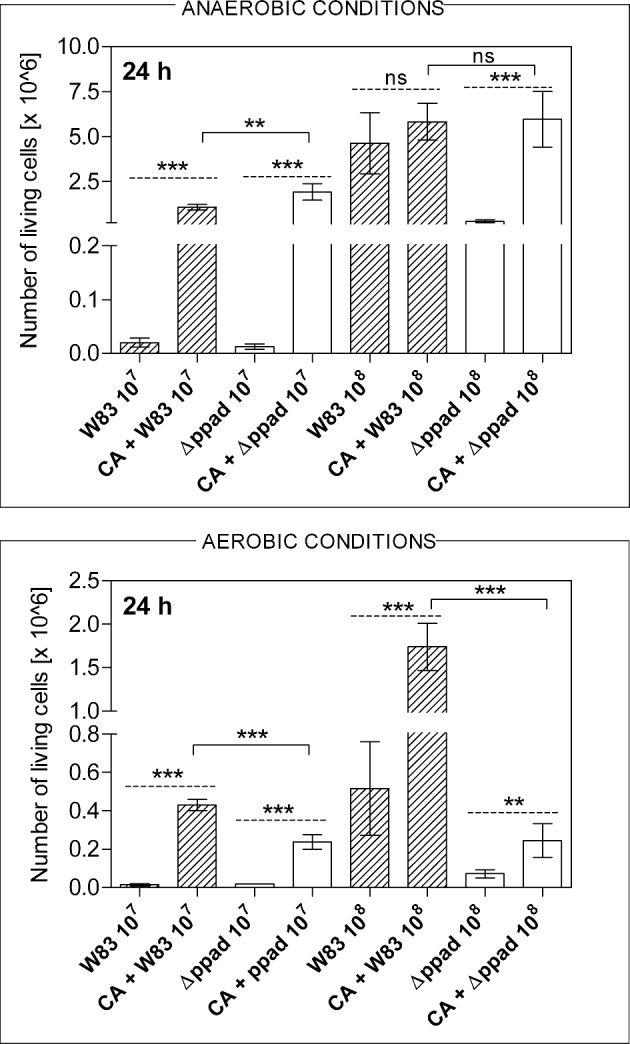
The viability of P. gingivalis cells after growth in mixed biofilm with C. albicans under anaerobic and aerobic conditions. After formation of dual-species biofilms by C. albicans (CA) and P. gingivalis strains W83 and Δppad, cells were mechanically detached from microplate wells and after dilutions transferred to blood agar plates. CFUs were counted after 7 days of anaerobic growth. Bars presented on the graph correspond to the mean values of three determinations ± SD. Statistical significance levels marked with **for P < 0.01, ***for P < 0.001 and ‘ns’ for ‘not significant’ were determined with one-way ANOVA test with Tukey's multiple comparison test. The solid line shows the comparison between two bacterial strains and the dashed line between the number of live bacterial cells in mono- or dual-species biofilm.
Considering the dependence of bacterial viability on the contact with fungal biofilm under anaerobic conditions, it can be noticed that the presence of fungi might also play a protective or supportive role for P. gingivalis Δppad strain, increasing the number of live bacterial cells collected from the dual-species biofilm (Fig. 3). In the case of wild-type W83 strain, such protection was observed only for samples with a lower number of bacterial cells.
Another interesting issue was the increased viability of C. albicans cells, confirmed with CFU assay, in the presence of 1 × 108P. gingivalis cells of Δppad strain, but not wild-type W83 strain (Fig. 4). Under both anaerobic and aerobic conditions, the fungal cell adhesion to the microplate wells, regardless of the presence of bacteria, did not differ (data not presented), similarly as did the number of the live C. albicans cells after 24 h of growth in the single-species biofilm or in the mixed-species biofilm with P. gingivalis cells of W83 strain.
Figure 4.
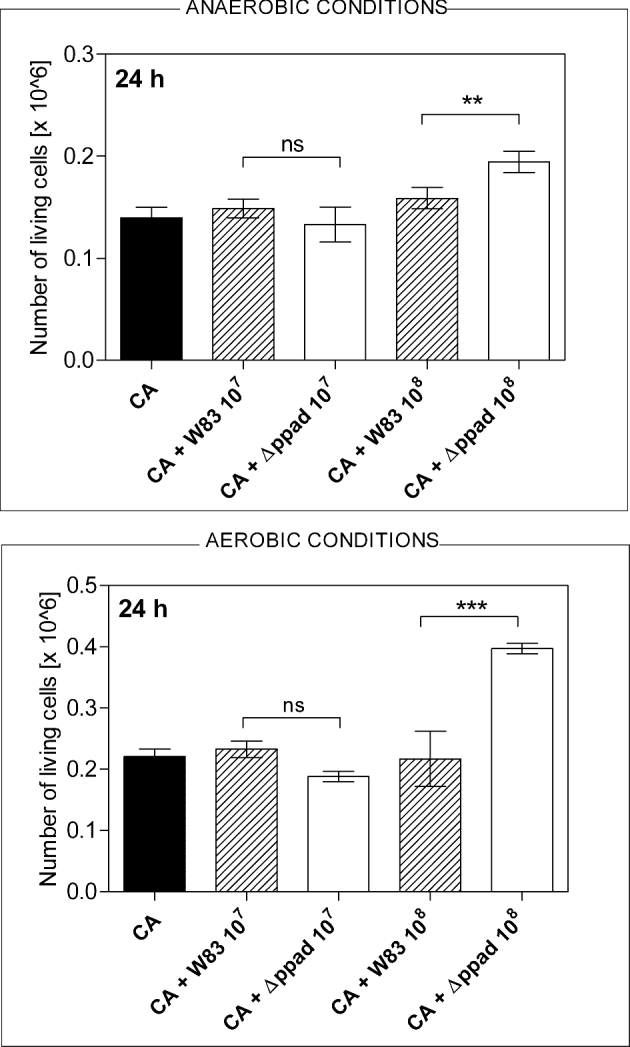
The number of live C. albicans cells (CA) counted after formation of biofilm with P. gingivalis strains W83 and Δppad under anaerobic and aerobic conditions. The CFU assay was carried out after the mechanical removal of adsorbed cells from the microplate wells and their further aerobic growth on agar plates for 24 h at 30°C. The mean values of three determinations ± SD are presented. One-way ANOVA test with Tukey's multiple comparison test was used to determine the statistical significance levels, which are marked with **for P < 0.01, ***for P < 0.001 and ‘ns’ for ‘not significant’.
Further important question was whether the molecules exposed at the surface of C. albicans cells, including various virulence factors and adhesins, might be modified by PPAD during the contact of both pathogens within the biofilm. Thus, the mass spectrometric identification of modified fungal proteins after cell surface shaving with trypsin (Karkowska-Kuleta et al.2015) was performed and used to indicate particular peptides, in which the arginine residue was converted to citrulline residue, resulting in a peptide mass increase by 1 Da per residue (Table 2). It was important to accurately analyze the spectra in order to exclude incorrect attributions of that mass change to deamidation of asparagine or glutamine residues. Nine different proteins were identified as potentially modified by PPAD, of which one only after co-culture under anaerobic conditions (pyruvate decarboxylase, Pdc1), whereas two only after C. albicans growth with P. gingivalis under aerobic conditions (heat shock protein, Ssb1 and pH-regulated antigen, Pra1). For each of the proteins listed in Table 2, at least one particular arginine residue was found to be modified to citrulline. The modifications of C. albicans proteins were identified only after exposure of fungal cells to bacterial PPAD produced during biofilm formation with P. gingivalis wild-type W83 strain. In the control samples, where dual-species biofilm was formed by C. albicans and P. gingivalis Δppad strain, as well as for the fungal monospecies biofilms, no protein was identified as having arginine modified to citrulline, even though the method of analysis was identical for all samples.
Table 2.
Mass spectrometry identification of C. albicans surface-exposed proteins after formation of dual-species biofilm. C. albicans cells (5×108) and P. gingivalis cells of wild-type W83 strain or Δppad strain (5×109) were mixed together and incubated in RPMI 1640 medium for 24 hours at 37°C under aerobic or anaerobic conditions to form mixed-species biofilm. Mono-species biofilm formed only by C. albicans cells, without bacteria, served as a control. Fungal surface-exposed proteins were identified with cell surface shaving with trypsin followed by analysis with the Dionex Ultimate 3000 UHPLC system coupled to an HCTultra ETDII mass spectrometer. The representative results from three independent experiments are presented, provided that particular peptide have been identified in each of them (m/z – mass to charge).
| Accession number | C. albicans protein name | Peptide | P. gingivalis strain | Observed m/z ratio (charge) | Calculated mass [Da] | Ion score | |
|---|---|---|---|---|---|---|---|
| aerobic conditions | anaerobic conditions | ||||||
| P30575 (ENO1_CANAL) | Enolase 1 | 319IQIVGDDLTVTNPTR333 | ― | 821.4640 (+2) | 1640.9134 | 108 | |
| W83 | 821.9680 (+2) | 1641.9214 | 65 | 76 | |||
| Δppad | 821.4160 (+2) | 1640.8174 | 83 | ||||
| P83776 (HXKB_CANAL) | Hexokinase-2 | 350IEEDPFENLSDVADLFR366 | ― | 1004.9900 (+2) | 2007.9654 | 31 | |
| W83 | 1005.4630 (+2) | 2008.9114 | 75 | 81 | |||
| Δppad | 1005.0610 (+2) | 2007.9425 | 103 | ||||
| P46273 (PGK_CANAL) | Phosphoglycerate kinase | 112DGEIFLLENLR122 | ― | 659.9270 (+2) | 1317.8394 | 34 | |
| W83 | 660.4390 (+2) | 1318.8634 | 66 | 59 | |||
| Δppad | 659.8570 (+2) | 1317.6994 | 53 | ||||
| P43067 (ADH1_CANAX) | Alcohol dehydrogenase 1 | 303DTAEAIDFFSR313 | ― | 636.3830 (+2) | 1270.7514 | 67 | |
| W83 | 636.8750 (+2) | 1271.7354 | 60 | 49 | |||
| Δppad | 636.3180 (+2) | 1270.6214 | 54 | ||||
| P83779 (PDC1_CANAL) | Pyruvate decarboxylase | 37IYEVEGMR44 | ― | 498.7190 (+2) | 995.4234 | 29 | |
| W83 | 499.2500 (+2) | 996.4854 | 47 | ||||
| Δppad | 498.8180 (+2) | 995.6214 | 19 | ||||
| Q59XX2 (MP65_CANAL) | Cell surface mannoprotein MP65 | 142SESQIASEIAQLSGFDVIR160 | ― | 684.0800 (+3) | 2049.2182 | 78 | |
| W83 | 1026.0470 (+2) | 2050.0794 | 73 | 109 | |||
| Δppad | 684.0370 (+3) | 2049.0892 | 61 | ||||
| Q5AIR7 (ENG1_CANAL) | Endo-1,3(4)-beta-glucanase 1 | 932DASNPSADDTYFPVSR948 | ― | 871.3610 (+2) | 1740.7591 | 54 | 23 |
| W83 | 871.8550 (+2) | 1741.7554 | 57 | ||||
| Δppad | 871.4090 (+2) | 1740.7870 | 26 | ||||
| 1082DQIAGFIDNVDSGWTGILR1100 | ― | n.o. | 2076.0276* | ||||
| W83 | 1039.5030 (+2) | 2076.9914 | 101 | 72 | |||
| P87222 (HSP75_CANAW) | Heat shock protein Ssb1 | 332SQVDEVVLVGGSTR345 | ― | 723.4650 (+2) | 1444.9154 | 59 | |
| W83 | 723.9760 (+2) | 1445.9374 | 30 | ||||
| Δppad | 723.4040 (+2) | 1444.7934 | 76 | ||||
| P87020 (PRA1_CANAL) | pH-regulated antigen | 168TNIFWAGDLLHR179 | ― | n.o. | 1441.7466* | ||
| W83 | 481.9460 (+3) | 1442.8162 | 51 | ||||
n.o. not observed
* theoretical mass for the specified peptide (unmodified)
Host responses to mixed C. albicans and P. gingivalis biofilm formation
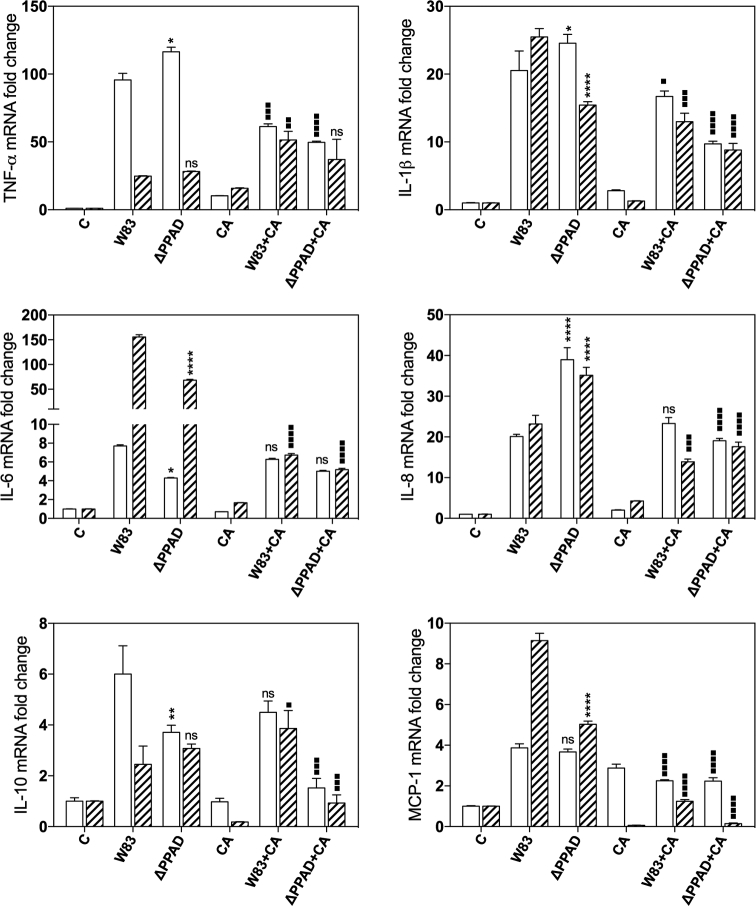
To determine the influence of mixed biofilm formation on the host cell response, we used the model of THP-1 cells differentiated into macrophages by PMA treatment (Qin 2012) and analyzed the expression of genes encoding selected cytokines to monitor the progress of infection. The responses of the human cells, contacting with only P. gingivalis or only C. albicans, served as controls (Fig. 5). Both P. gingivalis strains, W83 and Δppad, activated the responses of cytokine-coding genes, although the defect in PPAD production by P. gingivalis cells resulted in the generally lower expression level of genes that encode TNFα, IL-1β, IL-6, IL-10 and monocyte chemoattractant protein-1 (MCP-1). The opposite effect was observed for the expression of IL-8-encoding gene. The presence of hyphal form of C. albicans cells caused changes in the gene expression to the significantly lower level (ca. 10-fold) compared to bacterial responses. More meaningful changes were detected during formation of mixed biofilm, where the protection of P. gingivalis cells from the host recognition system was clearly visible. Both short (3 h) and long (24 h) responses of THP-1 cells to mixed infection, represented by the expression of selected genes, were significantly reduced—about 3-fold to 5-fold compared to unprotected bacterial cells. The protection effect was also observed during the biofilm formation between C. albicans cells and P. gingivalis mutant strain Δppad. The results confirmed previous observations and pointed at PPAD as an important virulence factor, recognized by the host and influencing the interaction with fungal cells.
Figure 5.
Expression profiles of genes that encode IL-1β, TNF-α, IL-6, IL8, IL-10 and MCP-1 in THP-1 monocyte-derived macrophages after contact with C. albicans and P. gingivalis cells for 3 h (open bars) or 24 h (crosshatch bars) at a multiplicity of infection of 1:1:100 measured by qRT-PCR. Data are expressed as the mean ± SD (three independent experiments). One-way ANOVA test with Tukey's multiple comparison test was used to determine the statistical significance levels. The significance levels were marked with */▪ for P < 0.05; **/▪▪ for P < 0.005, ***/▪▪▪ for P < 0.001, ****/▪▪▪▪ for P < 0.0001 and ‘ns’ for ‘not significant’. Asterisks were used to compare cell responses to wild and mutant bacterial strains, and squares to compare cell responses to mixed biofilm versus bacteria.
DISCUSSION
The maintenance of an ecological balance and an appropriate, physiological cooperation between all microorganisms that form the microbiota in the oral cavity contribute to the general health of the human host (Marsh 2010; Lof, Janus and Krom 2017). Several hundreds of different species of bacteria, fungi, viruses and protozoa create a complex community in the oral cavity during the formation of mixed-species biofilms on soft and hard tissues in the mouth (Dewhirst et al.2010; Wright et al.2013; Samaranayake and Matsubara 2017). Disturbing this complicated network of relationships between bacteria that are ‘early colonizers’ and ‘late colonizers’ in the oral cavity or microorganisms that ‘bridge’ the representatives of those two groups or occasionally contact with them during accidental infection might lead to the development of such common and onerous diseases such as caries or periodontitis (van Palenstein Helderman 1981; van Winkelhoff et al.2002; Filoche, Soma and Sissons 2007; He et al.2012).
One of the main infectious agents in periodontal disease is P. gingivalis, considered as a ‘keystone’ pathogen in the subgingival biofilm (Honda 2011; Bostanci and Belibasakis 2012). The changes in environmental conditions combined with the initial adhesion of P. gingivalis cells to other bacteria, including aerobic streptococci, might shift the microbiological balance in the oral cavity and facilitate further development of the pathogenic state related to gingivitis and periodontitis (Lamont, Hersey and Rosan 1992; Marsh 2010; Lof, Janus and Krom 2017). In addition to different bacterial species found within oral biofilms, other pathogenic microorganism, C. albicans, might also be involved in the interactions with P. gingivalis (Socransky et al.1998; Rautemaa and Ramage 2011; Camargo et al.2016). Since there are data presenting the importance of the interactions between these two pathogens during the development of certain oral infections (Tamai, Sugamata and Kiyoura 2011; Haverman et al.2017), it is important to investigate in detail the character of these interactions and the contribution of particular virulence factors to this contact.
Besides the fact that C. albicans can grow either under aerobic and anaerobic conditions (Webster and Odds 1987), it also possesses an ability to decrease the oxygen concentration in the immediate vicinity, thus facilitating the development of the microenvironment that protects and supports the growth of anaerobic bacteria (Fox et al.2014). In the current work, such an important capability was demonstrated with respect to P. gingivalis cells. We observed that under experimental conditions at the atmospheric oxygen concentration, the relative viability of bacteria was considerably higher, when they were co-incubated with C. albicans in a dual-species biofilm rather than in the monospecies biofilm. Porphyromonas gingivalis demonstrates the ability to adapt its metabolism to microaerophilic conditions at oxygen concentration about 6% (Lewis, Iyer and Anaya-Bergman 2009); thus, the localization of bacterial cells within the complex and heterogeneous structure of fungal biofilm might result in the successful growth and proliferation of bacteria. On the other hand, we cannot currently provide an explanation for the observed increased viability of C. albicans cells in biofilms formed with P. gingivalis cells deprived of PPAD. Probably, this might be caused by some compensative processes in bacterial cell metabolism, caused by the lack of this important protein, and undoubtedly this issue requires further, detailed studies.
Furthermore, we noticed the significant reduction in the level of adhesion to fungal biofilms after prolonged mutual contact, demonstrated in bacterial cells lacking the ability to citrullinate proteins. This phenomenon might be related to both autocitrullination of P. gingivalis surface-exposed proteins and/or modification of proteins derived from the cells of interacting partners. Recently, the extracellular citrullinome of P. gingivalis was investigated and several proteins, including important bacterial virulence factors, were found to be modified by PPAD, including arginine-specific cysteine proteinase (RgpA) and adhesive Mfa1 fimbrilin (Stobernack et al.2016). Such modifications of the bacterial cell surface proteins could also play a role during the formation of dual-species biofilm with C. albicans; however, the possibility of citrullination of fungal proteins should not be ignored. In the current work, a relatively novel proteomics-like approach—‘cell surface shaving’ with trypsin (Karkowska-Kuleta et al.2015)—was used after dual-species biofilm formation to identify fungal surface-exposed proteins. As both microorganisms were co-cultured for quite a long time, these fungal proteins could be first processed by P. gingivalis arginine-specific gingipains, resulting in the exposition of the C-terminal arginine residues prone to citrullination by PPAD (Wegner et al.2010b).
Enolase was found among C. albicans surface-exposed proteins, for which the potential citrullination could be suggested. This is an important ‘moonlighting’ protein, abundant glycolytic enzyme and well-known dominant antigen detected during disseminated candidiasis, and a binding protein for human plasminogen and components of the plasma kinin-generating system (Sundstrom and Aliaga 1992; Jong et al.2003; Karkowska-Kuleta et al.2011; Seweryn et al.2015). Candida albicans enolase is also responsible for the adhesion to polyvinyl chloride-made medical devices (Núñez-Beltrán, López-Romero and Cuéllar-Cruz 2017). A suggested ability of P. gingivalis PPAD to citrulllinate human enolase provides an interesting link between the inflammation during periodontal disease and the development of serious autoimmune disorder, rheumatoid arthritis (Kinloch et al.2005; Wegner et al.2010a).
In addition, two other C. albicans cytoplasm-derived enzymes, also found at the surface of fungal cells, were identified to be modified by bacterial PPAD. These included phosphoglycerate kinase (Pgk1) and alcohol dehydrogenase (Adh1), known as antigens involved also in binding of human plasminogen (Pitarch et al.2001; Crowe et al.2003). Moreover, the possible citrullination of the pH regulated antigen (Pra1) might be important for the interactions of fungi with the host during infection, as this protein participates in the regulation of host immune system activation through the interactions with immune cells and complement receptors and regulators (Luo et al.2009, 2010; Losse et al.2011; Soloviev, Jawhara and Fonzi 2011). Additional roles of Pra1 such as the binding to plasminogen and zinc sequestration during invasion on endothelial cells (Luo et al.2009; Citiulo et al.2012) might also be altered by potential citrullination of this protein by PPAD. Two other candidal enzymes, endo-1,3-β-glucanase, Eng1, and cell surface mannoprotein, MP65, play a role in maintaining the proper function of the fungal cell wall (Esteban et al.2005; Sandini et al.2007), and the latter protein is also involved in the interactions with the host, and in the biofilm formation (Pietrella et al.2006; Sandini et al.2011). Thus, the potential changes in the functionality of all of the abovementioned proteins caused by PPAD-dependent modification might significantly affect the interactions of C. albicans with bacteria or host cells during biofilm formation.
Numerous studies have demonstrated that during the periodontal infection P. gingivalis is able to induce the production of many inflammatory mediators from different types of host cells such as epithelial cells, fibroblasts, endothelial cells and macrophages (Gemmel and Seymour 1993; Quinchia-Rios et al.2008; Damgaard et al.2016). Results obtained in our current study fit to previously published data on the immune response of macrophages to infection with P. gingivalis wild-type cells (Gmiterek et al.2016; Lam et al.2016), including the reports of the stimulation of monocyte-derived macrophages to the secretion of proinflammatory cytokines and chemokines, such as TNF-α, IL-1β, IL-6, IL-8 and MCP-1, that subsequently recruit next immune cells to infiltrate infected tissues (Zhou and Amar 2006). We presented here, for the first time, markedly changed responses in macrophages that contacted the P. gingivalis mutant strain deprived of PPAD, thus providing a direct evidence for the significance of this enzyme as an important virulence factor. We found that the expression of the gene, encoding IL-1β, known to inhibit macrophage apoptosis, resulting in an impaired elimination of P. gingivalis from the infected host cells, was decreased in the host response to mutant strain. Similar effects were noticed for gene encoding IL-6 that is responsible for the differentiation of activated B cells into immunoglobulin-secreting plasma cells and may play an important role in regulating the immune response of patients with chronic periodontitis (Zhou and Amar 2006; Trindade et al.2012; Gmiterek et al.2016). On the other hand, the expression of the gene encoding TNF-α, the protein that coordinates many processes of macrophage activation in response to P. gingivalis, including the production of proinflammatory cytokines and surface expression of TLR2 (Papadopoulos et al.2013) seemed not to be influenced significantly by PPAD presence, as both strains generated similar responses of macrophages. Also, the expression of the gene encoding anti-inflammatory cytokine IL-10 in macrophages infected with mutant strain was decreased compared to parent strain. These results corroborate other study that presented IL-10 production by THP-1 cells upon the contact with wild-type strain 7436 and a strain defective in other P. gingivalis virulence factor, HmuY (Gmiterek et al.2016). We also identified changes in the expression of the gene that encodes the chemokine MCP-1, the most potent chemoattractant for monocytes. Its significantly higher levels were detected in gingival crevicular fluid, saliva and serum in subjects with chronic periodontitis compared to healthy controls (Gupta, Chaturvedi and Jain 2013; Zhu et al.2015). In our model, the responses of THP-1 to P. gingivalis Δppad cells were significantly decreased compared to wild-type P. gingivalis cells. Similarly, decreased responses of human cells were observed for Δppad cells that contact fibroblasts, where P. gingivalis was found to be involved in the modulation of the prostaglandin-dependent pathway. The host cells infected with wild-type P. gingivalis strain showed the upregulated expression of cyclooxygenase-2 and microsomal PGE synthase-1, two key enzymes that participate in PGE2 synthesis. But these effects were strongly reduced in cells infected with the Δppad strain as well as with the strain that expresses a catalytically inactive enzyme (Gawron et al.2014).
The opposite effect was observed in our study for the expression of IL-8- encoding gene, which seemed to increase in contact with Δppad mutant strain. This effect is confusing in line of data that both wild and mutant strains express comparable amount of gingipains, the enzyme involved in IL-8 degradation, and that PPAD exerted only a minor IL-8-citrullinating activity (Moelants et al.2014).
However, the most important results of our current study concerned the attenuated macrophage responses during the mixed infection with bacteria and C. albicans, and in particular, the decreased expression of genes that encode the analyzed cytokine and chemokine. Similar type of bacteria protection from host responses was observed previously for host cells upon contacts with mixed biofilms. However, depending on the type of contacting microorganisms, the responses can differ significantly (Peleg et al.2010). The interpretation of results presented hereby is challenging and needs more research, as we can expect many possible way of mutual pathogen interactions. One could concern antagonistic interactions where the virulence of bacteria is reduced by the contact with fungi, as was presented for mixed biofilm with P. aeruginosa (Lopez-Medina et al.2015). Other possibility is that the fungal cells may suppress local host immune response to allow bacteria to survive under unfavorable conditions (Roux et al.2009). Alternatively, fungal biofilm formation can serve as a camouflage for bacterial cells which hide in it against host recognition. However, further work is required to understand the relevance of C. albicans and P. gingivalis mixed biofilms for the predisposition to invasive infection and their susceptibility to host immune defense.
The association of the periodontitis with different severe systemic diseases, including cardiovascular diseases, Alzheimer's disease, diabetes or rheumatoid arthritis (Otomo-Corgel et al.2012), urges to thoroughly investigate any conditions that may contribute to the development of P. gingivalis-related infections associated with the formation of complex microbial communities within the dental plaque. It is likely that the citrullination of proteins by bacterial PPAD might be an important phenomenon during the formation of mixed-species biofilm together with C. albicans. This complex structure built by the fungal cells present in various morphological forms and containing the extracellular matrix might provide a protective environment for anaerobic bacteria in the oral cavity, thus facilitating and accelerating the development of the serious gingival diseases.
FUNDING
This work was supported by the National Science Centre of Poland (grant number 2015/17/B/NZ6/0 2078 to MRK) and National Institutes of Health (grant number NIH/NIDCR DE 022597 to JP).
Conflict of interest. None declared.
REFERENCES
- Bielecka E, Scavenius C, Kantyka T et al. . Peptidyl arginine deiminase from Porphyromonas gingivalis abolishes anaphylatoxin C5a activity. J Biol Chem 2014;289:32481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostanci N, Belibasakis GN. Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol Lett 2012;333:1–9. [DOI] [PubMed] [Google Scholar]
- Camargo GA, Abreu MG, Cordeiro Rdos S et al. . Prevalence of periodontopathogens and Candida spp. in smokers after nonsurgical periodontal therapy - a pilot study. Braz Oral Res 2016;30:e92. [DOI] [PubMed] [Google Scholar]
- Citiulo F, Jacobsen ID, Miramón P et al. . Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog 2012;8:e1002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JD, Sievwright IK, Auld GC et al. . Candida albicans binds human plasminogen: identification of eight plasminogen-binding proteins. Mol Microbiol 2003;47:1637–51. [DOI] [PubMed] [Google Scholar]
- Damgaard C, Kantarci A, Holmstrup P et al. . Porphyromonas gingivalis-induced production of reactive oxygen species, tumor necrosis factor-a, interleukin-6, CXCL8 and CCL2 by neutrophils from localized aggressive periodontitis and healthy donors: modulating actions of red blood cells and resolvin E1. J Periodontal Res 2016;52:246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau RP, Hajishengallis G, Curtis MA. Porphyromonas gingivalis as a potential community activist for disease. J Dent Res 2012;91:816–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J et al. . The human oral microbiome. J Bacteriol 2010;192:5002–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban PF, Ríos I, García R et al. . Characterization of the CaENG1 gene encoding an endo-1,3-beta-glucanase involved in cell separation in Candida albicans. Curr Microbiol 2005;51:385–92. [DOI] [PubMed] [Google Scholar]
- Filoche SK, Soma KJ, Sissons CH. Caries-related plaque microcosm biofilms developed in microplates. Oral Microbiol Immunol 2007;22:73–9. [DOI] [PubMed] [Google Scholar]
- Fox EP, Cowley ES, Nobile CJ et al. . Anaerobic bacteria grow within Candida albicans biofilms and induce biofilm formation in suspension cultures. Curr Biol 2014;24:2411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabarrini G, de Smit M, Westra J et al. . The peptidylarginine deiminase gene is a conserved feature of Porphyromonas gingivalis. Sci Rep 2015;5:13936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron K, Bereta G, Nowakowska Z et al. . Peptidylarginine deiminase from Porphyromonas gingivalis contributes to infection of gingival fibroblasts and induction of prostaglandin E2-signaling pathway. Mol Oral Microbiol 2014;29:321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmel E., Seymour GJ. Interleukin 1, interleukin 6 and transforming growth factor-ß production by human gingival mononuclear cells following stimulation with Porphyromonas gingivalis and Fusobacterium nucleatum. J Periodont Res 1993;28:122–9. [DOI] [PubMed] [Google Scholar]
- Gmiterek A, Kłopot A, Wójtowicz H et al. . Immune response of macrophages induced by Porphyromonas gingivalis requires HmuY protein. Immunobiology 2016;221:1382–94. [DOI] [PubMed] [Google Scholar]
- Gomes CC, Guimarães LS, Pinto LCC et al. . Investigations of the prevalence and virulence of Candida albicans in periodontal and endodontic lesions in diabetic and normoglycemic patients. J Appl Oral Sci 2017;25:274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M, Chaturvedi R, Jain A. Role of monocyte chemoattractant protein-1 (MCP-1) as an immune-diagnostic biomarker in the pathogenesis of chronic periodontal disease. Cytokine 2013;61:892–7. [DOI] [PubMed] [Google Scholar]
- Haverman TM, Laheij AMGA, de Soet JJ et al. . Candida and Porphyromonas gingivalis: the effect on wound closure in vitro. J Oral Microbiol 2017;9:1328266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Hu W, Kaplan CW et al. . Adherence to streptococci facilitates Fusobacterium nucleatum integration into an oral microbial community. Microb Ecol 2012;63:532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K. Porphyromonas gingivalis sinks teeth into the oral microbiota and periodontal disease. Cell Host Microbe 2011;10:423–5. [DOI] [PubMed] [Google Scholar]
- Janus MM, Crielaard W, Volgenant CM et al. . Candida albicans alters the bacterial microbiome of early in vitro oral biofilms. J Oral Microbiol 2017;9:1270613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong AY, Chen SH, Stins MF et al. . Binding of Candida albicans enolase to plasmin(ogen) results in enhanced invasion of human brain microvascular endothelial cells. J Med Microbiol 2003;52:615–22. [DOI] [PubMed] [Google Scholar]
- Karkowska-Kuleta J, Kedracka-Krok S, Rapala-Kozik M. et al. Molecular determinants of the interaction between human high molecular weight kininogen and Candida albicans cell wall: identification of kininogen-binding proteins on fungal cell wall and mapping the cell wall-binding regions on kininogen molecule. Peptides 2011;32:2488–96. [DOI] [PubMed] [Google Scholar]
- Karkowska-Kuleta J, Zajac D, Bochenska O et al. . Surfaceome of pathogenic yeasts, Candida parapsilosis and Candida tropicalis, revealed with the use of cell surface shaving method and shotgun proteomic approach. Acta Biochim Pol 2015;62:807–19. [DOI] [PubMed] [Google Scholar]
- Kinloch A, Tatzer V, Wait R et al. . Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res Ther 2005;7:R1421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam RS, O’Brien-Simpson NM, Holden JA et al. . Unprimed, M1 and M2 macrophages differentially interact with Porphyromonas gingivalis. PLoS ONE 2016;11:e0158629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambooij JM, Hoogenkamp MA, Brandt BW et al. . Fungal mitochondrial oxygen consumption induces the growth of strict anaerobic bacteria. Fungal Genet Biol 2017;109:1–6. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Hersey SG, Rosan B. Characterization of the adherence of Porphyromonas gingivalis to oral streptococci. Oral Microbiol Immunol 1992;7:193–7. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol R 1998;62:1244–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JP, Iyer D, Anaya-Bergman C. Adaptation of Porphyromonas gingivalis to microaerophilic conditions involves increased consumption of formate and reduced utilization of lactate. Microbiology 2009;155:3758–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MAO, Williams DW. Diagnosis and management of oral candidosis. Br Dent J 2017;223:675–81. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001;25:402–8 [DOI] [PubMed] [Google Scholar]
- Lof M, Janus MM, Krom BP. Metabolic interactions between bacteria and fungi in commensal oral biofilms. J Fungi 2017;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Medina E, Fan D, Coughlin LA et al. . Candida albicans inhibits Pseudomonas aeruginosa virulence through suppression of pyochelin and pyoverdine biosynthesis. PLoS Pathog 2015;11:e1005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losse J, Svobodová E, Heyken A et al. . Role of pH-regulated antigen 1 of Candida albicans in the fungal recognition and antifungal response of human neutrophils. Mol Immunol 2011;48:2135–43. [DOI] [PubMed] [Google Scholar]
- Luo S, Hartmann A, Dahse HM et al. . Secreted pH-regulated antigen 1 of Candida albicans blocks activation and conversion of complement C3. J Immunol 2010;185:2164–73. [DOI] [PubMed] [Google Scholar]
- Luo S, Poltermann S, Kunert A et al. . Immune evasion of the human pathogenic yeast Candida albicans: Pra1 is a Factor H, FHL-1 and plasminogen binding surface protein. Mol Immunol 2009;47:541–50. [DOI] [PubMed] [Google Scholar]
- McGraw WT, Potempa J, Farley D et al. . Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase. Infect Immun 1999;67:3248–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh PD. Microbiology of dental plaque biofilms and their role in oral health and caries. Dent Clin North Am 2010;54:441–54. [DOI] [PubMed] [Google Scholar]
- Marsh PD, Zaura E. Dental biofilm: ecological interactions in health and disease. J Clin Periodontol 2017;44:S12–22. [DOI] [PubMed] [Google Scholar]
- Moelants E, Loozen G, Mortier A et al. . Citrullination and proteolytic processing of chemokines by Porphyromonas gingivalis. Infect Immun 2014;82:2511–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez-Beltrán A, López-Romero E, Cuéllar-Cruz M. Identification of proteins involved in the adhesion of Candida species to different medical devices. Microb Pathog 2017;107:293–303. [DOI] [PubMed] [Google Scholar]
- O’Donnell LE, Millhouse E, Sherry L et al. . Polymicrobial Candida biofilms: friends and foe in the oral cavity. FEMS Yeast Res 2015;15:fov077. [DOI] [PubMed] [Google Scholar]
- Otomo-Corgel J, Pucher JJ, Rethman MP et al. . State of the science: chronic periodontitis and systemic health. J Evid Based Dent Pr 2012;12:20–8. [DOI] [PubMed] [Google Scholar]
- Papadopoulos G, Weinberg EO, Massari P et al. . Macrophage-specific TLR2 signaling mediates pathogen-induced TNF-dependent inflammatory oral bone loss. J Immunol 2013;190:1148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BA, Wu J, Hayes RB et al. . The oral fungal mycobiome: characteristics and relation to periodontitis in a pilot study. BMC Microbiol 2017;17:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrella D, Bistoni G, Corbucci C et al. . Candida albicans mannoprotein influences the biological function of dendritic cells. Cell Microbiol 2006;8:602–12. [DOI] [PubMed] [Google Scholar]
- Pitarch A, Díez-Orejas R, Molero G et al. . Analysis of the serologic response to systemic Candida albicans infection in a murine model. Proteomics 2001;1:550–9. [DOI] [PubMed] [Google Scholar]
- Peleg AY, Hogan DA, Mylonakis E et al. . Medically important bacterial-fungal interactions. Nat Rev Microbiol 2010;8:340–9. [DOI] [PubMed] [Google Scholar]
- Pyrc K, Milewska A, Kantyka T et al. . Inactivation of epidermal growth factor by Porphyromonas gingivalis as a potential mechanism for periodontal tissue damage. Infect Immun 2013;81:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis 2012;221:2–11. [DOI] [PubMed] [Google Scholar]
- Quinchia-Rios BH, Guerrero M, Abozeid S et al. . Down-regulation of epidermal growth factor receptor-dependent signaling by Porphyromonas gingivalis lipopolysaccharide in life-expanded human gingival fibroblasts. J Periodontal Res 2008;43:290–304. [DOI] [PubMed] [Google Scholar]
- Roux D, Dreyfuss JG, El-Benna J et al. . Candida albicans impairs macrophage function and facilitates Pseudomonas aeruginosa pneumonia in rat. Crit Care Med 2009;37:1062–7. [DOI] [PubMed] [Google Scholar]
- Rautemaa R, Ramage G. Oral candidosis-clinical challenges of a biofilm disease. Crit Rev Microbiol 2011;37:328–36. [DOI] [PubMed] [Google Scholar]
- Samaranayake L, Matsubara VH. Normal oral flora and the oral ecosystem. Dent Clin North Am 2017;61:199–215. [DOI] [PubMed] [Google Scholar]
- Sandini S, La Valle R, De Bernardis F et al. . The 65 kD amannoprotein gene of Candida albicans encodes a putative beta-glucanase adhesin required for hyphal morphogenesis and experimental pathogenicity. Cell Microbiol 2007;9:1223–38. [DOI] [PubMed] [Google Scholar]
- Sandini S, Stringaro A, Arancia S et al. . The MP65 gene is required for cell wall integrity, adherence to epithelial cells and biofilm formation in Candida albicans. BMC Microbiol 2011;11:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi JC, Scorzoni L, Bernardi T et al. . Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol 2013;62:10–24. [DOI] [PubMed] [Google Scholar]
- Seweryn K, Karkowska-Kuleta J, Wolak N et al. . Kinetic and thermodynamic characterization of the interactions between the components of human plasma kinin-forming system and isolated and purified cell wall proteins of Candida albicans. Acta Biochim Pol 2015;62:825–35. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA et al. . Microbial complexes in subgingival plaque. J Clin Periodontol 1998;25:134–44. [DOI] [PubMed] [Google Scholar]
- Soloviev DA, Jawhara S, Fonzi WA. Regulation of innate immune response to Candida albicans infections by αMβ2-Pra1p interaction. Infect Immun 2011;79:1546–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobernack T, Glasner C, Junker S et al. . Extracellular proteome and citrullinome of the oral pathogen Porphyromonas gingivalis. J Proteome Res 2016;15:4532–43. [DOI] [PubMed] [Google Scholar]
- Sundstrom P, Aliaga GR. Molecular cloning of cDNA and analysis of protein secondary structure of Candida albicans enolase, an abundant, immunodominant glycolytic enzyme. J Bacteriol 1992;174:6789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai R, Sugamata M, Kiyoura Y. Candida albicans enhances invasion of human gingival epithelial cells and gingival fibroblasts by Porphyromonas gingivalis. Microb Pathog 2011;51:250–4. [DOI] [PubMed] [Google Scholar]
- Trindade SC, Olczak T, Gomes-Filho IS et al. . Induction of interleukin (IL)-1β, IL-10, IL-8 and immunoglobulin G by Porphyromonas gingivalis in humans. J Periodontal Res 2012;47:27–32. [DOI] [PubMed] [Google Scholar]
- van Palenstein Helderman WH. Microbial etiology of periodontal disease. J Clin Periodontol 1981;8:261–80. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff AJ, Loos BG, van der Reijden WA et al. . Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J Clin Periodontol 2002;29:1023–8. [DOI] [PubMed] [Google Scholar]
- Webster CE, Odds FC. Growth of pathogenic Candida isolates anaerobically and under elevated concentrations of CO2 in air. J Med Vet Mycol 1987;25:47–53. [PubMed] [Google Scholar]
- Wegner N, Lundberg K, Kinloch A et al. . Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol Rev 2010a;233:34–54. [DOI] [PubMed] [Google Scholar]
- Wegner N, Wait R, Sroka A et al. . Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum 2010b;62:2662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CJ, Burns LH, Jack AA et al. . Microbial interactions in building of communities. Mol Oral Microbiol 2013;28:83–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Ling B, Dong L et al. . Theoretical insights into the protonation states of active site cysteine and citrullination mechanism of Porphyromonas gingivalis peptidylarginine deiminase. Proteins 2017;85:1518–28. [DOI] [PubMed] [Google Scholar]
- Zhou O, Desta T, Fenton M et al. . Cytokine profiling of macrophages exposed to Porphyromonas gingivalis, its lipopolysaccharide, or its FimA protein. Infect Immun 2005;73:935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Amar S. Identification of proteins differentially expressed in human monocytes exposed to Porphyromonas gingivalis and its purified components by high-throughput immunoblotting. Infect Immun 2006;74:1204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Lin X, Zheng P et al. . Inflammatory cytokine levels in patients with periodontitis and/or coronary heart disease. Int J Clin Exp Pathol 2015;8:2214–20. [PMC free article] [PubMed] [Google Scholar]