Abstract
The pathogenic Neisseria species, including Neisseria meningitidis and Neisseria gonorrhoeae, are obligate human pathogens that cause significant morbidity and mortality. The success of these pathogens, with regard to causing disease in humans, is inextricably linked to their ability to acquire necessary nutrients in the hostile environment of the host. Humans deploy a significant arsenal of weaponry to defend against bacterial pathogens, not least of which are the metal-sequestering proteins that entrap and withhold transition metals, including iron, zinc and manganese, from invaders. This review will discuss the general strategies that bacteria employ to overcome these metal-sequestering attempts by the host, and then will focus on the relatively uncommon ‘metal piracy’ approaches utilized by the pathogenic Neisseria for this purpose. Because acquiring metals from the environment is critical to microbial survival, interfering with this process could impede growth and therefore disease initiation or progression. This review will also discuss how interfering with metal uptake by the pathogenic Neisseriae could be deployed in the development of novel or improved preventative or therapeutic measures against these important pathogens.
Keywords: nutritional immunity, Neisseria gonorrhoeae, Neisseria meningitidis, transition metals, iron and zinc piracy
The pathogenic Neisseria species are obligate human pathogens that have evolved the capability to exploit the human armamentarium dedicated to sequestering transition metals away from bacterial pathogens, an effect known as nutritional immunity.
INTRODUCTION
Two pathogens in the species Neisseria exclusively infect humans and cause significant diseases. Neisseria gonorrhoeae causes the sexually transmitted infection (STI) gonorrhea and N. meningitidis causes meningococcal meningitis and septicemia. Despite extensive nucleotide sequence identity, the two pathogens cause significantly different diseases and differ dramatically in morbidity/mortality and treatment/prevention strategies.
Neisseria gonorrhoeae causes gonorrhea, which can manifest in a multitude of presentations ranging from urethritis in men and cervicitis in women to pelvic inflammatory disease, septic arthritis and even occasionally meningitis (Hook and Handsfield 2008). Auto-inoculation or vertical transmission to a neonate can result in conjunctivitis, which can lead to blindness, if untreated (Kohlhoff and Hammerschlag 2008). Often genital tract infections in women are asymptomatic (Hook and Handsfield 2008), enabling the infection to persist for longer periods of time and to ascend into the upper reproductive tract. Salpingitis in women can lead to permanent fallopian tube scarring and is a significant cause of infertility in sexually active women of child-bearing age (Westrom 1980). Incidence of gonococcal disease is estimated by the CDC to exceed 800,000 cases per year (CDC 2015), making gonorrhea the second most-common reportable infectious disease in the US. Treatment of this STI has been complicated by the dramatic rise in antimicrobial drug resistance (CDC 2007, 2011, 2012; Soge et al.2012; Unemo and Nicholas 2012; Hook and van Der Pol 2013; Barbee 2014), facilitated by the fact that N. gonorrhoeae is naturally competent and capable of facilely incorporating new genetic resistance determinants into its chromosome under selective pressure. Current recommendations (CDC 2015) for empirically treating uncomplicated gonococcal disease include administration of two drugs: ceftriaxone intramuscularly and azithromycin orally. Gonococcal infections do not result in protective immunity (Hedges et al.1998, 1999), so vulnerable populations are often repeatedly infected, resulting in increased morbidity. Furthermore, no effective vaccine has been developed to prevent gonococcal disease.
By contrast with manifestations caused by N. gonorrhoeae, diseases caused by the very closely related pathogen N. meningitidis often result in significant morbidity and mortality. Neisseria meningitidis disease can manifest as meningitis and/or meningococcemia and can culminate in multisystem organ failure, circulatory collapse and coma within hours of onset of initial symptoms (Thompson et al.2006). Even in survivors of meningococcal disease, the risk of debilitating sequelae, including deafness, blindness and limb amputation, is high (Pace and Pollard 2012). While N. gonorrhoeae typically colonizes the genital mucosal epithelium, from which it can ascend into the upper reproductive tract, N. meningitidis usually inhabits the nasopharyngeal epithelium, where it can remain and be carried by individuals without causing any disease manifestations (Caugant et al.1994). Crossing the nasopharyngeal epithelium can result in dissemination through the bloodstream, replication of the bacterium and ultimately meningococcemia. Circulating organisms can also permeate the blood–brain barrier, gaining access to the cerebrospinal fluid, where N. meningitidis can replicate rapidly. The resulting meningitis can develop rapidly, precipitously leading to coma and death without appropriate therapy (Nadel 2016). The incidence of meningococcal disease has decreased following targeted vaccination campaigns, with recent cases numbering in the hundreds in the last few years, according to CDC reporting data (CDC 2017). Antimicrobial drug resistance has not emerged in N. meningitidis as it has with N. gonorrhoeae. The mainstay of therapy for meningococcal disease remains penicillin-G, unless the bacterial etiology is unclear, in which case ceftriaxone is the empirical therapy of choice (Nadel 2016). Polysaccharide capsule-based vaccines have been available to protect against meningococcal disease caused by serogoups A, C, W and Y for decades. In the last few years, however, protein-based vaccines were developed to protect against serogroup B strains, which are endemic in industrialized countries. This breakthrough will likely lead to further decreases in incidence and therefore meningococcal morbidity and mortality (Atkinson, Gandhi and Balmer 2016).
While vaccine development efforts proceed towards improving meningococcal vaccines, no gonococcal vaccine has been developed, despite many years of efforts. Similarly, the arsenal of therapies to treat gonococcal disease is dwindling as well. Ideal targets for either vaccines or therapeutic intervention would be conserved in sequence, consistently expressed during infection, required for survival or virulence, and be easily targeted (for example, located on the cell surface). For the Neisseria species in particular, the first two criteria, being conserved in sequence and ubiquitously expressed, are difficult to accomplish. The Neisseriae are notorious for presenting to the host, and in particular to the host's immune system, surface antigens that are subject to both antigenic and phase variation. The mechanisms of phase and antigenic variation broadly fall into two categories: RecA-dependent and RecA-independent. Pilin variation falls into the former category and is accomplished by non-reciprocal gene exchange from one of many silent loci in the chromosome (pilS) into the only expressed locus (pilE) (Seifert et al.1994; Serkin and Seifert 1998). Recombination results in a pilE gene with a novel DNA sequence, leading to antigenic changes in the protein. Pili are necessary for adherence to mucosal surfaces, natural competence and twitching motility. Many other neisserial antigens are subject to variation by a process known as slipped-strand mispairing (Murphy et al.1989; Connell, Shaffer and Cannon 1990). During DNA replication, short repeat sequences can slip, causing the replicated strand to either increase or decrease in the number of repeats. Often these repeats are located in the coding region for surface antigens. Thus, a change in the number of repeats results in throwing the protein in or out of reading frame. The gonococcus has been referred to as a chameleon (Robinson et al.1989) since virtually all of the surface structures deployed on the outer membrane are subject to one or another form of high-frequency variation.
Attractive targets for vaccine or drug therapy include nutrient transporters (Cornelissen 2008) because they are conserved in sequence, expressed in vivo, required for survival and surface exposed. Nutrients acquired by these transporters include the transition metals iron (Fe) and zinc (Zn). Because these metals are necessary for a variety of physiological functions, targeting acquisition of these nutrients is anticipated to be detrimental to the in vivo survival of the pathogens. The nutrient acquisition systems deployed by the Neisseria species to acquire these necessary metals is the topic of this review.
Nutritional immunity is imposed by mammalian hosts
Living organisms, including bacteria, require transition metals to fulfill a variety of functions. Most bacteria employ Fe as an electron carrier for energy generation and they possess many Fe-S cluster proteins that function in critical redox reactions. Fe is also a cofactor in enzymes involved in oxidative stress tolerance (catalase, for example). Zn is also a cofactor for many enzymes, including oxidoreductases, hydrolases and transferases (Vallee and Falchuk 1993). The concentrations of Fe and Zn are maintained very low in human hosts, with the deployment of an armamentarium of resources (Fig. 1) that function to sequester these metals at such low levels that bacteria succumb to nutrient deprivation. Besides sequestering these metals to suppress microbial growth and replication, host metal-binding proteins (Fig. 1) also serve to maintain the solubility of metals and restrict them to non-reactive states. Iron, in particular, is highly insoluble at neutral pH and has the capacity to form toxic oxygen radicals via Fenton chemistry. The active effort by the human host to restrict the availability of transition metals to invading pathogens has been termed ‘nutritional immunity’ (Kochan 1973; Weinberg 1977, 1978). This term was initially applied to Fe, but has more recently been expanded to include other transition metals such as Zn and manganese (Mn) (Palmer and Skaar 2016). This review will focus on the sequestration of Fe and Zn, and the neisserial response to this effort, as our current understanding is limited to these two transition metals.
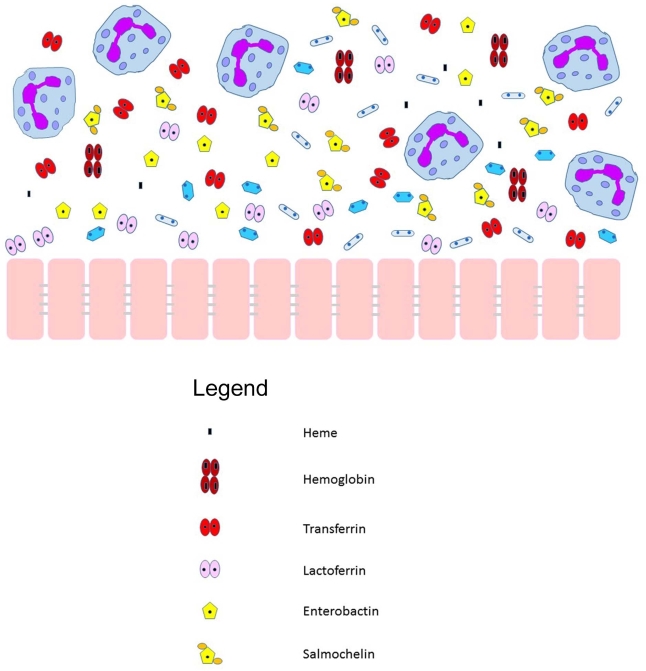
Figure 1.
An inflamed mucosa, including neutrophils and antimicrobial substances, encountered by the pathogenic Neisseriae. The mucosal membrane, with tight junctions, is shown in pink. Multinucleated cells are meant to represent neutrophils. The key for identification of other metal sequestration substances is shown below the image. Not drawn to scale.
Subsequent to infection by a pathogen, human hosts impose a ‘hypoferremic response’ in an attempt to sequester Fe (Cassat and Skaar 2013). This process includes extracting Fe out of the plasma, decreasing Fe absorption in the gut, deployment of neutrophils that harbor Fe-sequestering proteins (including lactoferrin) and elicitation of fever that decreases the ability of bacteria to produce Fe-chelating compounds (siderophores, see below). This response is coordinated by the hormone peptide, hepdicin. Similarly, ‘hypozincemia’ is imposed by the human host as a way to diminish free Zn levels that would otherwise be accessible to bacterial pathogens (Liuzzi et al.2005). This response, induced by LPS, is coordinated by the pro-inflammatory cytokine IL-6 and results in Zn sequestration via Zn transporters such as ZIP-14 (Liuzzi et al.2005). Neutrophils, deployed to the site of infection, bring Zn-sequestering S100 proteins, including calprotectin, which makes up ∼40% of the protein content in the neutrophil cytoplasm (Yui, Nakatani and Mikami 2003). Upon degranulation and extrusion of neutrophil extracellular traps (NETs), these Zn-sequestering proteins are in close proximity to bacterial pathogens and function to starve the invader of the nutrients required for growth and survival.
Proteins involved in nutritional immunity include those that sequester both Fe and Zn. Transferrin (Tf) is found at concentrations approximating 30 μM in the serum and is maintained only partially saturated (30%–35%) (Weinberg 1975). The stability constant for Tf binding to Fe is extremely high at 1030 (Davis, Saltman and Benson 1962) making bacterial access to the metal bound to this nutritional immunity protein exceedingly difficult. Tf maintains the free Fe levels in serum very low at approximately 10−24 M, far below the amount required for bacterial multiplication and survival (approximately 10−6 M) (Raymond, Dertz and Kim 2003). Similarly, lactoferrin (Lf) is found in secretions such as tears and milk and in high concentration in neutrophils. Lf is maintained at a lower saturation level, even at low pH, since the primary function of this protein is to absorb any excess Fe and thereby decrease the accessibility to this nutrient by bacterial invaders (Weinberg 2001). It has more recently been appreciated that the S100 proteins calprotectin (complex of A8 and A9), S100B, S100A7 and S100A12 serve a similar sequestering function for Zn (and also Mn and copper [Cu]) (for review, see Zackular, Chazin and Skaar 2015). The S100 proteins are found in high concentrations in neutrophils and on inflamed mucosal membranes (Zackular, Chazin and Skaar 2015).
Cumulatively, the active process of nutritional immunity, the human host's production of metal-sequestering proteins, ordinarily results in concentrations of the transition metals rendered below that needed for bacterial survival. However, the pathogenic Neisseria species are masters at overcoming nutritional immunity in that they are capable of using these metal-sequestering proteins directly as metal sources, without the deployment of siderophore intermediates.
Bacteria have evolved diverse approaches for overcoming nutritional immunity
Most bacteria combat Fe-specific nutritional immunity by production and secretion of low molecular weight Fe-chelating compounds called siderophores (Greek for ‘iron bearer’) (Neilands 1981). Three basic chemistries have been described for Fe coordination: catecholate, hydroxamate and carboxylic acid. Siderophores coordinate Fe with extreme high affinity, sufficient to compete with nutritional immunity proteins such as transferrin for the ferric Fe (Raymond, Dertz and Kim 2003). Both Gram-positive and Gram-negative bacteria secrete siderophores, but the process by which Fe-laden siderophores is internalized by these distinct groups of bacteria depends on the cell envelope structure. Gram-negative bacteria, with the outer membrane barrier beyond the periplasmic space, require an energized system to import Fe-siderophore complexes. The energy to power transport into the cell is provided by the TonB, ExbB and ExbD proteins (Skare et al.1993), which together harness the proton motive force across the cytoplasmic membrane and deliver the energy to the nutrient transporters, situated as β-barrels in the outer membrane (Noinaj et al.2011). These transporters share a common structure comprised of 22 transmembrane β-strands, 11 surface-exposed loops and an amino-terminal plug domain that folds up into the lumen of the β-barrel (Noinaj et al.2011). The extreme amino-terminus of the plug domain represents a point of physical contact between the transporter and TonB, which in some way dislodges or extracts the plug to accomplish transport across the outer membrane (Shultis et al.2006; Gumbart, Wiener and Tajkhorshid 2007). Fe-siderophore transporters are approximately 80–85 kDa in size and share significant sequence identity, particularly in the areas of transmembrane strands and the plug domain (Nau and Konisky 1989), which enables identification of genes that encode these transporters within genome sequence data.
Subsequent to transport across the outer membrane, a periplasmic binding protein (PBP) and ATP-binding cassette (ABC) transport system enables Fe assimilation through the periplasm, across the cytoplasmic membrane and into the cytoplasm. Similar PBP-dependent ABC transport systems are employed by Gram-positive bacteria for import of ferric siderophores into the cell (Sheldon and Heinrichs 2015).
The deployment and subsequent uptake of Fe-siderophores enables bacteria to overcome Fe-specific nutritional immunity. In addition, many bacteria are capable of internalizing siderophores for which they are not producers (for example, see Cornelis 2010). So called xenosiderophore uptake is common and presumably confers an advantage in mixed bacterial populations. Interference with Fe uptake by decreasing siderophore production, which occurs with increased temperature and the host's febrile response, or by sequestering the Fe-siderophore, would advantage the host and ‘intensify the iron famine of invaders’ (Weinberg 1975). In fact, counter measures are elicited by human hosts to incapacitate Fe-siderophores so that they cannot deliver their payload to bacterial pathogens. Anti-enterobactin antibodies are elicited during infection and anticipated to play a role in withholding siderophore-bound iron (Moore and Earhart 1981). In addition, Lipocalin 2 (also known as neutrophil gelatinase-associated lipocalin or NGAL) is a host protein, present in inflamed sites and in neutrophils, that accomplishes this feat (Flo et al.2004). Lipocalin binds to some Fe-siderophores, for example enterobactin, and prevents pathogens such as Escherichia coli from employing this siderophore as an Fe source (Raymond, Dertz and Kim 2003). But in the continuing arms race between invaders and their host, efficient bacterial pathogens have developed an advanced strategy to defeat Fe-siderophore sequestration by lipocalin 2. Salmonella Enterica produces not only enterobactin but also a glucosylated version of enterobactin called salmochelin (Muller, Valdebenito and Hantke 2009). The latter siderophore evades sequestration by lipocalin 2 due to the bulky sugar additions and thereby thwarts the host's siderophore scavenging mechanism. These so-called ‘stealth siderophores’ enable producers to efficiently evade Fe-specific nutritional immunity in the face of lipocalin 2 production.
Similar to the Fe situation, Zn and other metals can be chelated by siderophores as well. The best characterized example of a human pathogen producing a ‘zincophore’ is Yersinia pestis. While yersiniabactin is also an Fe-binding siderophore, recently this multifunctional siderophore has also been shown to bind to and enable internalization of Zn (Bobrov et al. 2014, 2017). Siderophores can also complex with other metals including Mn, Cu and Co. Again, analogous to the Fe-uptake systems described above, many bacteria produce a PBP-dependent, ABC transport system, encoded by the znuABC genes, which enables highly efficient internalization of Zn and Zn-laden siderophores (Bobrov et al. 2014, 2017; D’Orazio et al.2015). The Neisseria species are again somewhat unique in that two outer membrane transporters have also been shown to facilitate Zn passage across the outer membrane (see below). Other characterized Zn-uptake systems primarily rely on the high-affinity Zn-uptake system comprised of ZnuABC, with Zn diffusing through the outer membrane via porins. The Neisseria species produce a cytoplasmic membrane permease system homologous to ZnuABC (also called MntABC in the literature) (Lim et al.2008), but they furthermore produce cognate outer membrane transporters to accomplish the first step in dedicated Zn transport across the outer membrane.
The Neisseria species do not produce siderophores but instead hijack those secreted by other bacteria (xenosiderophores)
The pathogenic Neisseria species do not possess the genes that would enable them to produce and secrete siderophores for metal acquisition. However, they do encode a transport system that accomplishes uptake of catecholate-type siderophores including enterobactin, linearized salmochelin (S2) and dihydroxybenzoylserine acid (DHBS) (Hollander et al.2011). The crystal structure of meningococcal FetA suggests that the transporter actually binds to and perhaps directly internalizes ferric Fe (Saleem et al.2012), consistent with a broad specificity for different Fe-containing siderophores (Hollander et al.2011).
The fet operon (Carson et al.1999) encodes the components of the siderophore uptake system, including the outer membrane transporter (FetA), a PBP (FetB) and an ABC transport system (FetCDEF) (Table 1; Fig. 2). These genes are co-transcribed in a poly-cistronic operon that is differentially regulated by two different trans-acting factors. The fet operon is iron repressed (as are most iron uptake genes) by the repressor Fur. The operon is further positively regulated by an AraC-like regulator, MpeR (Hollander et al.2011). MpeR is iron repressed by virtue of the fact that Fur also represses mpeR expression in an Fe-dependent manner (Jackson et al.2010; Hollander et al.2011). Thus, optimal fet operon expression includes de-repression by low iron conditions and induction by MpeR activation. None of the recognized siderophore ligands however appear to serve as co-inducing signals with MpeR (Hollander et al.2011).
Table 1.
Metal transport systems involved in overcoming nutritional immunity.
| Name in Ngoa (alternative name) | Metal/ligand or function | Locus tag in NGO FA1090 | Homolog in Nmeb MC58 (alternative name) | Neisseria locus tag | MWc | Expression in vitro | Expression in vivo (women) | Virulence impacts | Distribution |
|---|---|---|---|---|---|---|---|---|---|
| TbpA/TbpB | Fe/Transferrin | NGO1495/NGO1496 | NMB0461/NMB0460 | NEIS1690/NEIS1691 | 100 kDa | Fe repressed | High 4/4 | Required for human infection | Most Neisseriae |
| LbpA/LbpB | Fe/Lactoferrin | NGO0260 (truncated) | NMB1540/NMB1541 | NEIS1468/NEIS1469 | 102 kDa | Fe repressed | Low 3/4 | Can substitute for Tf-Fe uptake system in human infection | Gene deletion in many Ngo; locus present in most Neisseriae |
| HpuA/HpuB | Heme/Hemoglobin | NGO2110/NGO2109 | None | NEIS1946/NEIS1947 | 90 kDa | Fe repressed | Low 2/4 | Phase ‘on’ during first half of menstrual cycle in infected women | Most Neisseriae; phase variable |
| FetA | Fe/catecholate Siderophores | NGO2093 | NMB1988 | NEIS1963 | 79 kDa | Fe repressed | 2/4 | None known | All Neisseriae; phase variable |
| TdfF | Fe? | NGO0021 | NMB1882 | NEIS0338 | 80 kDa | Fe repressedd | No | Contributes to intracellular survival | Only in pathogenic Neisseriae |
| TdfG | Unknown | NGO0553 | None | NEIS2646 | 136 kDa | Fe repressed | Low 2/4 | None known | Only in Ngo and N. elongata |
| TdfH | Zn/Calprotectin | NGO0952 | NMB1497 (CbpA) | NEIS1428 | 104 kDa | Zn repressed | 4/4 | Important for NET survival | Most Neisseriae |
| TdfJ | Zn/? | NGO1205 | NMB0964 (ZnuD) | NEIS0944 | 86 kDa | Fe induced; Zn repressed | 4/4 | Facilitates systemic Nme infection in mice | All Neisseriae |
| FbpA | Fe | NGO0217 | NMB0634 | NEIS0578 | 35 kDa | Fe repressed | High 4/4 | Decreased Fe uptake from Tf/Lf | Most Neisseriae |
| FbpB | Fe | NGO0216 | NMB0633 | NEIS0577 | 56 kDa | Fe repressed | High 4/4 | Decreased Fe uptake from Tf/Lf | Most Neisseriae |
| FbpC | Fe | NGO0215 | NMB0632 | NEIS0576 | 38 kDa | Fe repressed | High 4/4 | Decreased Fe uptake from Tf/Lf | Most Neisseriae |
| ZnuC (MntA) | Zn | NGO0170 | NMB0588 | NEIS530 | 28 kDa | Zn repressed | High 2/4 | Contributes to intracellular survival and resistance to oxidative stress | Most Neisseriae |
| ZnuB (MntB) | Zn | NGO0169 | NMB0587 | NEIS0529 | 29 kDa | Zn repressed | 3/4 | Contributes to intracellular survival and resistance to oxidative stress | Most Neisseriae |
| ZnuA (MntC) | Zn | NGO0168 | NMB0586 | NEIS528 | 34 kDa | Zn repressed | High 4/4 | Contributes to intracellular survival and resistance to oxidative stress | Most Neisseriae |
| TonB | Energization system | NGO1379 | NMB1730 | NEIS1650 | 29 kDa | Iron repressed | High 4/4 | Impaired metal uptake | All Neisseriae |
| ExbB | Energization system | NGO1378 | NMB1729 | NEIS1649 | 26 kDa | Iron repressed | High 4/4 | Impaired metal uptake | All Neisseriae |
| ExbD | Energization system | NGO1377 | NMB1728 | NEIS1648 | 15 kDa | Iron repressed | High 4/4 | Impaired metal uptake | All Neisseriae |
N. gonorrhoeae
N. meningitidis
Molecular weight
Not expressed in bacteriological medium.
Figure 2.
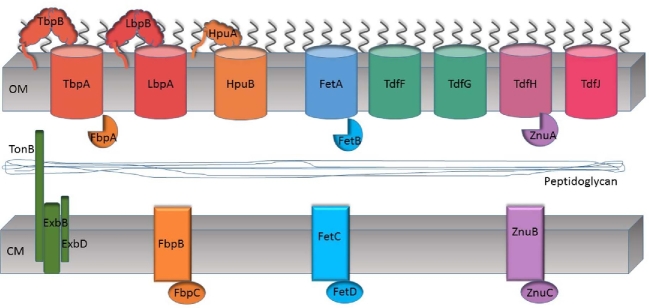
TonB-dependent transporters and ABC transport systems produced by the pathogenic Neisseria species. Barrel shapes in the outer membrane (OM) are meant to represent the eight TonB-dependent transporters produced by the Neisseriae. Three systems, Tbp, Lbp and Hpu, also include a lipoprotein component (TbpB, LbpB and HpuA, respectively), which are shown tethered to the outer membrane by a lipid moiety. Ligands for each system are described in the text. Black lines extending from the outer membrane are meant to represent lipooligosaccharide. The peptidoglycan layer is shown in the periplasm. The TonB, ExbB and ExpD proteins are shown tethered to or integral in the cytoplasmic membrane (CM). This system energizes transport through the outer membrane by the TonB-dependent transporters. Three ABC transport systems are also represented. FbpABC transports ferric iron; FetBCD transports catecholate siderophores; ZnuABC (sometimes referred to as MntABC in the literature) transports zinc and manganese.
The fetA gene is subject to slipped-strand mispairing and thus exhibits phase variation (Carson et al.2000). Unlike the situation described above in which repeat sequences are located in the coding region, in the case of fetA, the repeated C residues are located in the defined promoter region, namely between the -35 and -10 consensus sequences. A change in the number of C residues in this area, due to strand slippage during DNA replication, results in a promoter that expresses either high or low levels of FetA, depending upon the number of nucleotides between the −35 and −10 sequences. Seventeen or 18 nucleotides between promoter elements resulted in maximal expression, whereas 16 nucleotides resulted in greatly diminished expression. The rate of variation was measured as ∼1 × 10−2 in gonococcal strain FA1090 (Carson et al.2000). While the fet genes are present in all Neisseriae genomes (Marri et al.2010), the degree to which the genes are expressed depends therefore on the number of C residues in the promoter and the Fe condition of the medium, which controls expression through both Fur and MpeR. The FetA protein is highly conserved among members of the Neisseriae, with diversity being limited to a hypervariable region in an extracellular loop (Kortekaas et al. 2006, 2007; Saleem et al.2012). This restricted sequence variability does not occlude the Fe-binding pocket but perhaps, due to its antigenicity and variable character, serves as an immunological ‘decoy’ to attract antibodies that would not interfere with Fe-binding function (Saleem et al.2012). The limited variability in this protein antigen has been used epidemiologically to track meningococcal strains; however, the phase variable nature of the antigen suggests it is not an ideal vaccine target (Thompson, Feavers and Maiden 2003).
The Neisseria species subvert nutritional immunity by hijacking host metal scavenging proteins
Iron transporters
While the Neisseria species can take advantage of xenosi-derophores as Fe sources, they do not rely on this variably present environmental resource. Instead, to survive in the face of nutritional immunity, they encode genes that enable them to directly employ host-specific, Fe-containing proteins as metal sources. The best characterized Fe-uptake system possessed by the pathogenic Neisseriae is that which allows uptake of Fe from the host protein transferrin (Tf). Tf is produced by the liver, is the major Fe-transporting protein in the human body and is found on the serum in micromolar concentrations (Weinberg 1975). The neisserial Tf receptor system is absolutely specific for human Tf (hTf) (Cornelissen, Biswas and Sparling 1993) and exclusively binds to and extracts the iron from the C-lobe (Noinaj et al.2012b).
Unlike the more typical siderophore-Fe uptake systems, the Tf receptor system is comprised of two distinct proteins (Table 1; Fig. 2). Resembling the TonB-dependent siderophore transporters, the integral outer membrane component of the Tf-Fe uptake system is TbpA, which is larger than average TonB-dependent transporter at 100 kDa (Cornelissen et al.1992; Legrain et al.1993). The crystal structure of meningococcal TbpA demonstrates that the basic structure of TonB-dependent transporters has been maintained in this larger protein, with the shared topology comprising 22 transmembrane β-strands and an amino terminal plug domain (Noinaj et al.2012b). The additional amino acid residues in TbpA are localized to the 11 surface-exposed loops, resulting in a larger protein-interacting interface on the cell surface. The second component of the Tf-iron uptake system is TbpB, which is a fully surface-exposed protein that is attached to the outer leaflet of the outer membrane via an amino-terminal lipid moiety (Legrain et al.1993; Anderson, Sparling and Cornelissen 1994). Both TbpA and TbpB interact specifically with the C-lobe of hTf (Noinaj et al.2012b); however, TbpB has the additional specificity of interacting solely with the ferrated form of Tf (Cornelissen and Sparling 1996), enabling the receptor system to reel in the only ligand that is employable as an iron source (Noinaj, Buchanan and Cornelissen 2012a). Mutants unable to produce TbpA cannot employ hTf as an iron source (Cornelissen et al.1992; Legrain et al.1993); mutants lacking TbpB internalize Fe from hTf less efficiently (Anderson, Sparling and Cornelissen 1994). The latter phenotype is consistent with the role of TbpB in effectively acquiring the ferrated form of hTf and furthermore disposing of the deferrated ligand once Fe has been extracted (Noinaj, Buchanan and Cornelissen 2012a). Relevant to vaccine-development efforts (see below), the Tf-iron acquisition system is expressed by all N. gonorrhoeae and N. meningitidis strains, is not subject to phase or antigenic variation and is critical for the survival of the gonococcus in a human experimental infection model (Cornelissen et al.1998).
The tbp genes are encoded by a bi-cistronic operon (Ronpirin, Jerse and Cornelissen 2001) with the tbpB gene upstream of the tbpA gene (Anderson, Sparling and Cornelissen 1994). The genes are regulated by the Fe-sensitive Fur protein, resulting in iron abundance repressing expression of the genes (Velez Acevedo et al.2014). In addition, the genes are expressed such that tbpB transcripts outnumber tbpA transcripts by approximately 2:1 (Ronpirin, Jerse and Cornelissen 2001). Moreover, we have recently demonstrated that a very long, apparently untranslated RNA species is encoded upstream of tbpB, interruption of which leads to changes in tbp gene expression and stoichiometry (Velez Acevedo et al.2014). In addition to being iron regulated by the Fur repressor, expression of the tbp operon is further controlled by the response regulator MisR of the two-component regulatory system, which also includes the sensor kinase MisS (Kandler et al.2016).
All N. meningitidis and some N. gonorrhoeae strains (Marri et al.2010) also express a closely related Fe uptake system that enables the use of human lactoferrin (hLf) as an iron source. As with the Tf-Fe uptake system, two proteins participate: LbpA and LbpB (Biswas and Sparling 1995; Lewis et al.1998; Biswas et al.1999; Prinz et al.1999). LbpA is the TonB-dependent transporter component and LbpB is the lipid-modified surface-exposed component (Table 1; Fig. 2). Less detailed structural or functional information is known about this system. Unlike the Tf-Fe uptake system, the lbp genes are subject to phase variation by virtue of a slipped strand in the upstream gene in the operon (lbpB) (Biswas et al.1999). Approximately half of gonococci do not possess the genes to encode the Lf-Fe uptake system due to a large deletion (Anderson et al.2003). Strains unable to produce the Lbp proteins retain infectivity in experimental human infections and in naturally infected humans as lbp mutant strains have been isolated from clinical gonorrhea cases (strain FA1090, for example) (Jerse 1999). Nevertheless, a genetically engineered strain that was unable to employ hTf but was able to use hLf as an Fe source was created and shown to be infectious in the human experimental gonorrhea model, indicating that production of the Lbp proteins can substitute for the artificial loss of the Tbp proteins (Anderson et al.2003).
Two systems enable the Neisseriae to variously utilize protein-bound heme moieties. All gonococci have the capacity to produce the two-component hemoglobin utilization system comprised of HpuA and HpuB (Chen et al.1996; Chen, Elkins and Sparling 1998) (Table 1; Fig. 2). Unfortunately, the nomenclature is reversed for this system in that the hpuA gene lies upstream and encodes the lipoprotein whereas the hpuB gene lies downstream and encodes the TonB-dependent transporter (Lewis et al.1997). This system is also expressed by some meningococci and enables N. meningitidis to utilize hemoglobin bound to haptoglobin (Lewis et al.1998). In both Neisseria species, the hpuAB system is phase variable due to slipped-strand mispairing (Chen, Elkins and Sparling 1998; Lewis et al.1999). In vivo, the only variants isolated from humans with gonococcal infections are phase off for the hpu system, with the exception of infected women in the first half of their menstrual cycle (Anderson et al.2001). The latter observation suggests that the presence of hemoglobin in menstrual blood selects for variants that can produce this transport system.
The second hemoglobin-heme transport system is encoded by a single protein, produced only by some N. meningitidis strains (Richardson and Stojiljkovic 1999). HmbR is a TonB-dependent transporter, similar to TbpA, LbpA and HpuB, but in this case functions alone to enable some meningococcal strains to use hemoglobin as an iron source. The hmbR gene is present as a frame-shifted pseudogene in N. gonorrhoeae genomes (Harrison et al.2013). Highly virulent strains of N. meningitidis tend to possess both hpuAB and hmbR genes, or hmbR alone, suggesting hemoglobin utilization correlates with increased virulence (Tauseef et al.2011; Lucidarme et al.2013). Like the hpuAB genes, the meningococcal hmbR locus is phase variable by slipped-strand mispairing (Richardson and Stojiljkovic 1999). An hmbR mutant of N. meningitidis was not attenuated for growth in human blood, perhaps due to the production of the Tbps, which enables the use of hTf as a sole iron source in whole blood (Bidmos et al.2015).
Zinc transporters
As shown in Table 2 and Fig. 2, the pathogenic Neisseria species encode two outer membrane transporters characterized as Zn importers. Turner et al. (2001) first identified three TonB-dependent transporters of unknown function in the FA1090 genome database and gave them the appellations tdf for TonB-dependent function. It was surmised at the time that these transporters would enable the gonococcus to import other forms of Fe. However, we recently demonstrated that both TdfH (Turner et al.2001) and TdfJ (Cornelissen and Hollander 2011) (Tables 1 and 2) enable the gonococcus to import Zn (Jean et al.2016). Furthermore, production of TdfH enabled the gonococcus to bind to calprotectin (CP) and deliver the payload of Zn into the gonococcal cell (Jean et al.2016). We showed that TdfH production was Zn repressed in a Zur-dependent fashion and that expression of tdfH was MisRS regulated but not Fur regulated (Jean et al.2016). A similar ligand specificity and regulatory network was demonstrated for the meningococcal homologue of TdfH, which was renamed CbpA for CP-binding protein A (Stork et al.2013).
Table 2.
Comparison of Zn transporters produced by N. gonorrhoeae (Ngo) and N. meningitidis (Nme).
| Name | Ligand/metal | Zur regulation | Fur regulation | Other observations | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ngo | Nme | Ngo | Nme | Ngo | Nme | Ngo | Nme | Ngo | Nme |
| TdfH | CbpAa | Cp/Znb | Cp/Zna | Yesb | Yesc,a | Nob | Nod | Regulated by MisRSb,e | Regulated by MisRSa |
| TdfJ | ZnuDf | Zng,b | Zng,f,h | Yesb | Yesc,f | Yesb (iron activated) | Nod | Contains consensus heme-binding motif | Elicits bactericidal antibodiesf |
A second TonB dependent transporter, TdfJ, is expressed by all Neisseriae (Table 1). Kumar, Sannigrahi and Tzeng (2012) showed that the meningococcal homologue of TdfJ contained a consensus-like hemin-binding motif and did in fact sediment heme when overexpressed by E. coli. TdfJ and its meningococcal homologue are both Zn repressed, and we recently demonstrated that TdfJ, similar to TdfH, enables the gonococcus to accumulate Zn, but in this case Zn uptake is not enabled by the presence of CP (Jean et al.2016). Unlike its meningococcal homologue, TdfJ is iron induced (Jean et al.2016). The meningococcal homologue was recently crystallized by Calmettes et al. (2015) and strikingly the structures generated contained Zn even though additional metals were not added to the expression or crystallization protocols. Due to its contribution to Zn-dependent growth of N. meningitidis, this protein was renamed ZnuD by Stork et al. (2010). ZnuD has been postulated to be a potential vaccine candidate due to the fact that it elicited bactericidal antibodies against N. meningitidis and it is well conserved across the Neisseriae (Stork et al.2010).
Other poorly characterized TonB-dependent, outer membrane transporters
Two other TonB-dependent transporters, TdfF and TdfG (Table 1; Fig. 1), are associated with Fe, but have been incompletely characterized. The gene encoding TdfF is present in all pathogenic Neisseriae strains but in none of the commensal Neisseriae (Marri et al.2010), suggesting it plays an important role in pathogenesis. We demonstrated that production of TdfF was not detectable in bacteriological growth medium but could be detected by Western blot if gonococci were grown in epithelial cell culture media (Hagen and Cornelissen 2006). Expression of TdfF under these conditions was iron repressed. We furthermore demonstrated that a mutant unable to express tdfF was attenuated for growth within epithelial cells and this growth defect was reversed upon addition of supplemental Fe, suggesting that TdfF is an Fe transporter for metal accessed by the gonococcus inside of epithelial cells (Hagen and Cornelissen 2006). We have not yet identified a ligand for TdfF. Intracellular sources of Fe that could possibly be accessed by the Neisseriae include ferritin, Fe chelated to organic acids or to glutathione, Tf, Lf, heme and hemoglobin. Of these, only ferritin has been demonstrated to contribute to the intracellular survival of N. meningitidis (Larson, Howie and So 2004). Intracellular replication of N. meningitidis was demonstrated to be TonB-dependent (Larson et al.2002) and to result in degradation of ferritin (Larson, Howie and So 2004). Heme, hemoglobin, Tf and Lf did not contribute to intracellular survival based upon the replication of mutants unable to transport Fe from these ligands. Thus, it is conceivable that TdfF is the receptor that recognizes Fe derived from degraded ferritin and internalizes the metal in a TonB-dependent fashion, although the link between ferritin degradation and TdfF-dependent Fe uptake has not been directly demonstrated in either pathogenic species of Neisseriae.
TdfG is also Fe repressed (Turner et al.2001), but similarly, no ligand has been identified for this TonB-dependent transporter. This protein is expressed nearly exclusively by N. gonorrhoeae strains, and no other Neisseriae (Marri et al.2010), and is the largest predicted TonB-dependent transporter, at 136 kDa.
ABC transporters for metals
After traversing the outer membrane, metals are bound by a PBP, which shuttles the chelated metal across the periplasm and subsequently docks with the cognate cytoplasmic permease. The plasma membrane transporter is energized by ATP hydrolysis, enabling the metal to traverse the cytoplasmic membrane and enter the cytoplasm. Two transport systems have been well characterized as Fe and Zn uptake systems for the Neisseriae: FbpABC (Morse et al.1987; Chen et al.1993) and ZnuABC (Chen and Morse 2001; Lim et al.2008; Stork et al.2010), respectively (Table 1; Fig. 2). The latter system has also been referred to as MntABC. As anticipated, the Fbp system is Fe repressed while the Znu system is Zn repressed. Both systems demonstrate specificity for unchelated metals; however, we demonstrated that FbpA can also contribute to catechol-type siderophore internalization, perhaps by binding strongly to ferric Fe (Strange, Zola and Cornelissen 2011). In strains in which the Fet system is phase off or disabled by point mutation, catechol-type siderophores can still be internalized in an FbpA-dependent manner (Strange, Zola and Cornelissen 2011).
The Neisseria species are human-specific pathogens
The genus Neisseria includes approximately 20 species, several of which colonize the mucosal membranes of animals (Weyand 2017). However, the pathogenic Neisseria, including N. meningitidis and N. gonorrhoeae, are obligate human pathogens, having no reservoir in the environment or other animals. Having evolved with humans for millennia, the pathogenic Neisseria are exquisitely specific for many human proteins and ligands, a phenomenon that contributes to the host-restricted virulence of these pathogens. Given the specificity with which N. gonorrhoeae and N. meningitidis interact with their human hosts, modeling the diseases caused by these pathogens in animals has been problematic. Non-human primates have been employed for virulence studies (Weyand 2017), but these studies are expensive and fraught with ethical issues. Estradiol-treated female mice have also been employed to study lower genital tract colonization and clearance (Jerse et al.2011), but the host restriction imposed by the specificity of N. gonorrhoeae for many human ligands limits the type of experiments that are possible in this model. As an improvement to this mouse model, several transgenes have been added to several different breeds of male and female mice, enhancing the utility of the model by overcoming the host restriction to some degree. The human genes for transferrin (Zarantonelli et al.2007), lactoferrin (Ostan et al.2017), CEACAMs (Buckwalter et al.2017), factor H (Beernink et al.2011; Rossi, Konar and Beernink 2016) and CD46 (Wang et al.2017) have been integrated into the mouse genome by a variety of research groups in efforts to enhance infectivity and evaluate virulence factors.
The human-specific nature of the neisserial hTf interaction (Cornelissen, Biswas and Sparling 1993) was recently considered in an evolutionarily study by Barber and Elde (Barber and Elde 2014). The majority of the most rapidly evolving sites in hTf are in the C-lobe, which is the only lobe to which neisserial TbpA binds. Modest changes in hTf, even those currently present in the great apes, result in an inability of TbpA to recognize this very similar protein. This in turn results in resistance to infection by the pathogenic Neisseria and host restriction. These observations reinforce the idea that evolution is ongoing between bacterial pathogen and host, particularly with respect to nutritional immunity. The authors posit that bacterial ‘iron piracy’—a term first coined in 1994 in the title of a review of the transferrin-iron acquisition system from N. gonorrhoeae (Cornelissen and Sparling 1994)—can be overcome by rapid evolution of mammalian transferrins.
The pathogenic Neisseria species employ metal piracy to survive in vivo
As shown in Table 1, the majority of known or proposed players in metal transport in the pathogenic Neisseriae are expressed in vivo in women recently infected with N. gonorrhoeae. In the study published by McClure et al. (2015), gene expression was evaluated by RNA-seq in four women with recently acquired gonorrhea. Low levels of expression were detected for the lbp and hpu genes. Likewise, expression of fetA was only detected in 2/4 women and tdfG was also poorly expressed in 2/4 subjects. The only tdf transcript listed in Table 1 that was not detected at all in this study was tdfF. These results indicate that the lower female genital tract represents a low metal environment and furthermore that the majority of systems documented to facilitate metal uptake are well expressed and anticipated to be critical to survival in this environment. It is unclear at present whether the same is true of men with gonorrhea.
Also shown in Table 1 are virulence impacts of inactivating the metal transport systems in the pathogenic Neisseria species. The Tf-Fe acquisition system is expressed by all strains of both N. gonorrhoeae and N. meningitidis. We tested whether a mutant unable to produce the Tbps was infectious in a human male infection model almost two decades ago (Cornelissen et al.1998). Compared to the parental strain, FA1090, the tbp mutant strain was unable to elicit any signs or symptoms of urethritis in the male volunteers inoculated with this strain. The parental strain is naturally unable to utilize Lf as an iron source as a deletion in the genome replaces the lbp genes. Thus, the mutant created in our initial infection study was effectively unable to utilize either Lf or Tf. Subsequently, the Sparling group (Anderson et al.2003) replaced the naturally mutant lbp locus in FA1090 with the wild-type version from another strain. This resulted in an infectious strain but one that also has an iron phenotype that has never detected in nature. A strain that was Lf+/Tf+ had a competitive advantage over a strain that was Tf+/Lf– (Anderson et al.2003). The conclusion from these studies was that the lbp system can substitute for the tbp system, but that there is a selective force driving the loss of the lbp genes from the N. gonorrhoeae genome.
In a related study, N. gonorrhoeae samples from naturally infected human subjects were tested for whether or not the Hpu system was expressed (Anderson et al.2001). The Hpu system was phase off in all male subjects and in most female subjects, with the notable exception being in women in the first half of their menstrual cycle. The conclusion drawn from this study was that menses, and abundant hemoglobin, selects for strains that are able to internalize heme from hemoglobin using the Hpu receptor system (Anderson et al.2001). There is epidemiological evidence to suggest that production HmbR by N. meningitidis enhances virulence (Tauseef et al.2011; Harrison et al.2013; Lucidarme et al.2013). However, neither the HpuAB system nor the Fet system is necessary to colonize the female mouse genital tract (Jerse et al.2002). In fact, none of the characterized iron transport systems was necessary in the Balb/C, estradiol-treated mouse model of colonization (Jerse et al.2002). However, since neither hTf nor hLf are present this mouse model, the absence of dedicated receptors to internalize Fe from these proteins would not be anticipated to be detrimental for N. gonorrhoeae survival in this model.
Although gonococcal mutants unable to produce TdfH or TdfJ have not been tested for infectivity in humans, we recently demonstrated that TdfH is important for survival in the presence of NETs (Jean et al.2016). This defect can be overcome by supplementation with Zn and is presumably due to sequestration of Zn by Cp in NETs, which is overcome by the production of TdfH by N. gonorrhoeae. A mutant unable to produce the TdfJ homologue, ZnuD, was shown by Calmettes et al. (2015) to be defective for systemic infection by N. meningitidis in mice; however, localized infection of the nasopharynx was unimpaired in the mutant. This suggests that the ligand and/or key virulence function for TdfJ resides in the bloodstream.
While the Ton system, which energizes all TonB-dependent transporters, has not been evaluated for its virulence impacts in humans, one would expect that the inability to sequester metals at high affinity would result in decreased virulence in vivo. The fact that the Ton system is highly expressed in vivo (McClure et al.2015) is consistent with this proposition. The ABC transport system that facilitates import of ferric iron (FbpABC) has likewise not been tested for in vivo virulence impacts, but is anticipated to be critical for growth and survival in humans, which is a low iron environment. Finally, the ZnuABC system has also not been tested for virulence defects in humans either, but a mutant unable to produce any of these proteins was found to be defective in intracellular survival (Lim et al.2008) and was hypersensitive to oxidative stress (Tseng et al.2001). These in vitro phenotypes suggest that the ZnuABC system plays an important role in vivo as well.
Given the exquisite species specificity of the metal transport systems employed by the pathogenic Neisseria species, testing for virulence defects will result in the most relevant answers if conducted in humans. The human male infection model has great utility in this regard, but is limited in that only the initial phase of infection can be examined before human subjects must be treated with antibiotics. In addition, similar infection experiments cannot ethically be conducted in women and it is conceivable that some virulence factors could be gender specific in their importance to infection. Obviously, N. meningitidis infections could not be experimentally conducted with human subjects, and thus animal models must suffice. A promising advance related to animal modeling is the facility with which human transgenes can be introduced into the mouse genome. Expressing human CEACAMs, hTf and hLF in mice has recently enabled researchers to better model infection caused by these human-specific pathogens.
Sabotaging metal piracy systems could lead the way towards better therapies and prevention of diseases caused by the pathogenic Neisseria
Iron acquisition pathways, since they are critically important for growth and survival of most bacteria, have long been attractive candidates for development of efficacious vaccines or therapies. Recently, the yersiniabactin receptor has been shown to be a viable vaccine target in the prevention of urinary tract infections (UTIs) caused by E. coli (Brumbaugh, Smith and Mobley 2013). Similarly, vaccination with yersiniabactin or aerobactin, individually conjugated to proteins, demonstrated protective effects against E. coli in a mouse model of UTI (Mike et al.2016).
Two approaches have been considered when developing therapeutic agents targeting metal acquisition pathways. The first and oldest is to link siderophores to antibiotics. These so-called sideromycins directly link a metal-binding moiety to an antimicrobial agent. Several naturally occurring sideromycins are known, including the albomycins, produced by actinomycetes, which link a ferrichrome-type siderophore to a protein synthesis inhibitor (Ji, Juarez-Hernandez and Miller 2012). This ‘Trojan Horse’ approach has been coopted by researchers to design and biochemically synthesize antimicrobials that similarly ‘trick’ the bacterial pathogen into internalizing the inhibitor by virtue of the iron-binding moiety. Examples include the Pseudomonas siderophore pyoverdin conjugated to ampicillin or cephalexin (Ji, Juarez-Hernandez and Miller 2012). Additionally, pyochelin, another Pseudomonas siderophore, has been conjugated to fluoroquinolones (Ji, Juarez-Hernandez and Miller 2012). This approach has been applied to siderophores produced by other pathogens as well, including Plasmodium falciparum, M. tuberculosis and Acinetobacter baumanii (Ghosh et al.2017). The second approach is to target siderophore biosynthetic pathways using small molecule inhibitors (Lamb 2015). Interference with different steps in bacterial siderophore biosynthetic pathways is being considered as therapy against M. tuberculosis and Y. pestis (Ferreras et al.2005). Finally, small molecule inhibitors of heme utilization enzyme, HemO, have shown therapeutic activity against P. aeruginosa (Hom et al.2013).
Given the precedent from other pathogens, and the fact that the neisserial metal transporters are conserved, largely expressed in vivo and, where tested, important for virulence, these systems make attractive vaccine and drug targets for these bacterial pathogens. Neisseria gonorrhoeae in particular is currently impossible to prevent by vaccination and becoming increasingly difficult to treat. The Tf-iron acquisition system appears the most tractable target since it is expressed by all strains, not subject to phase or antigenic variation and critical for gonococcal survival in vivo. Sequence conservation, particularly in TbpA, suggests that a vaccine composed of this protein could potentially be cross-reactive among strains, or even cross-protective against N. meningitidis strains (Petousis-Harris et al.2017). It is conceivable that targeting only one metal transport system would allow growth and survival if one or more of the other metal transport systems remains functional. With respect to vaccine development, this concern is only valid if the only correlate of protection is blocking of ligand binding and metal uptake. If vaccination also results in other protective effects, such as fixing complement, opsonophagocytosis or aggregation and clearance, then blocking metal import in addition to these protective effects would simply be icing on the cake. We have demonstrated that vaccination of Balb/C mice (without supplementation or expression of hTf) with the gonococcal Tbps resulted in potentially protective activities, including production of serum antibodies that fixed complement and development of mucosal secretions that blocked hTf-Fe utilization by gonococci grown in vitro (Price, Russell and Cornelissen 2005; Price et al.2007). Meningococcal Tbps are similarly able to elicit bactericidal antibodies in the serum of immunized mice (Rokbi et al.1997). These observations in non-transgenic mice suggest that these proteins could be important components in an efficacious vaccine to prevent gonorrhea. With respect to treatment, crystal structures for the Tbps can guide small molecule screens for compounds that interfere with Tf-Fe utilization. As with the vaccination approach, targeting a single metal uptake system may be insufficient, but the same crystal structure based approach could be deployed against other metal uptake systems to ultimately identify cocktail treatment regimens that target multiple aspects of neisserial metal import. It is anticipated that Zn and other metal acquisition systems are equally as feasible as vaccine and drug targets as those pathways involved in Fe uptake.
CONCLUSIONS AND PERSPECTIVES
The human host presents a daunting environmental challenge for bacterial pathogens. Transition metals, necessary for a variety of biochemical reactions and energy generation, are aggressively sequestered by the host in an attempt to inhibit microbial growth and survival. Human proteins including transferrin, lactoferrin and calprotectin function as components of the host's armamentarium against invading pathogens, who must defend against ‘nutritional immunity’ in order to survive. Against this backdrop, bacteria have evolved a variety of metal sequestration strategies of their own; the ongoing evolutionary struggle continues to play out as the invader develops ever-more sophisticated strategies against the host.
While most bacteria have overcome this sequestration challenge by synthesizing and deploying siderophores for metal solubilization, chelation and transport, the pathogenic Neisseria species resort instead exclusively to ‘iron piracy’. These ultimate kleptomaniacs acquire the necessary metals directly from their host, by producing surface receptors dedicated to binding to and removing the metals from human metal-binding proteins. They can also steal Fe-siderophores from bacteria in the community, making them effective societal ‘cheaters’. The specificity of the pathogenic Neisseriae for human metal-binding proteins is hypothesized to at least contribute to, if not be responsible for, the strict host restriction of these pathogens.
The urgent need for vaccines to prevent neisserial diseases, and for better therapies to treat the afflictions caused by these pathogens, prompts us to consider whether interference with the sophisticated metal acquisition systems deployed for their in vivo survival could be translated into efficacious preventative and treatment strategies. Interference in Fe acquisition and homeostasis has proven successful in this regard for prevention of and therapy for other bacterial infectious diseases. Rational design of metal-import system based vaccines and therapeutics begins with complete crystal structures, a few of which are currently available. Outstanding questions remain however, including what routes of delivery and adjuvants induce the most effective immune responses, which antigens result in the most cross-reactive responses, and can protein-based vaccine components be employed to protect against diseases caused by both pathogenic Neisseria species? With respect to potential therapies, which metal transport systems are the most ‘druggable’, would these therapies be effective against Neisseria species only, and will interference with a single metal import system allow for bypass of the defect by upregulating or turning on another system in vivo? Vigorous, collaborative research in the next decade will hopefully be able to answer these outstanding questions and could potentially lead to novel prevention and treatment strategies that can be deployed against these very effective human-specific pathogens.
Acknowledgements
The author thanks members of her laboratory for providing comments on the manuscript.
FUNDING
This work was supported by grants from Virginia Commonwealth University School of Medicine and from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01 AI125421 and R01 AI127793).
Conflict of Interest. None declared.
REFERENCES
- Anderson JE, Hobbs MM, Biswas GD et al. Opposing selective forces for expression of the gonococcal lactoferrin receptor. Mol Microbiol 2003;48:1325–37. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Leonie PA, Miller WC et al. Selection for expression of the gonococcal hemoglobin receptor during menses. J Infect Dis 2001;184:1621–3. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Sparling PF, Cornelissen CN. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J Bacteriol 1994;176:3162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson B, Gandhi A, Balmer P. History of meningococcal outbreaks in the United States: implications for vaccination and disease prevention. Pharmacotherapy 2016;36:880–92. [DOI] [PubMed] [Google Scholar]
- Barbee LA. Preparing for an era of untreatable gonorrhea. Curr Opin Infect Dis 2014;27:282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber MF, Elde NC. Nutritional immunity. Escape from bacterial iron piracy through rapid evolution of transferrin. Science 2014;346:1362–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beernink PT, Shaughnessy J, Braga EM et al. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol 2011;186:3606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidmos FA, Chan H, Praekelt U et al. Investigation into the antigenic properties and contributions to growth in blood of the meningococcal haemoglobin receptors, HpuAB and HmbR. PLoS One 2015;10:e0133855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas GD, Anderson JE, Chen C-J et al. Identification and functional characterization of the Neisseria gonorrhoeae lbpB gene product. Infect Immun 1999;67:455–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas GD, Sparling PF. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect Immun 1995;63:2958–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrov AG, Kirillina O, Fetherston JD et al. The Yersinia pestis siderophore, yersiniabactin, and the ZnuABC system both contribute to zinc acquisition and the development of lethal septicaemic plague in mice. Mol Microbiol 2014;93:759–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrov AG, Kirillina O, Fosso MY et al. Zinc transporters YbtX and ZnuABC are required for the virulence of Yersinia pestis in bubonic and pneumonic plague in mice. Metallomics 2017;9:757–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbaugh AR, Smith SN, Mobley HL. Immunization with the yersiniabactin receptor, FyuA, protects against pyelonephritis in a murine model of urinary tract infection. Infect Immun 2013;81:3309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter CM, Currie EG, Tsang RSW et al. Discordant effects of licensed meningococcal serogroup B vaccination on invasive disease and nasal colonization in a humanized mouse model. J Infect Dis 2017;215:1590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmettes C, Ing C, Buckwalter CM et al. The molecular mechanism of Zinc acquisition by the neisserial outer-membrane transporter ZnuD. Nat Commun 2015;6:7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson SD, Stone B, Beucher M et al. Phase variation of the gonococcal siderophore receptor FetA. Mol Microbiol 2000;36:585–93. [DOI] [PubMed] [Google Scholar]
- Carson SDB, Klebba PE, Newton SMC et al. Ferric enterobactin binding and utilization by Neisseria gonorrhoeae. J Bacteriol 1999;181:2895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe 2013;13:509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant DA, Hoiby EA, Magnus P et al. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J Clin Microbiol 1994;32:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Trends in Reportable Sexually Transmitted Diseases in the United States, 2006: National Surveillance Data for Chlamydia, Gonorrhea, and Syphillis. Atlanta, Georgia, 2007, 1–7. [Google Scholar]
- CDC. Cephalosporin Susceptibility Among Neisseria gonorrhoeae Isolates—United States, 2000–2010. Morb Mortal Wkly Rep 2011;60:873–7. [PubMed] [Google Scholar]
- CDC. Update to CDC's Sexually Transmitted Diseases Treatment Guidelines, 2010: oral cephalosporins No longer a recommended treatment for gonococcal infections. Morb Mortal Wkly Rep 2012;61:590–954. [PubMed] [Google Scholar]
- CDC. Sexually transmitted diseases treatment guidelines. Morb Mortal Wkly Rep 2015;64:1–137. [Google Scholar]
- CDC. Notifiable Diseases and Mortality Tables. In. Atlanta, GA, 2017. [Google Scholar]
- Chen C-J, Sparling PF, Lewis LA et al. Identification and purification of a hemoglobin-binding outer membrane protein from Neisseria gonorrhoeae. Infect Immun 1996;64:5008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-Y, Berish SA, Morse SA et al. The ferric iron-binding protein of pathogenic Neisseria spp. functions as a periplasmic transport protein in iron acquisition from human transferrin. Mol Microbiol 1993;10:311–8. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Elkins C, Sparling PF. Phase variation of hemoglobin utilization in Neisseria gonorrhoeae. Infect Immun 1998;66:987–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Morse SA. Identification and characterization of a high-affinity zinc uptake system in Neisseria gonorrhoeae. FEMS Microbiol Lett 2001;202:67–71. [DOI] [PubMed] [Google Scholar]
- Connell TD, Shaffer D, Cannon JD. Characterization of the repertoire of hypervariable regions in the Protein II (opa) gene family of Neisseria gonorrhoeae. Mol Microbiol 1990;4:439–49. [DOI] [PubMed] [Google Scholar]
- Cornelis P. Iron uptake and metabolism in pseudomonads. Appl Microbiol Biot 2010;86:1637–45. [DOI] [PubMed] [Google Scholar]
- Cornelissen CN. Identification and characterization of gonococcal iron transport systems as potential vaccine antigens. Future Microbiol 2008;3:287–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen CN, Biswas GD, Sparling PF. Expression of gonococcal transferrin-binding protein 1 causes Escherichia coli to bind human transferrin. J Bacteriol 1993;175:2448–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen CN, Biswas GD, Tsai J et al. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J Bacteriol 1992;174:5788–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen CN, Hollander A. TonB-dependent transporters expressed by N. gonorrhoeae. Front Microbiol 2011;2:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen CN, Kelley M, Hobbs MM et al. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol 1998;27:611–6. [DOI] [PubMed] [Google Scholar]
- Cornelissen CN, Sparling PF. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol Microbiol 1994;14:843–50. [DOI] [PubMed] [Google Scholar]
- Cornelissen CN, Sparling PF. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J Bacteriol 1996;178:1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Orazio M, Mastropasqua MC, Cerasi M et al. The capability of Pseudomonas aeruginosa to recruit zinc under conditions of limited metal availability is affected by inactivation of the ZnuABC transporter. Metallomics 2015;7:1023–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B, Saltman P, Benson S. The stability constants of the iron-transferrin complex. Biochem Bioph Res Co 1962;8:56–60. [DOI] [PubMed] [Google Scholar]
- Ferreras JA, Ryu JS, Di Lello F et al. Small-molecule inhibition of siderophore biosynthesis in Mycobacterium tuberculosis and Yersinia pestis. Nat Chem Biol 2005;1:29–32. [DOI] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004;432:917–21. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Miller PA, Mollmann U et al. Targeted antibiotic delivery: selective siderophore conjugation with daptomycin confers potent activity against multidrug resistant Acinetobacter baumannii both in vitro and in vivo. J Med Chem 2017;60:4577–83. [DOI] [PubMed] [Google Scholar]
- Grifantini R, Sebastian S, Frigimelica E et al. Identification of iron-activated and -repressed Fur-dependent genes by transcriptome analysis of Neisseria meningitidis group B. P Natl Acad Sci USA 2003;100:9542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbart J, Wiener MC, Tajkhorshid E. Mechanics of force propagation in TonB-dependent outer membrane transport. Biophys J 2007;93:496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen TA, Cornelissen CN. Neisseria gonorrhoeae requires expression of TonB and the putative transporter TdfF to replicate within cervical epithelial cells. Mol Microbiol 2006;62:1144–57. [DOI] [PubMed] [Google Scholar]
- Harrison OB, Bennett JS, Derrick JP et al. Distribution and diversity of the haemoglobin-haptoglobin iron-acquisition systems in pathogenic and non-pathogenic. Neisseria Microbiol 2013;159:1920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges SR, Mayo MS, Mestecky J et al. Limited local and systemic antibody responses to Neisseria gonorrheae during uncomplicated genital infections. Infect Immun 1999;67:3937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges SR, Sibley DA, Mayo MS et al. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J Infect Dis 1998;178:742–51. [DOI] [PubMed] [Google Scholar]
- Hollander A, Mercante AD, Shafer WM et al. The iron-repressed, AraC-like regulator MpeR activates expression of fetA in Neisseria gonorrhoeae. Infect Immun 2011;79:4764–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom K, Heinzl GA, Eakanunkul S et al. Small molecule antivirulents targeting the iron-regulated heme oxygenase (HemO) of P. aeruginosa. J Med Chem 2013;56:2097–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook E, van Der Pol B. Evolving gonococcal antimicrobial resistance: research priorities and implications for management. Sex Transm Infect 2013;89:iv60–2. [DOI] [PubMed] [Google Scholar]
- Hook EWI, Handsfield HH. Gonococcal infections in the adult. In: Holmes KK, Sparling PF, Stamm WE et al. (eds). Sexually Transmitted Diseases, 4th ed New York, NY: McGraw-Hill, 2008, 627–45. [Google Scholar]
- Jackson LA, Ducey TF, Day MW et al. Transcriptional and functional analysis of the Neisseria gonorrhoeae Fur regulon. J Bacteriol 2010;192:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean S, Juneau RA, Criss AK et al. Neisseria gonorrhoeae evades calprotectin-mediated nutritional immunity and survives neutrophil extracellular traps by production of TdfH. Infect Immun 2016;84:2982–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse A. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect Immun 1999;67:5699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse AE, Crow ET, Bordner AN et al. Growth of Neisseria gonorrhoeae in the female mouse genital tract does not require the gonococcal transferrin or hemoglobin receptors and may be enhanced by commensal lactobacilli. Infect Immun 2002;70:2549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse AE, Wu H, Packiam M et al. Estradiol-treated female mice as surrogate hosts for Neisseria gonorrhoeae genital tract infections. Front Microbiol 2011;2 DOI: 10.3389/fmicb.2011.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C, Juarez-Hernandez RE, Miller MJ. Exploiting bacterial iron acquisition: siderophore conjugates. Future Med Chem 2012;4:297–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler JL, Acevedo RV, Dickinson MK et al. The genes that encode the gonococcal transferrin binding proteins, TbpB and TbpA, are differentially regulated by MisR under iron-replete and iron-depleted conditions. Mol Microbiol 2016;102:137–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I. The role of iron in bacterial infections, with special consideration of host-tubercle bacillus interaction. Curr Top Microbiol 1973;60:1–30. [DOI] [PubMed] [Google Scholar]
- Kohlhoff SA, Hammerschlag MR. Gonococcal and chlamydial infections in infants and children. In: Holmes KK, Sparling PF, Stamm WE et al. (eds). Sexually Transmitted Diseases, 4th edn New York, NY: McGraw Hill, 2008, 1613–27. [Google Scholar]
- Kortekaas J, Muller SA, Ringler P et al. Immunogenicity and structural characterisation of an in vitro folded meningococcal siderophore receptor (FrpB, FetA). Microbes Infect 2006;8:2145–53. [DOI] [PubMed] [Google Scholar]
- Kortekaas J, Pettersson A, van der Biezen J et al. Shielding of immunogenic domains in Neisseria meningitidis FrpB (FetA) by the major variable region. Vaccine 2007;25:72–84. [DOI] [PubMed] [Google Scholar]
- Kumar P, Sannigrahi S, Tzeng YL. The Neisseria meningitidis ZnuD zinc receptor contributes to interactions with epithelial cells and supports heme utilization when expressed in Escherichia coli. Infect Immun 2012;80:657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb AL. Breaking a pathogen's iron will: Inhibiting siderophore production as an antimicrobial strategy. Biochim Biophys Acta 2015;1854:1054–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson JA, Higashi DL, Stojiljkovic I et al. Replication of Neisseria meningitidis within epithelial cells requires TonB-dependent acquitision of host cell iron. Infect Immun 2002;70:1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson JA, Howie HL, So M. Neisseria meningiditis accelerates ferritin degradation in host epithelial cells to yield an essential iron source. Mol Microbiol 2004;53:807–20. [DOI] [PubMed] [Google Scholar]
- Legrain M, Mazarin V, Irwin SW et al. Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene 1993;130:73–80. [DOI] [PubMed] [Google Scholar]
- Lewis LA, Gipson M, Hartman K et al. Phase variation of HpuAB and HmbR, two distinct haemoglobin receptors of Neisseria meningitidis DNM2. Mol Microbiol 1999;32:977–89. [DOI] [PubMed] [Google Scholar]
- Lewis LA, Gray E, Wang Y-P et al. Molecular characterization of hpuAB, the haemoglobin-haptoglobin-utilization operon of Neisseria meningitidis. Mol Microbiol 1997;23:737–49. [DOI] [PubMed] [Google Scholar]
- Lewis LA, Rohde K, Gipson M et al. Identification and molecular analysis of lbpBA, which encodes the two-component meningococcal lactoferrin receptor. Infect Immun 1998;66:3017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KH, Jones CE, vanden Hoven RN et al. Metal binding specificity of the MntABC permease of Neisseria gonorrhoeae and its influence on bacterial growth and interaction with cervical epithelial cells. Infect Immun 2008;76:3569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi JP, Lichten LA, Rivera S et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. P Natl Acad Sci USA 2005;102:6843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucidarme J, Findlow J, Chan H et al. The distribution and ‘in vivo’ phase variation status of haemoglobin receptors in invasive meningococcal serogroup B disease: genotypic and phenotypic analysis. PLoS One 2013;8:e76932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marri PR, Paniscus M, Weyand NJ et al. Genome sequencing reveals widespread virulence gene exchange among human Neisseria species. PLoS One 2010;5:e11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure R, Nudel K, Massari P et al. The gonococcal transcriptome during infection of the lower genital tract in women. PLoS One 2015;10:e0133982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mike LA, Smith SN, Sumner CA et al. Siderophore vaccine conjugates protect against uropathogenic Escherichia coli urinary tract infection. P Natl Acad Sci USA 2016;113:13468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DG, Earhart CF. Specific inhibition of Escherichia coli ferrienterochelin uptake by a normal human serum immunoglobulin. Infect Immun 1981;31:631–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse SA, Mietzner TA, Bolen G et al. Characterization of the major iron-regulated protein of Neisseria gonorrhoeae and Neisseria meningitidis. A Van Leeuw 1987;53:465–9. [DOI] [PubMed] [Google Scholar]
- Muller SI, Valdebenito M, Hantke K. Salmochelin, the long-overlooked catecholate siderophore of Salmonella. Biometals 2009;22:691–5. [DOI] [PubMed] [Google Scholar]
- Murphy GL, Connell TD, Barritt DS et al. Phase variation of gonococcal protein II: regulation of gene expression by slipped-strand mispairing of a repetitive DNA sequence. Cell 1989;56:539–47. [DOI] [PubMed] [Google Scholar]
- Nadel S. Treatment of meningococcal disease. J Adolesc Health 2016;59:S21–28. [DOI] [PubMed] [Google Scholar]
- Nau CD, Konisky J. Evolutionary relationship between the TonB-dependent outer membrane transport proteins: nucleotide and amino acid sequences of the Escherichia coli colicin I receptor gene. J Bacteriol 1989;171:1041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands JB. Microbial iron compounds. Annu Rev Biochem 1981;50:715–31. [DOI] [PubMed] [Google Scholar]
- Noinaj N, Buchanan SK, Cornelissen CN. The transferrin-iron import system from pathogenic Neisseria species. Mol Microbiol 2012a;86:246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noinaj N, Easley NC, Oke M et al. Structural basis for iron piracy by pathogenic Neisseria. Nature 2012b;483:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noinaj N, Guillier M, Barnard TJ et al. TonB-dependent transporters: regulation, structure, and function. Annu Rev Microbiol 2011;64:43–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostan N, Morgenthau A, Yu RH et al. A comparative, cross-species investigation of the properties and roles of transferrin- and lactoferrin-binding protein B from pathogenic bacteria. Biochem Cell Biol 2017;95:5–11. [DOI] [PubMed] [Google Scholar]
- Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine 2012;30(Suppl 2):B3–9. [DOI] [PubMed] [Google Scholar]
- Palmer LD, Skaar EP. Transition metals and virulence in bacteria. Annu Rev Genet 2016;50:67–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlik MC, Hubert K, Joseph B et al. The zinc-responsive regulon of Neisseria meningitidis comprises 17 genes under control of a Zur element. J Bacteriol 2012;194:6594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petousis-Harris H, Paynter J, Morgan J et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet 2017;390:1603–10. [DOI] [PubMed] [Google Scholar]
- Price GA, Masri HP, Hollander AM et al. Gonococcal transferrin binding protein chimeras induce bactericidal and growth inhibitory antibodies in mice. Vaccine 2007;25:7247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GA, Russell MW, Cornelissen CN. Intranasal administration of recombinant Neisseria gonorrhoeae transferrin binding proteins A and B conjugated to the cholera toxin B subunit induces systemic and vaginal antibodies in mice. Infect Immun 2005;73:3945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz T, Meyer M, Pettersson A et al. Structural characterization of the lactoferrin receptor from Neisseria meningitidis. J Bacteriol 1999;181:4417–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond KN, Dertz EA, Kim SS. Enterobactin: an archetype for microbial iron transport. P Natl Acad Sci USA 2003;100:3584–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AR, Stojiljkovic I. HmbR, a hemoglobin-binding outer membrane protein of Neisseria meningitidis, undergoes phase variation. J Bacteriol 1999;181:2067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EN, Clemens CM, Schoolnik GK et al. Probing the surface of Neisseria gonorrhoeae: immunoelectron microscopic studies to localize cyanogen bromide fragment 2 in gonococcal pili. Mol MIcrobiol 1989;3:57–64. [DOI] [PubMed] [Google Scholar]
- Rokbi B, Mignon M, Maitre-Wilmotte G et al. Evaluation of recombinant transferrin-binding protein B variants from Neisseria meningitidis for their ability to induce cross-reactive and bactericidal antibodies against a genetically diverse collection of serogroup B strains. Infect Immun 1997;65:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronpirin C, Jerse AE, Cornelissen CN. The gonococcal genes encoding transferrin binding proteins (Tbp) A and B are arranged in a bicistronic operon but are subject to differential expression. Infect Immun 2001;69:6336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi R, Konar M, Beernink PT. Meningococcal factor H binding protein vaccine antigens with increased thermal stability and decreased binding of human factor H. Infect Immun 2016;84:1735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M, Prince SM, Patel H et al. Refolding, purification and crystallization of the FrpB outer membrane iron transporter from Neisseria meningitidis. Acta Crystallogr F 2012;68:231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HS, Wright CJ, Jerse AE et al. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J Clin Invest 1994;93:2744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serkin CD, Seifert HS. Frequency of pilin antigenic variation in Neisseria gonorrhoeae. J Bacteriol 1998;180:1955–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon JR, Heinrichs DE. Recent developments in understanding the iron acquisition strategies of gram positive pathogens. FEMS Microbiol Rev 2015;39:592–630. [DOI] [PubMed] [Google Scholar]
- Shultis DD, Purdy MD, Banchs CN et al. Outer membrane active transport: structure of the BtuB:TonB complex. Science 2006;312:1396–9. [DOI] [PubMed] [Google Scholar]
- Skare JT, Ahmer BMM, Seachord CL et al. Energy transduction between membranes: TonB, a cytoplasmic membrane protein, can be chemically cross-linked in vivo to the outer membrane receptor FepA. J Biol Chem 1993;268:16302–8. [PubMed] [Google Scholar]
- Soge OO, Harger D, Schafer S et al. Emergence of increased azithromycin resistance during unsuccessful treatment of Neisseria gonorrhoeae infection with azithromycin (Portland, OR, 2011). Sex Transm Dis 2012;39:877–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork M, Bos MP, Jongerius I et al. An outer membrane receptor of Neisseria meningitidis involved in zinc acquisition with vaccine potential. PLoS Pathog 2010;6:e1000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork M, Grijpstra J, Bos MP et al. Zinc piracy as a mechanism of Neisseria meningitidis for evasion of nutritional immunity. PLoS Pathog 2013;9:e1003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange HR, Zola TA, Cornelissen CN. The fbpABC operon is required for Ton-independent utilization of xenosiderophores by Neisseria gonorrhoeae strain FA19. Infect Immun 2011;79:267–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauseef I, Harrison OB, Wooldridge KG et al. Influence of the combination and phase variation status of the haemoglobin receptors HmbR and HpuAB on meningococcal virulence. Microbiol 2011;157:1446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EA, Feavers IM, Maiden MC. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiol 2003;149:1849–58. [DOI] [PubMed] [Google Scholar]
- Thompson MJ, Ninis N, Perera R et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet 2006;367:397–403. [DOI] [PubMed] [Google Scholar]
- Tseng HJ, Srikhanta Y, McEwan AG et al. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol Microbiol 2001;40:1175–86. [DOI] [PubMed] [Google Scholar]
- Turner PC, Thomas CE, Stojiljkovic I et al. Neisserial TonB-dependent outer-membrane proteins: detection, regulation and distribution of three putative candidates identified from the genome sequences. Microbiol 2001;147:1277–90. [DOI] [PubMed] [Google Scholar]
- Unemo M, Nicholas RA. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol 2012;7:1401–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev 1993;73:79–118. [DOI] [PubMed] [Google Scholar]
- Velez Acevedo RV, Ronpirin C, Kandler J et al. Identification of regulatory elements that control expression of the tbpBA operon in Neisseria gonorrhoeae. J Bacteriol 2014;196:2762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang D, Sjolinder M et al. CD46 accelerates macrophage-mediated host susceptibility to meningococcal sepsis in a murine model. Eur J Immunol 2017;47:119–30. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. Nutritional immunity. Host's attempt to withold iron from microbial invaders. JAMA 1975;231:39–41. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. Infection and iron metabolism. Am J Clin Nutr 1977;30:1485–90. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. Iron and infection. Microbiol Rev 1978;42:45–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ED. Human lactoferrin: a novel therapeutic with broad spectrum potential. J Pharm Pharmacol 2001;53:1303–10. [DOI] [PubMed] [Google Scholar]
- Westrom L. Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countries. Am J Obstet Gynecol 1980;138:880–92. [DOI] [PubMed] [Google Scholar]
- Weyand NJ. Neisseria models of infection and persistence in the upper respiratory tract. Pathog Dis 2017;75, DOI: 10.1093/femspd/ftx031. [DOI] [PubMed] [Google Scholar]
- Yui S, Nakatani Y, Mikami M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol Pharm Bull 2003;26:753–60. [DOI] [PubMed] [Google Scholar]
- Zackular JP, Chazin WJ, Skaar EP. Nutritional Immunity: S100 Proteins at the Host-Pathogen Interface. J Biol Chem 2015;290:18991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarantonelli ML, Szatanik M, Giorgini D et al. Transgenic mice expressing human transferrin as a model for meningococcal infection. Infect Immun 2007;75:5609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]