Abstract
Objective. To characterize a cohort of patients with RA who have interstitial lung disease (ILD) and to assess the utility of previously developed mortality staging systems [gender, age, lung physiology (GAP) and ILD-GAP].
Methods. All patients with RA and ILD seen at the Mayo Clinic from 1998 to 2014 were identified and manually screened for study inclusion. RA disease characteristics and pulmonary findings including high-resolution CT and pulmonary function testing were evaluated. Survival was estimated using Kaplan–Meier methods. GAP and ILD-GAP models were evaluated using c-statistics and standardized incidence ratios.
Results. The study included 181 patients with RA-associated ILD (96% Caucasian; 48% females; 37% never-smokers). The mean age at ILD diagnosis was 67.4 years (s.d. 9.9). The median time from RA diagnosis to ILD was 4.9 years (range −10.9–48.1). The median follow-up was 3.1 years (range 0.01–14.8). Age, RA disease duration and low initial diffusing capacity for carbon monoxide were predictive of premature mortality in multivariate modelling. Sex, smoking status, obstructive lung disease, seropositivity and erosive disease were not associated with mortality. The 5-year survival rate was 59.7% (95% CI 51.5, 69.2). Survival did not differ between usual interstitial pneumonia, non-specific interstitial pneumonia and organizing pneumonia (P = 0.42). The GAP model performed well in this cohort for both discrimination and calibration (c-statistic 0.71, standardized incidence ratio 0.97).
Conclusion. In this large single-centre cohort of patients with RA-ILD, most patients were seropositive and had a history of smoking. ILD most commonly occurred after the RA diagnosis. Mortality was high and did not differ among the types. The GAP model may be useful in assessing mortality risk.
Keywords: rheumatoid arthritis, usual interstitial pneumonia, non-specific interstitial pneumonia, organizing pneumonia, mortality, GAP model, ILD-GAP model
Rheumatology key messages
Patients who suffer from RA-associated interstitial lung disease have a poor prognosis.
Application of the gender, age, lung physiology model in RA may be useful to clinicians for prognosis.
Introduction
Interstitial lung disease (ILD) may develop as an extra-articular manifestation in patients with RA, either as a preceding entity or, more often, several years after the initial RA diagnosis, and can have a dramatic effect on morbidity and mortality [1–3]. Patients with RA may present with restrictive and/or obstructive lung disease, which is often clinically underrecognized [4]. Clinically significant restrictive lung disease occurs in ∼8–15% of patients with RA [5, 6].
There are two main recognized clinical and histopathological patterns of ILD, described as usual interstitial pneumonia (UIP) and non-specific interstitial pneumonia (NSIP), of which UIP is the more common among patients with RA [7, 8]. Although less common, organizing pneumonia (OP) associated with RA is often included among the types of ILD occurring in RA. While the lung disease course in patients with OP is often marked by a subacute illness with a duration of < 3 months, it can progress to fibrosis or may overlap with NSIP with striking interstitial inflammation [9].
ILD manifests clinically with a wide degree of variability. Dyspnoea and cough are among the most common symptoms. Histopathological patterns are most useful in the classification and differentiation of the various types of ILD, which may overlap [9]. In addition to clinical evaluation and direct tissue sampling, high-resolution CT (HRCT) has been shown to be sensitive for the detection and classification of ILD, while pulmonary function tests (PFTs) and bronchoalveolar lavage may be non-specific or even normal, and hence are less helpful for screening and diagnosis [7, 10]. Nonetheless, pulmonary physiology has been shown to independently predict mortality over the HRCT pattern [11].
Assessment of outcomes of patients with RA-related ILD (RA-ILD) is limited by a lack of long-term follow-up and little data on rates of change. Moreover, there is a lack of consensus about which outcome measures are best for assessing the course and progression of ILD [3]. The course of RA-related NSIP, for example, is heterogeneous, with some patients remaining relatively stable for years after diagnosis while others suffer from rapid deterioration [9]. Although also heterogeneous, the course of UIP has been generally associated with a worse outcome than NSIP [12].
Due to this large variation in clinical course for RA-ILD, predicting survival in this patient population is difficult and, therefore, application of a staging system may prove quite useful for clinicians. Assessment of a previously derived and validated staging system originally developed for idiopathic pulmonary fibrosis known as the gender, age, lung physiology (GAP) model and a secondary non-idiopathic ILD model (ILD-GAP) may present an opportunity to better predict mortality in the RA-ILD patient population [13, 14].
The aims of the present study were to characterize RA-ILD using a large single-centre cohort to evaluate mortality and to assess the performance of both the GAP and ILD-GAP models as mortality risk calculators in this population.
Methods
Study population
This retrospective cohort study was approved by the Mayo Clinic Institutional Review Board. Study subjects were identified through a unified single-centre electronic medical record system using the International Classification of Diseases, Ninth Revision (ICD-9) codes for ILD with diagnosis occurring between 1 January 1998 and 31 December 2014 [15]. Follow-up data were abstracted until death or 31 December 2015. All identified cases that fulfilled the 1987 ACR criteria for RA were manually reviewed for ILD diagnosis substantiation [16]. Patients with concomitant rheumatologic disease (such as SLE, vasculitis, etc.) were excluded. However, patients with secondary SS associated with RA were included. Individuals with ILD and concomitant obstructive lung disease, defined as obstructive airway diseases, emphysema, chronic bronchitis or asthma, were included.
Data collection
Patient characteristics recorded included sex, age, smoking history, PFT results and chest HRCT. If patients were on supplemental oxygen therapy, the oxygen requirement, in litres, at the last visit was recorded. If deceased, the date and cause of death were recorded.
PFT results were recorded in both volume (litres) and per cent predicted values abstracted at baseline (closest to ILD diagnosis). These included forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), total lung capacity and diffusing capacity for carbon monoxide (DLCO); the DLCO results were corrected for haemoglobin level when appropriate. If baseline DLCO values were unavailable, reasons for this were also manually abstracted. All HRCT interpretations were completed as part of clinical care and were interpreted by onsite radiologists with skill and training in this technique. Included HRCT scans were those performed closest to the time of the initial ILD diagnosis.
The GAP and ILD-GAP scores were calculated using age, sex and lung physiology variables (FVC, DLCO) at the ILD diagnosis [13, 14]. The ILD-GAP model is a modified version of the GAP model that adds a component for ILD subtype. The ILD-GAP model reduces the predicted risk estimates for patients with CTD-ILD, such as the RA-ILD patients in this study. In the evaluation of both the GAP model and the ILD-GAP model, only patients who had PFTs within 6 months of their ILD diagnosis were included; only tests done at the study centre were abstracted.
RA therapies and disease severity indicators were also collected by manual record review. Data abstracted on disease severity included RF and/or ACPA positivity, presence of rheumatoid nodules, erosions/destructive changes on radiographs and any additional extra-articular manifestations (ExRAs) other than ILD. Severe ExRAs were defined according to Malmö criteria and included pericarditis, pleuritis, Felty’s syndrome, glomerulonephritis, vasculitis, peripheral neuropathy, scleritis and episcleritis [17]. The ESR and CRP values closest to the diagnosis of ILD were also recorded when available and within 60 days. DMARDs and biologic use at any time prior to or after the ILD diagnosis, as well as glucocorticoid therapy at the index date, was recorded for all patients.
Statistical analysis
Descriptive statistics (means, percentages, etc.) were used to summarize the data. Comparisons between groups were performed using chi-square and rank sum tests. Survival rates were calculated using the Kaplan–Meier method. Cox models were used to examine the associations between baseline characteristics and mortality. Continuous variables were assessed for possible non-linear associations with mortality using smoothing splines.
The accuracy of risk predictions was assessed using calibration (i.e. agreement between the observed and predicted event rates) and discrimination (i.e. whether patients are correctly ranked from low to high risk). Calibration was assessed using standardized incidence ratios (SIRs), which are ratios of observed to expected events [18]. Discrimination was assessed using Harrell’s concordance (c) statistic [19]. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Of the 181 included patients, 87 (48%) were female and the majority were Caucasian (96%) (Table 1). The median follow-up time was 3.1 years (range 0.01–14.8). The mean age at ILD diagnosis was 67.4 years (s.d. 9.9) years with a median time of 4.9 years (range −10.9–48.1) from RA to ILD diagnosis. Sixty-seven patients were never-smokers (37%). Ninety-eight (54%) had UIP, 73 (40%) had NSIP and 10 (6%) had RA-related OP (Table 2).
Table 1.
Characteristics of 181 patients with ILD and RA
| Characteristic | Value |
|---|---|
| Age at ILD diagnosis, mean (s.d.), years | 67.4 (9.9) |
| Age at RA diagnosis, mean (s.d.), years | 58.5 (13.5) |
| Ethnicity, Caucasian, n (%) | 170 (96) |
| Sex, female, n (%) | 87 (48) |
| Smoking status, n (%) | |
| Never | 67 (37) |
| Former | 110 (61) |
| Current | 4 (2) |
| Length of follow-up from ILD diagnosis, median (range), years | 3.1 (0.01–14.8) |
| RA disease duration at ILD diagnosis, median (range), years | 4.9 (−10.9, 48.1) |
| RF positive, positive/tested, n (%) | 145/176 (82) |
| ACPA positive, positive/tested, n (%) | 105/136 (77) |
| Erosive disease, n (%) | 44 (24) |
| Extra-articular manifestations other than ILD, n (%) | 80 (44) |
| Medication use at ILD diagnosis, n (%) | |
| Glucocorticoid use at ILD diagnosis | 91 (51) |
| MTX exposure (ever) | 125 (69) |
| LEF exposure (ever) | 51 (28) |
| Biologic exposure (ever) | 129 (71) |
| Non-biologic exposure (ever) | 100 (55) |
| PFT at ILD diagnosis, mean (s.d.) | |
| TLC, per cent predicted | 73.3 (16.7) |
| FVC, per cent predicted | 72.3 (20.3) |
| FEV1, per cent predicted | 72.1 (20.7) |
| DLCO, per cent predicted | 55.7 (19.8) |
| Required supplemental oxygen, n (%) | 85 (50) |
| Oxygen requirement at last visit, mean (s.d.), l/m | 2.9 (1.6) |
Table 2.
Type of ILD in 181 patients with RA
| Characteristic | UIP (n = 98) | NSIP (n = 73) | OP (n = 10) | P-value |
|---|---|---|---|---|
| Age at ILD diagnosis, mean (s.d.), years | 68.1 (9.6) | 67.5 (10.0) | 58.9 (9.9) | 0.028 |
| Sex, female, n (%) | 43 (44) | 39 (53) | 5 (50) | 0.46 |
| RA disease duration at ILD diagnosis, median (range), years | 6.9 (−9.8–44.5) | 3.7 (−10.9–48.1) | 2.4 (−1.7–27.1) | 0.46 |
| Never smoker, n (%) | 34 (35) | 31 (42) | 2 (20) | 0.22 |
| ACPA positive, positive/tested, n (%) | 49/64 (77) | 52/65 (80) | 4/7 (57) | 0.39 |
| RF positive, positive/tested, n (%) | 80/95 (84) | 59/71 (83) | 6/10 (60) | 0.19 |
| ESR, median (IQR), mm/h | 35 (17–60) | 32.5 (20–48) | 36 (14.5–85) | 0.91 |
| CRP, median (IQR), mg/l | 16.7 (10–32.5) | 19.0 (4.8–52) | 18.2 (3–79) | 0.84 |
| Erosive disease, n (%) | 25 (26) | 16 (22) | 3 (30) | 0.77 |
| Pulmonary nodule by HRCT at ILD diagnosis, n (%) | 20 (20) | 31 (42) | 3 (30) | 0.008 |
| Cause of death, n (%) | 0.84 | |||
| Pulmonary disease | 15 (37) | 6 (21) | 0 | |
| Heart disease | 1 (2) | 1 (4) | 0 | |
| Renal disease | 1 (2) | 0 | 0 | |
| Infection | 7 (17) | 6 (21) | 2 (67) | |
| Cancer | 4 (10) | 4 (14) | 0 | |
| Stroke | 2 (5) | 1 (4) | 0 | |
| Other | 1 (2) | 0 | 0 | |
| Unknown | 10 (24) | 10 (36) | 1 (33) |
ACPA was present in 77% of 136 tested patients and RF was present in 82% of 176 tested patients. Erosive disease was documented in 44 (24%) patients and 80 (44%) had one or more ExRA prior to or at the time of ILD diagnosis. The most common ExRAs beyond ILD were subcutaneous rheumatoid nodules in 47 patients (26%) and secondary SS in 12 (7%). Severe ExRAs were present in 15 (8%) patients. The median ESR was 34 mm/h [interquartile range (IQR) 19–54; tested in 131 patients], while the median CRP was 18.1 mg/l (IQR 5–37.8; tested in 96 patients) with no significant differences between types of ILD (Table 2).
The baseline PFTs at the time of ILD diagnosis included the mean per cent predicted FVC of 72% (20), FEV1 of 72% (21), total lung capacity of 73% (17) and DLCO of 56% (20) (Table 1). The mean per cent predicted DLCO for UIP was 52% (19), for NSIP 60% (20) and for OP 68% (14) (P = 0.015) (Table 3). Eighty-five (50%) patients required supplemental oxygen therapy, with a mean requirement at last follow-up of 2.9 l/min (s.d. 1.6).
Table 3.
Baseline pulmonary function by type of ILD in 181 patients with RA
| Characteristic | UIP (n = 98) | NSIP (n = 73) | OP (n = 10) | P-value |
|---|---|---|---|---|
| TLC, mean (s.d.) | ||||
| Litres | 4.2 (1.1) | 4.5 (1.4) | 5.1 (0.8) | 0.048 |
| Per cent predicted | 70.3 (14.5) | 75.0 (18.8) | 89.3 (13.4) | 0.008 |
| FVC, mean (s.d.) | ||||
| Litres | 2.6 (0.8) | 2.7 (1.0) | 2.7 (1.0) | 0.79 |
| Per cent predicted | 71.0 (20.4) | 74.4 (20.9) | 68.0 (12.3) | 0.46 |
| FEV1, mean (s.d.) | ||||
| Litres | 2.0 (0.6) | 2.1 (0.7) | 1.9 (1.0) | 0.48 |
| Per cent predicted | 72.0 (20.3) | 74.1 (20.9) | 56.3 (19.5) | 0.08 |
| DLCO, mean (s.d.) | ||||
| Millilitres/minute | 12.1 (4.6) | 14.2 (5.9) | 17.4 (6.0) | 0.020 |
| Per cent predicted | 51.5 (18.8) | 59.6 (20.4) | 68.3 (13.8) | 0.015 |
| Requiring supplemental oxygen, n (%) | 51 (55) | 29 (43) | 5 (50) | 0.31 |
| Most recent oxygen requirement, l/min, mean (s.d.) | 2.8 (1.3) | 3.1 (2.0) | 3.2 (1.3) | 0.73 |
Chest imaging by HRCT closest to diagnosis showed that 54 (30%) patients had one or more pulmonary nodules while 25 (14%) had emphysema in addition to radiographic evidence of ILD. In the course of routine care, specialized chest radiologists qualitatively interpreted chest HRCT results as consistent with radiographic progression in 97 (65%) patients. The frequency of lung nodules detected by HRCT at the ILD diagnosis was highest in patients with NSIP (42%) compared with UIP (20%) and OP (30%) (P = 0.008; Table 2).
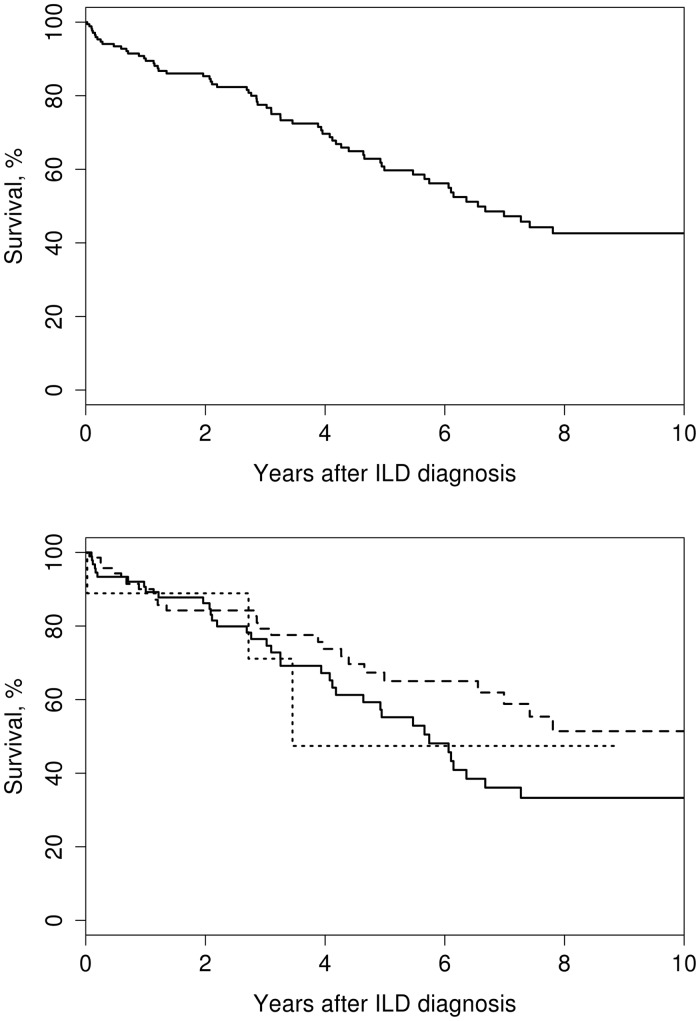
At the last follow-up, 72 patients had died. Overall mortality was high (Fig. 1, upper panel), with pulmonary disease accounting for 21 (29%) deaths, followed by infection [15 (21%)] and heart disease [8 (11%)]; the cause was not known in 21 (29%) patients (Table 2). Survival rates by the type of ILD at 5 years were 55.2% (95% CI 43.7, 69.8) for UIP, 65.0% (95% CI 53.6, 78.8) for NSIP and 47.4% (95% CI 18.5, 100) for OP. No difference was identified between the types of ILD (P = 0.42; Fig. 1, lower panel).
Fig. 1.
Overall survival of patients with RA-ILD
Overall survival of patients with RA-ILD (upper panel) and survival by the type of lung disease (lower panel; log rank P = 0.42). UIP (solid line), NSIP (dashed line), RA-related OP (dotted line).
Baseline risk factors significantly associated with mortality were age at ILD diagnosis [hazard ratio (HR) 1.34 (95% CI 1.11, 1.63) per 10 year increase] and RA disease duration at ILD diagnosis [age- and sex-adjusted HR 1.71 (95% CI 1.22, 2.39) per 10 year increase]. A lower baseline per cent predicted DLCO was associated with significantly higher mortality and a non-linear effect was found, which demonstrated that the mortality risk was much higher among patients with DLCO <40% predicted [HR 2.48 (95% CI 1.55, 3.95) per 10% predicted decrease] than among those with DLCO ⩾40% [HR 1.33 (95% CI 1.08, 1.65) per 10% predicted decrease]. A lower baseline per cent predicted FVC was also associated with significantly higher mortality [HR 1.20 (95% CI 1.04, 1.40) per 10% predicted decrease]. Sex, smoking status, obstructive lung disease, seropositivity, erosive disease and severe ExRAs were not associated with mortality (Table 4). A higher CRP at baseline was significantly associated with mortality [HR 1.07 (95% CI 1.00, 1.13) per 10 mg increase]. A multivariable Cox regression model was obtained including age, sex, RA disease duration, DLCO, FVC and CRP. CRP was subsequently removed from the model due to the amount of missing data and its lack of significance when adjusted for the other factors. Age, RA disease duration and DLCO remained significant in the multivariable model (Table 4).
Table 4.
Risk factors for mortality at ILD diagnosis in patients with RA
| Characteristic | Adjusteda univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age, yearsb | 1.34 (1.11, 1.63) | 0.003 | 2.27 (1.61, 3.21) | <0.001 |
| Male, sex | 1.16 (0.72, 1.86) | 0.55 | 1.33 (0.77, 2.30) | 0.30 |
| Ever smoker | 0.73 (0.44, 1.21) | 0.22 | ||
| NSIP (vs OP) | 0.43 (0.12, 1.47) | 0.18 | ||
| UIP (vs OP) | 0.62 (0.19, 2.08) | 0.62 | ||
| DLCO (% predicted)c | ||||
| For ≤40% | 2.48 (1.55, 3.95) | <0.001 | 2.67 (1.61, 3.21) | <0.001 |
| For >40% | 1.33 (1.08, 1,65) | 0.008 | 1.35 (1.08, 1.69) | 0.009 |
| FVC (% predicted)d | 1.20 (1.03, 1.40) | 0.014 | 0.95 (0.79, 1.14) | 0.56 |
| Obstructive lung disease | 1.27 (0.67, 2.40) | 0.46 | ||
| Seropositivity | 1.13 (0.51, 2.51) | 0.76 | ||
| Severe extra-articular manifestations | 0.87 (0.37, 2.08) | 0.76 | ||
| ESR, mm/hb | 1.06 (0.96, 1.18) | 0.25 | ||
| CRP, mg/lb | 1.07 (1.00, 1.13) | 0.036 | ||
| Erosive disease | 0.66 (0.35, 1.21) | 0.18 | ||
| RA disease duration, yearsb | 1.71 (1.22, 2.39) | 0.002 | 1.81 (1.16, 2.83) | 0.009 |
Adjusted for age and sex.
HR reported per 10 U increase.
Nonlinear effect.
HR reported per 10 U decrease.
At the time of ILD diagnosis, 91 (51%) patients were receiving glucocorticoids. The majority, 125 (69%), had used MTX and 51 (28%) had used LEF at some point during their disease course. A total of 84 (47%) patients used a TNF inhibitor, 32 (18%) used rituximab and 13 (7%) used abatacept. For treatment of ILD, 77 (43%) patients had used AZA and 12 (7%) used CYC (Table 1). Medication use at baseline did not differ significantly between types of ILD.
GAP and ILD-GAP scores could be calculated for 159 patients who had PFTs performed at the study site centre within 6 months of diagnosis. This included seven patients who were too ill to perform DLCO, but who completed the FVC measurement. There were no differences in baseline characteristics or in mortality rates between the 159 patients with calculated GAP scores and the 22 patients for whom GAP scores could not be calculated. A total of 30 patients died within 3 years of the ILD diagnosis. The GAP model predicted 31.0 deaths and demonstrated good calibration [SIR 0.97 (95% CI 0.68, 1.38)] and discrimination (c-statistic 0.71). It performed well in both sexes, across the range of ages, among both seropositive and seronegative patients and in all types of ILD (data not shown). Prediction of events by stage was best in those categorized as stage III [6 deaths compared with 5.5 predicted; SIR 1.08 (95% CI 0.49, 2.41)]. The GAP model overestimated mortality among stage I patients [7 deaths compared with 14.9 predicted; SIR 0.47 (95% CI 0.22, 0.99)] and underestimated mortality among stage II patients [17 deaths compared with 10.6 predicted; SIR 1.60 (95% CI 0.99, 2.57)]. Observed mortality risk at 1 year was 4.2% (95% CI 0.1, 8.1) for stage I, 27.6% (95% CI 11.3, 40.9) for stage II and 14.3% (95% CI 0.0, 36.7) for stage III.
The ILD-GAP score reduced the predicted mortality risk, so only 18.3 deaths were predicted within 3 years of ILD diagnosis. This demonstrated poor calibration [SIR 1.64 (95% CI 1.15, 2.35)]. The ranking of patients with the ILD-GAP score was similar to the GAP score, except for raising negative scores to zero, which resulted in an improved c-statistic of 0.74.
Discussion
In this large single-centre cohort of patients with RA-ILD, most were seropositive with a previous history of tobacco use, were diagnosed in the seventh decade of life after a relatively short duration of RA and had additional extra-articular manifestations of RA. The present cohort did not show as large a male predominance as previous studies (52 vs 59%) [6], which may be due in part to increasing smoking rates among females [20]. There were comparable extra-articular manifestations of RA (44 vs 40%) but less erosive disease (24 vs 35–39%) when compared with previous cohorts [6, 21, 22]. While the explanation for these differences is uncertain, they may be due in part to differences in case ascertainment and improvements in RA therapy in recent years, possibly including the introduction of biologic therapy [23].
UIP was more common than NSIP, and both were more common than OP, findings that other authors have also reported [7]. Even in this most recent period, these patients have a high mortality rate, with nearly 40% dying within 5 years. Of those who died, close to one-third of deaths were attributable to pulmonary disease. This study did not identify any difference in survival rates between types of ILD. This is in contrast to previous studies that have found that UIP carries a higher mortality rate than NSIP or OP [24]. Variable case ascertainment techniques, different baseline populations in these studies and a relatively low percentage of deaths directly attributable to pulmonary disease may account for these differences.
Baseline DLCO was generally lowest in patients with UIP and NSIP compared with patients with OP, while FEV1 and FVC were similar at baseline across these three types of ILD. Low DLCO is generally considered the most sensitive PFT parameter for early detection of ILD [25]. In addition, a lower per cent predicted DLCO at baseline was associated with higher mortality risk, similar to previous studies in RA-related ILD [11].
This is the first assessment of the GAP model in the RA-ILD population, and it had good discrimination and predictive value. In agreement with the GAP authors, this easy-to-use system may be a useful tool for clinicians, which may help to inform prognosis prediction and therapy management [13]. In contrast, the ILD-GAP model, which was developed to take into account an underlying CTD to better predict mortality in patients with ILD, did not perform as well in the present study [14]. This is most likely due to the large heterogeneity among patients with CTD who were used to develop the model. That is, the morbidity and mortality associated with different CTDs (e.g. RA vs SLE) are significantly different.
This study focused on patients encountered in clinical practice referred for evaluation by multiple specialists in a tertiary care centre. This is both a strength and a limitation of the current study. Only patients who presented with clinically evident ILD and RA were captured; routine screening for subclinical disease is not performed for the majority of patients with RA. Protocols for evaluation and follow-up were not standardized, but rather were part of routine care. The HRCTs were assessed by expert thoracic radiologists and reviewed with subsequent clinical management by expert subspecialty pulmonologists. The classification and the outcomes reflect actual clinical practice. There is high interobserver agreement in the characterization of the ILD pattern among expert radiologists and, in our experience, ‘blinded’ readings have not resulted in meaningful reclassification [10]. The centre’s electronic medical record is constantly updated as part of routine practice on patient mortality, even if the patients do not return for visits, minimizing loss of recording for deaths. The absence of ethnic diversity may hinder the generalizability to other populations. Replication of the GAP model’s performance in other studies with RA patients who have ILD is necessary.
Conclusion
Of the types of ILD occurring in RA, UIP is the most common, followed by NSIP and OP. Most patients are seropositive and have additional extra-articular manifestations of RA. Chest HRCT and low DLCO are sensitive indicators of RA-ILD. Progression of NSIP and UIP on HRCT is common. Assessment of the GAP model in this patient population demonstrated good discrimination, which may help to inform prognosis and patient management. RA-ILD is associated with decreased survival and remains a daunting clinical challenge.
Funding: This study was made possible by a Clinical and Translational Science Awards grant (UL1 TR000135) from the National Centre for Advancing Translational Sciences, a component of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Castelino FV, Varga J. Interstitial lung disease in connective tissue diseases: evolving concepts of pathogenesis and management. Arthritis Res Ther 2010;12:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nannini C, Medina-Velasquez YF, Achenbach SJ. et al. Incidence and mortality of obstructive lung disease in rheumatoid arthritis: a population-based study. Arthritis Care Res 2013;65:1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saketkoo LA, Mittoo S, Huscher D. et al. Connective tissue disease related interstitial lung diseases and idiopathic pulmonary fibrosis: provisional core sets of domains and instruments for use in clinical trials. Thorax 2014;69:428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suzuki A, Ohosone Y, Obana M. et al. Cause of death in 81 autopsied patients with rheumatoid arthritis. J Rheumatol 1994;21:33–6. [PubMed] [Google Scholar]
- 5. Doyle JJ, Eliasson AH, Argyros GJ. et al. Prevalence of pulmonary disorders in patients with newly diagnosed rheumatoid arthritis. Clin Rheumatol 2000;19:217–21. [DOI] [PubMed] [Google Scholar]
- 6. Bongartz T, Nannini C, Medina-Velasquez YF. et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010;62:1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee HK, Kim DS, Yoo B. et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest 2005;127:2019–27. [DOI] [PubMed] [Google Scholar]
- 8. Nannini C, Ryu JH, Matteson EL. Lung disease in rheumatoid arthritis. Curr Opin Rheumatol 2008;20:340–6. [DOI] [PubMed] [Google Scholar]
- 9. Travis WD, Costabel U, Hansell DM. et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Assayag D, Elicker BM, Urbania TH. et al. Rheumatoid arthritis-associated interstitial lung disease: radiologic identification of usual interstitial pneumonia pattern. Radiology 2014;270:583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Solomon JJ, Chung JH, Cosgrove GP. et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2016;47:588–96. [DOI] [PubMed] [Google Scholar]
- 12. Kim EJ, Elicker BM, Maldonado F. et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2010;35:1322–8. [DOI] [PubMed] [Google Scholar]
- 13. Ley B, Ryerson CJ, Vittinghoff E. et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012;156:684–91. [DOI] [PubMed] [Google Scholar]
- 14. Ryerson CJ, Vittinghoff E, Ley B. et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest 2014;145:723–8. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization. International Classification of Diseases, Ninth Revision. Geneva, Switzerland, World Health Organization, 1977.
- 16. Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 17. Turesson C, Jacobsson L, Bergstrom U. Extra-articular rheumatoid arthritis: prevalence and mortality. Rheumatology 1999;38:668–74. [DOI] [PubMed] [Google Scholar]
- 18. Crowson CS, Atkinson EJ, Therneau TM. Assessing calibration of prognostic risk scores. Stat Methods Med Res 2016;25:1692–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 2004;23:2109–23. [DOI] [PubMed] [Google Scholar]
- 20. Neuberger JS, Lai SM. Cigarette consumption and cigarette smoking prevalence among adults in Kansas. Prev Chronic Dis 2015;12:E93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis 2003;62:722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gabriel SE, Crowson CS, Kremers HM. et al. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis Rheum 2003;48:54–8. [DOI] [PubMed] [Google Scholar]
- 23. Dale J. Advances in the management of rheumatoid arthritis. Scott Med J 2015;60:108–14. [DOI] [PubMed] [Google Scholar]
- 24. Tsuchiya Y, Takayanagi N, Sugiura H. et al. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur Respir J 2011;37:1411–7. [DOI] [PubMed] [Google Scholar]
- 25. Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax 2001;56:622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]