Abstract
Clostridium difficile (Cd) is the leading cause of antibiotic-associated diarrhea. During an infection, Cd must compete with both the host and other commensal bacteria to acquire iron. Iron is essential for many cell processes, but it can also cause damage if allowed to form reactive hydroxyl radicals. In all organisms, levels of free iron are tightly regulated as are processes utilizing iron molecules. Genome-wide transcriptional analysis of Cd grown in iron-depleted conditions revealed significant changes in expression of genes involved in iron transport, metabolism and virulence. These data will aid future studies examining Cd colonization and the requirements for growth in vivo during an infection.
Keywords: iron, Clostridium difficile, transcriptome, nutrient acquisition
Changes in gene expression levels were measured in Clostridium difficile in response to iron starvation.
INTRODUCTION
Clostridium difficile (Cd) is a Gram-positive, spore-forming, bacterium that causes over 450 000 infections each year (Dieterle and Young 2017). Most Cd infections occur after antibiotic administration, which disrupt the intestinal microbiota (Rodriguez et al.2016). Infection occurs following ingestion of Cd spores, which then germinate into vegetative cells. This metabolically active form must acquire the necessary nutrients needed to colonize, grow, and cause disease. Iron is required for many different cell processes including metabolite biosynthesis, DNA replication, electron transfer, and gene regulation (Ezraty and Barras 2016). Due to its important role in many cell processes, the amount of free iron in the body is very low, approximately 10−18 M (Sheldon, Laakso and Heinrichs 2016). Since host iron storage mechanisms such as transferrin, lactoferrin, or hemeproteins, typically sequester free iron, bacterial pathogens have evolved mechanisms of effective iron acquisition and import (Skaar 2010; Parrow, Fleming and Minnick 2013; Ezraty and Barras 2016). Low iron environments serve as a signal for many pathogenic bacteria to induce expression of virulence factors, including iron acquisition machinery (Skaar 2010). Iron acquisition mechanisms are vital for multiple pathogens that colonize the gastrointestinal tract. The iron importer FeoB plays an important role in Helicobacter pylori virulence and both FeoB and TonB are important for growth of Salmonella in the murine gut (Tsolis et al.1996; Velayudhan et al.2000). Additionally, commensal Escherichia coli species reduce Salmonella colonization by competing for iron in the gut (Deriu et al.2013). We sought to better understand how Cd survives in an iron limited environment. Here, we report the transcriptional changes that occur in response to Cd growth in an iron-limited environment. We identified the induction of iron acquisition systems, iron stress adaptation, polyamine utilization, and metabolism in response to this signal. Understanding how C. difficile acquires iron during infection can lead to the identification and development of therapeutic strategies for starving or outcompeting this pathogen during infection.
RESULTS AND DISCUSSION
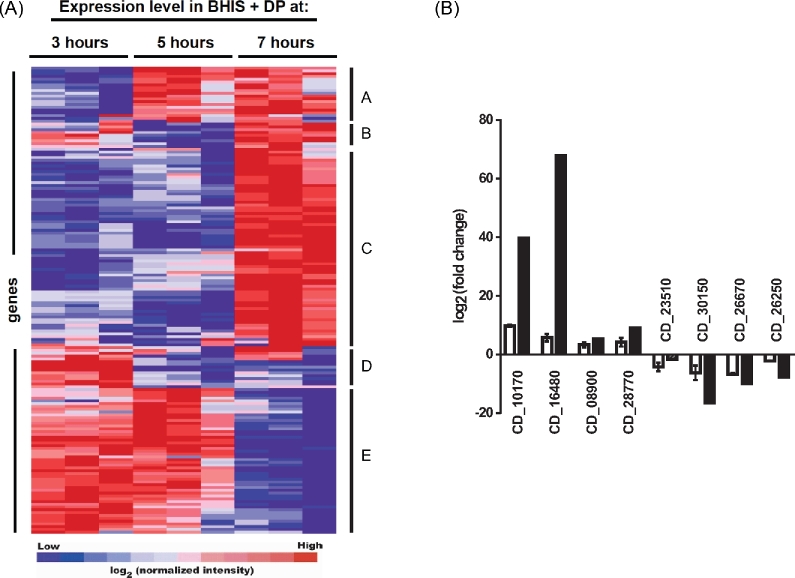
Due to the limited bioavailability of iron in the host gastrointestinal tract, we predicted that Cd is well adapted to surviving in an iron limited environment. To better understand how Cd adapts to low iron environments, transcriptional profiling of Cd grown in either nutrient-rich medium (BHIS − high iron) or in BHIS containing the iron chelator 2,2΄-dipyridyl (BHIS + DP − low iron). Iron starvation can be observed as a reduction in a growth rate at later time points in cultures containing DP (Fig. 1). Samples were grown in triplicate and a portion of each culture was removed at indicated time points (Fig. 1, arrows) for RNA isolation and subsequent analysis using a custom Cd Agilent. Expression of a given gene was considered significant if both a J5 score (Patel and Lyons-Weiler 2004) and fold change were greater than 2. Genes identified as significantly induced at any of the time points tested were analyzed using hierarchical clustering and the results are presented in Fig. 2a. Clear patterns of gene expression can be observed by clustering the significant genes. Cluster A includes genes that were induced at 5 h and remained expressed at higher levels through 7 h. A small group of genes were identified as significantly induced at 3 and 7 h only (cluster B). Clusters D and E represent genes with high expression at early time points, but lower levels at 7 h. As iron starvation became more evident based on reduced growth rates in the presence of dipyridyl, a greater number of significantly regulated genes were observed, with the most changes identified at 7 h (Fig. 2a, cluster C). Table 1 shows the 25 most induced genes at this time point. Significantly induced genes from each time point are presented in Tables S1–S3 (Supporting Information). Eight genes were chosen at random from among those most induced and repressed to confirm the microarray results using qPCR. Expression was determined as fold change relative to Cd630_36610. Fig. 2b shows the expression levels identified in both the microarray and qPCR data sets for each of these genes.
Figure 1.
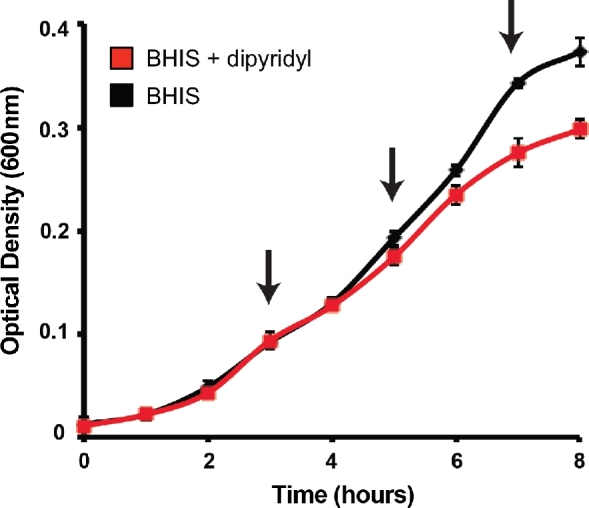
Growth of C. difficile 630 during iron starvation. Growth of C. difficile 630 in either BHIS (black) or BHIS + 75 μM 2΄2 ΄-dipyridyl (DP) (red). Growth was measured by OD600 and samples were removed for RNA isolation and subsequent transcriptome analysis at indicated time points (arrows). Data presented are mean + SD of three replicates.
Figure 2.
Global gene expression changes of C. difficile 630 in iron depleted media. (A) Hierarchical clustering of microarray data. Genes were identified as significant by the J5 test and fold-change across three independent microarray experiments. Statistically significant genes were grouped by hierarchical clustering using gene pattern and clustered using the Pearson correlation. (B) Validation of microarray data. Gene expression changes were measured by quantitative real-time PCR at 7 h from three independent cultures. The results of qPCR are represented with white bars, while corresponding values from the microarray experiments are represented with black bars. Data are presented as mean ± SEM of log2 transformed fold-change where fold change is the ratio of expression in iron depleted BHIS to BHIS alone.
Table 1.
Top 25 genes induced in iron depleted conditions at 7 h.
| Locus | J5 Score | Fold change | Abbr. | Description |
|---|---|---|---|---|
| CD630_24990 | 13.91 | 106.06 | – | Hypothetical protein CD630_24990 |
| CD630_05910 | 13.87 | 104.24 | – | ATPase, P-type, heavy metal translocating |
| CD630_14790 | 13.78 | 101.32 | feoB1 | Ferrous iron transport protein B |
| CD630_19990 | 13.75 | 99.64 | fldX | Flavodoxin |
| CD630_05920 | 12.81 | 73.14 | – | Hypothetical protein CD630_05920 |
| CD630_16480 | 12.37 | 37.88 | – | ABC transporter iron-family permease |
| CD630_16490 | 11.80 | 54.35 | – | ABC transporter iron-family ATP-binding protein |
| CD630_14800 | 11.15 | 41.96 | – | Hypothetical protein CD630_14800 |
| CD630_10170 | 10.98 | 39.65 | – | ABC transporter multidrug-family ATP-binding protein/permease |
| CD630_10180 | 10.90 | 38.74 | – | ABC transporter multidrug-family ATP-binding protein/permease |
| CD630_14850 | 10.75 | 36.61 | – | Hypothetical protein CD630_14850 |
| CD630_14770 | 9.56 | 24.61 | feoA | Ferrous iron transport protein |
| CD630_14780 | 9.32 | 22.62 | feoA | Ferrous iron transport protein |
| CD630_16470 | 9.27 | 22.44 | – | ABC transporter iron-family permease |
| CD630_16500 | 7.54 | 12.79 | – | ABC transporter iron-family extracellular substrate-binding protein |
| CD630_10870 | 7.38 | 11.94 | zupT | Zinc transporter ZupT |
| CD630_29900 | 7.34 | 11.70 | ssuB2 | ABC transporter sulfonate-family ATP-binding protein |
| CD630_29890 | 7.27 | 11.40 | ssuA2 | ABC transporter sulfonate-family extracellular solute-binding protein |
| CD630_28770 | 6.59 | 9.07 | fhuB | ABC transporter ferrichrome-specific permease |
| CD630_29920 | 6.50 | 8.12 | – | Hypothetical protein CD630_29920 |
| CD630_29910 | 6.46 | 8.07 | ssuC2 | ABC transporter sulfonate-family permease |
| CD630_28740 | 5.65 | 6.65 | – | Drug/sodium antiporter, MATE family |
| CD630_28780 | 4.95 | 5.30 | fhuD | ABC transporter ferrichrome-specific extracellular solute-binding protein |
| CD630_08900 | 4.93 | 5.22 | speE | Spermidine synthase |
| CD630_08910 | 4.89 | 5.16 | speB | Agmatinase (Agmatine ureohydrolase) (AUH) |
Many of the genes induced during growth of Cd in iron-depleted conditions have annotated roles in iron acquisition. In an anaerobic environment, the dominant form of iron is the ferrous form. Bacteria use ferrous iron permeases known as Feo transporters to transport free ferrous iron (Lau, Krewulak and Vogel 2016). These transporters are typically composed of a membrane bound unit (FeoB) and a cytoplasmic unit (FeoA). The genome of Cd630 encodes three Feo transporters, though only two were induced by iron starvation (Cd630_14770-14790 and Cd630_32730-32740). The third Feo transporter Cd630_15180-15170) shows homology to a Feo system found in Porphyromonas gingivalis that is involved in manganese import (Dashper et al.2005). Additionally, three ABC transporters predicted to import siderophores were induced in low iron conditions. Although no orthologs of known siderophore biosynthetic genes were identified in the genome of Cd630, we hypothesize that Cd utilizes siderophores produced by other members of the intestinal microbiota.
Bacteria have adapted multiple ways to respond to iron limitation. Flavodoxin, which was ∼100-fold induced in the array, can functionally replace ferredoxin as an election transfer protein in iron-limiting conditions (Yoch and Valentine 1972; Chandrangsu, Rensing and Helmann 2017). Proteins with Fe-S clusters are excellent sensors of redox- or iron-related stress due to the ability to access the redox state of iron (Py and Barras 2010). The expression of several genes involved in Fe-S formation and containing Fe-S clusters were induced and repressed suggesting the use of Fe-S clusters as redox sensors. Another mechanism of adaption is the utilization of alternative cations. Transition metals are important cofactors for the function of many enzymes (Waldron and Robinson 2009). Consequently, several mechanisms to import non-iron cations were induced in low iron including an annotated P-type ATPase, the zinc transporter ZupT, and magnesium ATPases. Although the role and specificity of these systems in Cd remains to be elucidated, ZupT and P-type ATPases from other organisms exhibit broad specificity and function in uptake of Zn2+, Fe2+, Co2+ and Mn2+ (Kuhlbrandt 2004; Grass et al.2005). In the absence of iron, other metal cations may be substituted for iron in biological processes and used as cofactors for virulence factors (Palmer and Skaar 2016). Overall, low iron serves as an environmental indicator signaling changes in energy production, signaling pathways and the transport of alternative transition metals that likely contributes to Cd survival during an infection.
Several genes associated with virulence in other pathogens were induced by iron starvation including those involved in polyamine biosynthesis and uptake, histidine biosynthesis and motility. Polyamines are low molecular weight polycations that, similar to iron, are essential for the growth of both prokaryotes and eukaryotes (Shah and Swiatlo 2008). Several reports have described polyamines themselves as a virulence factor, including evasion of killing by the immune system, siderophore production and biofilm production (Oves-Costales et al.2008; Shah and Swiatlo 2008; Carlson et al.2010). Additionally, polyamines chelate metal cations and have been suggested to co-transport iron in proliferating Caco-2 cells (Lescoat et al.2013) allowing for the possibility that polyamines are directly involved in iron acquisition in Cd.
Histidine biosynthesis genes were also induced in low iron conditions. Histidine has a high affinity for metals and is an amino acid involved in nitrogen metabolism and synthesis of purine nucleotides. Histidine could have a role in iron homeostasis due to its high affinity for iron. In Aspergillus fumigatus increased histidine was observed in low iron conditions and functioned to prevent heavy metal toxicity suggesting a role in both uptake and detoxification (Dietl et al.2016).
Bacterial flagellar systems are well-known virulence factors involved in adherence, nutrient acquisition, virulence factor translocation and activators of host toll-like receptor 5 (TLR5) (Duan et al.2013). Several flagella-associated genes were slightly induced during iron starvation. The role of flagella in Cd pathogenesis is unclear as some strains without flagella are still able to cause disease (Baban et al.2013; Stevenson, Minton and Kuehne 2015). It is possible that the expression of flagella occurs early after colonization as vegetative Cd begins acquiring nutrients including iron.
Many genes involved in iron homeostasis are regulated by the ferric uptake regulator (Fur). The data presented here are generally consistent with previous studies on Fur regulated components of iron acquisition in Cd. Ho and Ellermeier examined the transcriptional profile of Cd in a Fur mutant compared to wild type. Many of the iron acquisition genes induced in low iron conditions in the present study were also de-repressed by a fur mutation (Table S1–S3, Supporting Information) (Ho and Ellermeier 2015). Interestingly, we identified several genes induced in low iron that were not regulated by Fur including components of metabolism, polyamine biosynthesis, flagellar biosynthesis, and magnesium transport. Overall, many changes occur during Cd growth in iron limiting conditions. This work provides a greater understanding of how Cd compensates and adapts during an infection where competition for iron is fierce.
MATERIALS AND METHODS
Cd strain 630 was cultured in BHIS (brain–heart infusion broth supplemented with 0.5% yeast extract and 0.1% cysteine) unless otherwise indicated. Overnight cultures were grown anaerobically (Coy Laboratory Products, MI) at 30°C to minimize sporulation and bacterial death in cultures. These samples were then back diluted 1:10 in BHIS broth for 2 h to allow all cultures to reach early log phase and, therefore, active growth. Actively growing cultures were then diluted to an OD600 of 0.05 into BHIS or BHIS containing 2,2΄-dipyridyl (75 μM) and incubated at 37°C. 75uM dipyridyl was chosen as a concentration that showed iron limitation (late phase growth defect) without complete inhibition of growth (data not shown).
RNA isolation and analysis
At the indicated time points (3, 5 or 7 h) samples grown in BHIS or BHIS + DP were removed to isolate RNA using the following methods. Bacteria were collected using a 0.2 μM vacuum filter. Isolated bacteria were resuspended from the filter in cold nuclease free water. Preheated lysis buffer (2% SDS, 16mM EDTA and 200mM NaCl) was added to the resuspended bacteria and immediate incubated at 100°C for 3 min. Nucleic acid was isolated using two hot phenol extractions (65°C) followed by phenol:chloroform and chloroform extractions (22°C). Isolated nucleic acids were precipitated overnight at −20°C following the addition of 0.1x vol. ammonium acetate and 2.5 vol. ethanol. Resulting RNA was treated with DNase according to manufacturer's protocol (Turbo DNA-free, Ambion). RNA quantity was measured by spectrophotometry and quality confirmed using an Agilent Bioanalyzer. Triplicate samples were then assessed using a custom Cd Agilent microarray using manufacturer's protocols. The microarray was designed using Agilent's eArray platform and the genome of C. difficile 630. Probes corresponding to each ORF are included on the array in a minimum of four replicates. Full details of the array contents can be found with the deposited data.
Microarray data analysis
Analysis of microarray data was performed as previously described (Carlson et al.2009). The J5 value and fold-change for all genes with a significant change in transcription levels between conditions are reported in Tables 1 and S1–S3 (Supporting Information). Log transformed normalized intensity values for these genes were then input into GenePattern (Golub et al.1999) for hierarchical clustering visualization (Pearson correlation).
Quantitative PCR
All qPCR experiments were performed as previously described (Carlson et al.2009). The gene Cd630_36610 was used as the internal reference, as its expression was determined to remain unchanged between growth conditions tested.
AVAILABILITY OF SUPPORTING DATA
Microarray data will be deposited in the GEO database under accession number GSE109453.
SUPPLEMENTARY DATA
Supplementary data are available at FEMSPD online.
FUNDING
This work was supported by the Intramural Research Program of the Center for Biologics Evaluation and Research, Food and Drug Administration. This work was also supported by the Clostridium difficile Cooperative Research Center (U19 AI09087).
Conflict of interest. None declared.
Supplementary Material
Supplementary data are available at FEMSPD online.
REFERENCES
- Baban ST, Kuehne SA, Barketi-Klai A et al. The role of flagella in Clostridium difficile pathogenesis: comparison between a non-epidemic and an epidemic strain. PLoS One 2013;8:e73026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson PE Jr., Carr KA, Janes BK et al. Transcriptional profiling of Bacillus anthracis Sterne (34F2) during iron starvation. PLoS One 2009;4:e6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson PE Jr., Dixon SD, Janes BK et al. Genetic analysis of petrobactin transport in Bacillus anthracis. Mol Microbiol 2010;75:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrangsu P, Rensing C, Helmann JD. Metal homeostasis and resistance in bacteria. Nat Rev Microbiol 2017;15:338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashper SG, Butler CA, Lissel JP et al. A novel Porphyromonas gingivalis FeoB plays a role in manganese accumulation. J Biol Chem 2005;280:28095–102. [DOI] [PubMed] [Google Scholar]
- Deriu E, Liu JZ, Pezeshki M et al. Probiotic bacteria reduce Salmonella Typhimurium intestinal colonization by competing for iron. Cell Host Microbe 2013;14:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterle MG, Young VB. Reducing recurrence of C. difficile infection. Cell 2017;169:375. [DOI] [PubMed] [Google Scholar]
- Dietl AM, Amich J, Leal S et al. Histidine biosynthesis plays a crucial role in metal homeostasis and virulence of Aspergillus fumigatus. Virulence 2016;7:465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q, Zhou M, Zhu L et al. Flagella and bacterial pathogenicity. J Basic Microbiol 2013;53:1–8. [DOI] [PubMed] [Google Scholar]
- Ezraty B, Barras F. The ‘liaisons dangereuses’ between iron and antibiotics. FEMS Microbiol Rev 2016;40:418–35. [DOI] [PubMed] [Google Scholar]
- Golub TR, Slonim DK, Tamayo P et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science 1999;286:531–7. [DOI] [PubMed] [Google Scholar]
- Grass G, Franke S, Taudte N et al. The metal permease ZupT from Escherichia coli is a transporter with a broad substrate spectrum. J Bacteriol 2005;187:1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TD, Ellermeier CD. Ferric uptake regulator fur control of putative iron acquisition systems in Clostridium difficile. J Bacteriol 2015;197:2930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlbrandt W. Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol 2004;5:282–95. [DOI] [PubMed] [Google Scholar]
- Lau CK, Krewulak KD, Vogel HJ. Bacterial ferrous iron transport: the Feo system. FEMS Microbiol Rev 2016;40:273–98. [DOI] [PubMed] [Google Scholar]
- Lescoat G, Gouffier L, Cannie I et al. Involvement of polyamines in iron(III) transport in human intestinal Caco-2 cell lines. Mol Cell Biochem 2013;378:205–15. [DOI] [PubMed] [Google Scholar]
- Oves-Costales D, Kadi N, Fogg MJ et al. Petrobactin biosynthesis: AsbB catalyzes condensation of spermidine with N8-citryl-spermidine and its N1-(3,4-dihydroxybenzoyl) derivative. Chem Commun (Camb) 2008;14:4034–6. [DOI] [PubMed] [Google Scholar]
- Palmer LD, Skaar EP. Transition metals and virulence in bacteria. Annu Rev Genet 2016;50:67–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrow NL, Fleming RE, Minnick MF. Sequestration and scavenging of iron in infection. Infect Immun 2013;81:3503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Lyons-Weiler J. caGEDA: a web application for the integrated analysis of global gene expression patterns in cancer. Appl Bioinformatics 2004;3:49–62. [DOI] [PubMed] [Google Scholar]
- Py B, Barras F. Building Fe-S proteins: bacterial strategies. Nat Rev Microbiol 2010;8:436–46. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Van Broeck J, Taminiau B et al. Clostridium difficile infection: early history, diagnosis and molecular strain typing methods. Microb Pathog 2016;97:59–78. [DOI] [PubMed] [Google Scholar]
- Shah P, Swiatlo E. A multifaceted role for polyamines in bacterial pathogens. Mol Microbiol 2008;68:4–16. [DOI] [PubMed] [Google Scholar]
- Sheldon JR, Laakso HA, Heinrichs DE. Iron acquisition strategies of bacterial pathogens. Microbiol Spectr 2016;4:VMBF-0010-2015. DOI: 10.1128/microbiolspec.VMBF-0010-2015. [DOI] [PubMed] [Google Scholar]
- Skaar EP. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog 2010;6:e1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson E, Minton NP, Kuehne SA. The role of flagella in Clostridium difficile pathogenicity. Trends Microbiol 2015;23:275–82. [DOI] [PubMed] [Google Scholar]
- Tsolis RM, Baumler AJ, Heffron F et al. Contribution of TonB- and Feo-mediated iron uptake to growth of Salmonella typhimurium in the mouse. Infect Immun 1996;64:4549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayudhan J, Hughes NJ, McColm AA et al. Iron acquisition and virulence in Helicobacter pylori: a major role for FeoB, a high-affinity ferrous iron transporter. Mol Microbiol 2000;37:274–86. [DOI] [PubMed] [Google Scholar]
- Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol 2009;7:25–35. [DOI] [PubMed] [Google Scholar]
- Yoch DC, Valentine RC. Ferredoxins and flavodoxins of bacteria. Annu Rev Microbiol 1972;26:139–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at FEMSPD online.