Abstract
Menstrual toxic shock syndrome is associated with vaginal colonization by Staphylococcus aureus strains that encode toxic shock syndrome toxin 1 (tst+). Interestingly, a small proportion of women are colonized by S. aureus tst+ but do not have symptoms of toxic shock syndrome. Here we sought to determine if differences in the species composition of vaginal bacterial communities reflect a differential risk of colonization by S. aureus capable of producing toxic shock syndrome toxin 1 (TSST-1). The composition of vaginal communities of women that were or were not colonized with S. aureus tst+ were compared based on terminal restriction fragment length polymorphism (T-RFLP) profiles and sequences of cloned 16S rRNA genes. There were no detectable differences in community composition or species rank abundance between communities of women vaginally colonized with S. aureus tst+ as compared to those that were not. Phylogenetic analysis of cloned 16S rRNA gene sequences showed that the predominant members of communities of women colonized with S. aureus tst+ were indistinguishable from those of other healthy women. The data suggest that the numerically dominant members of vaginal communities do not preclude colonization and proliferation of S. aureus tst+ within indigenous microbial communities of the vagina.
Keywords: toxic shock syndrome, Staphylococcus aureus, microbial community, vagina
The numerically dominant members of vaginal communities do not preclude colonization and proliferation of Staphylococcus aureus tst+ within indigenous microbial communities of the vagina.
INTRODUCTION
Toxic shock syndrome (TSS) is a rare disease characterized by several symptoms including fever, rash, hypotension, desquamation and the involvement of multiple organ systems (Todd et al.1978). Menstrual TSS (mTSS) has been associated with menstruation and tampon use and although it is rare, the effects can be life threatening (Osterholm et al.1982; Hector et al.2001). The incidence of mTSS was reported to be 0.69 in 100 000 menstruating women per year (DeVries et al.2011) with ∼2% of cases resulting in fatality (Hajjeh et al.1999). Early investigations of the cause of mTSS identified toxic shock syndrome toxin 1 (TSST-1) as both necessary and sufficient to induce mTSS (Bergdoll et al.1981; Schlievert et al.1981; Bonventre et al.1983; Cohen et al.1983). It is thought that mTSS is associated with vaginal colonization by strains of Staphylococcus aureus that carry the gene for TSST-1, though recovery of S. aureus tst+ is not required for diagnosis of the disease (Reeves et al.1984; Musser et al.1990). TSST-1 is a superantigen that acts by binding to and cross-linking conserved regions in T-cell receptors and the major histocompatibility group II (MHC) proteins on antigen-presenting cells (Kotb 1995, 1998). Stimulation of the immune system by TSST-1 by this means is problematic because it bypasses the normal processing and presentation of antigens and results in activation of 5%–30% of all T cells, versus a conventional immune response, which typically results in 0.01% of T cells being activated (Dinges, Orwin and Schlievert 2000; Llewelyn et al.2004). The proliferation that occurs is accompanied by a cytokine cascade that produces the symptoms of mTSS (Callahan et al.1990; Misfeldt 1990; Herman et al.1991).
While the molecular mechanism of TSST-1 action is well understood, less is known about the steps leading to development of the disease. Previous work has suggested that three principal events are necessary for mTSS to occur (Parsonnet et al.2005). First, S. aureus tst+ must colonize and proliferate within the indigenous microbial community of the vagina. Second, sufficient TSST-1 must be produced and persist. This requires that the conditions that prevail in the vagina be conducive to expression of the tst gene, which is known to be regulated by environmental factors such as oxygen, carbon dioxide and pH (Ross and Onderdonk 2000) and influenced by specific bacterial taxa including Streptococcus agalactiae, Enterococcus spp. and Lactobacillus (MacPhee et al.2013). Once expressed, the toxin must persist and avoid degradation by proteases (Blake, Cook and Bashinski 1987; Dinges, Orwin and Schlievert 2000) or inactivation by IgM or IgG antibodies present in vaginal secretions (Kansal et al.2007). Third, TSST-1 must cross the vaginal epithelium and enter circulation where it can bind to T-cell receptors and MHC proteins and elicit the extraordinary release of cytokines that has been observed in mTSS patients. mTSS will not occur if any of these steps are interrupted (Bonventre et al.1988; Parsonnet et al.2005).
The species composition of indigenous bacterial communities in the vagina could also influence whether a woman is at risk to mTSS. One way is through the competitive exclusion of invasive species, including pathogens (Alpert, Bone and Holzapfel 2000; Hector et al.2001). This could occur through competition for resources or by blocking adherence to epithelial cell receptors (Zarate and Nader-Macias 2006). In addition, the bacterial populations found in the vagina may change the environment in which they reside and create conditions that preclude successful colonization by non-indigenous species. This could include creation of a low environmental pH (Whiting et al.1996) and the production by low molecular weight fatty acids that inhibit growth (Adams and Hall 1988). Finally, expression of the tst gene is unlikely to occur unless the indigenous communities maintain a pH and concentrations of glucose, O2 and CO2 that permit expression of the staphylococcal superantigen TSST-1 (Schlievert and Blomster 1983; Seidl, Bischoff and Berger-Bächi 2008). The ability of vaginal microbial communities to exclude S. aureus tst+ or preclude the expression of TSST-1 might vary among individuals since previous studies (Oakley et al.2008; Fettweis et al.2014; Ravel et al.2011; van de Wijgert et al.2014) have shown that the composition of these communities varies in terms of the kinds and relative abundances of bacterial species present. Nothing is known about the microbial communities indigenous to the vaginas of healthy women colonized by S. aureus tst+.
This study was done to determine if there was an association between the composition and structure of vaginal microbial communities and the presence of S. aureus tst+. These samples had been collected as part of a survey of 3012 healthy women to determine the prevalence of TSST-1 producing strains of S. aureus (Parsonnet et al.2005). The samples used for this study were all those obtained by Parsonnet et al., who were identified as being vaginally colonized with S. aureus tst+ as well as a control group consisting of women who were not vaginally colonized with TSST-1 positive strains of S. aureus. The vaginal communities of women in these two groups were compared on the basis of terminal restriction fragment length polymorphism (T-RFLP) profiles of 16S rRNA genes, and the predominant members of vaginal communities of women colonized with S. aureus tst+ were identified by phylogenetic analysis of cloned 16S rRNA genes.
METHODS
Sample collection
The samples used in this study were collected as part of a study to determine the prevalence of S. aureus carriage in reproductive age women in North America (Parsonnet et al.2005). Three thousand and twelve healthy, menstruating women between the ages of 13 and 40 were enrolled from five geographically separate sites in North America: Cincinnati, OH; East Brunswick, NJ; St. Petersburg, FL; Scottsdale, AZ; and Winnipeg, Manitoba, Canada. The demographics of the women sampled matched the racial profile of the 1990 US census (white = 80%, black = 12%, Hispanic = 5%, and Asian = 3%). Subjects were eligible for enrollment if they had regular menstrual cycles (21–35 days); used tampons at least occasionally; could read, write and understand English; did not bathe or shower within the 2 h prior to their scheduled visit; refrained from douching, vaginal medications, suppositories, feminine sprays, genital wipes or contraceptive spermicides for 48 h prior to their scheduled visit; and were willing to comply with all other protocol requirements. Subjects were not eligible if they were participating in another clinical study; were pregnant, actively trying to get pregnant or suspected they were pregnant; had a gynecological abnormality as judged by the study medical personnel; had a self-reported infection of the genitals within the past 6 weeks; had been medically diagnosed as having diabetes, kidney failure, hepatitis, AIDS (HIV positive) or TSS; or were using antimicrobial or antifungal drugs to treat a vaginal infection. Subjects completed a demographic questionnaire and classified themselves into one of four distinct racial groups: white, Black, Hispanic or Asian. Prior to sample collection informed consent was documented from each participant enrolled in this study. Upon collection of the vaginal sample, the attending health practitioner noted any signs of possible genital abnormalities.
Vaginal samples analyzed
Fifty-one vaginal swab samples were shipped on dry ice from Procter and Gamble (Cincinnati, Ohio) to the University of Idaho (Moscow, Idaho). They were stored at –80˚C until further analyses. Twenty one of the swabs constitute cases in which women were vaginally colonized with S. aureus tst+, while an additional thirty swabs were from women not vaginally colonized with S. aureus tst+ that constitute controls and belonged to one of three subgroups: 10 were from women not colonized vaginally, nasally or anally with S. aureus; 10 were from women colonized vaginally with S. aureus tst−; and 10 were from women whose nose or anus (but not the vagina) was colonized with S. aureus tst+. The samples were initially coded so that the investigators (JP and LF) were blinded for the first half of the study. The blind was broken for the selection of the 21 samples from individuals vaginally colonized with S. aureus tst+ that were used for the construction of clone libraries.
Genomic DNA isolation
Bacterial genomic DNA was isolated from vaginal swabs using previously described methods (Zhou et al.2004).
T-RFLP analysis
T-RFLP profiles of 16S rRNA genes were determined for each sample (Liu et al.1997). Internal regions of 16S rRNA genes were amplified by PCR using the fluorescently labeled primer pair, 8fm (AGAGTTTGATCMTGGCTCAG) and 926r (CCGTCAATTCCTTTRAGTTT) Invitrogen, Carlsbad, CA). Primer 8fm was labeled with VIC and 926r was labeled with 6-carboxy-flourescin (6-FAM; Applied Biosystems, Foster City, CA). The PCR reactions contained 1 μl of template DNA (∼100 ng), 5 units of AmpliTaq DNA polymerase (Applied Biosystems), 0.1 μM primer (8fm and 926r), 200 μM dNTP (GE Healthcare, Uppsala, Sweden), 5% by volume DMSO (Sigma-Aldrich, St. Louis, MO), 3 mM MgCl2 (Applied Biosystems) and 1x buffer (Applied Biosystems) in a final volume of 50 μl. The thermocycler (DNA Engine Dyad®, Bio-Rad, Hercules, CA) protocol was 5 min denaturation at 94°C followed by 35 cycles of 1 min at 94°C, 1 min at 55°C, 2 min at 72°C and a hold period for 10 min at 72°C.
The PCR amplicons from each sample were digested in two separate reactions, one of which included MspI (C∧CGG) and a second that included HaeIII (GG∧CC). Digestions were done as previously described (Zhou et al.2004). For each sample, equal volumes of these digestions were combined and the resulting mixture had four fluorescently labeled terminal restriction fragments derived from each phylotype. MspI and HaeIII have been theoretically and empirically shown to resolve the bacterial populations likely to be found in vaginal samples (Zhou et al.2004; Coolen et al.2005).
T-RFLP profiles for each sample were obtained by capillary electrophoresis by using an ABI PRISM 3100 DNA Analyzer and GeneScan software (Applied Biosystems) as previously described (Zhou et al.2004). CST ROX 25–1000 (BioVentures, Inc., Murfreesboro, TN) was used as an internal size standard.
Statistical analysis of T-RFLP profiles
Cluster analysis of T-RFLP data was used to identify communities with similar numerically abundant populations using methods described by Abdo et al. (2006). Fisher's exact test was used to test the hypothesis that the distribution of community types found in women colonized with S. aureus tst+ was significantly different from the distribution of communities found in women not vaginally colonized by the organism (Ott and Longnecker 2000).
We conducted a power analysis to determine the number of samples that would be needed to identify a significant difference between the distributions of samples from the two groups of women (those vaginally colonized and not vaginally colonized by S. aureus tst+). To perform this analysis, we used a resampling algorithm implemented in R (R Development Core Team 2008). This analysis used the observed data (see Table 1). For both groups frequency distributions were constructed using the observed community types in each group. We simulated drawing samples from both frequency distributions to test if there was a significant difference between the two sample sets. The process of drawing a set of samples from each distribution and comparing them was repeated 1000 times. Significance was determined using a Fisher's exact test. If the frequency of correctly rejecting the null hypothesis based on 50 samples was <80%, the sample size was increased by 5, and the process was repeated. This was done until the frequency of correctly rejecting the null hypothesis exceeded 80% (Cohen 1988).
Table 1.
The Fisher's exact test was used to determine if there was a significant difference between the frequency that samples occurred in each of the groups.
| Women vaginally colonized with S. aureus tst+ | Women without vaginal S. aureus tst+ | ||||
|---|---|---|---|---|---|
| Origin of sample | Observationsa | Expected frequencyb | Observations | Expected frequency | Total samples per group |
| Group 1 | 1 | 2.0588 | 4 | 2.9712 | 5 |
| Group 2 | 1 | 1.6471 | 3 | 2.3529 | 4 |
| Group 3 | 10 | 8.6471 | 11 | 12.3529 | 21 |
| Group 4 | 7 | 6.1765 | 8 | 8.8235 | 15 |
| Other | 2 | 2.4705 | 4 | 3.5294 | 6 |
| Total number of samples analyzed | 21 | 30 | 51 | ||
aObservations were counted by identifying the number of samples of a particular colonization status within each group identified in the cluster analysis.
bExpected frequencies were calculated by multiplying the total number of samples with the same colonization status by the number of samples identified in the corresponding group (Fig. 1) and then divided by the total number of samples.
Correspondence analysis was used to determine if there was a relationship between vaginal colonization by S. aureus tst+ and the presence and abundance of specific phylotypes in the samples (Greenacre 2007). The standardized T-RFLP data were used in conjunction with sample classification based on colonization status as identified by Parsonnet et al. (2005). An intraclass correlation was calculated for each DNA fragment category to determine if some DNA fragments are correlated with colonization or non-colonization.
16S rRNA gene clone library construction and sequence analysis
The 16S rRNA genes in samples from women vaginally colonized by S. aureus tst+ were amplified by PCR using ‘universal’ eubacterial bacterial primers 8fm and 926r, cloned and sequenced to identify the numerically dominant bacterial populations in each sample. The PCR conditions were the same as for T-RFLP except the 8fm and 926r primers were not fluorescently labeled. The PCR product was cleaned with the QIAquick PCR Purification Kit (Qiagen, Foster City, CA), eluted with 50 μl of water and the DNA concentration was spectrophotometrically measured (ND-1000, NanoDrop, Wilmington, DE).
The 3΄-A overhangs on the PCR products produced by AmpliTaq DNA polymerase that interfere with blunt-end cloning were removed using T4 DNA polymerase (1 U) in 45 μl reactions that contained 5 μl of NeBuffer2 and 0.2 μl of 100 μM dNTP (New England Biolabs, Ipswich, MA) per 100 ng of PCR product. The mixtures were incubated for 15 min at 12°C, held at 4°C while 4 μl of 125 mM EDTA was added. Afterwards, the reactions were heated to 75°C for 20 min to inactivate the enzyme then kept at 4°C until they were used in cloning reactions.
Prior to cloning the amplicons were cleaned with the QIAquick PCR Purification Kit (Qiagen), eluted with 50 μl of water and the DNA concentration was spectrophotometrically measured (ND-1000, NanoDrop). The cleaned products were cloned using the Zero Blunt® TOPO® PCR cloning kit (Invitrogen) as recommended by the manufacturer. For each reaction 1 μl of vector was added, 1 μl of 1:4 dilution of the salt solution, the appropriate volume of PCR product and water to a volume totaling 6 μl. The PCR product to vector ratio was ∼8:1. The mixture was incubated at room temperature for 5 min., and 2 μl of this mixture was used to transform 25 μl of electrocompetent Escherichia coli cells that had been added to 25 μl of deionized nano-pure water. Electroporation was done at 2.5 mV on a Gene Pulser® (Bio-Rad) in pre-chilled 1 mm cuvettes, and afterwards 250 μl of SOC medium was added to each cuvette. The cells were transferred to an Eppendorf tube that was incubated for 1 h at 37°C with agitation, then plated on Luria-Bertani (LB) plates with 50 μg/ml of kanamycin (Fisher Scientific, Pittsburg, PA) and incubated overnight at 37°C. Colonies that grew on the kanamycin plates were randomly picked and grown in deep-well microtiter plates with LB + 50 μg/ml of kanamycin for 24 h at 37°C with shaking. The cells in each well were pelleted by centrifugation at 6200 rpm for 45 min on a Sigma 4–15 laboratory centrifuge (Sigma, Germany), and washed twice with 0.9% NaCl. The washed cells were stored at –80°C in 0.9% NaCl.
Plasmid DNA was isolated from at least 100 clones from each sample using QIAprep 96 Turbo BioRobot kits (Qiagen) using the recommended protocol and stored at –80˚C. The cloned inserts were sequenced using 10 μl reactions that contained 2 μl of Big-Dye (Applied Biosystems), 0.5 μl of 3.2 μM 926r primer (Invitrogen) and 4 μl of template. The reactions were done in a thermocycler (DNA Engine Dyad, Bio-Rad) using the following program: 1 min at 94°C, 24 cycles of 95°C for 1 min, 55°C for 1 min and 72°C for 1 min with a final extension of 72°C for 5 min. Sequencing products were cleaned by ethanol precipitation by adding 3 μl of 125 μM EDTA followed by 30 μl of 100% ethanol. The precipitated DNA was collected by centrifugation for 10 min at 1650 rpm at 4°C using a Sigma 4–15 laboratory centrifuge (Sigma, Germany), and washed with 70% ethanol. The samples were dried at 55°C then 10 μl of Hi Di formamide (Applied Biosystems) was added. The DNA sequences were determined using an ABI 3730 PRISM Genetic Analyzer using the standard protocol.
The sequence data were analyzed as previously described (Zhou et al.2007) to verify that each sequence surpassed minimum standards for length and quality. Clones were assigned to phylotypes by comparing their 16S rRNA gene sequences to those of known organisms in the RDPII (Cole et al.2003) using the BLAST algorithm (Altschul et al.1990). The genus and species names were used if the sequence similarity to a type species was >97%; the genus only was used if the sequence similarity was <97%, but >90%. The uncultured bacterium was described if the sequence similarity of clones to known organisms was <90%. The sequences from vaginal communities and closely related organisms were identified using HiTSA (http://www.ibest.uidaho.edu/tools/hitsa/index.php).
RESULTS AND DISCUSSION
The two primary goals of this study were to compare the vaginal microbial communities of women vaginally colonized with S. aureus tst+ with those of women not vaginally colonized and to identify the predominant species present in the communities of women colonized with S. aureus tst+. The T-RFLP profiles provided the means to compare communities based on the numerically dominant members of the vaginal communities, while phylogenetic analysis of cloned 16S rRNA genes was used to identify abundant members of the vaginal communities sampled.
Comparison of community profiles
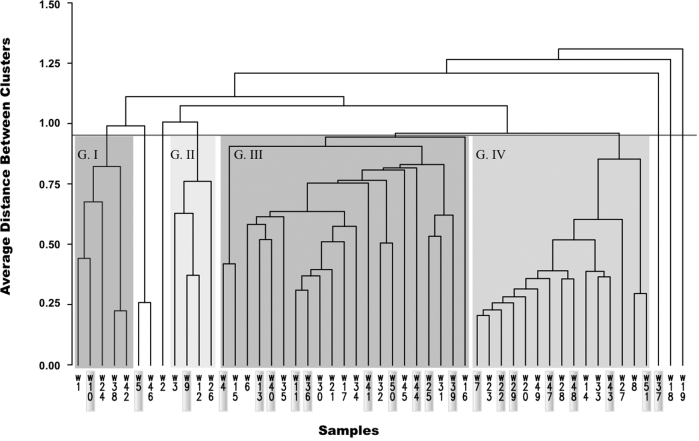
The T-RFLP profiles of 16S rRNA genes in samples from 21 women vaginally colonized with S. aureus tst+ were compared with those of 30 women not vaginally colonized with S. aureus to determine if there were fundamental differences in the numerically abundant bacterial species present. Hierarchical clustering of the T-RFLP profiles of samples from all the women resulted in four significant clusters and six samples that did not cluster with two or more other samples (Fig. 1). The S. aureus tst+ samples were distributed among all the clusters found in this analysis. Statistical tests were used to determine if the observed distribution of sequences among clusters was significant. Using Fisher's exact test, communities colonized with S. aureus tst+ were tested for a significant difference in the distribution among community types when compared to the communities that were not colonized. The cluster identity of each sample was used to construct a contingency table that contained the number of samples observed in each cluster and the expected number of samples that would be in each category if there was not a difference in colonization by S. aureus tst+ (Table 1). The results of Fisher's exact test showed that there was not a significant difference in the rank abundance of community types between vaginal communities that were colonized by S. aureus tst+ and those that were not (P = 0.7582). Also, there was not a specific community type that seemed to exclude S. aureus tst+. Taken together, there is no evidence to suggest a significant effect of microbial community structure on vaginal colonization with S. aureus tst+.
Figure 1.
A dendrogram showing the similarity of vaginal microbial communities from women vaginally colonized and those not vaginally colonized by S. aureus tst+. Samples highlighted in the gray boxes represent vaginal community profiles from women who were vaginally colonized with S. aureus tst+. G I–IV represent the statistically significant clusters identified.
However, it is possible that too few women colonized by S. aureus tst+ were sampled, and more would have to be included to detect a statistically significant difference in the distribution of community types found in cases and controls. A post hoc power analysis was performed to estimate the number of samples that would be needed to detect a difference in the distribution of microbial communities from women vaginally colonized and not vaginally colonized with S. aureus tst+. This was done by simulating data sampling using the distribution of community types observed for women colonized and not colonized with S. aureus tst+. The results suggest that 135 samples from women vaginally colonized with S. aureus tst+ or more would be needed to detect a statistically significant difference between the distributions with 80% power. We were unable to include more samples of women colonized with S. aureus tst+ because this is a rare event. Of 3012 women sampled, only 32 were colonized by S. aureus tst+. The results of the power analysis using a conservative assumption that the observed distribution of community types was true suggest that more than 135 women colonized by S. aureus tst+ would need to be sampled, which would require screening nearly 20 000 women. Alternatively, it could indeed be true that no significant differences exist between the vaginal communities of women colonized by S. aureus tst+ as compared to women who are not colonized by with S. aureus. This would imply that bacterial populations indigenous to the vagina are not an important determinant of whether colonization by S. aureus tst+ occurs.
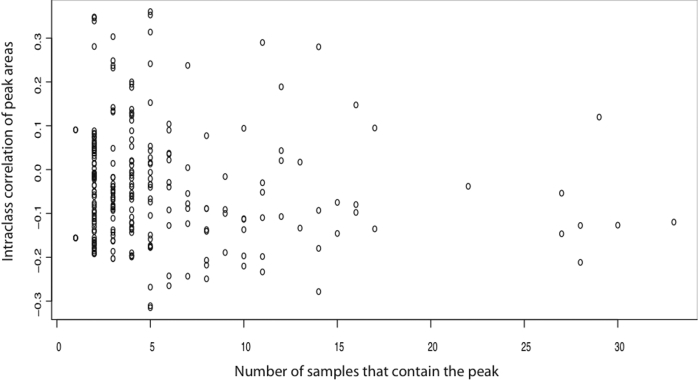
A second possibility is that while the overall community composition may not differ, the abundances of one or more populations in the community may be positively or negatively correlated with vaginal colonization by S. aureus tst+. To explore this possibility, an intraclass correlation analysis was done to identify the numerically abundant phylotypes whose abundances covaried with the presence of S. aureus tst+. Figure 2 shows that no single phylotype was highly correlated with colonization status, suggesting that the numerically abundant members of indigenous microbial communities neither encouraged or inhibited colonization by S. aureus tst+.
Figure 2.
Intraclass correlation between DNA fragment size and colonization status. This analysis was used to identify DNA fragments in T-RFLP profiles of 16S rRNA genes that are correlated with colonization by S. aureus tst+. Each circle shows the number of samples in which the fragment occurred and how well the fragment was correlated to colonization status. We would expect some of the fragments (circles) to have a higher correlation value if they were indicative of colonization or to have a lower correlation if indicative of precluding colonization.
Composition of vaginal communities colonized with S. aureus tst+
While the T-RFLP method is very useful for comparing communities on the basis of numerically abundant phylotypes, the method is limited in so far as a single DNA fragment may be composed of more than one phylotype. To confirm that this was not the case for these samples, libraries were constructed and the sequences of cloned 16S rRNA genes were determined. This was only done on the samples from women colonized with S. aureus tst+ because the composition of communities from normal healthy women have been previously described (Zhou et al.2007; Fettweis et al.2014; Ravel et al.2011). The results of the analyses for women colonized with S. aureus tst+ are shown in Table S1 (Supplementary Data). The results were unremarkable. The most common clone among all of the samples was Lactobacillus iners, which accounted for over 40% of all clones sequenced. Atopobium vaginae, Streptococcus species, L. crispatus, L. jensenii and L. gasseri in addition to L. iners collectively account for more than 70% of all clones sequenced. The predominance of lactobacilli is consistent with the findings of previous studies (Oakley et al.2008; Fettweis et al.2014; Ravel et al.2011; van de Wijgert et al.2014). Finally, it should be noted that no species were common to all the samples. These data show there were no detectable differences in the composition of vaginal communities of women vaginally colonized with S. aureus tst+ as compared to those that were not as determined by culture-independent methods.
There are several possible explanations for the inability to detect differences in the distribution of community types among these two groups of women. It could be that actual differences in the community composition were below the detection threshold of the techniques used in this study. Indeed, we know that there were differences in the community composition that were not detected by the methods used because we did not detect S. aureus in all the samples colonized with S. aureus. Since the PCR primers and methods used are known to amplify 16S rRNA genes from S. aureus, this suggests the organism was comparatively rare in these communities, and when present it constituted <1% of the total community. While the detection threshold may have limited the ability to observe differences in less abundant community members, the results do indicate that there were no significant differences in predominant taxa of vaginal communities in women of the two groups and unusual or unexpected species were not observed.
The samples for this study were collected from women that had not and were not experiencing mTSS so they may be viewed as ‘carriers’ of the organism. The work of Parsonnet et al. (2005) showed that approximately 1 in 100 women are vaginally colonized with S. aureus tst+, suggesting that the incidence of carriage is common, while the incidence of disease is very low (approximately 1 case in 100,000 women per year). This implies that although a fair number of women are colonized by the pathogen, the conditions required for the expression of virulence are insufficient, or that TSST-1 is neutralized and/or degraded before it can stimulate a catastrophic cytokine cascade (Kimber et al.2013). The abundance of S. aureus tst+ in these ‘carriers’ was not determined, nor is it known if the women were persistently colonized since the samples were taken as part of a cross-sectional study with one sampling event. Nonetheless, the results of this study and that of Parsonnet et al. (2005) indicate that vaginal colonization by S. aureus tst+ is a poor predictor of overall risk to mTSS.
The reasons why mTSS does not occur in women vaginally colonized with S. aureus tst+ are unknown. It has previously been postulated that serum antibodies that bind the TSST-1 toxin and prevent it from binding to MHC proteins are critical to preventing the disease (Bonventre et al.1983; Schlievert and Blomster 1983). This is largely consistent with studies on antibody titers against TSST-1 in reproductive age women; direct evidence to support this was demonstrated by Kansal et al. (2007). These studies have shown that toxin specific antibodies are absent in women who contracted mTSS, which suggests that circulating antibodies are important for protection (Bonventre et al.1984; Christensson and Hedstrom 1985; Stolz et al.1985; Noleto et al.1986). In addition, the finding that TSST-1 specific antibodies inhibit the development of TSS in rabbit models seems to confirm the importance of specific antibodies (Best et al.1988; Bonventre et al.1988). Moreover, two recent studies show that there were high titers of antibodies specific to TSST-1 present in healthy women who were colonized by S. aureus tst+ (Parsonnet et al.2005; Kansal et al.2007) but had not contracted mTSS, suggesting these antibodies were neutralizing and/or surrogate markers of protection. In a study of 39 healthy individuals, Kansal et al. (2007) showed that 35 of the 39, including all 20 who were colonized with S. aureus tst+, had titers of neutralizing antibody to TSST-1 that are considered protective. The data from this study suggest that high titers of neutralizing antibodies are common, and may be protective against mTSS. Importantly, however, there are instances of healthy women who have antibody titers that are low and comparable to those of women that have experienced mTSS (Kansal et al.2007). This suggests that perhaps antibody titer alone cannot explain why some women colonized with S. aureus tst+ do not develop mTSS (Kimber et al.2013). Further examination of host susceptibility factors in the pathogenesis of the disease may provide more insight.
CONCLUSION
Using cultivation-independent methods to characterize the species composition of vaginal communities, we were unable to detect differences between the numerically predominant bacterial taxa in vaginal communities of healthy women that were colonized with S. aureus tst+ and those that were not. We cannot, however, rule out the possibility that small differences exist that were not detected using the methods we employed. As seen in other studies, there were differences in the composition of vaginal communities in both groups of women, but there was no specific community type that seemed to exclude S. aureus tst+. Taken together, there is no evidence to suggest a significant effect of microbial community structure on vaginal colonization with S. aureus tst+ and the circumstances that allow for colonization of the vagina by S. aureus tst+ remain unknown.
DECLARATIONS
Ethics approval and consent to participate
Study protocol and informed-consent document were reviewed and approved by the Institutional Review Board of Hill Top Research Inc. Subjects read and signed informed-consent forms prior to the collection of any information or clinical sampling as described in Parsonnet et al. (2005).
Consent for publication
All samples were entirely de-identified.
Availability of data and material
The datasets obtained and analyzed during the current study are available from the corresponding author on reasonable request.
SUPPLEMENTARY DATA
Supplementary data are available at FEMSPD online.
Acknowledgements
We would also like to thank Dr Zaid Abdo, Dr Haruo Suzuki and Dr Christopher Williams for their assistance in statistical analysis; Dr Celeste Brown, G. Maria Schneider and Dr Xia Zhou for technical assistance and data analysis; and Ursel Schütte and Yuli Song for invaluable assistance and helpful comments.
AUTHOR'S CONTRIBUTIONS
MAH, LJF and CCD conceived the study. MAH supervised the clinical study and JDP performed all the samples and statistical analyses. LJF and JDP wrote the manuscript.
FUNDING
This research was funded by The Procter & Gamble Company, Cincinnati, OH. The DNA Sequence Analysis Core Facility at the University of Idaho is supported by a Center of Biomedical Research Excellence grant (P20 RR016448) from the National Institutes of Health to LJF.
Conflict of Interest. LJF has consulted for The Procter & Gamble, Company. CCD and MAH were employees of The Procter & Gamble Company during the course of this study.
REFERENCES
- Abdo Z, Schuette UM, Bent SJ et al. Statistical methods for characterizing diversity of microbial communities by analysis of terminal restriction fragment length polymorphisms of 16S rRNA genes. Environ Microbiol 2006;8:929–38. [DOI] [PubMed] [Google Scholar]
- Adams MR, Hall CJ. Growth inhibition of food-borne pathogens by lactic and acetic acids and their mixtures. Int J Food Sci Technol 1988;23:287–92. [Google Scholar]
- Alpert P, Bone E, Holzapfel C. Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect Plant Ecol 2000;3:52–66. [Google Scholar]
- Altschul SF, Gish W, Miller W et al. Basic local alignment search tool. J Mol Biol 1990;215:403–10. [DOI] [PubMed] [Google Scholar]
- Best GK, Scott DF, Kling JM et al. Protection of rabbits in an infection model of toxic shock syndrome (TSS) by a TSS toxin-1-specific monoclonal antibody. Infect Immun 1988;56:998–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergdoll MS, Crass BA, Reiser RF et al. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet 1981;371:1017–21. [DOI] [PubMed] [Google Scholar]
- Blake E, Cook C Jr, Bashinski J. Evidence that “vaginal peptidase” is a bacterial gene product. J Forensic Sci 1987;32:888–99. [PubMed] [Google Scholar]
- Bonventre PF, Linnemann C, Weckbach LS et al. Antibody responses to toxic-shock-syndrome (TSS) toxin by patients with TSS and by healthy staphylococcal carriers. J Infect Dis 1984;150:662–6. [DOI] [PubMed] [Google Scholar]
- Bonventre PF, Thompson MR, Adinolfi LE et al. Neutralization of toxic shock syndrome toxin-1 by monoclonal antibodies in vitro and in vivo. Infect Immun 1988;56:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre PF, Weckbach L, Staneck J et al. Production of staphylococcal enterotoxin F and pyrogenic exotoxin C by Staphylococcus aureus isolates from toxic shock syndrome-associated sources. Infect Immun 1983;40:1023–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan JE, Herman A, Kappler JW et al. Stimulation of B10.BR T cells with superantigenic staphylococcal toxins. J Immunol 1990;144:2473–9. [PubMed] [Google Scholar]
- Christensson B, Hedstrom SA. Serological response to toxic shock syndrome toxin in Staphylococcus aureus infected patients and healthy controls. Acta Path Micro Im B 1985;93:87–90. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Erlbaum, 1988. [Google Scholar]
- Cohen ML, Graves LM, Hayes PS et al. Toxic shock syndrome: modification and comparison of methods for detecting marker proteins in Staphylococcus aureus. J Clin Microbiol 1983;18:372–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JR, Chai B, Marsh TL et al. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res 2003;31:442–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen MJL, Post E, Davis CC et al. Characterization of microbial communities found in the human vagina by analysis of terminal restriction fragment length polymorphisms of 16S rRNA genes. Appl Environ Microb 2005;71:8729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AS, Lesher L, Schlievert PM et al. Staphylococcal toxic shock syndrome 2000–2006: epidemiology, clinical features, and molecular characteristics. PLoS One 2011;6:e22997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 2000;13:16–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettweis JM, Brooks JP, Serrano MG et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 2014;160:2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenacre MJ. Correspondence Analysis in Practice, 2nd ed Chapman & Hall/CRC, Baton Rouge, Florida, 2007. [Google Scholar]
- Hajjeh RA, Reingold A, Weil A et al. Toxic shock syndrome in the United States: surveillance update, 1979–1996. Emerg Infect Dis 1999;5:807–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector A, Dobson K, Minns A et al. Community diversity and invasion resistance: an experimental test in a grassland ecosystem and a review of comparable studies. Ecol Res 2001;16:819–31. [Google Scholar]
- Herman A, Kappler JW, Marrack P et al. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol 1991;9:745–72. [DOI] [PubMed] [Google Scholar]
- Kansal R, Davis C, Hansmann M et al. Structural and functional properties of antibodies to the superantigen TSST-1 and their relationship to menstrual toxic shock syndrome. J Clin Immunol 2007;27:327–38. [DOI] [PubMed] [Google Scholar]
- Kimber I, Nookala S, Davis CC et al. Toxic shock syndrome: characterization of human immune responses to TSST-1 and evidence for sensitivity thresholds. Toxicol Sci 2013;134:49–63. [DOI] [PubMed] [Google Scholar]
- Kotb M. Bacterial pyrogenic exotoxins as superantigens. Clin Microbiol Rev 1995;8:411–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotb M. Superantigens of Gram-positive bacteria: structure—function analyses and their implications for biological activity. Curr Opin Microbiol 1998;1:56–65. [DOI] [PubMed] [Google Scholar]
- Liu WT, Marsh TL, Cheng H et al. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl Environ Microb 1997;63:4516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewelyn M, Sriskandan S, Peakman M et al. HLA class II polymorphisms determine responses to bacterial superantigens. J Immunol 2004;172:1719–26. [DOI] [PubMed] [Google Scholar]
- MacPhee RA, Miller WL, Gloor GB et al. Influence of the vaginal microbiota on toxic shock syndrome toxin 1 production by Staphylococcus aureus. Appl Environ Microb 2013;79:1835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt ML. Microbial “superantigens”. Infect Immun 1990;58:2409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser JM, Schlievert PM, Chow AW et al. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. P Natl Acad Sci USA 1990;87:225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noleto ALS, Cesar EC, Bergdoll MS. Antibodies to Staphyloccoal enterotoxins and toxic shock syndrom toxin 1in sera of patients and healthy people in Rio de Janeiro, Brazil. J Clin Microbio 1986;24:809–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley BB, Fiedler TL, Marrazzo JM et al. Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Appl Environ Microb 2008;74:4898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholm MT, Davis JP, Gibson RW et al. Tri-state toxic-shock syndrome, results of a multistate case-control study. J Infect Dis 1982;145:431–40. [DOI] [PubMed] [Google Scholar]
- Ott R, Longnecker M. An Introduction to Statistical Methods and Data Analysis. Belmont, CA: Duxbury Press,2000. [Google Scholar]
- Parsonnet J, Hansmann MA, Delaney ML et al. Prevalence of toxic shock syndrome toxin 1-producing Staphylococcus aureus and the presence of antibodies to this superantigen in menstruating women. J Clin Microbiol 2005;43:4628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team R A Language and Environment for Statistical Computing. 2008http://www.r-project.org/ (date last accessed, 26 February 2018). [Google Scholar]
- Ravel J, Gajer P, Abdo Z et al. Vaginal microbiome of reproductive-age women. P Natl Acad Sci USA 2011;108 Suppl 1:4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MW, Pine L, Feeley JC et al. Presence of toxic shock toxin in toxic shock and other clinical strains of Staphylococcus aureus. Infect Immun 1984;46:590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Onderdonk AB. Production of toxic shock syndrome toxin 1 by Staphylococcus aureus requires both oxygen and carbon dioxide. Infect Immun 2000;68:5205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert PM, Blomster DA. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J Infect Dis 1983;147:236–42. [DOI] [PubMed] [Google Scholar]
- Schlievert PM, Shands KN, Dan BB et al. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis 1981;143:509–16. [DOI] [PubMed] [Google Scholar]
- Seidl K, Bischoff M, Berger-Bächi B. CcpA mediates the catabolite repression of tst in Staphylococcus aureus. Infect Immun 2008;76:5093–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz SJ, Davis JP, Vergeront JM et al. Development of serum antibody to toxic shock toxin among individuals with toxic shock syndrome in Wisconsin. J Infect Dis 1985;151:883–9. [DOI] [PubMed] [Google Scholar]
- Todd J, Fishaut M, Kapral F et al. Toxic-shock syndrome associated with phage-group-staphylococci. Lancet 1978;312:1116–8. [DOI] [PubMed] [Google Scholar]
- van de Wijgert JHHM, Borgdorff H, Verhelst R et al. The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One 2014;9:e105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting RC, Sackitey S, Calderone S et al. Model for the survival of Staphylococcus aureus in nongrowth environments. Int J Food Microbiol 1996;31:231–43. [DOI] [PubMed] [Google Scholar]
- Zarate G, Nader-Macias ME. Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. Lett Appl Microbiol 2006;43:174–80. [DOI] [PubMed] [Google Scholar]
- Zhou X, Bent SJ, Schneider MG et al. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology 2004;150:2565–73. [DOI] [PubMed] [Google Scholar]
- Zhou X, Brown CJ, Abdo Z et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J 2007;1:121–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.