Abstract
Concerns regarding resource expenditures have been expressed about the 2012 NIA-AA Sponsored Guidelines for neuropathologic assessment of Alzheimer disease (AD) and related dementias. Here, we investigated a cost-reducing Condensed Protocol and its effectiveness in maintaining the diagnostic performance of Guidelines in assessing AD, Lewy body disease (LBD), microvascular brain injury, hippocampal sclerosis (HS), and congophilic amyloid angiopathy (CAA). The Condensed Protocol consolidates the same 20 regions into 5 tissue cassettes at ∼75% lower cost. A 28 autopsy brain–retrospective cohort was selected for varying levels of neuropathologic features in the Guidelines (Original Protocol), as well as an 18 consecutive autopsy brain prospective cohort. Three neuropathologists at 2 sites performed blinded evaluations of these cases. Lesion specificity was similar between Original and Condensed Protocols. Sensitivities for AD neuropathologic change, LBD, HS, and CAA were not substantially impacted by the Condensed Protocol, whereas sensitivity for microvascular lesions (MVLs) was decreased. Specificity for CAA was decreased using the Condensed Protocol when compared with the Original Protocol. Our results show that the Condensed Protocol is a viable alternative to the NIA-AA guidelines for AD neuropathologic change, LBD, and HS, but not MVLs or CAA, and may be a practical alternative in some practice settings.
Keywords: Alzheimer disease, Cost and congophilic amyloid angiopathy, Hippocampal sclerosis, Lewy body disease, Microvascular brain injury
INTRODUCTION
Alzheimer disease (AD) is currently estimated to afflict 5.4 million people in the United States (1). By the year 2050, the number of cases is expected to increase to between 11 and 16 million Americans (1). As part of an effort to update diagnostic guidelines for AD, in 2012 the National Institute on Aging (NIA) in collaboration with the Alzheimer’s Association (AA) sponsored a panel of experts to revise the previous guidelines, which were published in 1997 (2). The outcome of this work is called the NIA–AA guidelines for the neuropathologic assessment of AD and related illnesses (3, 4). When these revised guidelines were presented at the 90th annual meeting of the American Association of Neuropathologists in 2014, concern was expressed by numerous attendees that resources in some academic departments, in forensic settings, and in private practice could not support the extensive sampling recommended by the NIA–AA guidelines.
The rationales for designing an alternative protocol were to streamline sampling and staining, and thereby reduce attendant effort and costs, while maintaining the diagnostic performance of the original guidelines. We developed an alternative approach called the Condensed Protocol in which samples from the same 20 brain regions as in the original 2012 NIA–AA guidelines are consolidated into 5 tissue cassettes for a single round of histochemical or immunohistochemical (IHC) stains; this bypasses the tiered structure whereby primary results are obtained prior to additional staining for secondary and tertiary analyses (3, 4). In this study, we determined the sensitivity and specificity of the Condensed Protocol compared with the Original Protocol evaluated in blinded fashion by 3 neuropathologists at 2 different sites using both retrospective and prospective cohorts.
MATERIALS AND METHODS
This study was approved by the IRB at the University of Washington (UW) and University of Kentucky (UK).
Retrospective Case Selection
Thirty research brains from the UW Alzheimer’s Disease Research Center (ADRC) Neuropathology Core that already had been evaluated exactly according to Original Protocol described in the 2012 NIA–AA guidelines (3, 4) were selected to highlight the 3 types of ranked neuropathologic features: AD neuropathologic change, Lewy body disease (LBD), and microvascular lesions (MVLs). Cases were selected for a high level of 1 type of neuropathologic change while allowing co-morbid lesion severity to vary. Two of the LBD cases subsequently were excluded because IHC for α-synuclein (α-syn) failed when performing the Condensed Protocol. The final retrospective study set for analysis comprises these 28 cases.
Prospective Case Selection
We also evaluated prospectively an additional 20 consecutive brain autopsies collected through the UW ADC Neuropathology Core; 2 of these cases were excluded because they ultimately were diagnosed as Niemann–Pick disease or progressive supranuclear palsy and were, therefore, not relevant for testing the NIA–AA assessments in the condensed protocol. Thus, there was a final prospective study set of 18 cases.
Tissue and Slide Preparation
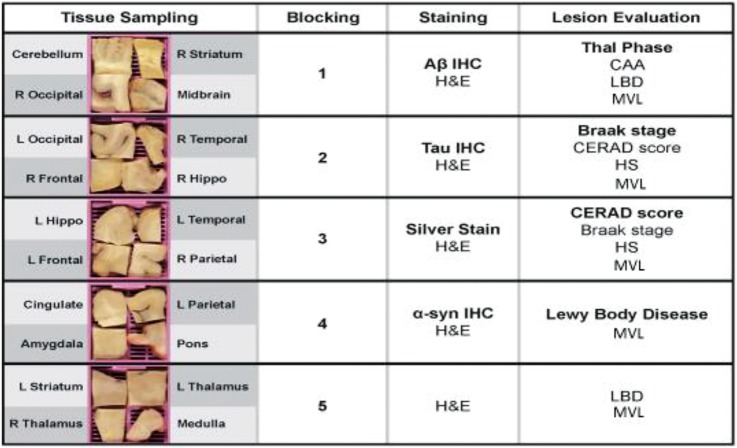
All tissue sampling, tissue block preparation, and slide preparation were performed by the UW ADRC using well-established histochemical and IHC protocols, all of which are “preferred” or “acceptable alternatives” by NIA–AA guidelines (3, 4). Each of the 5 tissue blocks was strategically sampled to meet specific diagnostic targets (Fig. 1). Hematoxylin and eosin (H&E) combined with Luxol fast blue staining was performed on sections from each block. In addition, special stains were performed as follows: Immunohistochemistry for β-amyloid ([Aβ], antibody: 6E10; Biolegend, San Diego, CA) was performed on sections of right striatum, midbrain, right occipital cortex and cerebellum. Immunohistochemistry for neurofibrillary tangles and other pathological tau deposits (antibody: Phospho-PHF-tau pSer202 + Thr205 (AT8); Thermo Fisher Scientific, Waltham, MA) were performed on sections of left occipital cortex, right temporal cortex, right frontal cortex, and right hippocampus. Bielschowsky preparation was performed on sections of left hippocampus, left temporal cortex, left frontal cortex, and right parietal cortex. Immunohistochemistry for Lewy bodies (antibody: anti-α-synuclein LB 509; Abcam, Cambridge, MA) was performed on sections of cingulate gyrus, amygdala, left parietal cortex, and pons. No special stains were performed on the fifth block, which contained sections of left striatum, right thalamus, left thalamus, and medulla. These combinations allow simultaneous staining rather than the tiered approach of the Original guidelines (3, 4). (Fig. 1 and Pathologyoutlines.com, for further details). In cases where 1 hippocampus side was entirely submitted for frozen tissue banking at time of autopsy, contralateral hippocampus was submitted in blocks 2 and 3 for evaluation. In some cases in the retrospective cohort, brainstem paraffin blocks were melted and re-embedded to collect midbrain, pons, and medulla for the Condensed Protocol.
FIGURE 1.
Condensed Protocol. Diagram shows tissue sampling, blocking, staining, and lesion evaluation for the 5 tissue cassettes used in the Condensed Protocol. CERAD, Consortium to Establish a Registry for Alzheimer’s Disease. Hippo, hippocampus; L, left; R, right.
Neuropathologic Evaluation
This study was a collaborative effort among neuropathologists at the UW and the UK ADC. Neuropathologic evaluation either followed exactly the original 2012 NIA–AA guidelines (Original Protocol) or the Condensed Protocol. Original Protocol evaluations were performed independently at the time of diagnostic workup. Condensed Protocol evaluations were performed subsequently and independently by 3 neuropathologists who were blinded to all other evaluations. For the Condensed Protocol, 9 slides (5 H&E-stained slides, 3 IHC stained slides, and 1 Bielschowsky slide) per case, along with score sheets were distributed to the two evaluating neuropathologists at UW and the 1 neuropathologist at UK (Fig. 1). All stained slides and score sheets were then returned to UW upon completion of the evaluation.
Statistics
Sensitivity and specificity for AD neuropathologic change, LBD, and MVLs were computed across the 138 Condensed Protocols (3 evaluations per case). Ninety-five percent confidence intervals were estimated using a bootstrap, blocking on specimen. In brief, the bootstrapping method generated 5000 datasets where the data on the 30 specimens were randomly sampled with replacement. For each bootstrapped dataset, sensitivity and specificity were computed. Using these 5000 pairs of sensitivity and specificity estimates, 95% confidence intervals were calculated using the adjusted percentile method (5). Inter-rater reliability was estimated using the kappa statistic accompanied by bootstrapped-adjusted percentile confidence intervals as described above. Kappa statistics with bootstrapped confidence intervals were computed using Stata 14.1 (StataCorp, College Station, TX). All other analyses were carried out using R 3.2.3 (6). Sensitivity and specificity for hippocampal sclerosis (HS) and congophilic amyloid angiopathy (CAA) were calculated from 2 × 2 tables that were evaluated as “present” or “absent” for each protocol.
RESULTS
We devised a Condensed Protocol and compared it with the published consensus NIA–AA guidelines (here called the “Original” protocol) to assess sensitivity and specificity of our proposed alternative approach with respect to AD neuropathologic change, LBD, MVLs, HS, and CAA. The Condensed protocol eliminates tiered sampling by combining sampled brain regions that ultimately undergo the same staining method into a single cassette (Fig. 1); however, histopathologic scoring methods remained identical between the 2 protocols. This strategy expedited evaluation by eliminating the need to return to the wet tissue multiple times. Estimates of the comparative effort (# slides) and cost of the 2 Protocols at the University of Washington are presented in Table 1. The Original and Condensed Protocols were used to evaluate in blinded fashion 50 research brain autopsies collected though the UW ADRC: 30 retrospective and 20 prospective cases; of these, 4 were excluded because of technical reasons in 2 cases (retrospective) and because 2 (prospective) were less common neurodegenerative diseases not relevant to the current study. Results from the Original Protocol for the 50 assessed cases are presented in Table 2. Results from the Original Protocol for the 46 cases included in our analysis were used as the “gold standard” for assessing the performance of the Condensed Protocol.
TABLE 1.
Effort (Number of Slides) and Cost Comparison for Original Versus Condensed Protocols at the University of Washington from 2014
| H&E Slides (n) | IHC Slides (n) | Bielschowsky Slides (n) | UW Pathology Cost | |
|---|---|---|---|---|
| Original Protocol | 20 | 13 | 3 | $1204.66 |
| Condensed Protocol | 5 | 3 | 1 | $303.39 |
| Condensed versus Original | 75% less | 77% less | 67% less | 78% less |
TABLE 2.
Results of Neuropathologic Evaluation Using Original Protocol for Retrospective and Prospective Cases
| Neuropathologic Rankings |
Prospective (n) | Retrospective (n) | ||
|---|---|---|---|---|
| AD | LB | MVL | ||
| None/low | None | None | 3 | |
| Moderate | 2a | |||
| Severe | 1 | 4 | ||
| Transitional | Severe | 1 | ||
| Neocortical | None | 2 | ||
| Moderate | 1 | |||
| Intermediate | None | None | 3 | |
| Moderate | 1 | |||
| Severe | 5 | |||
| Transitional | Moderate | 1 | ||
| Neocortical | None | 1 | ||
| Moderate | 3 | |||
| Severe | 1 | |||
| High | None | None | 4 | 2 |
| Moderate | 3 | |||
| Severe | 1 | |||
| Transitional | None | 4 | ||
| Moderate | 1 | |||
| Severe | 1 | |||
| Neocortical | None | 1 | 2 | |
| Not AD | 2b | |||
| Total assessed | 20 | 30 | ||
| Total analyzed | 18 | 28 | ||
aOldest 2 retrospective cases where α-synuclein (LB 509) IHC failed in the Condensed Protocol work up. Both were excluded from analysis
bOne case of Niemann Pick disease and 1 case of progressive supranuclear palsy. Both were excluded from analysis.
Sensitivity and specificity were calculated for the retrospective and prospective cohorts combined (Table 3). Ranked endpoints of AD, LBD, and MVLs were analyzed using both strict (AD High, LBD Neocortical, and MVL Severe) and relaxed (AD High or Intermediate, LB Any, and MVL Severe or Moderate) parameters. Specificity using the Condensed Protocol for all lesion types in all groups was ≥85%, regardless of strict or relaxed parameters; in many instances, it exceeded 95%. Sensitivity using the Condensed Protocol varied more widely. Sensitivity for AD neuropathologic change was overall excellent (≥80%), and even greater with relaxed parameters. Sensitivity for LBD was poorer (<60%) for ranked evaluation but improved to overall 80% when analyzed as “any” versus “none”. There was higher sensitivity in the prospective cases for LBD when compared with the retrospective cases (not shown), supporting degradation of IHC signal for samples that had remained for long periods in formalin before being embedded which necessitated exclusion of the 2 oldest LBD Neocortical cases from the 30 original retrospective cohort. Sensitivity for MVL detection was overall poor with either strict or relaxed parameters, although relaxed parameters did trade reduction in specificity for some increase in sensitivity.
TABLE 3.
Sensitivity and Specificity and Kappa Scores for Inter-Rater Reliability (95% Confidence Intervals) to Compare the Condensed Protocol vs. Original Protocol
| Sensitivity | Specificity | Kappa | |
|---|---|---|---|
| Hi AD | 84% | 88% | 0.79 (0.65, 0.94) |
| (67%, 94%) | (73%, 96%) | ||
| HI + Inter AD | 97% | 81% | 0.71 (0.51, 0.90) |
| (92%, 99%) | (59%, 94%) | ||
| Neocortical | 47% | 95% | 0.54 (0.33, 0.76) |
| LB | (22%, 72%) | (87%, 99%) | |
| Any LB | 80% | 98% | 0.84 (0.71, 0.98) |
| (61%, 94%) | (92%, 100%) | ||
| Severe MVLs | 21% | 97% | 0.36 (0.22, 0.50) |
| (5%, 38%) | (88%, 100%) | ||
| Severe + Mod | 53% | 84% | 0.29 (0.05, 0.53) |
| MVLs | (33%, 70%) | (74%, 91%) |
HS and CAA were also evaluated as present or absent. By the Original Protocol, HS was identified in 5 cases in the retrospective group and 2 in the prospective group; CAA was identified in 11 cases in the retrospective group and 12 in the prospective group. Sensitivity (86%) and specificity (84%) were excellent for evaluation of HS using the Condensed Protocol. Sensitivity (87%) was excellent for evaluation of CAA by the condensed protocol; however, specificity was quite low (32%) with 47 false positive scores out of 138 (46 cases × 3 evaluators).
DISCUSSION
We evaluated an alternative to the full workup recommendation in the revised NIA–AA guidelines for the neuropathologic evaluation of AD as a possible option to reduce resources needed by neuropathologists outside of a research context. The Condensed Protocol, which reduces effort and cost by ∼75% compared with the Original Protocol, maintained excellent specificity in each category of neuropathologic change. However, these advantages must be balanced against varying reductions in sensitivity for detection of ranked lesions of AD, LBD, and MVLs. Indeed, sensitivity for AD neuropathologic changes remained excellent, whereas sensitivity for LBD detection was moderate, and for MVL detection it was poor. Although adequate for evaluating AD neuropathologic change, we view the reduced sensitivity for LBD and MVL as a potentially significant limitation of the Condensed Protocol because numerous studies have shown the impact of these diseases (often in combination with AD) on cognitive impairment and dementia.
In addition, although the current NIA–AA guidelines do not include evaluation for HS or CAA, they do recommend sampling for it. These lesions, recorded as present or absent, maintained excellent sensitivities. Specificity for HS remained high, whereas specificity for CAA was poor, likely due to differences in assessing CAA in the Original Protocol (using Congo Red stain) and in the Condensed Protocol (using Aβ IHC) as well as the focal nature of these lesions and possible lack of sampling at the initial time of analysis. The Original Protocol did not consider pathologic workup for other forms of dementia such as frontotemporal lobar degenerations or prion disease; rather, it refers neuropathologists to other consensus protocols if the case does not have AD neuropathologic change. Although the sampling of the Condensed Protocol may be adequate first step in screening for other causes of dementia, we have not yet determined the sensitivity and specificity of Condensed Protocol sampling for lesions other than AD, MVL, and LBD.
Sensitivity of the Condensed Protocol was poor for the detection of MVLs, likely due to decreased sampling. Indeed, the total area of tissue evaluated microscopically is greatly reduced in the Condensed Protocol. Microinfarcts and microhemorrhages occur much less frequently as individual pathologic lesions compared with plaques, neurofibrillary tangles and even Lewy bodies, and they are not grossly visible. Thus, they cannot be sampled deliberately. It is not surprising, therefore, that sensitivity for detection of MVLs was substantially reduced in the Condensed Protocol. Depending on the context, poor sensitivity for MVL detection could be viewed as a major shortcoming of the Condensed protocol because MVLs, that is microvascular brain injury, is a common contributor to dementia in older individuals, particularly in population-based settings (7, 8). Comprehensive evaluation of vascular brain injury is, therefore, important. A potential compromise might be supplemental sampling for MVLs with H&E stains in regions recommended by the NIA–AA guidelines, perhaps focused on vascular border zone regions, which would modestly increase effort and cost but also would likely improve sensitivity for detecting MVLs in individuals with dementia.
Evaluation of IHC performed with the Condensed Protocol was somewhat challenging. For retrospective cases, this was most significant for α-syn IHC (antibody LB509) and less so for Aβ IHC (antibody 6E10), particularly in the striatum. Because there was an interval between sampling of tissue for the Original and Condensed Protocols for the retrospective cases, we speculate that degradation of antigen for IHC with time may account in part for the reduced sensitivity observed. If that is true, then sensitivity for the Condensed Protocol for retrospective cases may be underestimates, particularly for LBD, and would be expected to be increased in prospective cases where Original and Condensed Protocol sampling was performed simultaneously. Other issues encountered during this study included multiple tissue pieces in a single cassette in the Condensed Protocol creating more “edge” artifacts in IHC slides. This made interpretation of these slides more difficult than in the Original Protocol and required additional pathologist effort at times. There was also variation between our 2 ADCs in staining practices. Therefore, familiarity with certain stains performed at UW ADC varied among evaluators. The kappa statistic for detection of high AD neuropathologic change using the Condensed Protocol was similar to a larger 10-site study that included both UW and UK ADC Neuropathology cores (9), with kappa differing by less than 10% between these 2 studies. Kappa for detection of any LBD in the present study was excellent at 0.84. Kappa for severe MVLs was poor at 0.29, perhaps related to both sampling bias (smaller sample area) and less clear consensus guidelines for evaluation of these lesions (3, 4). To improve assessment of cerebrovascular pathology, more normal-sized samples could be evaluated via H&E, which could be relatively inexpensive.
In summary, we have designed and tested a Condensed Protocol for NIA–AA guidelines for the neuropathologic evaluation of AD that yields ∼75% reduction in effort and cost and maintains excellent specificity for the ranked neuropathologic features, but has variable sensitivity for detection of AD neuropathologic change (excellent), LBD (moderate), and MVLs (poor). Specificity for HS and CAA, recorded as present or absent, was acceptable for HS but was poor for CAA, likely due to methodological differences. TDP-43 evaluation is most likely to be relevant for differentiating AD from CARTS (cerebral age-related TDP-43 with sclerosis), which is an area where further work is required regardless of the protocol used (10). The advantages and disadvantages of the Condensed versus Original Protocols need to be weighed by neuropathologists in different practice settings such as forensic, private practice, and academic. Our Condensed Protocol may be most appropriate where the focus is on determining AD neuropathologic change by the most efficient means, recognizing its limitations in evaluating common comorbidities.
Disclosure/conflict of interest: The authors have no duality or conflicts of interest to declare.
Funding: P50 AG005136, P30 AG028383, P50 AG047366, University of Washington Accountable Care for Anatomic Pathology Pilot Grant, and the Nancy and Buster Alvord Endowment.
REFERENCES
- 1. Alzheimer's Association. 2015 Alzheimer's disease facts and figures. Alzheimers Dement 2015;11:332–84. [DOI] [PubMed] [Google Scholar]
- 2. Dickson DW. Neuropathological diagnosis of Alzheimer's disease: a perspective from longitudinal clinicopathological studies. Neurobiol Aging 1997;18:S21–6. [DOI] [PubMed] [Google Scholar]
- 3. Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 2012;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davison AC, Hinkley DV, Bootstrap Methods and Their Applications. Chapter 5.3. Cambridge: Cambridge University Press; 1997 [Google Scholar]
- 6. R Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2014, Vienna: Available at http://www.R-project.org/. [Google Scholar]
- 7. Brundel M, de Bresser J, van Dillen JJ, et al. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab 2012;32:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kapasi A, Schneider JA. Vascular contributions to cognitive impairment, clinical Alzheimer's disease, and dementia in older persons. Biochim Biophys Acta 2016; 1862:878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montine TJ, Monsell SE, Beach TG, et al. Multisite assessment of NIA-AA guidelines for the neuropathologic evaluation of Alzheimer's disease. Alzheimers Dement 2016;12:164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson PR, Trojanowski JQ, Abner EL, et al. “New old oathologies”: AD, PART, and cerebral age-related TDP-43 with sclerosis (CARTS). J Neuropathol Exp Neurol 2016;75:482–98. [DOI] [PMC free article] [PubMed] [Google Scholar]