Abstract
Study Objectives
Napping is a useful countermeasure to the negative effects of acute sleep loss on alertness. The efficacy of naps to recover from chronic sleep loss is less well understood.
Methods
Following 2 baseline nights (10 hours' time-in-bed), participants were restricted to 7 nights of 5-hour sleep opportunity. Ten adults participated in the No-Nap condition, and a further 9 were assigned to a Nap condition with a daily 45-minute nap opportunity at 1300 h. Sleepiness was assessed using the multiple sleep latency test and a visual analogue scale at 2-hour intervals. Both objective and subjective indexes of sleepiness were normalized within subject as a difference from those at baseline prior to sleep restriction. Mixed-effects models examined how the daytime nap opportunity altered sleepiness across the day and across the protocol.
Results
Short daytime naps attenuated sleepiness due to chronic sleep restriction for up to 6–8 hours after the nap. Benefits of the nap did not extend late into evening. Subjective sleepiness demonstrated a similar short-lived benefit that emerged later in the day when objective sleepiness already returned to pre-nap levels. Neither measure showed a benefit of the nap the following morning after the subsequent restriction night.
Conclusions
These data indicate a short daytime nap may attenuate sleepiness in chronic sleep restriction, yet subjective and objective benefits emerge at different time scales. Because neither measure showed a benefit the next day, the current study underscores the need for careful consideration before naps are used as routine countermeasures to chronic sleep loss.
Keywords: countermeasure, MSLT, napping, sleepiness, sleep loss, sleep restriction
Statement of Significance
This study shows that daytime naps provide short-term attenuation of sleepiness caused by chronic sleep restriction. The effects of these naps do not extend beyond 6–8 hours, and are undetectable the following day. Therefore, this study highlights the importance of recognizing both the benefits of naps to rescue alertness and the limits to these benefits. Investigations into different nap lengths may determine the amount of sleep needed to maintain alertness across successive days under chronic sleep restriction conditions.
INTRODUCTION
Nearly 30% of the US population report obtaining 6 or fewer hours of sleep each night.1 This figure has increased over the past two decades, likely due to increased work and social pressures.2, 3 Apart from affecting alertness and performance, cumulative sleep loss is associated with several adverse health outcomes including cardiovascular, metabolic and mood disorders, obesity, and cancer.2 These issues raise a clear need for a countermeasure to the negative effects of chronic sleep restriction. While daytime naps may logically meet this need, little evidence exists as to whether, and for how long, daytime naps can attenuate the negative consequences of cumulative reduced sleep.
A well-established consequence of chronic sleep restriction is a cumulative decline in alertness and performance.4–6 In fact, the impact of chronic sleep restriction can be as severe as acute total sleep deprivation. For example, participants restricted to 4 hours’ time-in-bed (TIB) for 6 days showed performance impairment equivalent to one night of total sleep loss.6 Similarly, Carskadon and Dement4 showed that following 7 days of 5 hours' TIB, sleep onset latency (SOL) was significantly reduced, with one-third of participants experiencing pathological levels of sleepiness (SOL <5 minutes).7 Moreover, while performance deficits coupled with increased physiological sleepiness is common, subjective awareness of sleepiness saturates at lower levels during chronic sleep restriction.6 Thus, chronic sleep restriction poses a unique hazard resulting from physiological sleepiness and performance impairment occurring with a simultaneous lack of subjective awareness.
In light of the consequences of chronic sleep restriction, naps have long been proposed as a potential countermeasure to the effects of cumulative sleep loss.8 The benefits of daytime naps following habitual sleep,9, 10 acute total sleep deprivation,11 and acute partial sleep restriction12-17 have been consistently demonstrated. Afternoon naps as short as 10 minutes have the potential to restore alertness and performance to well-rested levels following sleep restriction;15–17 longer naps tend to provide a dose-dependent benefit.11, 18, 19
Following one night of sleep restriction (typically 4–5 hours' TIB), studies generally report improvements in self-rated sleepiness for up to 1–2 hours post-nap,13–17 although one study claimed subjective benefits lasting up to 4 hours.12 Naps of at least 30 minutes duration have also shown increased sleep onset latency15–17 and reduced physiological markers of sleepiness in the electroencephalogram12, 13 for 1–2 hours post-nap. These studies suggest that naps, at least in the short term, have the ability to “rescue” alertness in the face of acute sleep loss. Unclear, however, is how long the benefit from napping persists under conditions of chronic sleep loss, and the duration across multiple days of sleep restriction is yet to be investigated.
An initial report by Hayashi and colleagues10 studied the effects of a 20-minute nap at noon for 5 consecutive days of unrestricted sleep (on average about 7 hours). Naps taken later in the week were better able to suppress sleepiness in the afternoon, suggesting that under saturated sleep conditions, repetitive use of naps can have cumulative benefits. Napping was also effective at ameliorating the impact of sleep restriction following a week of cumulative sleep loss,4 although this prior study did not investigate napping during the restriction protocol itself.
Together, the literature to date suggests that daytime naps taken across consecutive days of sleep restriction may be able to alleviate the accumulation of sleepiness associated with chronic sleep loss. Indeed, a recent study of adolescents restricted to 5 nights of 5 hours’ TIB showed that a 1-hour afternoon nap opportunity was able to attenuate performance decline across the restriction period but did not restore performance to baseline levels.20 While the authors report that subjective sleepiness improved following a nap, this study did not include a physiological measure of sleepiness, such as the multiple sleep latency test (MSLT).21
Thus, the aim of this study is to explore the duration and profile of objective and subjective sleepiness following a 45-minute afternoon nap opportunity across successive days of sleep restriction in healthy young adults. We hypothesized that the nap would improve subsequent alertness on restriction days compared to not napping. Furthermore, we hypothesized that this effect would be strongest for measures taken shortly following the nap (e.g., within 6 hours), diminishing with further time awake.
METHODS
Participants
This study is an expansion of a prior investigation of the impact of chronic sleep restriction upon objective and subjective sleepiness.4 The No Nap group is drawn from that prior report, while participants in the Nap group were recruited subsequent to this original study. In total, the sample consisted of 19 young adults participating in the in-laboratory Stanford Sleep Camp across three summers. Following informed consent, 10 participants (5 females; aged 17–24 [mean: 20] years) formed the No Nap group completing seven consecutive nights of chronic sleep restriction, while 9 participants (4 females; aged 18–26 [mean: 21] years) entered the Nap group, completing the same protocol with an additional 45-minute nap opportunity during the days of restricted sleep. No systematic differences were present between the two groups with respect of habitual sleep.
Study Design
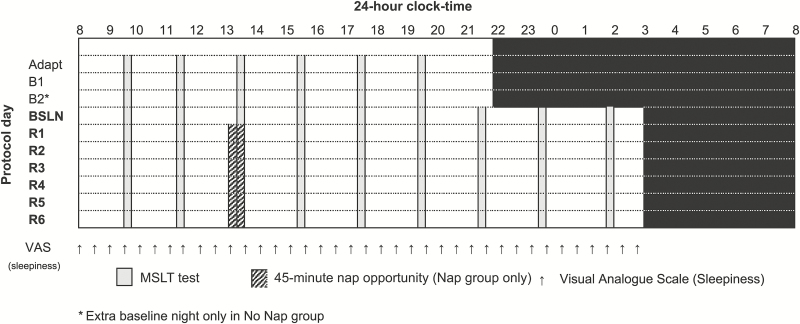
Participants completed an intensive 13-day in-laboratory protocol involving nocturnal sleep recordings and daytime measurement of sleepiness. Figure 1 illustrates the portion of the study protocol germane to the current analysis.
Figure 1.
Study protocol. Study protocol schematic illustrating the in-laboratory procedures. Each row indicates a day of the experimental protocol (BSLN: baseline day; R1-6: restriction days). Black shading denotes sleep episodes. Vertical gray bars indicate timing of multiple sleep latency test (MSLT); hashed bars indicate afternoon nap opportunity for participants in the Nap group. Vertical arrows illustrate half-hourly visual analogue scales (VAS) measuring subjective sleepiness.
Participants lived in the laboratory for the entirety of the study in a dormitory setting on the Stanford campus. Participants were continuously monitored in a light- and temperature-controlled laboratory environment during testing and sleep periods. Participants had access to regular meals, snacks, and free time when not testing although were restricted from caffeine while in the laboratory. Participants were not permitted to engage in strenuous physical exercise during the protocol. Participants were allowed to walk outside the laboratory in-between testing, monitored by research staff.
Following an initial adaptation night in the laboratory, participants completed two baseline days (three in the No Nap group) with a 10-hour sleep schedule (bedtime: 2200 h; rise time: 0800 h). During the day, the MSLT21 was completed 6 times at 2-hour intervals. In the evening of the final baseline day, the first night of sleep restriction extended bedtime to 0300 h, reducing the sleep opportunity to 5 hours. All participants slept on this reduced schedule for the next 6 nights. On the days following reduced sleep (Restriction Days 1–6), the same MSLT protocol was continued into extended wakefulness, with the final MSLT occurring at 0130 h. During sleep restriction, participants in the Nap group were given a 45-minute nap opportunity at 1300 h, supplanting the regularly scheduled 1330 h MSLT period. Those in the No Nap group continued the MSLT protocol as normal during these days. Bedtime on the seventh day of the sleep restriction protocol was restored to 2200 h, and participants were given two nights of recovery sleep before exiting the study.
A complete description of the sleep restriction results can be found in the earlier report of the No Nap group.4 The current study focuses on the first 6 days of testing following sleep restriction: consisting of nine MSLT tests across a 19-hour interval. The seventh restriction day only involved six MSLT tests, as bedtime was advanced to begin a recovery protocol; as such, this day is not examined in the current analysis.
As the Nap group was given two nights of unrestricted baseline sleep prior to the first day of wake extension while the No Nap group was given an additional third night, our analyses target the final night of unrestricted sleep, as the baseline condition (BSLN) for each group. Thus, it is sleep from this final unrestricted night and sleepiness metrics from the subsequent first day of wake extension that are taken as baseline measurements for each group for purposes of comparing to the 6 days of testing following restricted sleep.
Sleep Monitoring
All nocturnal and daytime sleep (including MSLT testing) was monitored by polysomnography using Grass Model 7 polygraphs. Electroencephalography included two central (C3; C4) and two occipital (O1; O2) derivations referenced to the contralateral mastoid. Electrooculography was placed at the right and left outer canthi, and electromyography was recorded from chin and submental electrodes. Recordings were conducted with a high-pass filter at 0.3 Hz, a low-pass filter at 35 Hz, and a paper speed of 10 mm/sec. All sleep was scored in 30-second epochs according to the standardized procedures of Rechtschaffen and Kales.22
Measures of Sleepiness
Sleepiness throughout the protocol was assessed in two ways: (1) the objective MSLT21 and (2) a visual analogue scale (VAS) for sleepiness. The VAS is a 100-mm visual analogue scale where one pole corresponds to “very wide awake” and the other pole to “very sleepy.” Participants were instructed to view the line as a continuum between these two extremes and to place a mark at the point describing their current sleepiness. VAS data were scored by measuring the mark along the 100-mm line. Thus, a score of 0 indicates complete alertness and a score of 100 indicates complete sleepiness.
Each day, subjective assessments of sleepiness occurred immediately following morning awakening and at 30-minute intervals throughout the remainder of the day (see Figure 1 for timing relative to sleep episodes). Participants were not permitted to see previous scores at the time of each assessment.
The MSLT was conducted each day according to standard procedures,21 at 2-hour intervals throughout the day beginning at 0930 h (see Figure 1). On days where bedtime was 0300 h (during chronic sleep restriction), MSLTs continued until a ninth test at 0130 h. As in our prior studies, the MSLT involved participants lying quietly in their darkened bedroom where they were asked to “please close your eyes, lie quietly, and try to fall asleep.” The MSLT episode was measured using the same polysomnography techniques as with nocturnal sleep. The tests were terminated after three consecutive 30-second epochs of sleep (usually, but not always, Stage 1 non-rapid eye movement [NREM] sleep). Sleep latency formed the objective measure of sleepiness and was defined as the elapsed time from lights out to the first of the three consecutive scored epochs of sleep.
Statistical Methods
Statistical analyses were completed in Stata SE 14 (StataCorp LP, College Station, Texas) using linear mixed-effects models. Each model utilized restricted maximum likelihood estimation. Statistical testing used Satterthwaite’s approximation of degrees of freedom to adjust inference for small sample sizes.
Sleepiness scores (both MSLT and subjective ratings) are presented as differences from baseline to account for any unexpected variability in sleepiness at the start of the study between the two groups. A two-step analytical approach was followed for each data component. First, the entirety of the data was plotted (e.g., Figure 2). Based on clear clustering of effects to specific times of day, subsequent analytical models divided the day into three equal segments with scores averaged to reduce the number of data points and improve our ability to systematically test time-of-day × group interactions in this small sample. Thus, this binning procedure increases our statistical power to detect within-subject effects, in contrast to treating each individual time point (particularly for subjective sleepiness separate). Nevertheless, post hoc between-subject t-tests are included for each original time point (displayed in figures) for the most complete description of the data possible.
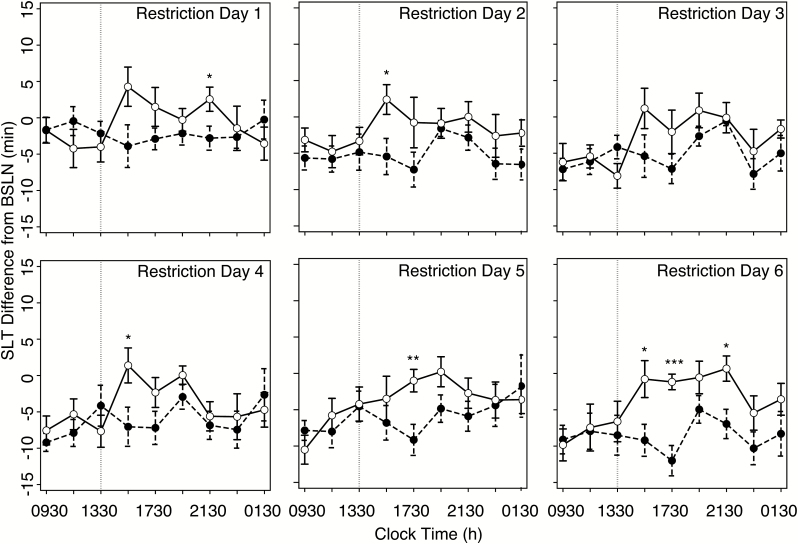
Figure 2 .
MSLT across restriction days. MSLT-indexed sleep latencies across 6 days of the protocol for No Nap (black circles) and Nap (white circles), respectively. MSLT values expressed as differences from the baseline rested day (BSLN). Vertical lines indicate timing of afternoon nap. Error bars indicate standard error of mean (SEM). Pairwise t-tests at each MSLT test differences between group: *p < .05; **p < .01; ***p < .001.
RESULTS
Nocturnal Sleep
Sleep measures for each nocturnal sleep episode are reported in Table 1. Sleep on the baseline night prior to sleep restriction did not differ significantly between groups on any measure, with the exception more time spent in Stage 1 sleep in the No Nap group.
Table 1.
Nocturnal Sleep Statisticsa.
| Sleep on baseline night, min | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nap | No Nap | t | p | |||||||
| TST | 541 (43) | 527 (42) | 0.72 | .48 | ||||||
| SL | 21 (17) | 33 (34) | −1.00 | .33 | ||||||
| WASO | 30 (33) | 28 (40) | 0.094 | .93 | ||||||
| Stage 1 | 39 (15) | 57 (21) | −2.13 | .048 | ||||||
| Stage 2 | 270 (39) | 280 (35) | −0.62 | .54 | ||||||
| SWS | 95 (20) | 81 (34) | 1.03 | .31 | ||||||
| REM | 132 (26) | 108 (29) | 1.90 | .074 | ||||||
| Sleep on restriction nights, min | ||||||||||
| R1 | R2 | R3 | R4 | R5 | R6 | FDayXGrp | p | |||
| TSTb | ||||||||||
| Nap | 291 (11) | 289 (12) | 296 (4) | 293 (9) | 296 (5) | 296 (7) | 0.23 | .95 | ||
| No Nap | 285 (7) | 284 (9) | 289 (7) | 288 (7) | 290 (7) | 292 (4) | ||||
| SLb | ||||||||||
| Nap | 5 (6) | 6 (6) | 4 (4) | 3 (5) | 3 (3) | 2 (3) | 0.32 | .90 | ||
| No Nap | 10 (7) | 11 (8) | 7 (6) | 8 (7) | 8 (7) | 6 (4) | ||||
| WASOb | ||||||||||
| Nap | 4 (7) | 6 (8) | 1 (1) | 4 (8) | 3 (4) | 3 (5) | 1.39 | .24 | ||
| No Nap | 1 (2) | 1 (1) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | ||||
| Stage 1b | ||||||||||
| Nap | 17 (13) | 15 (9) | 10 (6) | 11 (4) | 10 (6) | 9 (5) | 0.23 | .95 | ||
| No Nap | 21 (8) | 17 (11) | 14 (6) | 16 (9) | 14 (7) | 14 (8) | ||||
| Stage 2 | ||||||||||
| Nap | 115 (27) | 115 (24) | 115 (13) | 112 (27) | 110 (25) | 116 (31) | 0.34 | .88 | ||
| No Nap | 127 (29) | 118 (24) | 121 (28) | 114 (33) | 120 (25) | 124 (26) | ||||
| SWS | ||||||||||
| Nap | 92 (20) | 90 (19) | 93 (17) | 96 (26) | 91 (19) | 95 (19) | 0.45 | .82 | ||
| No Nap | 81 (29) | 86 (31) | 92 (34) | 94 (29) | 89 (30) | 90 (26) | ||||
| REMb | ||||||||||
| Nap | 62 (15) | 67 (23) | 76 (12) | 73 (13) | 84 (17) | 72 (17) | 0.72 | .61 | ||
| No Nap | 56 (18) | 64 (10) | 62 (12) | 64 (20) | 67 (13) | 64 (15) | ||||
Abbreviations: TST = total sleep time; SL = sleep latency [elapsed time from lights out to first of three consecutive epochs of sleep]; WASO = wake after sleep onset; SWS = slow wave sleep [NREM Stages 3 + 4]; REM = rapid eye movement sleep. Interaction terms reflect statistics from mixed-effects models, as in the main article. No sleep parameters during the restriction protocol demonstrated main-effects of group.
aData are presented as means (standard deviation).
bMain-effects of day on sleep parameters across the protocol.
The same table presents nocturnal sleep architecture for both the Nap and the No Nap groups across sleep restriction. Examining total sleep time reveals the success of our protocol in limiting sleep to near 50% of baseline levels, in all participants. While the effect of sleep restriction is clear across the six protocol days, no significant interaction of Day and Group was present in any sleep parameter (either in duration or in architecture; all p's ≥ .24). Therefore, there was no impact of the nap on nocturnal sleep.
Daytime Nap Opportunity
Table 2 presents sleep architecture for the daytime nap opportunity provided to those participants in the Nap group. Neither total sleep time nor the stage architecture of the nap varied across the protocol (all p's ≥ .30). Moreover, across the entirety of the protocol, nap sleep was predominately Stage 2 sleep, with limited slow-wave sleep, and negligible amounts of rapid eye movement sleep.
Table 2.
MSLT-derived Objective Sleepiness on the Baseline Day (minutes)a.
| Nap | No Nap | |
|---|---|---|
| 0930 | 14 (7) | 16 (5) |
| 1130 | 15 (6) | 16 (5) |
| 1330 | 13 (7) | 16 (5) |
| 1530 | 12 (6) | 13 (6) |
| 1730 | 15 (7) | 20 (0) |
| 1930 | 17 (5) | 19 (4) |
| 2130 | 15 (6) | 19 (1) |
| 2330 | 12 (7) | 18 (4) |
| 0130 | 7 (6) | 13 (8) |
Abbreviation: MSLT, multiple sleep latency test.
aData are reported as mean (SD).
Objective Sleepiness (MSLT)
The primary dependent variable in this report is sleepiness during chronic sleep restriction measured by the MSLT. Sleep latencies on the final baseline day, reported in Table 3, were largely consistent between groups and within normative levels. Exploratory analyses revealed a significant main effect of time of day (F8, 136 = 5.42, p < .001), yet no significant interaction of time-of-day and group (F8, 136 = 0.99, p = .45). Moreover, no overall difference in sleepiness was identified between the two groups (F1, 17 = 3.95, p = .064). Despite no significant baseline differences in the sleepiness between groups, all data from restriction days are presented as difference scores from the corresponding baseline MSLT to account for any minor variability between the groups.
Table 3.
Afternoon Nap Sleep Statistics (Nap Group Only)a.
| R1 | R2 | R3 | R4 | R5 | R6 | F Day | p | |
|---|---|---|---|---|---|---|---|---|
| TST | 32 (13) | 29 (13) | 35 (7) | 37 (13) | 31 (14) | 37 (6) | 1.25 | .30 |
| Stage 1 | 4 (3) | 5 (2) | 5 (3) | 3 (2) | 4 (3) | 4 (3) | 0.68 | .64 |
| Stage 2 | 20 (11) | 19 (10) | 21 (5) | 21 (10) | 18 (12) | 22 (6) | 0.33 | .89 |
| SWS | 7 (8) | 6 (8) | 9 (5) | 12 (8) | 7 (6) | 10 (9) | 1.01 | .42 |
| REM | 1 (2) | 0 (0) | 0 (0) | 1 (2) | 1 (3) | 1 (2) | 0.75 | .59 |
Abbreviations: TST = total sleep time; SWS = slow wave sleep [NREM Stages 3 + 4]; REM = rapid eye movement sleep.
aData are presented as means (standard deviation). Statistics reported from mixed-effects models, as in the main article.
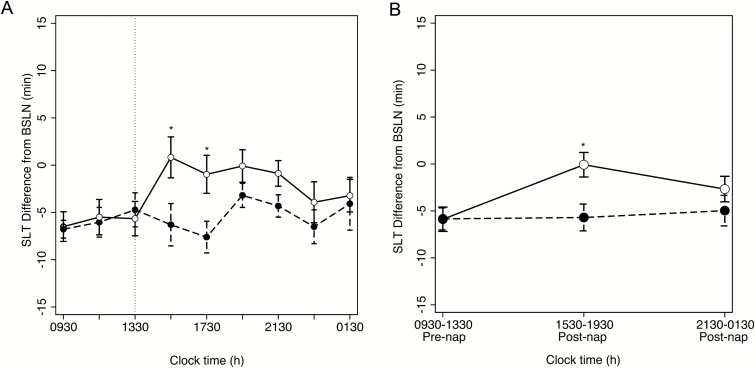
Figure 2 presents sleep latencies expressed as a difference from baseline rested conditions (see Supplementary Figure S1 for raw MSLT sleep latencies) for each group across the 6 days of sleep restriction, before and after the daytime nap opportunity in the Nap group. Figure 3A displays these same data averaged across restriction days. A clear benefit of the nap is seen in elongated sleep latencies in the Nap group, compared to the No Nap group, following the daytime nap opportunity.
Figure 3.
MSLT across hours of the day. MSLT-indexed sleep latencies averaged across protocol days for No Nap (black circles) and Nap (white circles) groups, respectively. (A) Sleep latencies plotted for each time of day as differences from baseline, as in Figure 2. Vertical lines indicate timing of afternoon nap. (B) Sleep latencies collapsed into three segments, reported as marginal means from mixed-effects analysis reported in the manuscript. Error bars indicate standard error of mean (SEM). *p < .05.
To investigate this effect in greater detail, the nine MSLT tests each day were collapsed into three equally spaced segments (Figure 3B): 0930–1330 h (including the sleep latency of the afternoon nap for Nap group participants), 1530–1930 h, and 2130–0130 h. A three-way, mixed-effects analysis identified a main effect of restriction day (F5, 85 = 12.02, p < .001, indicating that mean sleep latency decreased across the 6 days of sleep restriction irrespective of group; however, no interaction between restriction day and group was observed (F5, 85 = 1.67, p = .15).
While a main effect of time-of-day was identified (F2, 34 = 3.95, p = .029), a significant interaction with group (F2, 34 = 3.44, p < .044) indicated that the groups demonstrated differential diurnal patterns of sleepiness as a function of an afternoon nap opportunity (Figure 3B). To deconstruct this significant interaction, simple-effect analyses revealed that from 0930 h to 1330 h, prior to the nap, the two groups were statistically indistinguishable in alertness (F1, 39.38 ≈ 0, p = .98). On the other hand, the Nap group demonstrated statistically longer sleep latencies, compared to No Nap group in the 1530–1930 h segment immediately following the nap (F1, 39.38 = 8.28, p = .0064). This difference was not found late in the day where the groups did not differ statistically (F1, 39.38 = 1.39, p = .25). Thus, on an objective test of sleepiness, an afternoon nap rescued sleepiness in the Nap group to levels indistinguishable from those under rested conditions (t(8) = −0.06, p = .95). However, this benefit was short lived, present neither later in the day nor the following the morning.
Subjective Sleepiness (VAS)
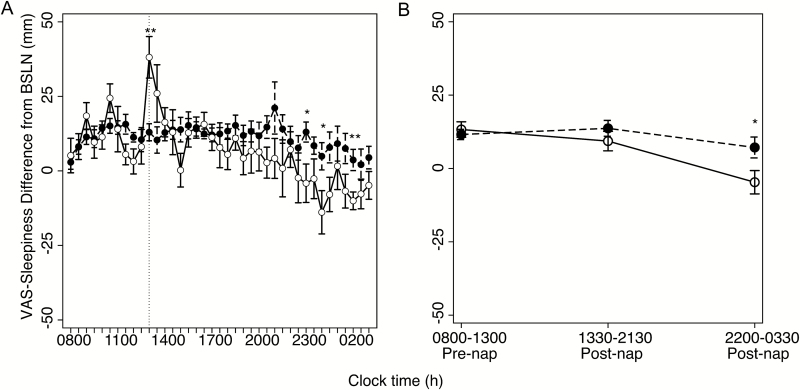
Figure 4 illustrates VAS-measured sleepiness, presented as a difference from baseline, for each group collapsed across the 6 days of sleep restriction. A clear divergence in subjective sleepiness can be observed late during the protocol day, with the Nap group perceiving a delayed rescue of alertness during the extended waking period.
Figure 4.
Subjective sleepiness across hours of the day. Visual analogue scale (VAS)-measured subjective sleepiness averaged across protocol days for No Nap (black circles) and Nap (white circles) groups, respectively. (A) Sleepiness plotted for each time of day as differences from baseline, as in Figures 2 and 3A. Vertical lines indicate timing of afternoon nap. (B) Sleepiness collapsed into three segments, as in Figure 3B, reported as marginal means from mixed-effects analyses reported in the manuscript. Error bars indicate standard error of mean (SEM). *p < .05.
To quantify this effect, the same procedures as with the MSLT were followed: the VAS scores were binned into three segments—prior to the nap (0800–1300 h), after the nap (1330–2130 h), and into the night (2200–0330 h)—and submitted to a homologous mixed-effects model. As with the MSLT data, a significant interaction was observed between time-of-day and group (F2, 34 = 3.73, p = .034) indicating that the afternoon nap differentially impacted subjective sleepiness across the day in the Nap group. Simple effect analyses revealed that unlike the MSLT result, the timing of diminished subjective sleepiness occurred at night, approximately 8–13.5 hours after the nap opportunity (F1, 41.63 = 7.48, p = .0091). Despite this delayed return of alertness to rested levels for the Nap group (t(8) = −1.18, p = .27), morning sleepiness was indistinguishable between the Nap and No Nap groups (F1, 41.63 = 0.17, p = .68).
DISCUSSION
Our primary finding shows a short-lived amelioration of sleepiness during chronic sleep restriction for objective and subjective sleepiness following an afternoon nap, with alertness levels returned to baseline levels within several hours of the nap. This return to baseline appeared at different rates for objective and subjective measures, with objective sleepiness returning to baseline levels sooner. Furthermore, no benefit for either measure of sleepiness was observed the next morning following additional exposure to sleep restriction.
Previous reports have focused on napping as an effective countermeasure to sleepiness induced by sleep deprivation and acute sleep restriction.11–17 The current study adds to this literature by demonstrating that these benefits are present and consistent following a daytime nap across 6 days of chronic sleep restriction. The daytime nap in this instance returned sleep onset latency to baseline levels for 6–8 hours after the nap and improved subjective sleepiness later in the evening. Importantly, however, neither objective nor subjective reductions in sleepiness were observed the next morning following a night of restricted sleep. Thus, while napping may temporarily “rescue” alertness, such recovery neither “pays forward” to later in the night or the next morning as long as sleep continues to be restricted.
Temporal Profile of Nap Benefits
A fundamental aim of the current study was to assess how long lasting any gains from a 45-minute daytime nap may be for objective measures of sleepiness and subjective alertness. In keeping with prior discrepancies between subjective and objective measures of alertness,6, 23 we observed different temporal profiles between the MSLT and the visual analogue scale of subjective sleepiness. Objective decreases in sleepiness, compared to the No Nap group, were observed 2 hours after the nap. Sleepiness returned to levels similar to those observed in the No Nap group after 6 hours.
Thus, by late in the evening Nap and No Nap patients were indistinguishable in physiological sleepiness. Subjective report of increased alertness following the nap, however, was only observable later in the evening, at a time when physiologically measured sleepiness was no longer different between the two groups. This disconnect between objective and subjective measures of sleepiness has been demonstrated previously6, 20, 23 and highlights the need to measure both aspects of sleepiness when assessing the benefits of napping strategies.
In considering the benefit of the nap, it is critical to acknowledge that the physiological benefits of recovery were observed immediately, yet participants did not perceive an immediate benefit. Moreover, participants overestimated their benefits later in the night. Because Nap group participants failed to perceive an early benefit, and erroneously perceived rescue later in the day, this study highlights a distinct window for sleepiness-related risk. Our findings speak to a potential danger in perceived recovery following a nap not matching actual alertness levels. Thus, the timing of safety-critical tasks to any prescribed nap in the context of short sleep schedules is of paramount importance when examining the effectiveness of the nap. Subjective perceptions of sleepiness are important for decisions regarding behavior (e.g., whether to drive, continue work, and so on). Unfortunately, performance measures such as the psychomotor vigilance task24 were not available in the current data set. However, work by Dinges and colleagues25 identified overlapping time courses of impairment between the psychomotor vigilance test and the MSLT. Thus, one may hypothesize that if performance measures were present in the current data, they would roughly follow the pattern observed in the MSLT. How the profiles of performance recovery and the MSLT compare in timing, however, poses an intriguing empirical question for future work.
Length of Nap Benefits
Regardless of the sleepiness measure and the time frame in which a return to baseline was observed, no benefit to sleepiness was observed the morning following a successive night of restricted sleep. Moreover, no systematic differences in macro-architecture were observed in nocturnal sleep throughout the protocol in the Nap group. Thus, it appears that the afternoon nap in the current study was unable to provide any measurable relief to homeostatic sleep pressure accrual throughout chronic sleep restriction and, therefore, no long-lasting benefit to alertness. One consideration in interpreting this lack of long-lasting remission in sleepiness is the length of the nap opportunity itself. In this study, participants slept for approximately 30 minutes each day in a 45-minute opportunity. This nap length appears to be too short to sustain long-term benefits or reduce overall sleepiness following a 5-hour restricted sleep opportunity. The nap in the current study was predominantly composed of NREM Stage 2 sleep, perhaps lacking the depth of slow-wave sleep needed to alter the accrual of sleep pressure across the protocol. Our results echo those of Lo et al.,20 in which a 1-hour nap opportunity across chronic sleep restriction days (5 hours’ TIB) bestowed a benefit on the psychomotor vigilance task up to only 5 hours after the nap opportunity. In the report by Lo, some neurocognitive measures showed a significant improvement relative to the No Nap group in the second period of sleep restriction, yet this was not consistent across neurocognitive measures, nor were these benefits observed during the first period of sleep restriction. Additionally, when comparing our results to those reported by Lo and colleagues, a number of methodological considerations must be accounted for. First, while Lo utilized the discrete Karolinska Sleepiness Scale,26 we used a continuous visual analogue instrument. Second, our participants were assessed for subjective sleepiness every half-hour while awake, while whole KSS scores were taken 3 times each day during Lo. Third, our study restricted sleep by delaying bedtime while Lo advanced waketime in addition to delaying bedtime; thus, differences in homeostatic load (time since waking) exist between the two protocols (exaggerated by the nap in Lo being scheduled for 1400 h, an hour later than in our protocol). Finally, the second period of restriction in Lo makes their design distinctly different from ours. Ultimately, while these factors make the two studies difficult to directly compare, they complement each other. When taken together they highlight the utility of a nap in combating sleep restriction the same day, while the same short (<1-hour) nap cannot reliably improve either performance or physiological sleepiness the following day.
A longer nap, perhaps more capable of sustained slow-wave sleep, or one placed later in the day, may be better able to sustain benefits the following morning. Our study’s nap opportunity occurred at 1300 h, 5 hours after rising, whereas Lo and colleagues20 provided a nap 6 hours after rising at 1400 h. A nap placed at 1500 h may be even more efficient in both its circadian timing and its extension of homeostatic load to adequately dissipate sleep pressure in a way that would persist to the following morning. However, a potential consequence of such a nap may be unforeseen changes in nocturnal sleep. In the current study, our early nap, containing little slow-wave sleep, did not impact nocturnal sleep. An additional concern with a longer nap opportunity may be the increased risk of sleep inertia. Since the physiological impact on sleepiness in the current study was short lived, the potential for it to be masked by sleep inertia may occlude any gains in alertness. Taken together, while a nap has a short-term benefit, understanding the dose dependency and temporal dynamics of these benefits will ultimately require future research combining the physiological measurement of sleepiness with enriched performance testing.
No Cumulative Effect Across 6 Days
As noted earlier, nocturnal sleep did not differ across the protocol in the Nap and No Nap groups, nor did we observe any changes across the protocol in the composition of the daytime nap itself, and the benefit of the nap for sleepiness did not change significantly across the protocol days. One might have expected a cumulative effect of nearly 30 minutes more sleep each day, just as deficits from short sleep accrue with time. While the formal test for an interaction between this group difference and time in the protocol was not significant, an intriguing trend, on inspection of the data, reveal that the groups begin to diverge on the sixth day following sleep restriction, with benefits of napping becoming more prominent. Whether this divergence results from a cumulative benefit beginning to manifest in the Nap group, or from the saturating impact of chronic sleep restriction in the No Nap group, is unclear. Longer chronic sleep restriction studies are needed to investigate the potential for daytime naps to provide a cumulative benefit under extended sleep restriction conditions. An alternative possibility is that the use of visual scoring techniques limit our ability to detect changes in sleep microstructure across the protocol. For example, minor dissipation of sleep homeostatic pressure by the afternoon nap may alter slow wave dynamics during the subsequent nocturnal sleep episodes. Unfortunately, in contrast to more recent investigations27 of the impact of naps on oscillatory activity during sleep, our paper polygraph records preclude the use of modern signal-processing techniques such as spectral analysis.
In summary, in the context of repeated nights of restricted sleep, early afternoon naps “rescue” objectively measured sleepiness for about 4–6 hours but provide no benefit late in the evening and the subsequent morning. In contrast, such naps are perceived by participants to benefit alertness late in the evening, after objective gains have already diminished. The mismatch of objective and perceived benefits to alertness causes concern for operational safety in cases where such restricted schedules are introduced in the real world, yet a 45-minute afternoon nap may appear an attractive countermeasure.
FUNDING
JMS was funded by T32MH019927 and K01MH109854; CJH was funded by an Australian Government Endeavour Research Fellowship. Research was supported by MH28461, NS13211, and MH05804 to WCD.
INSTITUTION WHERE WORK WAS PERFORMED
The data were collected at Stanford University, Palo Alto, California. The data were analyzed and the manuscript written at Brown University, Providence, Rhode Island.
CONFLICTS OF INTEREST
None disclosed.
Supplementary Material
ACKNOWLEDGMENTS
Drs. Saletin and Hilditch contributed to this work equally. The authors thank the participants who generously donated their time to this study. They additionally thank the student technicians who worked on the study as members of the Stanford Summer Sleep Camp Staff.
REFERENCES
- 1. Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. 2009; 169(9): 1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luyster FS, Strollo PJ Jr, Zee PC, Walsh JK; Boards of Directors of the American Academy of Sleep Medicine and the Sleep Research Society Sleep: a health imperative. Sleep. 2012; 35(6): 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012; 22(10): 939–943. [DOI] [PubMed] [Google Scholar]
- 4. Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981; 18(2): 107–113. [DOI] [PubMed] [Google Scholar]
- 5. Belenky G, Wesensten NJ, Thorne DR et al. . Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003; 12(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 6. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003; 26(2): 117–126. [DOI] [PubMed] [Google Scholar]
- 7. Dement WC, Carskadon M, Richardson G. Excessive daytime sleepiness in the sleep apnea syndrome. Sleep Apnea Syndromes. 1978: 23–46. [Google Scholar]
- 8. Dinges DF, Orne MT, Whitehouse WG, Orne EC. Temporal placement of a nap for alertness: contributions of circadian phase and prior wakefulness. Sleep. 1987; 10(4): 313–329. [PubMed] [Google Scholar]
- 9. Takahashi M, Fukuda H, Arito H. Brief naps during post-lunch rest: effects on alertness, performance, and autonomic balance. Eur J Appl Physiol Occup Physiol. 1998; 78(2): 93–98. [DOI] [PubMed] [Google Scholar]
- 10. Hayashi M, Fukushima H, Hori T. The effects of short daytime naps for five consecutive days. Sleep Research Online. 2003; 5(1): 13–7. [Google Scholar]
- 11. Mulrine HM, Signal TL, van den Berg MJ, Gander PH. Post-sleep inertia performance benefits of longer naps in simulated nightwork and extended operations. Chronobiol Int. 2012; 29(9): 1249–1257. [DOI] [PubMed] [Google Scholar]
- 12. Gillberg M, Kecklund G, Axelsson J, Akerstedt T. The effects of a short daytime nap after restricted night sleep. Sleep. 1996; 19(7): 570–575. [DOI] [PubMed] [Google Scholar]
- 13. Horne JA, Reyner LA. Counteracting driver sleepiness: effects of napping, caffeine, and placebo. Psychophysiology. 1996; 33(3): 306–309. [DOI] [PubMed] [Google Scholar]
- 14. Takahashi M, Arito H. Maintenance of alertness and performance by a brief nap after lunch under prior sleep deficit. Sleep. 2000; 23(6): 813–819. [PubMed] [Google Scholar]
- 15. Tietzel AJ, Lack LC. The short-term benefits of brief and long naps following nocturnal sleep restriction. Sleep. 2001; 24(3): 293–300. [DOI] [PubMed] [Google Scholar]
- 16. Tietzel AJ, Lack LC. The recuperative value of brief and ultra-brief naps on alertness and cognitive performance. J Sleep Res. 2002; 11(3): 213–218. [DOI] [PubMed] [Google Scholar]
- 17. Brooks A, Lack L. A brief afternoon nap following nocturnal sleep restriction: which nap duration is most recuperative? Sleep. 2006; 29(6): 831–840. [DOI] [PubMed] [Google Scholar]
- 18. Lumley M, Roehrs T, Zorick F, Lamphere J, Roth T. The alerting effects of naps in sleep-deprived subjects. Psychophysiology. 1986; 23(4): 403–408. [DOI] [PubMed] [Google Scholar]
- 19. Bonnet MH. The effect of varying prophylactic naps on performance, alertness and mood throughout a 52-hour continuous operation. Sleep. 1991; 14(4): 307–315. [DOI] [PubMed] [Google Scholar]
- 20. Lo J, Lee S, Teo L, Lim J, Gooley J, Chee M. Neurobehavioral impact of successive cycles of sleep restriction with and without naps in adolescents. Sleep. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carskadon M, Dement W. Effects of a daytime nap on sleepiness during sleep restriction. Sleep Res. 1986; 15:69. [Google Scholar]
- 22. Rechtschaffen A, Kales A.. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/Brain Research Institute, University of California; 1968. [Google Scholar]
- 23. Centofanti SA, Hilditch CJ, Dorrian J, Banks S. The impact of short night-time naps on performance, sleepiness and mood during a simulated night shift. Chronobiol Int. 2016; 33(6): 706–715. [DOI] [PubMed] [Google Scholar]
- 24. Dorrian J, Rogers NL, Dinges DF. Psychomotor vigilance performance: Neurocognitive assay sensitive to sleep loss. In: Kushida C, ed. Sleep deprivation: clinical issues, pharmacology and sleep loss effects. New York: Marcel Dekker; 2005: 39–70. [Google Scholar]
- 25. Dinges DF, Pack F, Williams K et al. . Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997; 20(4): 267–277. [PubMed] [Google Scholar]
- 26. Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990; 52(1–2): 29–37. [DOI] [PubMed] [Google Scholar]
- 27. Ong JL, Lo JC, Gooley JJ, Chee MWL. EEG changes accompanying successive cycles of sleep restriction with and without naps in adolescents. Sleep. 2017; 40(4). doi: 10.1093/sleep/zsx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.