Abstract
Many studies have suggested that inappropriate plasma usage is common. An important factor contributing to futile plasma administration in most patients is the nonlinear relationship between coagulation-factor levels and the volume of plasma transfused. In this review, a validated mathematical model and data from the literature will be used to illuminate 3 key properties of plasma transfusion. Those properties are as follows: the effect of plasma transfusion on international normalized ratio (INR) is transient; for the same volume of transfused plasma, a greater reduction in INR is observed at higher initial INRs; and the effect of plasma transfusion on INR correction (ie, the difference between initial and final INRs) diminishes as more plasma is transfused. Frequent misunderstanding of these properties may contribute to inappropriate plasma usage. Therefore, this review will assist physicians in navigating these common pitfalls. Stronger understanding of these principles may result in a reduction of inappropriate plasma transfusions, thus potentially enhancing patient safety and reducing healthcare costs.
Keywords: blood transfusion; guidelines; mathematical model; plasma usage, coagulopathy management, pre-operative management
The practice of blood transfusion is decreasing in the United States, largely as a result of successful blood usage initiatives (ie, patient blood management [PBM]). However, this practice remains a common therapeutic modality.1,2 To date, PBM programs have focused primarily on the reduction of red blood cell (RBC) transfusions.1 However, plasma transfusion has increased in recent years. Also, the findings from multiple recent studies3‐5 suggest that most of these plasma transfusions may be inappropriate and/or not indicated.
Currently, many physicians struggle with the management of preprocedural/preoperative, laboratory-defined coagulopathic manifestations (ie, the presence of a prolonged of prothrombin time [PT], international normalized ratio [INR], or activated partial thromboplastin time [APTT] in a stable patient who is not bleeding).5 The clinical benefit of using INR as an indicator of coagulopathic manifestations or, more specifically, of correcting an elevated INR preprocedurally is highly debatable. Nevertheless, transfusing plasma for this purpose is a common practice in both academic and community-based hospitals.6‐9 Given that the transfusion of plasma can present significant risks, under- and overdosing should be avoided.10,11
Many studies reported a high rate of inappropriate plasma transfusion in Canada, the United Kingdom, and the United States.3‐5 One plausible explanation is a lack of a clear understanding of the complex properties related to the transfusion of plasma (ie, the impermanent effect of plasma transfusion on coagulation-factor levels and the nonlinear relationship between INR reduction and the volume of plasma transfused [Figures 1 and 2]).12 To assist physicians in dosing plasma, 2 of us previously developed and reported a mathematical formula that reliably estimates INR after plasma transfusion (see Supplemental section [part A] for the equation and a brief summary of its derivation).12 This formula was successfully validated using selected data from a randomized clinical trial assessing plasma transfusion efficacy for critically ill patients in the Netherlands between May 2010 and June 2013.13
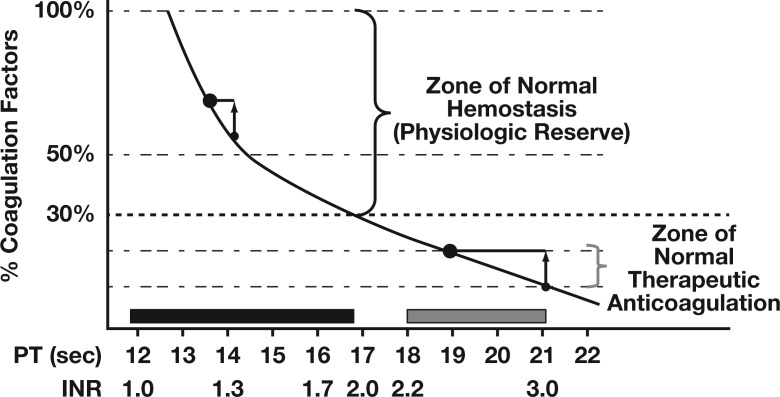
Figure 1.
The general nonlinear relationship between concentrations of coagulation factors and the prothrombin (PT)/international normalized ratio (INR). The local position of the curve will vary between laboratories due to the technical aspects of the assays. Adapted with permission from Callum JL and Dzik WH, The Use of blood components prior to invasive bedside procedures: A critical appraisal. In Mintz PD, ed. Transfusion Therapy: Clinical Principles and Practice. 3rd ed. Bethesda, MD: AABB; 2010.14
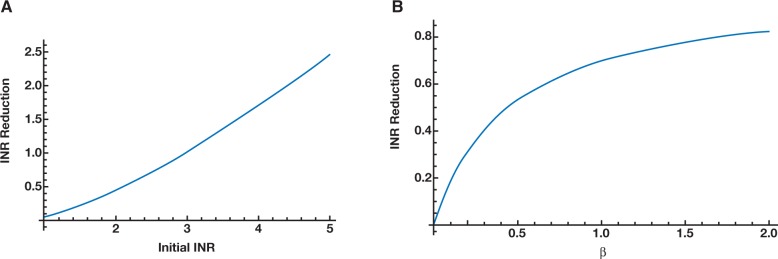
Figure 2.
Using mathematical formulae from Pham et al12 and transfusion data from Müller et al13 (at t = 0), ε as a function of INRinitial (using β [plasma volume transfused as a proportion of initial plasma volume for the patient] = 0.24) (A); ε as a function of β at INRinitial = 1.8 (B).
Physicians often encounter situations when coagulopathic reversal may be urgently needed. For that reason, the goal of this short review is to demonstrate 3 key properties of plasma transfusion using our validated mathematical formula and practical observations from the medical literature. A full understanding of these properties by physicians will enable more accurate predictions of the effect of plasma transfusion on achieving a targeted INR, thus reducing inappropriate plasma usage.
The Effect of Plasma Transfusion on INR is Transient
To investigate the long-term effect of plasma transfusion, we examined the steady-state limit (t → ∞) of our INR-estimation formula. By assuming no additional synthesis of coagulation factors, we mathematically demonstrated that in the long run, post-transfusion INR will ultimately exceed the initial INR. As a result, the beneficial effect of plasma transfusion on INR is inherently transient (see Supplemental section [part B]). This mathematical result is consistent with the findings reported by Spector and colleagues approximately 50 years ago.15 Those researchers showed that coagulation factor levels increased after plasma transfusion but that these changes were temporary: levels rapidly declined within 2 to 4 hours and returned to baseline between 6 and 24 hours after transfusion. The implications of this mathematical relationship on clinical practice are that the effect of plasma transfusion on INR is short-lived and therefore, frequent transfusions may be necessary to maintain adequate levels of coagulation factors. Further, if plasma is used to correct the INR preoperatively, transfusion should take place immediately before the procedure, to maximize the effect.
With the Same Plasma-Volume Transfusion, a Greater Reduction in INR is Observed at Higher Initial INRs
To investigate this property of our equation, we examined the effect of initial INR on the INR reduction (ε) while keeping the volume of plasma transfusion constant. Based on this assumption, we were able to prove mathematically that as the initial INR increases, the INR reduction also increases (see Supplemental section [part C] and Figure 2A). This mathematical result is consistent with clinical observations reported in the medical literature: the higher the initial INR, the more effective plasma transfusion is at reducing the INR.3,13,16 On a practical level, this result suggests that with an average recommended dose of plasma transfusion between 10 and 15 mL per kg,17 plasma transfusion should not be used as a therapeutic modality for an initial INR of less than 1.8 to 2.0. The reduction in such a case would be minimal, although the patient would be exposed to multiple units of plasma.10,11 Alternative therapies, such as prothrombin complex concentrates, may be considered after weighing the thrombotic risk versus the benefit of INR reduction; however, it should be noted that in the United States, prothrombin-complex concentrates are only licensed by the United States Food and Drug Administration (FDA) for use for the treatment of bleeding patients who have experienced warfarin overdose.18
Given an Initial INR, the Effect of Plasma Transfusion on INR Correction Diminishes as More Plasma is Transfused
To investigate this property, we examined the effect of the volume of plasma transfusion on the ε value while keeping the initial INR constant. Mathematically, we proved that as more plasma is transfused, the effect on INR reduction decreases (see Supplemental section [part D] and Figure 2B). This mathematical result is consistent with clinical observations, such as those by Triulzi et al5: the effect of additional plasma on INR reduction was minimal after more than 4 units of plasma were transfused. Further, as shown in Figure 2B, the steepest part of the INR response curve (ie, where plasma transfusion is most effective) is found for transfusion volumes that are 20% to 40% of the initial plasma volume for the patient (β ([plasma volume transfused as a proportion of initial plasma volume for the patient]) values between 0.2 and 0.4), which translates in practical terms to approximately 10 to 15 mL plasma transfusion per kg body weight. This finding is consistent with the recommended standard dose of plasma transfusion in the literature.17 Moreover, the effect of INR reduction is significantly reduced when β is greater than 0.5; thus, transfusing plasma at a dose higher than 20 mL per kg typically does not confer any clinically significant benefit.19 Such a course may unnecessarily place the patient at risk of developing transfusion-associated circulatory overload (TACO), in addition to other adverse events associated with blood transfusion.10,11
Discussion
In summary, our mathematical model agreed well with published experimental data3‐5,12,13,15,16,19 and demonstrates the usefulness of mathematical modeling in medicine.9,12,20‐22 A review of the 3 important properties of plasma transfusion discussed herein can provide a practical interpretation of these features from a clinical perspective. Specifically, these features suggest that if plasma is used to correct INR preoperatively, the maximum effect will be achieved by transfusing immediately before the procedure;15 therapeutic plasma transfusion should be avoided for initial INRs less than 1.8 to 2.0 due to minimal benefit;17 and plasma transfusions for the purpose of INR correction should ideally use the recommended dose of 10 to 15 mL per kg but should not exceed 20 mL per kg because transfusing higher plasma volumes confers no significant benefit.17 When appropriate usage of our mathematical model suggests that reaching a target INR by plasma transfusion is impractical for a patient (ie, in the setting of large plasma-transfusion volumes),12 a more cost effective therapy, such as prothrombin complex concentrates, should be considered.9
These principles, as supported by our mathematical model, provide further explanation for the numerous report findings that demonstrate the futility of plasma transfusion in the setting of laboratory-defined coagulopathic manifestations (ie, the presence of an elevated PT level, INR, and/or APTT level) in patients in stable health who are not bleeding. Nevertheless, these observations and reviews by themselves are likely insufficient to change clinical practice because beliefs regarding the benefit of using plasma in the management of preprocedural/preoperative, laboratory-defined coagulopathic manifestations in patients in stable health who are not bleeding are based in long-held assumptions and/or common clinical practice. However, Tavares et al23 outlined a successful model for the reduction of inappropriate plasma transfusions. The proposed model suggested that change should be initiated slowly, by first introducing/establishing evidence-based guidelines for how and/or when transfusion is appropriate and then encouraging adherence passively through education and dissemination of information. This period should be followed by slowly introducing escalating policy restrictions to ensure compliance and adherence.23 This model has been shown to be an effective means of physician education that has reduced unnecessary plasma transfusions and overall cost.23,24
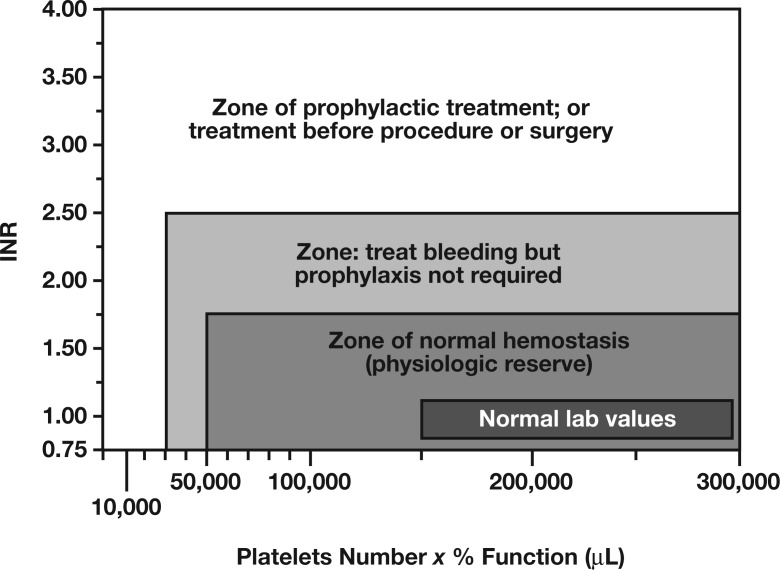
Although not covered in detail in this review, another key concept in the management of preprocedural/preoperative coagulopathic manifestations is the importance of platelets in the hemostatic process. For instance, the bleeding risk increases significantly when both thrombocytopenia and coagulopathic manifestations are present (Figure 3).14 Further, the hemostatic process is complex and depends on the interaction between multiple factors. Thus, an assessment for bleeding-risk stratification must take into account other variables in addition to coagulation-factors levels, as reflected by the PT/INR and/or APTT test results.
Figure 3.
Bleeding risk as a function of platelet count and amount of coagulation factors. Adapted with permission from Callum JL and Dzik WH, The use of blood components prior to invasive bedside procedures: A critical appraisal. In: Mintz PD, ed. Transfusion Therapy: Clinical Principles and Practice. 3rd ed. Bethesda, MD: AABB; 2010.14
Conclusions
Physicians often encounter situations when INR reversal may be urgently needed in patients who are bleeding and/or as a prophylaxis before invasive procedures. Plasma transfusion is only one of several therapeutic options and, in many situations, is not the ideal therapeutic modality for patient treatment. Understanding the key features of plasma transfusions, from the theoretical and practical perspectives, will enable healthcare professionals to more effectively stratify patients who might benefit from plasma usage, as opposed to those who might require alternative methods of coagulation-factor supplementation and/or simple monitoring. Doing so potentially enhances patient safety and reduces overall healthcare costs.
Supplemental material can be found at online www.labmedicine.com.
Supplementary Material
Glossary
Abbreviations
- PBM
patient blood management
- RBC
red blood cell
- PT
prothrombin time
- INR
international normalized ratio
- APTT
activated partial thromboplastin time
- FDA
United States Food and Drug Administration
- TACO
transfusion-associated circulatory overload
References
- 1. Chung KW, Basavaraju SV, Mu Y, et al. Declining blood collection and utilization in the United States. Transfusion. 2016;56:2184-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N.. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion. 2016;56:2173-2183. [DOI] [PubMed] [Google Scholar]
- 3. Stanworth SJ, Grant-Casey J, Lowe D, et al. The use of fresh-frozen plasma in England: high levels of inappropriate use in adults and children. Transfusion. 2011;51:62-70. [DOI] [PubMed] [Google Scholar]
- 4. Tinmouth A, Thompson T, Arnold DM, et al. Utilization of frozen plasma in Ontario: a provincewide audit reveals a high rate of inappropriate transfusions. Transfusion. 2013;53:2222-2229. [DOI] [PubMed] [Google Scholar]
- 5. Triulzi D, Gottschall J, Murphy E, et al. ; for the NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III). A multicenter study of plasma use in the United States. Transfusion. 2015;55:1313-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Townsend JC, Heard R, Powers ER, Reuben A.. Usefulness of international normalized ratio to predict bleeding complications in patients with end-stage liver disease who undergo cardiac catheterization. Am J Cardiol. 2012;110:1062-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Segal JB, Dzik WH, Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45:1413-1425. [DOI] [PubMed] [Google Scholar]
- 8. Yang L, Stanworth S, Hopewell S, Doree C, Murphy M.. Is fresh-frozen plasma clinically effective? An update of a systematic review of randomized controlled trials. Transfusion. 2012;52:1673-1686. [DOI] [PubMed] [Google Scholar]
- 9. Pham HP, Sireci AN, Kim CH, Schwartz J.. Cost-effectiveness analysis of plasma versus recombinant factor VIIa for placing intracranial pressure monitors in pretransplant patients with acute liver failure. Clin Appl Thromb Hemost. 2014;20:607-614. [DOI] [PubMed] [Google Scholar]
- 10. Harvey AR, Basavaraju SV, Chung KW, Kuehnert MJ.. Transfusion-related adverse reactions reported to the National Healthcare Safety Network Hemovigilance Module, United States, 2010 to 2012. Transfusion. 2015;55:709-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delaney M, Wendel S, Bercovitz RS, et al. ; for the Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Transfusion reactions: prevention, diagnosis, and treatment. Lancet. 2016;33810061:2825-2836. [DOI] [PubMed] [Google Scholar]
- 12. Pham HP, Müller MC, Williams LA III, Juffermans NP.. Mathematical model and calculation to predict the effect of prophylactic plasma transfusion on change in international normalized ratio in critically ill patients with coagulopathy. Transfusion. 2016;56:926-932. [DOI] [PubMed] [Google Scholar]
- 13. Müller MC, Arbous MS, Spoelstra-de Man AM, et al. Transfusion of fresh-frozen plasma in critically ill patients with a coagulopathy before invasive procedures: a randomized clinical trial (CME). Transfusion. 2015;55:26-35. [DOI] [PubMed] [Google Scholar]
- 14. Callum JL, Dzik WH.. The use of blood components prior to invasive bedside procedures: a critical appraisal In: Mintz PD, ed. Transfusion Therapy: Clinical Principles and Practice Bethesda. 3rd ed Bethesda, MD: AABB; 2010. [Google Scholar]
- 15. Spector I, Corn M, Ticktin HE.. Effect of plasma transfusions on the prothrombin time and clotting factors in liver disease. N Engl J Med. 1966;275:1032-1037. [DOI] [PubMed] [Google Scholar]
- 16. Holland LL, Brooks JP.. Toward rational fresh frozen plasma transfusion: The effect of plasma transfusion on coagulation test results. Am J Clin Pathol. 2006;126:133-139. [DOI] [PubMed] [Google Scholar]
- 17. Yazer MH. The how’s and why’s of evidence based plasma therapy. Korean J Hematol. 2010;45:152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarode R, Milling TJ Jr, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128:1234-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Szczepiorkowski ZM, Dunbar NM.. Transfusion guidelines: when to transfuse. Hematology Am Soc Hematol Educ Program. 2013;2013:638-644. [DOI] [PubMed] [Google Scholar]
- 20. Pham HP, Kim CH, Schwartz J.. Phenotypically matched vs. traditional screen method for preparing red blood cell units in patients with abnormal placentation: a decision analysis approach. Vox Sang. 2014;107:399-406. [DOI] [PubMed] [Google Scholar]
- 21. Okerberg CK, Williams LA III, Kilgore ML, et al. Cryoprecipitate AHF vs. fibrinogen concentrates for fibrinogen replacement in acquired bleeding patients—an economic evaluation. Vox Sang. 2016;111:292-298. [DOI] [PubMed] [Google Scholar]
- 22. Pham HP, Williams LA III. Plasma vs. cryoprecipitate for fibrinogen replacement in therapeutic plasma exchange procedures. J Clin Apher. 2015;30:382-383. [DOI] [PubMed] [Google Scholar]
- 23. Tavares M, DiQuattro P, Nolette N, Conti G, Sweeney J.. Reduction in plasma transfusion after enforcement of transfusion guidelines. Transfusion. 2011;51:754-761. [DOI] [PubMed] [Google Scholar]
- 24. Sarode R, Refaai MA, Matevosyan K, Burner JD, Hampton S, Rutherford C.. Prospective monitoring of plasma and platelet transfusions in a large teaching hospital results in significant cost reduction. Transfusion. 2010;50:487-492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.