Abstract
Macrophages are first-line responders against microbes. The success of Mycobacterium tuberculosis (Mtb) rests upon its ability to convert these antimicrobial cells into a permissive cellular niche. This is a remarkable accomplishment, as the antimicrobial arsenal of macrophages is extensive. Normally bacteria are delivered to an acidic, degradative lysosome through one of several trafficking pathways, including LC3-associated phagocytosis (LAP) and autophagy. Once phagocytozed, the bacilli are subjected to reactive oxygen and nitrogen species, and they induce the expression of proinflammatory cytokines, which serve to augment host responses. However, Mtb hijacks these host defense mechanisms, manipulating host cellular trafficking, innate immune responses, and cell death pathways to its benefit. The complex series of measures and countermeasures between host and pathogen ultimately determines the outcome of infection. In this review, we focus on the diverse effectors that Mtb uses in its multipronged effort to subvert the innate immune responses of macrophages. We highlight recent advances in understanding the molecular interface of the Mtb–macrophage interaction.
Keywords: tuberculosis, phagosome maturation, LC3-associated phagocytosis, autophagy, apoptosis, inflammasome
The authors focus on the diverse effectors that Mycobacterium tuberculosis uses in its multi-pronged effort to subvert the innate immune responses of macrophages.
INTRODUCTION
In 1882, Robert Koch announced that Mycobacterium tuberculosis (Mtb) causes tuberculosis (TB). In the same year, Metchnikoff made the surprising finding that host phagocytes ingest and kill bacteria. Thus, protagonist and antagonist were identified, and more than 100 years later, the story continues to unfold. Work from many investigators has provided a detailed, molecular understanding of the antimicrobial arsenal of Metchnikoff's phagocytes. How Mtb is able to subvert these host defenses remains an area of ongoing investigation. Here, we focus on the effectors that enable Mtb to impair macrophage function, thereby undermining the innate and adaptive immune response. It has been known for decades that one of the principle ways in which Mtb does this is by preventing its own delivery to the lysosome (Armstrong and Hart 1971). Recent studies have revealed two distinct lysosomal trafficking pathways that involve LC3-marked organelles (Huang and Brumell 2014; Martinez et al.2015). To prevent its degradation, Mtb must be able to undermine these distinct, lysosomal trafficking pathways. In addition, Mtb encounters many cellular and subcellular host environments that it has to navigate (Ehrt and Schnappinger 2009; Srivastava, Ernst and Desvignes 2014). Here, we focus our discussion on how Mtb directly manipulates host cellular trafficking, innate immune responses, and cell death pathways in macrophages. There is considerably less known about how Mtb directly manipulates other myeloid cells, such as dendritic cells and neutrophils; therefore, we concentrate on macrophages. There are already many excellent reviews on these topics, so we specifically emphasize the role of Mtb effectors and recent findings. Lastly, we have tried to assimilate many studies into a cohesive picture. In doing so, we have not been able to give all studies the attention they deserve, and we have glossed over details. We direct the interested reader to more comprehensive reviews and the primary literature.
FIRST ENCOUNTERS
Once inhaled, Mtb is taken up by alveolar macrophages. Macrophages are phenotypically diverse (Jakubzick, Randolph and Henson 2017), and alveolar macrophages, the first to encounter Mtb, are adapted to minimize lung injury. This may make alveolar macrophages particularly poorly equipped to control Mtb (Hussell and Bell 2014; Rajaram et al.2014). Moreover, mycobacteria selectively recruit permissive monocytes instead of more antimicrobial populations to the site of infection (Cambier et al.2014). Numerous phagocytic receptors can mediate uptake of Mtb into macrophages (Philips and Ernst 2012; Stamm, Collins and Shiloh 2015). The particular phagocytic receptors that Mtb engages may influence the subsequent ability of macrophages to control infection. In fact, shortly after D’Arcy Hart demonstrated that Mtb can evade lysosomal degradation (Armstrong and Hart 1971), he showed that antibody opsonized bacteria, which bind the Fc-γ receptor, traffic to the lysosome (Armstrong and Hart 1975). Conversely, mannose-capped lipoarabinomannan (ManLAM) in the mycobacterial envelop binds mannose receptor (MR), which can impair phagosome maturation (Kang et al.2005). Recent work demonstrates that human MR activates the protein tyrosine phosphatase SHP-1, which blocks phagosome maturation by limiting the activity of class III phosphatidylinositol 3-kinase (PI3K) on Mtb phagosomes (Rajaram et al.2017). Thus, even before entering the phagocyte, Mtb may set itself up for success by selectively parasitizing more permissive cells and interacting with cell surface receptors that promote its intracellular survival.
MTB’S INTRACELLULAR LIFESTYLE
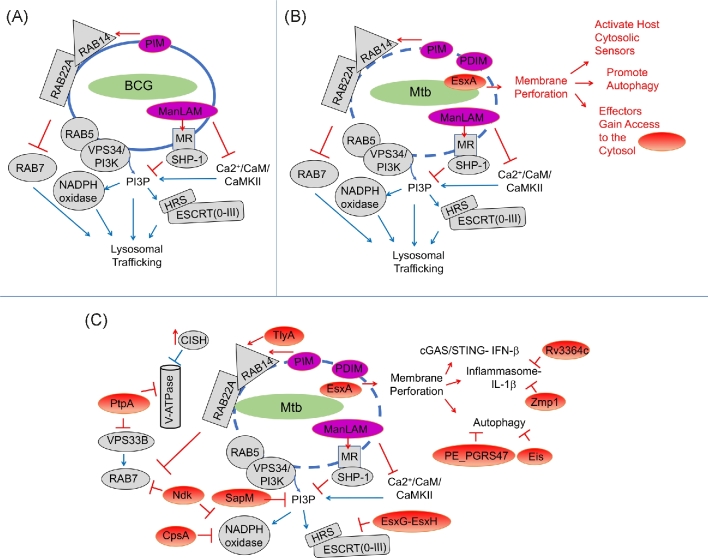
Normally after uptake, a phagosome immediately matures from a comparatively inert compartment to an antimicrobial phagolysosome (Flannagan, Cosío and Grinstein 2009). The phagosome acidifies, acquires lysosomal hydrolases and antimicrobial peptides, and restricts essential nutrients such as iron, while generating a toxic burst of zinc (Botella et al.2011). In addition, microorganisms activate pattern recognition receptors (PRRs), which augment the antimicrobial capacity of the phagosome by promoting NADPH oxidase recruitment (Nunes, Demaurex and Dinauer 2013). The NADPH oxidase generates reactive oxygen species (ROS) to kill microorganisms and promotes a distinct phagosome maturation pathway called LC3-associated phagocytosis (Martinez et al.2015). Mtb and related species such as M. marinum prevent these normal maturation events. Multiple effectors enable Mtb to modulate phagosome maturation, undermining the normal antimicrobial capacity of lysosomal trafficking. The mycobacterial vacuole is characterized by the prolonged retention of early endosomal markers that are normally cleared from the maturing phagosomal surface, while the NADPH oxidase and numerous late endosomal and lysosomal markers are diminished or absent, including the vacuolar-H(+ )-ATPase (V-ATPase) (Sturgill-Koszycki et al.1994) (Fig. 1). This early endosome-like arrest allows the bacteria to acquire iron via recycling endosomes, while avoiding the degradative environment of the lysosome.
Figure 1.
Mtb effectors manipulate host lysosomal trafficking. (A) Mtb and BCG impair lysosomal trafficking. Complex glycolipids in the mycobacterial cell wall such as PIM and ManLAM can impair acquisition of PI3P and alter RAB protein dynamics. Early endosomal RAB proteins (RAB5, RAB22A, RAB14) are retained, and recruitment of activated RAB7 is impaired. (B) Mtb permeabilizes the phagosome, which depends upon EsxA and PDIM. This promotes host defense by activating cytosolic sensors and autophagy, and also allows bacterial effectors to gain access to the cytosol. (C) Once bacterial effectors enter the cytoplasm, they further inhibit host pathways. SapM hydrolyzes PI3P, and PtpA, NdkA, CpsA and EsxG-EsxH target proteins involved in lysosomal trafficking, including the V-ATPase, VPS33B, RAB7, NADPH oxidase and HRS/ESCRT. Addition effectors manipulate inflammasome signaling and IL-1β production. Host proteins are shown in grey, whereas bacterial protein effectors are in red and bacterial lipids are magenta. The action (activating or inhibitory arrow) of the bacterial or host factor is also shown in red or blue, respectively.
MTB PERFORATES THE PHAGOSOME
One of the most critical determinants of bacterial fate in the macrophage is the Mtb ESX-1 type VII secretion system (recently reviewed in Wong 2017). Disruption of this secretion system in the vaccine strain BCG is the major reason that BCG is attenuated. ESX-1 secretes a number of effectors, including a heterodimer composed of EsxA/ESAT-6 and EsxB/CFP-10 (Champion et al.2006). EsxA is implicated in rupturing the phagosome. Whether the EsxA protein itself has direct membranolytic activity is controversial (de Jonge et al.2007; Conrad et al.2017), but numerous studies demonstrate that mycobacterial mutants defective in esxA do not perforate the phagosome, and they have less membrane lytic activity (Hsu et al.2003; Gao et al.2004; van der Wel et al.2007; Simeone et al.2012; Watson, Manzanillo and Cox 2012) (Fig. 1B). The nature of the membranolytic activity is not entirely clear, but it appears that mycobacteria cause gross membrane disruption in a contact-dependent manner (Myrvik, Leake and Wright 1984; Conrad et al.2017; Schnettger et al.2017). Recent work shows that the mycobacterial lipid phthicerol dimycocerosate (PDIM) works in concert with EsxA in mediating phagosomal damage (Augenstreich et al.2017; Barczak et al.2017; Quigley et al.2017). Like mutants defective in esxA, PDIM mutants are attenuated, and they fail to promote host cell death or induce type I interferon, both of which are correlated with phagosomal damage. It is possible that additional bacterial factors also promote phagosomal damage, such as the sphingomyelinase SpmT/Rv0888, which, like ESX-1, contributes to the hemolytic activity of Mtb (Speer et al.2015).
Damage to the Mtb phagosome is central to the pathogens' success and also provokes a host response. ESX-1-dependent membrane damage happens early after bacterial uptake, and host cytosolic sensors detect bacterial products and respond within the first hours of infection (Stanley et al.2007; Wong and Jacobs 2011; Manzanillo et al.2012; Subbian et al.2013; Collins et al.2015; Simeone et al.2015; Wassermann et al.2015; Watson et al.2015). Presumably the ability of Mtb to perforate the phagosome is so critical to its success because that is how Mtb delivers the majority of its effectors to the cytosol (Fig. 1C). For example, recent work showed that the TB necrotizing toxin, TNT, is trapped in Δesx-1 mutant phagosomes, whereas it gains access to the cytoplasm during wild-type infection. Once in the cytosol, TNT can hydrolyze host nicotinamide adenine dinucleotide (NAD+) and causes necrosis (Sun et al.2015). Thus, the role of EsxA in host cell death or other cellular phenotypes might be quite indirect, reflecting a failure to deliver effectors to the cytoplasm or a secondary consequence of endomembrane damage. Phagosomal damage may also provide access to important nutrients to the bacilli, and over time, increasing membrane damage eventually allows bacilli translocate to the cytosol (van der Wel et al.2007; Houben et al.2012; Simeone et al.2012). What determines whether the bacteria lightly perforate the phagosome to ‘access’ the cytosol versus fully rupture the organelle and translocate into the cytosol is unclear. The degree of damage depends not only upon the bacterial strain, but also host cell type. Thus, host determinants that influence phagosome acidification, NRAMP (natural resistance associated macrophage proteins) function, and phospholipase A2 activity might influence the degree of membrane damage (Simeone et al.2015; Jamwal et al.2016). Interestingly, recent work suggests that host RAB20, by promoting formation of a spacious vacuole, antagonizes ESX-1 mediated-membrane damage (Schnettger et al.2017). While the nature of the damage itself and the host factors that modulate it are not well elucidated, what is clear is that the consequences of perforating the phagosome are dramatic: bacterial effectors are set loose, and the host responds to the damage by altering cellular trafficking and activating inflammatory signaling. Bacterial effectors undoubtedly modulate this ensuing host response. Thus, a complex series of measures and countermeasures are set in motion that will ultimately determine the outcome of infection.
MTB BLOCKS PHAGOSOME MATURATION
Mtb has a multipronged approach to subvert lysosomal trafficking pathways, undermining the functions of RAB proteins, PI3K, the vacuolar-H(+ )-ATPase (V-ATPase) and the NADPH oxidase (Fig. 1). Small GTP-binding RAB proteins control vesicular transport between organelles (Prashar et al.2017), with RAB5 and RAB7 serving as master regulators of transport along the endocytic and phagocytic pathways. Normally, nascent phagosomes recruit RAB5, which is subsequently replaced by late endocytic RAB7 during the maturation process (an event termed Rab conversion) (Rink et al.2005). RAB7 recruits downstream effectors to confer fusion competence with lysosomes. In contrast, the mycobacterial phagosome is characterized by altered RAB dynamics (Via et al.1997; Fratti et al.2001; Vergne et al.2004; Seto, Tsujimura and Koide 2011).
At least two bacterial effectors interfere with RAB conversion. Nucleoside diphosphate kinase (Ndk) possesses GTPase activating protein (GAP) activity toward RAB5 and RAB7 in vitro (Sun et al.2010). In addition, the Mtb secreted tyrosine phosphatase PtpA dephosphorylates Vps33B (Bach et al.2008). Based upon its homology to yeast Vps33p, VPS33B was initially thought to be a part of the endosomal tethering complexes, CORVET/HOPS (class C core vacuole/endosome tethering and homotypic fusion and protein sorting). These complexes are recruited to RAB5 and RAB7 containing membranes, where they function in endosome-endosome and endosome-lysosome fusion, respectively (Balderhaar and Ungermann 2013). In mammals there are two VPS33 proteins, and VPS33B may not be a member of CORVET/HOPS, but rather a mammalian-specific endosomal-targeting complex involved in lysosomal trafficking (van der Kant et al.2015). Remarkably, when absorbed on latex beads, both recombinant NdkA and PtpA were shown to impair lysosomal trafficking of the beads (Bach et al.2008; Sun et al.2010). This is hard to reconcile with the cytosolic location of their targets. In the case of PtpA, when adsorbed on beads, the recombinant protein was able to enter the cytosol, although it is not yet clear how this happens. For NdkA, the protein is required for intracellular survival of BCG, suggesting that either NdkA acts inside the phagosome or it also has some way to access the cytosol in the absence of ESX-1.
Mtb manipulates RAB proteins so that the mycobacterial phagosome maintains its interaction with early endosomes. For example, phagosomes containing live BCG retain RAB14 and RAB22A, which are cleared from phagosomes that contain heat killed BCG. The retention of RAB14 and RAB22A may be important in allowing the mycobacterial phagosome to maintain its early endosome-like state (Kyei et al.2006; Roberts et al.2006; Seto, Tsujimura and Koide 2011). Since these studies were done with BCG, which does not perforate the phagosome, the observed alterations in RAB trafficking do not depend upon delivery of ESX-1-delivered cytosolic effectors. Perhaps select proteins are still able to access the cytosol in BCG infections, which is also suggested by the PtpA and NdkA experiments mentioned above. In addition, the secreted protein TlyA, which may have membrane destabilizing activity of its own, has been shown to recruit RAB14 (Mittal et al.2014). Moreover, mycobacterial cell envelope glycolipids intercalate into host membranes (Beatty et al.2000), where they modulate trafficking irrespective of ESX-1 or phagosomal damage. For example, the mycobacterial cell envelope glycolipid phosphatidylinositol mannoside (PIM) has been shown to promote phagosome-early endosome fusion by altering RAB programing (Vergne et al.2004). At the same time, ManLAM can disrupt a calcium-dependent calmodulin signaling pathway, thereby impairing phosphatidylinositol 3-phosphate (PI3P) production and phagosome maturation (Fratti et al.2003). In fact, ManLAM coated beads were shown to preferentially retain RAB14 (Shui et al.2011). Thus, ESX-1-independent effectors, such as PIM and ManLAM, likely work in concert with cytosolic effectors during Mtb infection.
The class III PI3K VPS34 also plays an essential role in phagosome maturation. VPS34 generates PI3P on early endosomes, which is critical in recruiting early endosomal proteins involved in protein sorting, membrane trafficking and multiprotein complex assembly. PI3P is also important in autophagy initiation, as discussed below. The mycobacterial phagosome accumulates less PI3P than phagosomes containing latex beads or dead mycobacteria (Chua and Deretic 2004; Vieira et al.2004; Purdy et al.2005; Vergne et al.2005). In addition to ManLAM, discussed above, which can impair PI3P production, secreted SapM is thought to remove it, by acting as a PI3P phosphatase (Vergne et al.2005). Mtb requires SapM to arrest phagosome maturation. Consistent with the idea that SapM acts in the cytosol, when it is deleted in BCG, there is no effect (Festjens et al.2011; Saikolappan et al.2012; Puri, Reddy and Tyagi 2013).
One important PI3P binding protein is hepatocyte growth factor-regulated tyrosine kinase substrate (HGS/HRS). Normally, HRS is localized to early endosomes, where it recruits the endosomal sorting complex required for transport (ESCRT), which directs receptors to multivesicular bodies for lysosomal degradation. Mtb phagosomes do not efficiently recruit HRS. The failure to recruit HRS is not explained just by the diminished PI3P found on Mtb phagosomes (Mittal, unpublished); Mtb takes additional measures to impair ESCRT. We found that the Mtb secreted effector EsxH binds HRS and inhibits ESCRT activity. EsxH is secreted as a heterodimer with EsxG by the ESX-3 type VII secretion system. We found that EsxG-EsxH is critical for virulence, and it impairs phagosome maturation and inhibits MHC II antigen presentation likely by targeting ESCRT (Mehra et al.2013; Portal-Celhay et al.2016; Tufariello et al.2016). Given the variety of cell biological processes that ESCRT is involved in, including exosome secretion (Hanson and Cashikar 2012), it seems likely that EsxG-EsxH also perturbs additional cellular functions to promote virulence.
Recent work shows that Mtb disrupts the vacuolar-H(+)-ATPase (V-ATPase) both directly and by co-opting a host degradative pathway. In the later mechanism, Mtb infection induces the expression of host cytokine-induced SRC homology 2 (SH2) domain protein (CISH), a member of the suppressor of cytokine signaling (SOCS) family. CISH localizes to Mtb phagosomes and selectively targets subunit A of the V-ATPase for ubiquitination and degradation (Queval et al.2017). In addition, PtpA binds subunit H of the V-ATPase. By directly binding both VPS33B, as discussed above, and the V-ATPase, PtpA may prevent delivery of V-ATPase-containing vesicles to Mtb phagosomes (Wong et al.2011, 2018).
The NADPH oxidase generates ROS that have important antimicrobial activities, but also can be toxic to the host. Therefore, this multiprotein complex is selectively targeted to phagosomes containing microorganisms and its activity is tightly regulated. NADPH oxidase is composed of two integral membrane subunits that form flavocytochrome b558 and a trimeric cytosolic complex (composed of p40phox, p47phox and p67phox). PRRs promote recruitment of flavocytochrome b558 to the maturing phagosome from recycling endosomes (Casbon et al.2009). The cytosolic subunits subsequently assemble with the membrane components, and the catalytic activity of the assembled complex depends upon recruitment of GTP-bound RAC1/2 (Nunes, Demaurex and Dinauer 2013). Mtb appears to have taken advantage of the multiple levels of NADPH oxidase regulation to undermine each step. We recently found that the Mtb protein CpsA, a LytR-CpsA-Psr (LCP) domain-containing protein, inhibits recruitment of the flavocytochrome b558 complex and downstream cytosolic components to mycobacterial phagosomes (Köster et al.2017). The p40phoxsubunit of the cytosolic complex binds PI3P through its PX domains (Ellson et al.2001; Kanai et al.2001). Therefore, SapM and ManLAM, by blocking PI3P, would also be predicted to impair recruitment of the trimeric cytosolic complex, although this requires experimental validation. Lastly, in addition to acting on RAB5 and RAB7 as discussed above, NdkA can serves as GAP to inactivate RAC1, thereby disrupting the final activation step required for ROS (Sun et al.2013). Thus, the concerted activity of at least four Mtb effectors likely undermines the highly regulated trafficking, assembly and activation of NADPH oxidase. In addition, Mtb express ROS detoxifying enzymes such as superoxide dismutase (SodA) and the catalase KatG. Mtb's successful evasion of the NADPH oxidase may explain why NADPH oxidase plays a limited role in host defense against Mtb, at least in mice (Ng et al.2004). In people, mutations in NADPH oxidase cause chronic granulomatous disease, which results in increased susceptibility to mycobacterial diseases, including Mtb (Deffert, Cachat and Krause 2014). Therefore, even though Mtb has enzymes that detoxify ROS as well as multiple strategies to inhibit the NADPH oxidase, it must do so imperfectly. Thus, the battle over ROS appears to be at the crux of a fragile detente.
Recent work has shown that in addition to direct antimicrobial activity, the NADPH oxidase plays an important role in lysosomal trafficking by promoting a non-canonical LC3 trafficking pathway called LC3-associated phagocytosis (LAP). This pathway is different than autophagy (discussed below). While both use a common set of autophagy-related (ATG) proteins and result in LC3 deposition into the vacuolar membrane, LAP involves a single-membrane organelle and depends upon the NADPH oxidase (Mehta et al.2014; Martinez et al.2015), whereas canonical autophagy generates a double membrane compartment and does not depend upon NADPH oxidase. We showed that CpsA, by interrupting the recruitment of NADPH oxidase on mycobacteria containing phagosome, blocks lysosomal delivery by the LAP pathway (Köster et al.2017, 2018). It is likely that other molecules also block LAP. We speculate that NdkA might block LAP by interfering with NADPH oxidase assembly and activation, as discussed above, and Eis and NuoG, discussed below, may also block LAP by interfering with host ROS (Kim et al.2012; Gengenbacher et al.2016), although the role of these effectors in LAP has not yet been experimentally tested.
There are numerous other Mtb proteins that have been implicated as effectors that block phagosome maturation, notably PknG (Walburger et al.2004) and LpdC (Deghmane et al.2007). It is possible that in some of these cases, their role in virulence is related to an intrinsic function in bacterial metabolism (Venugopal et al.2011; Khan et al.2017; Rieck et al.2017). Bacterial mutants that cannot survive in the intracellular environment will not be able to maintain their intracellular niche and will secondarily be delivered to a lysosome. This has made it hard to distinguish proteins that have a direct role in disrupting lysosomal trafficking from those that play some other role in intracellular survival; it is quite possible that in some cases, one protein does both. This probably reflects the manner in which Mtb has had to engineer virulence factors given a lack of horizontal gene transfer. It appears to be a recurring theme that Mtb virulence factors have evolved from proteins that play a basic role in bacterial physiology in non-pathogenic, environmental mycobacteria. A prominent example is EsxA-EsxB, which plays a role in conjugation in non-pathogenic mycobacteria (Flint et al.2004). Similarly, while EsxG-EsxH is required for iron uptake in both M. smegmatis and M. tuberculosis, only the Mtb protein has the ability to interact with HRS and inhibit ESCRT (Mehra et al.2013). Accordingly, in vivo EsxG-EsxH plays a critical role in virulence that cannot be provided by the M. smegmatis protein and which is separable from its function in metal homeostasis (Portal-Celhay et al.2016; Tufariello et al.2016). In the case of CpsA, it belongs to a protein family involved in cell envelope homeostasis, but CpsA itself is found in pathogenic mycobacteria and appears to play a distinct role in virulence (Köster et al.2017). Thus, Mtb effectors may have multiple functions, which can be difficult to unravel. One way to distinguish whether the primary defect of a mutant is in arresting phagosome maturation is to determine whether the intracellular growth defect can be rescued by experimentally blocking phagosome maturation (for example, by silencing Rab7).
It is also worth keeping in mind that mutations that alter the mycobacterial envelop may also indirectly affect trafficking by altering bacterial interactions with pathogen recognition receptors such as MR, Toll-like receptors and C-type lectin receptors, which influence subsequent trafficking (Stamm, Collins and Shiloh 2015). This may explain some of the differences seen when heat killed bacilli have been used as a comparator to live mycobacteria, as heat killed Mtb might drive different trafficking pathways, such as LAP (see above), by virtue of exposing pathogen-associated molecular patterns (PAMPs) that are normally masked by the mycobacterial envelop. In addition, several Mtb effectors seem to only work in human cells, which has contributed to contradictory findings (Grundner, Cox and Alber 2008; Rajaram et al.2017). Thus, Mtb uses multiple, overlapping, partially redundant mechanisms to impair phagosome maturation. While we have highlighted a handful of the Mtb effectors that are likely to do so, there are undoubtedly many more and how they work in concert to promote the intracellular niche of Mtb remains to be elucidated.
MTB EVADES AUTOPHAGY
Perforating the phagosome is essential for Mtb virulence, but it comes with significant consequences, including activating autophagy, another degradative pathway that the bacilli have to evade to survive intracellularly. When Mtb gains access to host cytosol, it activates a cytosolic surveillance pathway, which results in induction of Type1 interferon and autophagy (Watson, Manzanillo and Cox 2012; Collins et al.2015; Wassermann et al.2015; Watson et al.2015). Autophagy is a homeostatic process that generates double membrane compartments to turn over cytoplasmic contents, clearing damaged organelles and protein aggregates in the lysosome; it can also be activated to clear invading microorganisms. When the double membrane autophagosome sequesters a microbe, the process is called xenophagy (recently reviewed Mitchell and Isberg 2017). Damaged phagosomes expose host glycans located in phagosomal lumen to the cytosol, which are detected by cytosolic galectins (Thurston et al.2012; Chauhan et al.2016). At the same time, bacterial DNA is detected in the cytosol and activates the cGAS-STING pathway (Watson, Manzanillo and Cox 2012; Collins et al.2015; Wassermann et al.2015; Watson et al.2015). The bacteria that are marked for destruction recruit E3 ligases, Parkin, SMURF1 and TRIM16 (Manzanillo et al.2013; Franco et al.2017; Kumar et al.2017). Galectins and ubiquitin serve to recruit selective autophagy adaptors, such as NBR1, NDP52 and p62, which in turn recruit LC3. ATG proteins coordinate the formation of a double membrane compartment, the autophagosome, which fuses with the lysosome. Thus, by damaging the phagosome, Mtb makes itself vulnerable to a distinct degradative, lysosomal pathway.
However, under basal conditions in vitro, LC3 trafficking makes a very limited contribution to clearing Mtb (Gutierrez et al.2004; Watson, Manzanillo and Cox 2012; Sakowski et al.2015), and in mice, it fails to control bacterial replication (Kimmey et al.2015). The limited efficacy of xenophagy in clearing Mtb points to evasion mechanisms on the part of the bacilli. Although our understanding is incomplete, it appears that Mtb impairs both autophagy initiation and the ability of autophagosomes to fuse with lysosomes (referred to as autophagy flux). Since autophagosome-lysosome fusion depends upon some of the same machinery as late endosome-lysosome fusion, such as Rab7, certain Mtb effectors should be able to block both. In fact, careful analysis of Mtb xenophagy showed that virulent Mtb impairs autophagy flux in macrophages and dendritic cells (Romagnoli et al.2012; Chandra et al.2015). The ability of Mtb to block autophagy flux depends upon ESX-1, suggesting that cytosolic effectors are responsible. In addition, as is seen during phagosome-lysosome arrest imposed by Mtb, the block to autophagosome-lysosome fusion occurs at the level of Rab7 (Chandra et al.2015), consistent with the idea that there are common effectors undermining both.
Mtb also seems to impair autophagy initiation, since the majority of invading bacilli do not recruit LC3. How exactly they do this is not completely clear. One strategy exploited by Mtb is to modulate the expression of ATG genes. For example, we found that Mtb induces miR-33, which inhibits autophagy, lysosomal function and host lipid metabolism, thereby facilitating intracellular bacterial survival (Ouimet et al.2016). In addition, Mtb upregulates expression of miR-155 in dendritic cells, which dramatically reduces ATG3 protein levels and autophagosome formation (Etna et al.2018). Since autophagy initiation depends upon PI3P, the effectors discussed above that impair PI3P accumulation would also be predicted to impair autophagy. Consistent with this idea, ManLAM coated beads block LC3 trafficking (Shui et al.2011). In addition, there are two Mtb secreted proteins that are implicated as more direct anti-autophagy effectors, PE_PGRS47 and enhanced intracellular survival (Eis). PE_PGRS47 was identified in a genome wide screen for loci that modulate antigen presentation. Subsequent analysis revealed that Mtb mutants lacking PE_PGRS47 have a reduced capacity to inhibit autophagy (Saini et al.2016). Eis has an N-acetyltransferase activity that has been shown to modulate macrophage autophagy (Shin et al.2010), as well as host cell death pathways as discussed below. Among other targets, Eis can acetylate DUSP16/MKP-7, a JNK-specific phosphatase (Kim et al.2012). JNK is required for activation of Beclin1 (BECN1), so Eis may suppress autophagy initiation by blocking BECN1 activation. It has also been suggested that Eis blocks autophagy by upregulating IL-10, a cytokine previously shown to block autophagy (Harris et al.2007; Duan et al.2016). Thus, several potential players have been identified, but we lack a clear understanding of how Mtb directly manipulate autophagy; the mechanism of action of PE_PGRS47 may shed further light on this matter. LC3 is routinely used as a marker of autophagy, but LC3 is conjugated to phagosomes during LAP, and autophagy and LAP require overlapping cellular machinery; therefore, it will be important for future studies to distinguish these related processes.
MTB MANIPULATES THE INFLAMMASOME
Interleukin-1β (Il-1β) and interleukin 18 (IL-18) are important cytokines for host immune defense against Mtb (McElvania Tekippe et al.2010; Mayer-Barber et al.2010, 2011). They also have to be appropriately regulated to prevent overly exuberant inflammation and tissue damage (Hernandez-Cuellar et al.2012; Mishra et al.2013). Production of these cytokines depends upon the inflammasome. The inflammasome is a multimeric complex that detects pathogens and activates an inflammatory cascade, producing mature IL-1β and IL-18 and triggering a distinct cell death pathway termed pyroptosis. The inflammasome is composed of NOD-like receptors (NLRs), apoptosis-associated speck-like protein containing a caspase recruiting domain (ASC/PYCARD) and the caspase-1 protease. NLRs are intracellular cytosolic PRRs that recognize PAMPs and host-derived danger-associated molecular patterns. ASC/PYCARD acts as a scaffold to bridge the NLR with caspase-1, which proteolytically processes the pro-Il1-β and pro-IL-18 into their mature forms. There are many NLRs that are activated by different bacterial products, including the NLRP3-inflammasome, which is activated by a variety of cellular insults, and the AIM2-inflammasome, which detects cytosolic DNA. Only the NLRP3-inflammasome responds to Mtb in macrophages. Membrane damage alone is sufficient to trigger the NLRP3-inflammasome, and in the case of Mtb infection, IL-1β production depends upon ESX-1, so this is another consequence of ESX-1 that the bacilli need to manage (Carlsson et al.2010; McElvania Tekippe et al.2010; Mayer-Barber et al.2010, 2011; Mishra et al.2010; Wong and Jacobs 2011; Dorhoi et al.2012).
AIM2 detects cytosolic DNA. It is surprising that the AIM2 inflammasome does not respond to Mtb because Mtb DNA is able to trigger the cGAS-STING pathway, as discussed above. This suggests that there are Mtb effectors that block the AIM2 inflammasome. Consistent with this idea, Mtb was shown to inhibit the AIM2 inflammasome in a manner that depended upon the ESX-1 system (Briken, Ahlbrand and Shah 2013; Shah et al.2013); the effector and target remain to be identified. One bacterial factor that has been implicated in modulating the inflammasome is the Zn2+ metalloprotease Zmp1 (Master et al.2008), although there are also conflicting findings (Wong and Jacobs 2011). Rv3364c may also impair IL-1β production. This protein translocates to the macrophage cytosol and inhibits caspase-1, possibly through cathepsin G inactivation (Danelishvili et al.2011). Taken together, it seems likely that by damaging the phagosome, Mtb triggers the inflammasome, an innate defense mechanism to restrict growth of invading pathogens. However, it only provides partial protection against Mtb, which likely fine-tunes the system to its advantage.
MTB IMPAIRS APOPTOSIS
There is considerable complexity and cross talk between cell death pathways during Mtb infection. One idea that has emerged is that apoptosis is host-protective, while necrosis favors the bacilli (Molloy, Laochumroonvorapong and Kaplan 1994; Balcewicz-Sablinska et al.1998; Oddo et al.1998; Keane, Remold and Kornfeld 2000; Keane, Shurtleff and Kornfeld 2002; Sly et al.2003). Necrosis depends upon the ESX-1 secretion system and the NAD+ glycohydrolase TNT, which induces necrotic cell death by depleting NAD+ (Sun et al.2015). Mtb also potentiates necrosis by causing mitochondrial inner membrane disruption (Chen, Gan and Remold 2006) and inhibiting plasma membrane repair (Divangahi et al.2009). Unlike necrosis, apoptosis minimizes inflammation, and Mtb-infected macrophages that undergo apoptosis can be rapidly ingested by neighboring uninfected macrophages through a process called efferocytosis (Martin et al.2012). Mtb released during necrosis can infect and proliferate in a subsequent round of macrophage infections. In contrast, when a macrophage ingests apoptotic bodies harboring Mtb bacilli, the bacilli cannot manipulate macrophage trafficking, and they are delivered to the lysosome, leading to bacterial control. In addition, efferocytosis by dendritic cells promotes priming and cross presentation (Schaible et al.2003; Winau et al.2006; Divangahi et al.2010; Blomgran et al.2012). Thus, overall, an anti-apoptotic, pro-necrotic program appears to favor the bacilli. However, the cell death pathways that are elicited and the consequences are likely to be context specific, and Mtb may modulate these pathways to its advantage (Moraco and Kornfeld 2014).
One way in which Mtb inhibits apoptosis is by limiting host ROS, as revealed in the studies of NuoG, SodA and Eis. NuoG was identified as a virulence determinant in Mtb using a gain-of-function genetic screen in M. smegmatis for suppressors of apoptosis. NuoG is a subunit of the type I NADH dehydrogenase, and it suppresses apoptosis in infected host cells by neutralizing ROS generated by macrophage NADPH oxidase (Velmurugan et al.2007; Miller et al.2010). The apoptosis pathway induced by the ΔnuoG mutant is dependent upon TNF-α signaling and caspase-8 activation. Similarly, bacterial mutants defective in superoxide dismutase (SodA) or its secretion (SecA2) enhance caspase-dependent apoptosis (Edwards et al.2001; Hinchey et al.2007). Mtb Eis is a secreted protein with an N-acetyltransferase activity that can inactivate aminoglycoside antibiotics (Chen et al.2011). As discussed above, Eis impairs autophagy. It was also reported to inhibit apoptosis in infected cells. Although Δeis mutants induce more host cellular ROS, suggesting that they induce apoptosis in a similar manner to the ΔnuoG and ΔsodA mutants, the cell death pathway appears to be distinct and is caspase-independent (Shin et al.2010). Eis has pleiotropic effects, altering cell death, autophagy, phagosome maturation and cytokine production, reflecting several host and bacterial targets for acetylation, including the dual-specificity protein phosphatase 16 (DUSP16/MKP-7) (Kim et al.2012), histone H3 (Duan et al.2016) and the bacterial nucleoid-associated protein HU (Ghosh et al.2016). Similarly, PtpA discussed above for its role in trafficking can impair apoptosis by dephosphorylating GSK3-α and TRIM27 (Poirier, Bach and Av-Gay 2014; Wang et al.2016). There are a number of additional bacterial genes shown to be anti-apoptotic, including PknE, SigH, MPT64 and Rv3654c (Mustafa et al.2007; Jayakumar, Jacobs and Narayanan 2008; Danelishvili et al.2011; Dutta et al.2012; Wang et al.2014), and how they all work together remains to be determined.
ADAPTIVE IMMUNITY IMPOSES A DETENTE
The majority of studies examining the activity of Mtb effectors have focused exclusively on naïve macrophages. However, the antimycobacterial activity of macrophages is modulated by a variety of inflammatory cytokines and other exogenous signals, such as vitamin D (MacMicking, Taylor and McKinney 2003; Gutierrez et al.2004; Singh et al.2006; Yuk et al.2009). Once an adaptive immune response develops and effector T cells arrive at the site of infection, they secrete cytokines which make macrophages better able to control bacterial replication. In fact, when antigen-specific T cells are experimentally elicited in the lungs prior to infection, animals are protected (Griffiths et al.2016). IFN-γ, TNF-α and IL-1β enhance bacterial clearance by activating autophagy, promoting the production of antimicrobial peptides, enhancing the production of reactive oxygen and reactive nitrogen species, and forcing phagosome maturation (reviewed in MacMicking 2014). Therefore, the adaptive immune response insures that the majority of infected individuals will contain the bacilli and never develop active disease.
However, not to be outdone, Mtb undermines these host responses, preventing sterilizing immunity. There is a long delay in T-cell priming, creating a large window of opportunity for Mtb to grow prior to the arrival of antigen-specific T cells (Wolf et al.2008). Moreover, prolonged Toll-like receptor 2 activation by Mtb can downregulate expression of major histocompatibility complex (MHC) class II molecules that would normally be induced by IFN-γ signaling in macrophages (Harding and Boom 2010). Even in cells that already express MHC class II, by targeting ESCRT, EsxG-EsxH impairs antigen processing and loading onto MHCII (Portal-Celhay et al.2016). Moreover, PE_PGRS47, mentioned above, also inhibits antigen presentation (Saini et al.2016), and Hip1, a serine protease, impairs dendritic cell function by cleaving GroEL2 (Madan-Lala et al.2014; Georgieva et al.2018). Lastly, physical access of T cells to infected macrophages is impaired by the granuloma structure and macrophage production of indoleamine 2,3-dioxygenase (Cronan et al.2016; Gautam et al.2017). There are certain to be other mechanisms that Mtb uses to undermine the adaptive immune response and persist in the face of robust T-cell responses.
CONCLUSION
The outcome of Mtb infection depends on the struggle between the host antimicrobial defense arsenal and sophisticated bacterial countermeasures. Mtb and humans have evolved with each other for millennia (Gagneux 2012), and they are well matched in this battle. Most often, the struggle ends in détente; the tubercle bacilli is contained, but not eradicated. To cause infection and persist in the host, Mtb relies upon numerous effectors that facilitate its survival in the hostile host environment. These effectors enable Mtb to interfere with cellular trafficking and immune recognition, inhibit phagosome acidification, block phagosomal–lysosomal fusion, impair apoptosis and autophagy, delay antigen presentation and modulate inflammasome activation. Even for the effectors that have been identified, we are far from understanding how they coordinate this assault. There are undoubtedly numerous yet to be identified effectors and cellular targets. For example, recent work has revealed that Mtb effectors may reprogram host gene expression by direct epigenetic modification (Sharma et al.2015; Yaseen et al.2015), a new area that warrants further study. We are gaining an increasing appreciation for how Mtb influences host cellular metabolism and its link with immune function (Shi, Eugenin and Subbian 2016), but understand little about how Mtb modulates these responses (Ouimet et al.2016). How Mtb manipulates dendritic and neutrophil function is poorly understood. The role of bacterial and host extracellular vesicles in immune evasion remains to be fully explored (Prados-Rosales et al.2014; Athman et al.2015). By understanding the molecular basis of the host–pathogen interface, we can fulfill the legacy of Koch and Metchnikoff, and in doing so, we will open up new therapeutic opportunities. By disrupting critical Mtb effectors, we should be able to restore the natural antimicrobial capacity of the host to clear Mtb. Such treatments would not depend on bacterial replication like most conventional antibiotics; therefore, they might effectively shorten therapy by clearing slow growing and non-replicating persisters. Given the alarming increase in multidrug-resistant TB, it is imperative to develop these new opportunities for intervention.
Acknowledgements
We thank M. G. Chheda (Washington University in St. Louis School of Medicine; WUSTL) for comments on the manuscript.
FUNDING
This work was supported by funds from National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) (R01 AI087682, R01 AI130454, R21 AI128427) and WUSTL to JAP.
Conflict of interest. None declared.
REFERENCES
- Armstrong JA, Hart PD. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med 1971;134:713–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JA, Hart PD. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med 1975;142:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athman JJ, Wang Y, McDonald DJ et al. Bacterial membrane vesicles mediate the release of Mycobacterium tuberculosis lipoglycans and lipoproteins from infected macrophages. J Immunol 2015;195:1044–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augenstreich J, Arbues A, Simeone R et al. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell Microbiol 2017;19:e12726. [DOI] [PubMed] [Google Scholar]
- Bach H, Papavinasasundaram KG, Wong D et al. Mycobacterium tuberculosis virulence is mediated by PtpA dephosphorylation of human vacuolar protein sorting 33B. Cell Host Microbe 2008;3:316–22. [DOI] [PubMed] [Google Scholar]
- Balcewicz-Sablinska MK, Keane J, Kornfeld H et al. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J Immunol 1998;161:2636–41. [PubMed] [Google Scholar]
- Balderhaar HJ, Ungermann C. CORVET and HOPS tethering complexes - coordinators of endosome and lysosome fusion. J Cell Sci 2013;126:1307–16. [DOI] [PubMed] [Google Scholar]
- Barczak AK, Avraham R, Singh S et al. Systematic, multiparametric analysis of Mycobacterium tuberculosis intracellular infection offers insight into coordinated virulence. PLoS Pathog 2017;13:e1006363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WL, Rhoades ER, Ullrich HJ et al. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic 2000;1:235–47. [DOI] [PubMed] [Google Scholar]
- Blomgran R, Desvignes L, Briken V et al. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host Microbe 2012;11:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella H, Peyron P, Levillain F et al. Mycobacterial P1-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 2011;10:248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briken V, Ahlbrand SE, Shah S. Mycobacterium tuberculosis and the host cell inflammasome: a complex relationship. Front Cell Infect Microbiol 2013;3:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier CJ, Takaki KK, Larson RP et al. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature 2014;505:218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson F, Kim J, Dumitru C et al. Host-detrimental role of Esx-1-mediated inflammasome activation in mycobacterial infection. PLoS Pathog 2010;6:e1000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casbon AJ, Allen LA, Dunn KW et al. Macrophage NADPH oxidase flavocytochrome B localizes to the plasma membrane and Rab11-positive recycling endosomes. J Immunol 2009;182:2325–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion PA, Stanley SA, Champion MM et al. C-terminal signal sequence promotes virulence factor secretion in Mycobacterium tuberculosis. Science 2006;313:1632–6. [DOI] [PubMed] [Google Scholar]
- Chandra P, Ghanwat S, Matta SK et al. Mycobacterium tuberculosis inhibits RAB7 recruitment to selectively modulate autophagy flux in macrophages. Sci Rep 2015;5:16320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan S, Kumar S, Jain A et al. TRIMs and galectins globally cooperate and TRIM16 and galectin-3 co-direct autophagy in endomembrane damage homeostasis. Dev Cell 2016;39:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Gan H, Remold HG. A mechanism of virulence: virulent Mycobacterium tuberculosis strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in macrophages leading to necrosis. J Immunol 2006;176:3707–16. [DOI] [PubMed] [Google Scholar]
- Chen W, Biswas T, Porter VR et al. Unusual regioversatility of acetyltransferase Eis, a cause of drug resistance in XDR-TB. P Natl Acad Sci USA 2011;108:9804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua J, Deretic V. Mycobacterium tuberculosis reprograms waves of phosphatidylinositol 3-phosphate on phagosomal organelles. J Biol Chem 2004;279:36982–92. [DOI] [PubMed] [Google Scholar]
- Collins AC, Cai H, Li T et al. Cyclic GMP-AMP synthase is an innate immune DNA sensor for Mycobacterium tuberculosis. Cell Host Microbe 2015;17:820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad WH, Osman MM, Shanahan JK et al. Mycobacterial ESX-1 secretion system mediates host cell lysis through bacterium contact-dependent gross membrane disruptions. P Natl Acad Sci USA 2017;114:1371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan MR, Beerman RW, Rosenberg AF et al. Macrophage epithelial reprogramming underlies mycobacterial granuloma formation and promotes infection. Immunity 2016;45:861–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danelishvili L, Everman JL, McNamara MJ et al. Inhibition of the plasma-membrane-associated serine protease cathepsin G by Mycobacterium tuberculosis Rv3364c suppresses caspase-1 and pyroptosis in macrophages. Front Microbiol 2011;2:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge MI, Pehau-Arnaudet G, Fretz MM et al. ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J Bacteriol 2007;189:6028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffert C, Cachat J, Krause KH. Phagocyte NADPH oxidase, chronic granulomatous disease and mycobacterial infections. Cell Microbiol 2014;16:1168–78. [DOI] [PubMed] [Google Scholar]
- Deghmane AE, Soualhine H, Soulhine H et al. Lipoamide dehydrogenase mediates retention of coronin-1 on BCG vacuoles, leading to arrest in phagosome maturation. J Cell Sci 2007;120:2796–806. [DOI] [PubMed] [Google Scholar]
- Divangahi M, Chen M, Gan H et al. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol 2009;10:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divangahi M, Desjardins D, Nunes-Alves C et al. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat Immunol 2010;11:751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorhoi A, Nouailles G, Jörg S et al. Activation of the NLRP3 inflammasome by Mycobacterium tuberculosis is uncoupled from susceptibility to active tuberculosis. Eur J Immunol 2012;42:374–84. [DOI] [PubMed] [Google Scholar]
- Duan L, Yi M, Chen J et al. Mycobacterium tuberculosis EIS gene inhibits macrophage autophagy through up-regulation of IL-10 by increasing the acetylation of histone H3. Biochem Bioph Res Co 2016;473:1229–34. [DOI] [PubMed] [Google Scholar]
- Dutta NK, Mehra S, Martinez AN et al. The stress-response factor SigH modulates the interaction between Mycobacterium tuberculosis and host phagocytes. PLoS One 2012;7:e28958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KM, Cynamon MH, Voladri RK et al. Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am J Resp Crit Care 2001;164:2213–9. [DOI] [PubMed] [Google Scholar]
- Ehrt S, Schnappinger D. Mycobacterial survival strategies in the phagosome: defence against host stresses. Cell Microbiol 2009;11:1170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellson CD, Gobert-Gosse S, Anderson KE et al. PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40(phox). Nat Cell Biol 2001;3:679–82. [DOI] [PubMed] [Google Scholar]
- Etna MP, Sinigaglia A, Grassi A et al. Mycobacterium tuberculosis-induced miR-155 subverts autophagy by targeting ATG3 in human dendritic cells. PLoS Pathog 2018;14:e1006790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festjens N, Bogaert P, Batni A et al. Disruption of the SapM locus in Mycobacterium bovis BCG improves its protective efficacy as a vaccine against M. tuberculosis. EMBO Mol Med 2011;3:222–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannagan RS, Cosío G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol 2009;7:355–66. [DOI] [PubMed] [Google Scholar]
- Flint JL, Kowalski JC, Karnati PK et al. The RD1 virulence locus of Mycobacterium tuberculosis regulates DNA transfer in Mycobacterium smegmatis. P Natl Acad Sci USA 2004;101:12598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco LH, Nair VR, Scharn CR et al. The ubiquitin ligase Smurf1 functions in selective autophagy of Mycobacterium tuberculosis and anti-tuberculous host defense. Cell Host Microbe 2017;22:421–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti RA, Backer JM, Gruenberg J et al. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol 2001;154:631–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti RA, Chua J, Vergne I et al. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. P Natl Acad Sci USA 2003;100:5437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneux S. Host-pathogen coevolution in human tuberculosis. Philos T Roy Soc B Biol Sci 2012;367:850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao LY, Guo S, McLaughlin B et al. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol Microbiol 2004;53:1677–93. [DOI] [PubMed] [Google Scholar]
- Gautam US, Foreman TW, Bucsan AN et al. In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium tuberculosis. P Natl Acad Sci USA 2017;115:201711373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengenbacher M, Nieuwenhuizen N, Vogelzang A et al. Deletion of nuoG from the vaccine candidate Mycobacterium bovis BCG ΔureC::hly improves protection against tuberculosis. mBio 2016;7:e00679–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva M, Sia JK, Bizzell E et al. Mycobacterium tuberculosis GroEL2 modulates dendritic cell responses. Infect Immun 2018;86:e00387–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Padmanabhan B, Anand C et al. Lysine acetylation of the Mycobacterium tuberculosis HU protein modulates its DNA binding and genome organization. Mol Microbiol 2016;100:577–88. [DOI] [PubMed] [Google Scholar]
- Griffiths KL, Ahmed M, Das S et al. Targeting dendritic cells to accelerate T-cell activation overcomes a bottleneck in tuberculosis vaccine efficacy. Nat Commun 2016;7:13894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundner C, Cox JS, Alber T. Protein tyrosine phosphatase PtpA is not required for Mycobacterium tuberculosis growth in mice. FEMS Microbiol Lett 2008;287:181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004;119:753–66. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Bi 2012;28:337–62. [DOI] [PubMed] [Google Scholar]
- Harding CV, Boom WH. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat Rev Microbiol 2010;8:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, De Haro SA, Master SS et al. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity 2007;27:505–17. [DOI] [PubMed] [Google Scholar]
- Hernandez-Cuellar E, Tsuchiya K, Hara H et al. Cutting edge: nitric oxide inhibits the NLRP3 inflammasome. J Immunol 2012;189:5113–7. [DOI] [PubMed] [Google Scholar]
- Hinchey J, Lee S, Jeon BY et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest 2007;117:2279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben D, Demangel C, van Ingen J et al. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell Microbiol 2012;14:1287–98. [DOI] [PubMed] [Google Scholar]
- Hsu T, Hingley-Wilson SM, Chen B et al. The primary mechanism of attenuation of Bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. P Natl Acad Sci USA 2003;100:12420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Brumell JH. Bacteria–autophagy interplay: a battle for survival. Nat Rev Microbiol 2014;12:101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol 2014;14:81–93. [DOI] [PubMed] [Google Scholar]
- Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol 2017;17:349–62. [DOI] [PubMed] [Google Scholar]
- Jamwal SV, Mehrotra P, Singh A et al. Mycobacterial escape from macrophage phagosomes to the cytoplasm represents an alternate adaptation mechanism. Sci Rep 2016;6:23089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar D, Jacobs WR, Narayanan S. Protein kinase E of Mycobacterium tuberculosis has a role in the nitric oxide stress response and apoptosis in a human macrophage model of infection. Cell Microbiol 2008;10:365–74. [DOI] [PubMed] [Google Scholar]
- Kanai F, Liu H, Field SJ et al. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat Cell Biol 2001;3:675–8. [DOI] [PubMed] [Google Scholar]
- Kang PB, Azad AK, Torrelles JB et al. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med 2005;202:987–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol 2000;164:2016–20. [DOI] [PubMed] [Google Scholar]
- Keane J, Shurtleff B, Kornfeld H. TNF-dependent BALB/c murine macrophage apoptosis following Mycobacterium tuberculosis infection inhibits bacillary growth in an IFN-gamma independent manner. Tuberculosis 2002;82:55–61. [DOI] [PubMed] [Google Scholar]
- Khan MZ, Bhaskar A, Upadhyay S et al. Protein kinase G confers survival advantage to Mycobacterium tuberculosis during latency-like conditions. J Biol Chem 2017;292:16093–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, An DR, Song J et al. Mycobacterium tuberculosis Eis protein initiates suppression of host immune responses by acetylation of DUSP16/MKP-7. P Natl Acad Sci USA 2012;109:7729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmey JM, Huynh JP, Weiss LA et al. Unique role for ATG5 in neutrophil-mediated immunopathology during M. tuberculosis infection. Nature 2015;528:565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Chauhan S, Jain A et al. Galectins and TRIMs directly interact and orchestrate autophagic response to endomembrane damage. Autophagy 2017;13:1086–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyei GB, Vergne I, Chua J et al. Rab14 is critical for maintenance of Mycobacterium tuberculosis phagosome maturation arrest. Embo J 2006;25:5250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster S, Klevorn T, Papavinasasundaram K et al. Consequence of enhanced LC3-trafficking for a live, attenuated M. tuberculosis vaccine. Vaccine 2018;36:939–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster S, Upadhyay S, Chandra P et al. Mycobacterium tuberculosis is protected from NADPH oxidase and LC3-associated phagocytosis by the LCP protein CpsA. P Natl Acad Sci USA 2017;114:E8711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElvania Tekippe E, Allen IC, Hulseberg PD et al. Granuloma formation and host defense in chronic Mycobacterium tuberculosis infection requires PYCARD/ASC but not NLRP3 or caspase-1. PLoS One 2010;5:e12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking JD. Cell-autonomous effector mechanisms against Mycobacterium tuberculosis. Cold Spring Harb Perspect Med 2014;4:a018507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science 2003;302:654–9. [DOI] [PubMed] [Google Scholar]
- Madan-Lala R, Sia JK, King R et al. Mycobacterium tuberculosis impairs dendritic cell functions through the serine hydrolase Hip1. J Immunol 2014;192:4263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanillo PS, Ayres JS, Watson RO et al. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature 2013;501:512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanillo PS, Shiloh MU, Portnoy DA et al. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe 2012;11:469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CJ, Booty MG, Rosebrock TR et al. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe 2012;12:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Malireddi RK, Lu Q et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat Cell Biol 2015;17:893–906. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Master SS, Rampini SK, Davis AS et al. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe 2008;3:224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Barber KD, Andrade BB, Barber DL et al. Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity 2011;35:1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Barber KD, Barber DL, Shenderov K et al. Caspase-1 independent IL-1beta production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol 2010;184:3326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra A, Zahra A, Thompson V et al. Mycobacterium tuberculosis type VII secreted effector EsxH targets host ESCRT to impair trafficking. PLoS Pathog 2013;9:e1003734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, Henault J, Kolbeck R et al. Noncanonical autophagy: one small step for LC3, one giant leap for immunity. Curr Opin Immunol 2014;26:69–75. [DOI] [PubMed] [Google Scholar]
- Miller JL, Velmurugan K, Cowan MJ et al. The type I NADH dehydrogenase of Mycobacterium tuberculosis counters phagosomal NOX2 activity to inhibit TNF-alpha-mediated host cell apoptosis. PLoS Pathog 2010;6:e1000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BB, Moura-Alves P, Sonawane A et al. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol 2010;12:1046–63. [DOI] [PubMed] [Google Scholar]
- Mishra BB, Rathinam VA, Martens GW et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β. Nat Immunol 2013;14:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G, Isberg RR. Innate Immunity to intracellular pathogens: balancing microbial elimination and inflammation. Cell Host Microbe 2017;22:166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal E, Kumar S, Rahman A et al. Modulation of phagolysosome maturation by bacterial tlyA gene product. J Biosci 2014;39:821–34. [DOI] [PubMed] [Google Scholar]
- Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular Bacillus Calmette-Guerin. J Exp Med 1994;180:1499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraco AH, Kornfeld H. Cell death and autophagy in tuberculosis. Semin Immunol 2014;26:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa T, Wiker HG, Mørkve O et al. Reduced apoptosis and increased inflammatory cytokines in granulomas caused by tuberculous compared to non-tuberculous mycobacteria: role of MPT64 antigen in apoptosis and immune response. Clin Exp Immunol 2007;150:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrvik QN, Leake ES, Wright MJ. Disruption of phagosomal membranes of normal alveolar macrophages by the H37Rv strain of Mycobacterium tuberculosis. A correlate of virulence. Am Rev Respir Dis 1984;129:322–8. [PubMed] [Google Scholar]
- Ng VH, Cox JS, Sousa AO et al. Role of KatG catalase-peroxidase in mycobacterial pathogenesis: countering the phagocyte oxidative burst. Mol Microbiol 2004;52:1291–302. [DOI] [PubMed] [Google Scholar]
- Nunes P, Demaurex N, Dinauer MC. Regulation of the NADPH oxidase and associated ion fluxes during phagocytosis. Traffic 2013;14:1118–31. [DOI] [PubMed] [Google Scholar]
- Oddo M, Renno T, Attinger A et al. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J Immunol 1998;160:5448–54. [PubMed] [Google Scholar]
- Ouimet M, Koster S, Sakowski E et al. Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat Immunol 2016;17:677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips JA, Ernst JD. Tuberculosis pathogenesis and immunity. Annu Rev Pathol Mech 2012;7:353–84. [DOI] [PubMed] [Google Scholar]
- Poirier V, Bach H, Av-Gay Y. Mycobacterium tuberculosis promotes anti-apoptotic activity of the macrophage by PtpA protein-dependent dephosphorylation of host GSK3α. J Biol Chem 2014;289:29376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portal-Celhay C, Tufariello JM, Srivastava S et al. Mycobacterium tuberculosis EsxH inhibits ESCRT-dependent CD4+ T-cell activation. Nat Microbiol 2016;2:16232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados-Rosales R, Weinrick BC, Piqué DG et al. Role for Mycobacterium tuberculosis membrane vesicles in iron acquisition. J Bacteriol 2014;196:1250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prashar A, Schnettger L, Bernard EM et al. Rab GTPases in immunity and inflammation. Front Cell Infect Microbiol 2017;7:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy GE, Owens RM, Bennett L et al. Kinetics of phosphatidylinositol-3-phosphate acquisition differ between IgG bead-containing phagosomes and Mycobacterium tuberculosis-containing phagosomes. Cell Microbiol 2005;7:1627–34. [DOI] [PubMed] [Google Scholar]
- Puri RV, Reddy PV, Tyagi AK. Secreted acid phosphatase (SapM) of Mycobacterium tuberculosis is indispensable for arresting phagosomal maturation and growth of the pathogen in guinea pig tissues. PLoS One 2013;8:e70514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queval CJ, Song OR, Carralot JP et al. Mycobacterium tuberculosis controls phagosomal acidification by targeting CISH-mediated signaling. Cell Rep 2017;20:3188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J, Hughitt VK, Velikovsky CA et al. The cell wall lipid PDIM contributes to phagosomal escape and host cell exit of Mycobacterium tuberculosis. mBio 2017;8:e00148–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram MV, Ni B, Dodd CE et al. Macrophage immunoregulatory pathways in tuberculosis. Semin Immunol 2014;26:471–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram MVS, Arnett E, Azad AK et al. M. tuberculosis -initiated human mannose receptor signaling regulates macrophage recognition and vesicle trafficking by FcRγ-Chain, Grb2, and SHP-1. Cell Rep 2017;21:126–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieck B, Degiacomi G, Zimmermann M et al. PknG senses amino acid availability to control metabolism and virulence of Mycobacterium tuberculosis. PLoS Pathog 2017;13:e1006399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink J, Ghigo E, Kalaidzidis Y et al. Rab conversion as a mechanism of progression from early to late endosomes. Cell 2005;122:735–49. [DOI] [PubMed] [Google Scholar]
- Roberts EA, Chua J, Kyei GB et al. Higher order Rab programming in phagolysosome biogenesis. J Cell Biol 2006;174:923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli A, Etna MP, Giacomini E et al. ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy 2012;8:1357–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikolappan S, Estrella J, Sasindran SJ et al. The fbpA/sapM double knock out strain of Mycobacterium tuberculosis is highly attenuated and immunogenic in macrophages. PLoS One 2012;7:e36198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini NK, Baena A, Ng TW et al. Suppression of autophagy and antigen presentation by Mycobacterium tuberculosis PE_PGRS47. Nat Microbiol 2016;1:16133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakowski ET, Koster S, Portal Celhay C et al. Ubiquilin 1 promotes IFN-γ-induced xenophagy of Mycobacterium tuberculosis. PLoS Pathog 2015;11:e1005076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible UE, Winau F, Sieling PA et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med 2003;9:1039–46. [DOI] [PubMed] [Google Scholar]
- Schnettger L, Rodgers A, Repnik U et al. A Rab20-dependent membrane trafficking pathway controls M. tuberculosis replication by regulating phagosome spaciousness and integrity. Cell Host Microbe 2017;21:619–28e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto S, Tsujimura K, Koide Y. Rab GTPases regulating phagosome maturation are differentially recruited to mycobacterial phagosomes. Traffic 2011;12:407–20. [DOI] [PubMed] [Google Scholar]
- Shah S, Bohsali A, Ahlbrand SE et al. Cutting edge: Mycobacterium tuberculosis but not nonvirulent mycobacteria inhibits IFN-β and AIM2 inflammasome-dependent IL-1β production via its ESX-1 secretion system. J Immunol 2013;191:3514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Upadhyay S, Srilalitha M et al. The interaction of mycobacterial protein Rv2966c with host chromatin is mediated through non-CpG methylation and histone H3/H4 binding. Nucleic Acids Res 2015;43:3922–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Eugenin EA, Subbian S. Immunometabolism in tuberculosis. Front Immunol 2016;7:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Jeon BY, Lee HM et al. Mycobacterium tuberculosis eis regulates autophagy, inflammation, and cell death through redox-dependent signaling. PLoS Pathog 2010;6:e1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shui W, Petzold CJ, Redding A et al. Organelle membrane proteomics reveals differential influence of mycobacterial lipoglycans on macrophage phagosome maturation and autophagosome accumulation. J Proteome Res 2011;10:339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone R, Bobard A, Lippmann J et al. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog 2012;8:e1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone R, Sayes F, Song O et al. Cytosolic access of Mycobacterium tuberculosis: critical impact of phagosomal acidification control and demonstration of occurrence in vivo. PLoS Pathog 2015;11:e1004650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SB, Davis AS, Taylor GA et al. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science 2006;313:1438–41. [DOI] [PubMed] [Google Scholar]
- Sly LM, Hingley-Wilson SM, Reiner NE et al. Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J Immunol 2003;170:430–7. [DOI] [PubMed] [Google Scholar]
- Speer A, Sun J, Danilchanka O et al. Surface hydrolysis of sphingomyelin by the outer membrane protein Rv0888 supports replication of Mycobacterium tuberculosis in macrophages. Mol Microbiol 2015;97:881–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Ernst JD, Desvignes L. Beyond macrophages: the diversity of mononuclear cells in tuberculosis. Immunol Rev 2014;262:179–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm CE, Collins AC, Shiloh MU. Sensing of Mycobacterium tuberculosis and consequences to both host and bacillus. Immunol Rev 2015;264:204–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley SA, Johndrow JE, Manzanillo P et al. The type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol 2007;178:3143–52. [DOI] [PubMed] [Google Scholar]
- Sturgill-Koszycki S, Schlesinger PH, Chakraborty P et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 1994;263:678–81. [DOI] [PubMed] [Google Scholar]
- Subbian S, Bandyopadhyay N, Tsenova L et al. Early innate immunity determines outcome of Mycobacterium tuberculosis pulmonary infection in rabbits. Cell Commun Signal 2013;11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Singh V, Lau A et al. Mycobacterium tuberculosis nucleoside diphosphate kinase inactivates small GTPases leading to evasion of innate immunity. PLoS Pathog 2013;9:e1003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Siroy A, Lokareddy RK et al. The tuberculosis necrotizing toxin kills macrophages by hydrolyzing NAD. Nat Struct Mol Biol 2015;22:672–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Wang X, Lau A et al. Mycobacterial nucleoside diphosphate kinase blocks phagosome maturation in murine RAW 264.7 macrophages. PLoS One 2010;5:e8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston TL, Wandel MP, von Muhlinen N et al. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 2012;482:414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufariello JM, Chapman JR, Kerantzas CA et al. Separable roles for Mycobacterium tuberculosis ESX-3 effectors in iron acquisition and virulence. P Natl Acad Sci USA 2016;113:E348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kant R, Jonker CT, Wijdeven RH et al. Characterization of the mammalian CORVET and HOPS complexes and their modular restructuring for endosome specificity. J Biol Chem 2015;290:30280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wel N, Hava D, Houben D et al. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 2007;129:1287–98. [DOI] [PubMed] [Google Scholar]
- Velmurugan K, Chen B, Miller JL et al. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog 2007;3:e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal A, Bryk R, Shi S et al. Virulence of Mycobacterium tuberculosis depends on lipoamide dehydrogenase, a member of three multienzyme complexes. Cell Host Microbe 2011;9:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I, Chua J, Lee HH et al. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. P Natl Acad Sci USA 2005;102:4033–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I, Fratti RA, Hill PJ et al. Mycobacterium tuberculosis phagosome maturation arrest: mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol Biol Cell 2004;15:751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via LE, Deretic D, Ulmer RJ et al. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem 1997;272:13326–31. [DOI] [PubMed] [Google Scholar]
- Vieira OV, Harrison RE, Scott CC et al. Acquisition of Hrs, an essential component of phagosomal maturation, is impaired by mycobacteria. Mol Cell Biol 2004;24:4593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walburger A, Koul A, Ferrari G et al. Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science 2004;304:1800–4. [DOI] [PubMed] [Google Scholar]
- Wang J, Teng JL, Zhao D et al. The ubiquitin ligase TRIM27 functions as a host restriction factor antagonized by Mycobacterium tuberculosis PtpA during mycobacterial infection. Sci Rep 2016;6:34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu S, Tang Y et al. MPT64 protein from Mycobacterium tuberculosis inhibits apoptosis of macrophages through NF-kB-miRNA21-Bcl-2 pathway. PLoS One 2014;9:e100949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann R, Gulen MF, Sala C et al. Mycobacterium tuberculosis differentially activates cGAS- and inflammasome-dependent intracellular immune responses through ESX-1. Cell Host Microbe 2015;17:799–810. [DOI] [PubMed] [Google Scholar]
- Watson RO, Bell SL, MacDuff DA et al. The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to Induce type i interferons and activate autophagy. Cell Host Microbe 2015;17:811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RO, Manzanillo PS, Cox JS. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host dna-sensing pathway. Cell 2012;150:803–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winau F, Weber S, Sad S et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity 2006;24:105–17. [DOI] [PubMed] [Google Scholar]
- Wolf AJ, Desvignes L, Linas B et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med 2008;205:105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D, Bach H, Sun J et al. Mycobacterium tuberculosis protein tyrosine phosphatase (PtpA) excludes host vacuolar-H+-ATPase to inhibit phagosome acidification. P Natl Acad Sci USA 2011;108:19371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D, Li W, Chao JD et al. Protein tyrosine kinase, PtkA, is required for Mycobacterium tuberculosis growth in macrophages. Sci Rep 2018;8:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KW. The role of ESX-1 in Mycobacterium tuberculosis pathogenesis. Microbiol Spectr 2017;5:TBTB2-0001-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KW, Jacobs WR. Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis. Cell Microbiol 2011;13:1371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaseen I, Kaur P, Nandicoori VK et al. Mycobacteria modulate host epigenetic machinery by Rv1988 methylation of a non-tail arginine of histone H3. Nat Commun 2015;6:8922. [DOI] [PubMed] [Google Scholar]
- Yuk JM, Shin DM, Lee HM et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009;6:231–43. [DOI] [PubMed] [Google Scholar]