Abstract
The SLC39A family of metal transporters was identified through homologies with the Zrt- and Irt-like (ZIP) proteins from yeast and plants. Of all the ZIP transporters, ZIP14 is arguably the most robustly characterized in terms of function at the integrative level. Mice with a global knockout of Zip14 are viable, thus providing the opportunity to conduct physiologic experiments. In mice, Zip14 expression is highly tissue specific, with the greatest abundance in the jejunum > liver > heart > kidney > white adipose tissue > skeletal muscle > spleen > pancreas. A unique feature of Zip14 is its upregulation by proinflammatory conditions, particularly increased interleukin 6 (IL-6) and nitric oxide. The transcription factors AP-1, ATF4, and ATF6α are involved in Zip14 regulation. ZIP14 does not appear to be zinc-regulated. The Zip14 knockout phenotype shows multiple sites of ZIP14 function, including the liver, adipose tissue, brain, pancreas, and bone. A prominent feature of the Zip14 ablation is a reduction in intestinal barrier function and onset of metabolic endotoxemia. Many aspects of the phenotype are accentuated with age and accompany increased circulating IL-6. Studies with 65Zn, 59Fe [nontransferrin-bound iron (NTBI)] and 54Mn show that ZIP14 transports these metals. At a steady state, the plasma concentrations of zinc, NTBI, and manganese are such that zinc ions are the major substrate available for ZIP14 at the cell surface. Upregulation of ZIP14 accounts for the hypozincemia and hepatic zinc accumulation associated with acute inflammation and sepsis and is required for liver regeneration and resistance to endoplasmic reticulum (ER) stress. Zip14 ablation in mice produces a defect in manganese excretion that leads to excess manganese accumulation in the brain that produces characteristics of Parkinsonism.
Keywords: metal transporter, zinc, iron, manganese, nontransferrin-bound iron, inflammation
Introduction
Coordination of the functions of the nutritionally required trace metals by mammals was significantly stimulated by the identification of specific transporters. Relevant to this review was the initial description of the SLC39A family of transporters based on homologies with the Zrt- and Irt-like (ZIP) proteins from yeast and plants (1). These are commonly referred to as the ZIP transporters. In the following 2 decades, numerous reviews have provided updates on progress in our understanding of the roles the ZnT and ZIP proteins play in trace metal transport. That progress was achieved through the utilization of an array of approaches and has helped define roles for these transporters in dietary acquisition, systemic metabolism, and more recently, targeted functions at specific subcellular sites. This review will leave that pattern and instead will focus on one metal transporter (i.e., ZIP14; SLC39A14). Of all of the ZIP transporters, ZIP14 is arguably the most robustly characterized in terms of physiologic function at the integrative level. This has arisen in part because the Zip14 null mutation in mice is not lethal, thus providing the opportunity to conduct physiologic experiments with the use of these models.
Identification and Initial Characterization
SLC39A14 (ZIP14) was first identified in an effort to accumulate information on the coding sequences of uncharacterized human genes (2). In that screening study, cDNA clones that carry unreported sequences at the 5′-ends were isolated from human cDNA libraries and the sizes of their inserts were then compared with those corresponding transcripts by Northern hybridization. After sequencing those inserts, ZIP14 was named KIAA0062, among 40 newly identified coding sequences. Of the tissue RNAs sampled, KIAA0062 was most highly expressed in the liver. With the use of differential display, it was found to be induced by IL-1α in human epithelial cells (3).
Sequence alignment studies showed that ZIP14 has 9 homologs, all with sequence homology to the protein produced by the estrogen-related gene LIV-1 (4). It was originally proposed that these proteins should be called the LZT subfamily of the ZIP (ZRT/IRT-related protein) transporter family proteins because of this sequence homology (4). These homologs are now referred to as group IV of the 14-member ZIP family (5). Analyses of ZIP14 secondary structure prediction showed that it has 8 transmembrane domains, a long extracellular N-terminus, a short extracellular C-terminus, and numerous histidine-rich repeats (6). Multiple alignment analyses also showed an important conserved motif (HEXPHE). This motif localized in TM domain V is similar to the HXXXE motif in TM domain V of other ZIP proteins of this subfamily. The motif is thought to be part of the intramembrane metal-binding site, which forms the pore region required for zinc transport function (6). The one exception in the subfamily is ZIP14, which has a glutamic acid replacement of the first histidine residue of the conserved HEXPHE motif. This replacement may allow for the transport of ions in addition to zinc (7). ZIP14 was shown to run as a trimer in an SDS-PAGE with the use of nonreducing conditions (4). Similarly, numerous other studies have shown that immune-detectable ZIP14 can migrate as multiples of the molecular mass of the primary structure of 52 KDa. The ability of the ZIP transporter family to function primarily as dimers has been documented (8). This ability to form complexes is consistent with the formation of ion channels. In the future, these multiple forms may be shown to reflect specific functions for ZIP14 monomers and dimers or specific interactions with other ZIP proteins or other protein partners. Such interactions are under investigation.
Tissue Specificity of Zip14 Expression
The earliest reports on ZIP14 documented marked tissue specificity of expression. At the mRNA level, an array of human tissues of undefined origin showed the greatest mRNA abundance in liver followed by pancreas > thyroid > heart (9). In mice, we showed that the greatest Zip14 mRNA abundance was in the duodenum followed by jejunum > liver > heart > kidney > white adipose tissue (WAT) > skeletal muscle > spleen > pancreas (10, 11). These mice were at steady state—that is, under normal dietary and environmental conditions and no treatments. Separate experiments showed that mouse Zip14 mRNA levels in liver and brain are refractory to changes in dietary zinc intake (12). The marked sensitivity of Zip14 expression in mice to proinflammatory conditions will be discussed in the sections that follow.
ZIP14 and Metal Transport
The transport properties of human ZIP14 were shown in 2005 with the use of human ZIP14 transfected cells (9). This activity agrees with the plasma membrane localization of ZIP14 (9, 13). Concurrently, the zinc transport function of the mouse ortholog of ZIP14 was shown with the use of mouse Zip14 transfected HEK293 human kidney cells and with multiple approaches to measure zinc uptake in the presence of 10 µM extracellular zinc (13). In the same study, the plasma membrane localization of ZIP14 was shown with the use of liver tissue sections and isolated murine liver parenchymal cells. We further reasoned, as discussed below, that physiologic regulation of ZIP14 expression could influence the metabolism of zinc and perhaps other metals. Focusing on iron, we showed that ZIP14 could transport nontransferrin-bound iron (NTBI) (10). Later, it was shown with the use of canine kidney epithelial cells that ZIP14 could transport cadmium and manganese via metal/bicarbonate symporter activity (14). Kinetic studies that used the Xenopus oocyte system provided further evidence that ZIP14 could transport multiple metals, albeit with different affinities (15).
ZIP14 and Zinc Transport
ZIP14-mediated zinc transport in the liver
Hypozincemia is one of the consequences of an acute inflammatory response and occurs concurrently with hypoferremia (16). Hypozincemia is thought to be part of a host defense mechanism; the exact physiologic role of hypozincemia is not known. Two possibilities are as follows: 1) deprivation in the availability of zinc for utilization by pathogenic micro-organisms (17) or 2) the provision of more zinc for cellular needs. During acute inflammation, a large number of acute-phase response (APR) proteins are synthesized by the liver. A reduction in circulating zinc was observed in IL-1α–injected rats (18) and was concurrent with an increase in liver zinc uptake. This differential was likely the result of enhanced zinc transporter expression.
To delineate which transporter or transporters were responsible for hepatic zinc uptake during acute inflammation, differential expression of Zip and ZnT transporter mRNAs were measured in the liver of mice after the administration of LPS (13). Hepatic metallothionein (Mt) mRNA levels were significantly higher in LPS-injected mice than in control mice, suggesting that hepatic zinc uptake was increased and activated metal responsive transcription factor 1 (MTF-1). The most responsive zinc transporter mRNA was found to be Zip14, with a 3.1-fold increase in mRNA levels in response to the LPS injection. Among the cytokines that are produced in response to an LPS injection, IL-6 is viewed as the main proinflammatory cytokine responsible for downstream effects of LPS (19, 20). Studies with IL-6 knockout mice showed that, after LPS, elevated hepatic Zip14 and Mt expression and hypozincemia were not detected, suggesting that Zip14 expression was IL-6 dependent. (13). Similarly, when primary hepatocytes that were isolated from wild-type (WT) and inducible NO synthase (iNOS) knockout mice were treated with IL-1β (21), ZIP14 expression and zinc uptake were increased in WT hepatocytes, whereas there was no change in iNOS knockout hepatocytes. These experiments suggested that NO might be a cytokine-stimulated downstream mediator of Zip14 expression. Treatment of isolated mouse hepatocytes with the NO donor, S-nitroso-N-acetylpenicillamine, resulted in increased ZIP14 abundance at the plasma membrane. Chromatin immunoprecipitation showed that NO increased transcription of Zip14 via activation of transcription factor AP-1 (21). These results showed 3 important concepts: 1) Zip14 was a cytokine-regulated gene, 2) ZIP14 must be the main transporter to facilitate hepatic zinc uptake during inflammation, and 3) Zip14 transcription is activated by AP-1.
Functional significance of ZIP14-mediated zinc transport is also implicated in other studies. For example, repeated psychological stress in male rats caused sequential increases in serum cortisol, IL-6, and liver Zip14 mRNA expression that were coincident with an elevated liver zinc concentration and hypozincemia (22). In another study, Zip14 expression and zinc uptake were upregulated in the liver of magnesium-deficient male rats (23). Those authors speculated that the Zip14 upregulation was due to the increase in serum IL-6 and IL-1β caused by magnesium deficiency (24). In contrast, decreased hepatic ZIP14 expression, along with hepatic zinc deficiency and increased serum and urinary zinc, was shown in male mice in response to chronic alcohol exposure (25).
We also explored a role in liver regeneration (LR), because this process is initiated by proinflammatory cytokines (26, 27). IL-6–induced expression of hepatocyte growth factor (HGF) is a major regulator of LR. During LR, we found that there was an influx of hepatic zinc early after partial hepatectomy that was concurrent with increased ZIP14 expression (28). After partial hepatectomy, Zip14 knockout mice did not accumulate zinc and experienced delayed and perhaps defective LR as shown by depressed proliferation markers. The increased hepatic zinc was shown to depress tyrosine-protein phosphatase non–receptor type 1 (PTP1B) activity. PTP1B regulates c-Met phosphorylation, which is required for regeneration after activation by HGF. PTP1B regulates c-Met phosphorylation, thus regulating ERK1/2, a downstream regulator of proliferation (29). It has long been known from in vitro studies that zinc is a potent inhibitor of PTP1B activity [reviewed in (30)]. We proposed that ZIP14-transported zinc functions to inhibit PTP1B activity and thus regulates the hepatocyte proliferation signaling pathway during LR. To our knowledge, these data represent the first demonstration of a link between function and ZIP14-mediated zinc transport (Figure 1B).
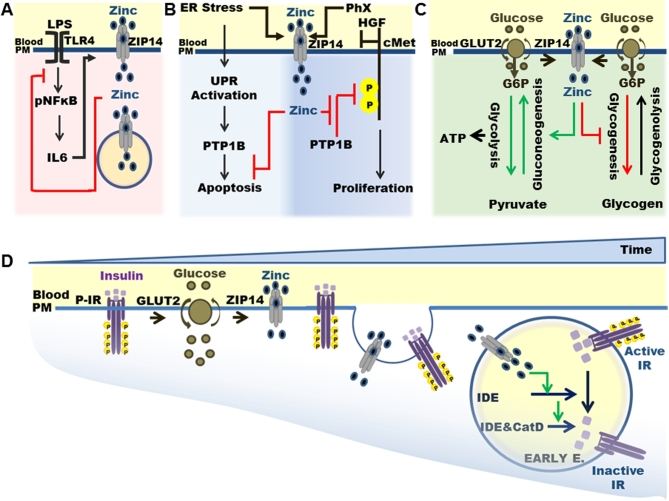
FIGURE 1.
Function of ZIP14-mediated zinc transport in different physiologic events. (A) An inflammation-induced increase in intracellular zinc concentration via ZIP14-mediated transport provides a negative feedback by inhibiting TLR4 signaling for NF-κB activation. (B) ZIP14-mediated zinc transport is needed for modulation of PTP1B phosphatase activity during regulatory pathways for hepatic ER stress and regeneration. (C) Hepatic ZIP14 expression and zinc uptake are upregulated during glucose uptake. Increased intracellular zinc involves differential regulation of hepatic glucose metabolism. (D) Hepatic ZIP14 along with transporter-bound zinc is endocytosed with active IR. ZIP14-bound (then released) zinc in early endosomes is necessary for the inactivation of IR via activation of zinc-requiring enzymes, IDE, and CatD required for disassociation and degradation of insulin. Green and red arrows indicate positive and negative regulation of zinc, respectively. CatD, cathepsin D; cMet, tyrosine-protein kinase Met; Early E., early endosome; ER, endoplasmic reticulum; GLUT2, glucose transporter 2; G6P, glucose-6-phosphate; HGF, hepatocyte growth factor; IDE, insulin-degrading enzyme; IR, insulin receptor; PHx, partial hepatectomy; P-IR, phosphorylated insulin receptor; PM, plasma membrane; pNFKB, phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells; PTP1B, tyrosine-protein phosphatase nonreceptor type 1; TLR4, Toll-like receptor 4; UPR, unfolded protein response; ZIP14, Zrt- and Irt-like 14.
When the Zip14 knockout mouse model became available (31), we revisited the hypothesis that ZIP14 was the main transporter to facilitate hepatic zinc uptake during LPS-induced acute inflammation. We found that, after LPS administration, hypozincemia was fully prevented and hepatic zinc uptake was impaired in female Zip14 knockout mice (32). Similar to the response of ZIP14 expression to LPS, polymicrobial sepsis in mice of mixed sexes leads to expression of multiple ZIP transporters, with ZIP14 having the most sustained expression on a temporal basis (33). Features of polymicrobial sepsis, such as serum IL-6, TNF-α, and IL-10 concentrations, are markedly elevated in Zip14 knockout mice. The knockout mice also exhibited reduced white blood cell counts. Such experiments suggest that defects in ZIP14 function extend beyond the liver. These findings collectively prove our long-standing hypothesis that ZIP14-mediated tissue zinc uptake is the primary driving force needed for the hypozincemia that occurs with acute endotoxemia (13) (Figure 1A).
Our initial characterization of Zip14 knockout mice showed a phenotype with multiple metabolic abnormalities, suggesting that ZIP14-mediated zinc transport influences metabolic pathways (32). ZIP14 is highly expressed in the liver, the major metabolic organ responsible for the utilization and production of glucose and for energy balance. Hepatic glucose metabolism is regulated by insulin. Upon insulin binding to the insulin receptor (IR; active), insulin-bound IR is internalized by endocytosis. Insulin is then released from IR (inactivation) and degraded by zinc-dependent endosomal enzymes, insulin-degrading enzyme (IDE) and cathepsin D (CatD). In experiments that used female mice, we observed that during hepatic glucose uptake, zinc was distributed to multiple sites in hepatocytes through sequential translocation of ZIP14 from the plasma membrane to early and late endosomes, suggesting ZIP14 internalization along with IR (34) (Figure 1C, D). Hepatic endosomes from Zip14 knockout mice were zinc-depleted based on analysis with the fluorophore FluoZin3. Activities of IDE and CatD were impaired; hence, IR inactivation was prevented (Figure 1D).
In Zip14 knockout mice, cytosolic zinc content was less than in WT mice, whereas the liver glycogen content was significantly greater (34). When fed a zinc-supplemented diet, the liver glycogen concentration in Zip14 knockout mice returned to the same level as in WT mice. This suggests that ZIP14-mediated hepatic zinc transport during glucose uptake might provide a negative feedback for glycogen synthesis. By measuring the activity of the enzymes glycogen synthase kinase 3 and glycogen synthase (regulate glycogen metabolism), we determined that, in HepG2 cells, the transient increases in cytosolic zinc concentration during glucose uptake, and additional zinc treatment, both caused suppression of glycogen synthesis. Zip14 knockout mice exhibited impaired gluconeogenesis and glycolysis, most likely related to low cytosolic zinc concentrations (34). Expression of the enzymes of the gluconeogenic pathway, phosphoenolpyruvate (Pepck) and glucose-6-phosphatase (G6Pase), was lower in Zip14 knockout mice. When mice were fed a zinc-supplemented diet, the expression of Pepck and G6Pase was enhanced in both WT and Zip14 knockout mice. Moreover, the pyruvate tolerance test showed that impaired gluconeogenesis in Zip14 knockout mice was rescued by dietary zinc supplementation. Glucose metabolism is largely dependent on mitochondria to generate energy. The mitochondrial zinc content was reduced in HepG2 cells during glucose uptake (34). The constant mitochondrial zinc content appeared to be regulated by the reciprocal action of ZIP14 and ZIP8, because there was no change between WT and Zip14 knockout genotypes in mitochondrial ATP concentration. In contrast, cytosolic ATP was lower in Zip14 knockout mice due to impaired glycolysis. Impaired glycolysis in the knockout mice was recovered by dietary zinc supplementation. The conclusion was that ZIP14-mediated zinc transport contributed to the regulation of endosomal insulin receptor activity and glucose homeostasis in hepatocytes. These experiments answer questions about the influence of zinc on glucose homeostasis that go back decades (35–37). We believe that this study shows the potential to modulate zinc transport for therapeutic strategies.
Zinc is essential for both maintaining normal endoplasmic reticulum (ER) function and resolving ER stress (38). Induction of zinc deficiency through injection of the metal chelator TPEN [N,N,N′,N′-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine] or injection of the ER stress inducer tunicamycin resulted in increased expression of Zip mRNAs, including Zip14, along with markers of ER stress in livers of mice (39). It was proposed that ZIP14-mediated zinc transport influences superoxide dismutase 1 conformation, leading to progression to ER stress. Our experiments failed to show that a nutritional zinc deficiency in mice causes hepatic ER stress (40). During both a pharmacologically (tunicamycin) or high-fat-diet–induced hepatic ER stress, an increase in ZIP14 expression was followed by an elevation of hepatic zinc uptake in WT mice, whereas Zip14 knockout mice exhibited impaired hepatic zinc uptake (41). The impaired hepatic zinc uptake in Zip14 knockout mice during ER stress coincided with a greater expression of proapoptotic pathway ER stress proteins (pelF2α, ATF4, and CHOP) and a lower expression of the ER chaperone protein GPR94. Furthermore, in Zip14 knockout mice, liver sections showed a greater number of Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling assay (TUNEL)-positive cells, indicative of apoptosis, and also increased serum alanine amino transferase, indicative of liver damage, respectively. When ER stress was induced, the Zip14 knockout mice showed greater levels of hepatic steatosis. PTP1B is a zinc-regulated phosphatase (28, 30) with expression and activity that are increased during ER stress to help modulate the hepatic ER stress response (42–44). We proposed that ZIP14 transport activity provides zinc ions to inhibit PTP1B activity (41). In support of that notion, PTP1B activity was greater in Zip14 knockout mice due to a deficiency of zinc in a cellular pool that interacts with the enzyme (Figure 1B). The effect of zinc on the ER stress response was confirmed with the use of HepG2 cells. Similar to data derived from mouse liver, HepG2 cells treated with tunicamycin had impaired hepatic zinc uptake; a greater amount of phospho (p)-elF2α, ATF4, and CHOP; and higher PTP1B activity and apoptosis when Zip14 was knocked down by small, interfering RNA (siRNA). Zinc treatment rescued the knocked-down phenotype in tunicamycin-treated HepG2 cells. Chromatin immunoprecipitation studies showed that ER stress caused an increase in Zip14 gene expression via activation of the transcription factors ATF4 and ATF6α. These results collectively show the importance of zinc trafficking and functional ZIP14 transporter activity for adaptation to hepatic ER stress associated with chronic metabolic disorders.
ZIP14-mediated zinc transport and function in the intestine
The highest Zip14 mRNA expression of those tissues examined in mice was in the duodenum and jejunum (10). Zinc is required for intestinal health and cell renewal as well as for barrier function. Therefore, we asked whether ZIP14-mediated zinc transport has a role in intestinal zinc metabolism, barrier function, or both? A most important finding in that regard was that intestinal ZIP14 was localized at the basolateral membrane of enterocytes along most of the villus (45). When 65Zn was administered via subcutaneous injection to label the endogenous zinc pool, a greater accumulation of 65Zn was found in the small intestines of Zip14 knockout mice than in WT mice. This unexpected result led to the hypothesis that ZIP14 might be associated with endosomes; hence, the absence of ZIP14 might prevent endosomal zinc mobilization, thus trapping zinc in the endosomes. To test this concept, crude endosomes were isolated from the small intestine of WT and Zip14 knockout mice. The presence of ZIP14 was confirmed, and higher zinc concentrations were found in endosomes isolated from Zip14 knockout mice. These results suggested that zinc was trapped in endosomes of enterocytes of the Zip14 knockout mice, causing an intracellular zinc-deficient environment. The mechanism for the accumulation of endosomal zinc in the absence of ZIP14 is currently under investigation. Zinc deficiency can lead to intestinal inflammation (46). Interestingly, expressions of IL-6 and TNF-α were greater in the jejunum of Zip14 knockout mice. Inflammation has been reported to cause an increase in intestinal permeability (47). Intestinal permeability was assessed in Zip14 knockout mice with the use of both oral fluorescein isothiocyanate–dextran and serum endotoxin content. Amounts of both fluorescein isothiocyanate–dextran in plasma and endotoxin in serum were greater in Zip14 knockout mice, indicating impaired intestinal barrier function with leakage into the systemic circulation. Intestinal barrier function is regulated by tight junction proteins (TJPs) (48–50). The reduced threonine phosphorylation of the TJP occludin and the respective decrease and increase in abundance of TJPs claudin 1 and 2 in the intestine of the knockout mice are highly supportive of a role for ZIP14-mediated zinc transport in the maintenance of intestinal barrier function (45). In addition, in light of the current finding that zinc is an important regulator of the gut microbiota (46), basolaterally localized ZIP14 might be required to supply zinc to the intestine for renewal of the gut epithelium or microbial host defense and intestinal barrier function.
ZIP14-mediated zinc transport and function in adipose tissue
Zip14 upregulation during adipocyte differentiation in vitro was one of the first findings about this gene (51). To our knowledge, however, there had not been any research on adipose tissue and ZIP14 until we reported that ZIP14 alters zinc trafficking in adipose tissue with functional outcomes in vivo (11). At steady state, mRNA expression of Zip14 is low in WAT; however, upregulation of Zip14 mRNA in response to LPS-induced inflammation is the highest in any tissue examined (11, 32).The Zip14 knockout phenotype includes elevated serum endotoxin, most likely due to impaired intestinal barrier function, and increased fat or lean body composition (32, 45). The marked induction of ZIP14 in WAT during inflammation, along with the knockout phenotype of increased adiposity and metabolic endotoxemia at steady state, led to the hypothesis that ZIP14 could be critical to the inflammatory response of adipose tissue and ultimately to the metabolic activity of WAT (Figure 1A). Characterization of the phenotypic profile of Zip14 knockout adipose tissue showed enhanced activation of MyD88, NF-κB, and STAT3, along with higher IL-6, TNF-α, and IL-1β and lower adiponectin mRNA expression (11). Furthermore, expression of preadipocytic markers Pre-1 and Pref-1, along with adipogenic enzymes Acaca and Acyl Hmgcr, were greater, whereas the differentiation marker Pparγ along with lipolytic markers hormone-sensitive lipase and zinc α2-glycoprotein were lower in Zip14 knockout adipose tissue. Higher leptin concentrations were found in the adipose tissue and serum of knockout mice. Overall, the Zip14 null mutation appeared to enhance WAT inflammation and adiposity and depress adipocyte differentiation. Accordingly, decreased IR phosphorylation at steady state was found in Zip14 knockout adipose tissue, indicating the development of insulin resistance. Of note, antibiotic treatment reversed the adipose tissue inflammation and insulin-resistance phenotype of Zip14 knockout, supporting the idea that metabolic endotoxemia was the underlying cause of this aspect of the phenotype (11).
Zinc metabolism in WAT was altered in Zip14 knockout mice. After oral 65Zn administration, greater zinc accumulation was observed in WAT of the knockout genotype; however, Mt mRNA expression was lower, indicating less available cytosolic zinc (11). Similar results were obtained in 3T3-L1 cells when Zip14 was knocked down by siRNA. These findings in adipocytes support the “zinc trap” hypothesis associated with Zip14 deletion, which we showed previously in mouse intestine (45), and described in the section above.
Zip14 mRNA expression was found to be higher in the adipose tissue of obese individuals than in healthy controls (52). When obese individuals were provided with a calorie-restricted diet, Zip14 expression diminished. The expression pattern of Zip14 correlated with adipose zinc concentration. Those data support the notion that adipose inflammation induces ZIP14 expression in WAT.
ZIP14-mediated zinc transport and function in other tissues
Zinc is required for normal systemic growth (53). Mice with global Zip14 deletions are smaller than their WT counterparts (54, 55). Zip14 knockout mice also exhibited wry neck, slightly radiolucent bones, decreased bone volume and trabecular number, and increased trabecular separation. ZIP14 was highly expressed in the proliferative zone of the growth plate, specifically in primary pituitary cells and chondrocytes of WT mice. Concurrently, intracellular zinc concentrations were significantly decreased in the proliferative zone of the Zip14 knockout mice growth plate (54). Chondrocytes and pituitary cells are important for bone elongation (56) and growth hormone (GH) production (57), respectively. Experiments with primary chondrocytes showed that zinc transported by ZIP14 was required to maintain the steady state level of parathyroid hormone 1 receptor (PTH1R)–mediated signaling by inhibiting and enhancing PDE and cAMP activity, respectively. Pituitary cells from Zip14 knockout mice exhibited both low cAMP and zinc concentrations. Accordingly, GH and insulin-like growth factor I (IGF-I) expression and secretion were lower in these cells than in WT cells. GH and IGF-I are regulated by the activity of GH-releasing hormone receptor (GHRHR). Both PTH1R in chondrocytes and GHRHR in pituitary cells are G protein–coupled receptors (GPCRs); thus, these data collectively indicated that ZIP14-mediated zinc transport was selectively involved in the GPCR signaling pathways (54).
In addition to impaired growth, Zip14 knockout mice also showed metabolic endotoxemia and altered glucose regulation (11, 32, 34, 45). Such phenotypes are associated with aging. Subsequently, we compared the phenotype of young and old male WT and Zip14 knockout mice (55). Zip14 deletion reduced growth and increased serum endotoxin in both young and old mice compared with WT mice. Accordingly, higher serum IL-6 was detected in both young and old knockout mice. Of note, aging greatly amplified the differential in serum IL-6 in the Zip14 knockout mice. IL-6 mRNA levels in the spleen of the old knockout mice were the highest when compared with all groups; this suggests that splenic macrophages could be one site of origin of the elevated IL-6. In support of these data, the highest amount of activated (phosphorylated) NF-κB and STAT3, master regulators of IL-6 production and secretion, were found in the spleen of old knockout mice.
Both age and Zip14 deletion influenced bone morphology as measured by micro-computed tomography scanning. Cortical bone volume decreased, whereas bone surface area increased in old knockout mice, indicating a critical reduction in bone mass by Zip14 ablation (55). Furthermore, greater loss of femoral trabecular bone was observed in the Zip14 knockout mice. The old knockout mice showed significant reductions in trabecular bone based on numerical indexes of bone mineral density.
In keeping with these results, when ear mesenchymal stem cells (EMSCs) from Zip14 knockout mice were cultured in osteogenic medium, there was less mineralization when compared with their WT counterparts, based on Alizarin Red staining (55). In addition, by using these EMSCs we showed that pSmad1/5 (a key transducer of the bone morphogenetic protein pathway) was downregulated in Zip14 knockout mice (55). ZIP14 upregulation under inflammatory conditions was reported in multiple organisms and tissues, such as mouse lung (58), porcine spleen (59), sheep pulmonary artery endothelial cells (60), primary human macrophages (61), and human peripheral blood mononuclear cells (62), but functional studies were not conducted.
ZIP14 and Iron Transport
Many of the ZIP family member metal transporters have been shown to transport iron as well as other cations (63–66). ZIP14 is unique in the ZIP family in that it has a glutamic acid replacement of the first histidine residue of the conserved HEXPHE motif. It has been suggested that this replacement may allow for the transport of metal ions in addition to zinc (7). Focusing on iron, we showed that ZIP14 could transport NTBI (10). Overexpression of mouse Zip14 in HEK293 human kidney and Sf9 insect cells resulted in greater iron accumulation by these cells. Accordingly, lower iron accumulation was found in AML12 hepatocytes when Zip14 was knocked down by siRNA. We postulated that in pathologic conditions where NTBI concentrations are detectable (e.g., hemochromatosis and repeated transfusions), ZIP14 could lead to excessive hepatic iron accumulation (10).
In vitro support for NTBI transport by ZIP14 was that stable expression of the hereditary hemochromatosis protein (HFE) in HepG2 hepatocytes suppressed NTBI uptake through decreasing ZIP14 stability (67). Overexpression of ZIP14 in HeLa cells caused an increase in NTBI uptake. When HFE expression was induced, with the use of a tetracycline promoter, ZIP14-mediated NTBI uptake by these cells was diminished. Association of ZIP14 with transferrin-bound iron (Fe-TF) assimilation in endosomes was shown in HEK293 cells (67). Partial co-localizations of 3-FLAG ZIP14 and TF and early endosome protein EEA1 were shown in 3-FLAG tagged Zip14 knock-in HepG2 cells. With Zip14 knock-down in HepG2 cells, 59Fe accumulation from 59Fe-TF was lower, suggesting that ZIP14 contributes to the uptake of Fe-TF. However, the same group in their later work showed that hepatic Fe-TF uptake was not different between Zip14 knockout and WT mice (68).
Dietary iron regulation of ZIP14 expression has been shown (69). ZIP14 protein concentrations were upregulated in dietary iron–loaded rat liver and pancreas. Similarly, ZIP14 protein concentrations were higher and lower when stable Zip14-transfected HepG2 cells were iron loaded and depleted, respectively (70). Iron depletion promoted deglycosylation of the ZIP14 protein, and thus proteasome degradation resulted. In contrast, iron overload prevented internalized ZIP14 degradation. Hence, ZIP14 regulation by iron appears to be post-translational.
We conducted the initial evaluation of iron metabolism in Zip14 knockout mice (adults aged >8 wk). Total serum and liver iron concentrations were not different between genotypes. However, greater absorption of orally administered 59Fe without gastrointestinal tract and greater uptake of 59Fe by the liver was found in the knockout mice (32). In those studies, 59Fe absorption and liver 59Fe uptake were calculated as a percentage of whole-body radioactivity corrected for body weight or liver weight, respectively. When total body (without gastrointestinal tract) 59Fe absorption and liver 59Fe uptake were calculated only as a percentage of whole0body radioactivity of young (4- to 6-wk-old) mice, there was no difference between genotypes (68). Total body and liver 59Fe uptake from intravenously injected 59Fe-NTBI were both lower in Zip14 knockout mice, suggesting involvement of ZIP14 in NTBI uptake.
Mutations in both HFE and HFE2 (hereditary hemochromatosis) genes cause an increase in iron absorption and subsequent iron accumulation in body tissues. Jenkitasemwong et al. (68) tested the function of ZIP14 in hereditary hemochromatosis, through crosses of Zip14 knockouts with Hfe knockout as well as Hfe2 knockout mice to create double knockout (DKO) mice. In both Hfe and Hfe2 knockouts, hepatic nonheme iron and 59Fe accumulation were greater when compared with WT mice. However, in the DKO mice, hepatic 59Fe accumulation was not seen in mice of the Hfe and Hfe2 knockout genotypes. After dietary iron overload, the elevation in hepatic nonheme iron in WT mice was not observed in Zip14 DKO mice. These data collectively indicate that ZIP14 contributes to NTBI uptake by murine hepatocytes in diet-induced iron overload and in hereditary hemochromatosis.
Zip14 knockout mice displayed impaired pancreatic 59Fe uptake from intravenously injected 59Fe-NTBI, whereas there was no difference in transferrin-bound iron (TBI) uptake (68). Mice with double knockout of Zip14 with Hfe and Hfe2 showed that only the Hfe DKO and at a smaller magnitude the Hfe2 DKO caused an increase in 59Fe accumulation in the pancreas. Iron accumulation was in pancreatic acinar cells in Hfe2 DKO and in inter- and perilobular regions in Hfe2 DKO mice. Dietary iron–loaded mice showed a similar pattern of iron deposition. These data suggest that both deletion of Zip14 and dietary iron overload in mice contribute to iron accumulation in the pancreas. ZIP14 was found in βlox5 cells, a human β cell line (71). Increased transport of NTBI by ZIP14-overexpressing βlox5 cells was shown. Moreover, when Zip14 was knocked down in both βlox5 and primary human islets, NTBI transport was impaired (71). An association of ZIP14 with iron metabolism has been implicated in multiple organisms and tissues, but with little mechanistic explanation (72–78).
ZIP14 and Manganese Transport
The transport of manganese ions by ZIP14 was shown in vitro with the use of transfected HEK293 cells and fetal fibroblasts overexpressing ZIP14 (14). Knockdown of Zip14 with siRNA reduced manganese uptake by murine kidney tubular cells (79), but not in rat basophilic leukemia cells (80). The murine ear accumulates manganese when injected subcutaneously and also expresses Zip14 mRNA, but a direct relation to transport was not established (81). An analysis of metal transport with the use of a Xenopus oocyte heterologous system expressing mouse ZIP14 compared the transport of manganese with other metals (15). The affinity of ZIP14 for zinc and manganese was such that, at 2 μM zinc, a manganese concentration of 20 μM in 1 mM l-ascorbic acid did not inhibit 65Zn uptake by the oocytes. Conversely, at 2 μM manganese, a concentration of 20 μM zinc completely inhibited 54Mn uptake. Manganese concentrations of this magnitude in plasma of animals would be nonphysiologic; hence, these results may not be reflective of true ZIP14 metal trafficking activity in vivo.
ZIP14 inducibility by IL-6 as a factor in ZIP14-mediated manganese transport was introduced with in vitro studies that used SH-SY5Y neuronal cells (82). IL-6–stimulated neuronal cells had a 3-fold greater manganese accumulation than nonstimulated cells. A direct link between ZIP14 expression and manganese transport was not shown through siRNA inhibition or other methods.
A major advance in the understanding of the manganese transporting relevance of ZIP14 was the observation that mutations in ZIP14 are concurrent with manganese accumulation in humans with early-onset Parkinsonism dystonia (83). Companion studies in zebrafish showed that these ZIP14 mutations led to excess manganese accumulation and behavioral characteristics corresponding to Parkinsonism. They proposed a model in which ZIP14 facilitates manganese transport from the liver to the biliary excretory route. Furthermore, they showed that mutant ZIP14 has a diminished manganese transporting capability, which leads to tissue manganese accumulation by the zebrafish. We investigated the manganese transporting capabilities in vivo by using a mouse Zip14 knockout model (84). Our results showed that these knockout mice present with markedly impaired locomotor function, increased intensity of MRI images of the brain indicative of manganese accumulation, impaired manganese (54Mn) elimination, and increased tissue manganese accumulation. Manganese accumulation was markedly greater in male knockout mice, which parallels the greater incidence of Parkinsonism in human males (85). We concluded that normal ZIP14 transport function is necessary for manganese homeostasis in mice fed a commercial feed pellet–type diet. 54Mn radiotracer experiments showed that the intestine may be a route for some manganese excretion, because ZIP14 is localized to the basolateral membrane of enterocytes from the proximal small intestine (45). These conclusions are in line with the characterization of mammalian manganese homeostasis produced by Cotzias and colleagues (86) in the 1960s. Moreover, our data show that ZIP14 is not required for manganese uptake into neuronal tissues. However, systemic ZIP14 transport activity is essential to prevent manganese-related signatures of neurodegeneration, including, for example, elevated translocator protein (TSPO), activated NF-κB, and increased TNF-α mRNA in brain. A model based on ZIP14-mediated manganese uptake by hepatocytes with subsequent biliary excretion was proposed to explain the data (84). We further speculated that ZIP14 could be a component of the high-affinity transport system identified for manganese in rat hepatocytes (87). Subsequently, similar studies with another Zip14 knockout murine model, in which the sex of the mice was not reported, showed similar motor dysfunction and manganese accumulation in the brain and most other tissues (88). A liver-specific Zip14 knockout did not result in manganese accumulation in the brain or other tissues. Upon feeding a highly nonphysiologic amount of dietary manganese (2400 mg/kg), excess manganese was detected in the brain cortex and pancreas of these liver-specific Zip14 knockout mice (88).
The function of ZIP14-mediated metal transport in neuronal tissues is currently unknown (89). We know at this juncture that ZIP14 is not essential for manganese uptake into the brain. That does not exclude the potential for ZIP14-mediated delivery of zinc and possibly NTBI and manganese into the brain. The identification of ZIP14 (SLC39A14), by exome sequencing, as among 32 genes in which variants were associated with intellectual disability, is of significant interest (90). That finding may suggest a beneficial role for ZIP14 in neuronal metal transport.
ZIP14 and Transport of Other Metals
Cadmium is a nonessential, toxic environmental contaminant that is poorly absorbed. Cadmium was found to be transported by ZIP14 in vitro by transfected kidney cells and fetal fibroblasts overexpressing ZIP14 (14). Cadmium exhibited a lower affinity and transport velocity than manganese. Moreover, cadmium uptake could be inhibited by manganese. In rat basophilic leukemia (RBL-2H3) cells, which are very sensitive to cadmium, Zip14 knockdown with siRNA did not influence cadmium uptake (80). In contrast, the same approach decreased cadmium uptake by mouse kidney proximal cells as did siRNA knockdown of Zip8 and Dmt1 (79). It was proposed that ZIP14 is involved in the renal reabsorption and retention of cadmium. ZIP14 and other metal transporter mRNAs are upregulated in the rat placenta by cadmium in a dose-dependent manner (91). Induction of Zip14 mRNA by LPS was correlated with hepatic cadmium accumulation in mice when cadmium was given by injection (92). Feeding a zinc-deficient diet to those mice accentuated the cadmium accumulation after LPS treatment. Oral cadmium administration to Zip14 knockout mice decreased hepatic cadmium retention compared with WT mice (93). The reverse situation was found for cadmium accumulation in proximal small intestine, kidney, and lung. No mechanistic explanation was provided by the authors, except that acute shock of the cadmium dose caused upregulation of multiple metal transporter genes based on transcript data.
Cadmium concentrations of erythrocytes from a female Andean population were associated with polymorphisms in ZIP14 (SLC39A14). Specifically, higher concentrations were found in women carrying the GT or TT genotypes of rs4872479 than those carrying GG (94). Such association studies may be of value in studies relating ZIP14 transport functions and phenotype in humans. Experiments with the Xenopus oocytes expressing mouse Zip14, as described above, did not provide evidence for the transport of copper (either Cu1+ or Cu2+) (15).
ZIP14 and Cancer
ZIP14 downregulation in human hepatoma relative to adjacent normal tissue in liver biopsy specimens was detected by microarray (95). This finding supported the long-known (96) observation that zinc concentrations were lower in human hepatomas (97). By using a tissue microarray, the presence and absence of ZIP14 protein in plasma membrane was shown in normal hepatocytes and hepatoma cells, respectively (98). A decrease in ZIP14 was concurrent with the decrease in zinc in hepatomas. A meta-analysis of whole transcriptomes of hepatocellular carcinoma (HCC) was conducted to explore ZIP14 as a potential biomarker and therapeutic target for HCC (99). Similar to the above microarray studies, ZIP14 was downregulated in human HCC samples compared with adjacent normal tissue. Furthermore, aberrant splicing in ZIP14 was found to be associated with HCC.
Transcript variants of Zip14 were previously reported (14). Transcript variants of Zip14 were the result of alternative splicing of either exon 4A or exon 4B. Mouse Zip14 exons 4A and 4B are both 170-bp long and share 67% nucleotide identity. Transcripts of ZIP14A and ZIP14B encode 2 different proteins, both with 489 amino acids but different molecular masses. Differing expression of exons 4A and 4B between normal and colorectal cancer (CRC) tumor samples was reported (100). Even though there was no difference in total ZIP14 expression, the 4A-to-4B ratio was significantly lower in adenomas and carcinomas when compared with normal tissues. Due to differential expression of ZIP14 splicing variants in cancer tissue, ZIP14 was suggested as a biomarker for CRC. This idea was further explored by evaluating ZIP14 variants in both CRC biopsy specimens and healthy tissues (101). In that study, high relative expression of ZIP14B compared with ZIP14A in CRC was confirmed. However, ZIP14B expression was also present in healthy tissue, suggesting that the cancer-specificity of ZIP14B was mainly confined to the colon and rectum.
ZIP14 protein and mRNA levels in human prostate cancer (PCa) tissues were lower than those in adjacent noncancerous prostate tissue (102). The decreased expression of ZIP14 protein more frequently occurred in PCa tissues at the advanced clinical stage. Furthermore, the expression of ZIP14 mRNA was identified as one of the predictors of recurrence-free survival in patients with PCa. In vitro, the overexpression of ZIP14 suppressed cell proliferation, invasion, and migration in the PCa cell line LNCaP. Suppression was reversed by the knockdown of ZIP14 (102).
Conclusions and Perspectives
Although the ZIP14 gene was identified >20 y ago, the past decade has seen major developments toward an understanding of this transporter's biological relevance. An appreciation of this relevance requires an understanding of the strengths and limitations of the in vivo and in vitro methodologies used for research on ZIP14. This is particularly important within the context of the transport potential of multiple metal ions. Factors that must be considered include the steady state compared with induced state of ZIP14 expression, normal plasma concentrations of the metal substrates (zinc, iron, manganese, cadmium, etc.), plasma-binding ligands for those metals and their binding affinities, physiologic conditions that influence those concentrations, comparisons of total tissue metal concentration compared with intracellular metal distributions, as well as phenotypic metal-related abnormalities associated with ZIP14 deletion or mutations. The latter are a likely reflection of biological roles.
To compare the transport options for ZIP14, we considered the metal substrates available to ZIP14 in the systemic circulation. In normal subjects, the plasma zinc concentration is ∼15 μM, with zinc ions primarily loosely bound to albumin (103); plasma NTBI is either undetectable or no greater than 0.04 µM (104, 105); and plasma manganese is ∼0.07 μM and is tightly bound to transferrin and α2-macroglobulin (106). In some inherited disorders of iron metabolism, as well as in diabetes and occuring transiently after transfusion and iron supplementation (105, 107, 108), NTBI concentrations may increase in plasma and provide iron (Fe2+) as a substrate for ZIP14 (10, 68). Portal blood may have somewhat greater concentrations of each metal. Nevertheless, under normal dietary conditions, cells are exposed to plasma, where the concentrations of these metal ions are markedly different [Zn2+>>>>Mn2+>Fe2+ (NTBI)]. We propose that Zn2+ has an abundance advantage of ∼400-fold compared with Fe2+ or Mn2+. This stoichiometric relation likely explains why, during situations in which ZIP14 expression is increased (e.g., stress, acute infection, or sepsis), liver zinc concentrations increase but those for iron and manganese do not. Nevertheless, organellar concentrations of these metals may be markedly different through intracellular trafficking and targeted ZIP14 transport activity (34, 67).
We have reviewed the evidence for the multiple faces of ZIP14 (Figure 2). We argue that the primary biological role of ZIP14 is to regulate intracellular zinc homeostasis and functions, such as modulation of cell signaling pathways. Furthermore, we argue that transport roles for NTBI and manganese are secondary, with pathologic or excretory and protective roles, respectively. These roles may be magnified under proinflammatory conditions.
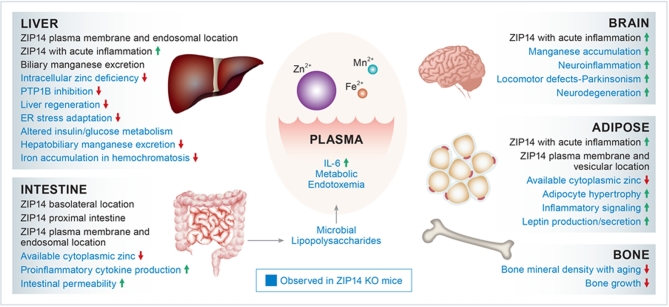
FIGURE 2.
Metabolic and intracellular functions of metal transporter ZIP14 (Slc39a14). At steady state, ZIP14 is highly expressed in the jejunum of mice followed by liver > heart > kidney > white adipose tissue > skeletal muscle > spleen > pancreas. Proinflammatory stimuli increase ZIP14 expression in the liver, adipose tissue, and muscle. ZIP14-mediated zinc transport influences cell signaling related to cell proliferation, apoptosis prevention, and suppression of inflammatory pathways. ZIP14 also transports nontransferrin-bound iron and manganese. Circles are approximations of plasma concentrations of Zn2+, Fe2+, and Mn2+ available as substrates for ZIP14 at the cell surface. ZIP14 transport activity can influence the intracellular concentrations of these ions through endocytotic trafficking. Phenotypic changes produced in mice through deletion of Zip14 are shown in blue. ER, endoplasmic reticulum; KO, knockout; PTP1B, tyrosine-protein phosphatase nonreceptor type 1; ZIP14, Zrt- and Irt-like 14.
Acknowledgments
The authors’ responsibilities were as follows—TBA and RJC: wrote and reviewed the manuscript and gave the final decision for publication; and both authors: read and approved the final manuscript.
Notes
Research from the Cousins Laboratory described herein was supported by NIH grant R01 DK 94244.
Abbreviations used:
- CRC
colorectal cancer
- DKO
double knockout
- ER
endoplasmic reticulum
- Fe-TF
transferrin-bound iron
- GH
growth hormone
- HCC
hepatocellular carcinoma
- HFE
hereditary hemochromatosis protein
- IR
insulin receptor
- LR
liver regeneration
- Mt
metallothionein
- NTBI
nontransferrin-bound iron
- PCa
prostate cancer
- PTP1B
tyrosine-protein phosphatase nonreceptor type 1
- TJP
tight junction protein
- siRNA
small, interfering RNA
- SLC39A
solute carrier 39A
- WAT
white adipose tissue
- WT
wild-type
- ZIP
Zrt- and Irt-like.
References
- 1. Eng BH, Guerinot ML, Eide D, Saier MH. Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins. J Membr Biol 1998;166:1–7. [DOI] [PubMed] [Google Scholar]
- 2. Nomura N, Nagase T, Miyajima N, Sazuka T, Tanaka A, Sato S, Seki N, Kawarabayasi Y, Ishikawa K, Tabata S. Prediction of the coding sequences of unidentified human genes. II. The coding sequences of 40 new genes (KIAA0041-KIAA0080) deduced by analysis of cDNA clones from human cell line KG-1. DNA Res 1994;1:223–9. [DOI] [PubMed] [Google Scholar]
- 3. Zhu H, Cong JP, Shenk T. Use of differential display analysis to assess the effect of human cytomegalovirus infection on the accumulation of cellular RNAs: induction of interferon-responsive RNAs. Proc Natl Acad Sci USA 1997;94:13985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor KM, Nicholson RI. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim Biophys Acta 2003;1611:16–30. [DOI] [PubMed] [Google Scholar]
- 5. Schmitt-Ulms G, Ehsani S, Watts JC, Westaway D, Wille H. Evolutionary descent of prion genes from the ZIP family of metal ion transporters. PLoS One 2009;4:e7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta Mol Cell Res 2006;1763:711–22. [DOI] [PubMed] [Google Scholar]
- 7. Heitzer M, Hallmann A. An extracellular matrix-localized metal-loproteinase with an exceptional QEXXH metal binding site prefers copper for catalytic activity. J Biol Chem 2002;277:28280–6. [DOI] [PubMed] [Google Scholar]
- 8. Kambe T, Tsuji T, Hashimoto A, Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev 2015;95:749–84. [DOI] [PubMed] [Google Scholar]
- 9. Taylor KM, Morgan HE, Johnson A, Nicholson RI. Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett 2005;579:427–32. [DOI] [PubMed] [Google Scholar]
- 10. Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA 2006;103:13612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Troche C, Beker Aydemir T, Cousins RJ. Zinc transporter Slc39a14 regulates inflammatory signaling associated with hypertrophic adiposity. Am J Physiol Endocrinol Metab 2016;310:E258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lichten LA, Ryu M-S, Guo L, Embury J, Cousins RJ. MTF-1-mediated repression of the zinc transporter Zip10 is alleviated by zinc restriction. PLoS One 2011;6:e21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA 2005;102:6843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, Nebert DW. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol 2008;73:1413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pinilla-Tenas JJ, Sparkman BK, Shawki A, Illing AC, Mitchell CJ, Zhao N, Liuzzi JP, Cousins RJ, Knutson MD, Mackenzie B. Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am J Physiol Cell Physiol 2011;301:C862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moshage H. Cytokines and the hepatic acute phase response. J Pathol 1997;181:257–66. [DOI] [PubMed] [Google Scholar]
- 17. Jurado RL. Iron, infections, and anemia of inflammation. Clin Infect Dis 1997;25:888–95. [DOI] [PubMed] [Google Scholar]
- 18. Cousins RJ, Leinart AS. Tissue-specific regulation of zinc metabolism and metallothionein genes by interleukin 1. FASEB J 1988;2:2884–90. [DOI] [PubMed] [Google Scholar]
- 19. Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1 beta mediates leptin induction during inflammation. Am J Physiol 1998;274:R204–8. [DOI] [PubMed] [Google Scholar]
- 20. Siewert E, Dietrich CG, Lammert F, Heinrich PC, Matern S, Gartung C, Geier A. Interleukin-6 regulates hepatic transporters during acute-phase response. Biochem Biophys Res Commun 2004;322:232–8. [DOI] [PubMed] [Google Scholar]
- 21. Lichten LA, Liuzzi JP, Cousins RJ. Interleukin-1beta contributes via nitric oxide to the upregulation and functional activity of the zinc transporter Zip14 (Slc39a14) in murine hepatocytes. Am J Physiol Gastrointest Liver Physiol 2009;296:G860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tian X, Zheng Y, Li Y, Shen Z, Tao L, Dou X, Qian J1, Shen H. Psychological stress induced zinc accumulation and up-regulation of ZIP14 and metallothionein in rat liver. BMC Gastroenterol 2014;14:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kotani M, Kim KH, Ishizaki N, Funaba M, Matsui T. Magnesium and calcium deficiencies additively increase zinc concentrations and metallothionein expression in the rat liver. Br J Nutr 2013;109:425–32. [DOI] [PubMed] [Google Scholar]
- 24. Weglicki WB, Phillips TM. Pathobiology of magnesium deficiency: a cytokine/neurogenic inflammation hypothesis. Am J Physiol 1992;263:R734–7. [DOI] [PubMed] [Google Scholar]
- 25. Sun Q, Li Q, Zhong W, Zhang J, Sun X, Tan X, Yin X, Sun X, Zhang X, Zhou Z. Dysregulation of hepatic zinc transporters in a mouse model of alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 2014;307:G313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fausto N, Riehle KJ. Mechanisms of liver regeneration and their clinical implications. J Hepatobiliary Pancreat Surg 2005;12:181–9. [DOI] [PubMed] [Google Scholar]
- 27. Taub R, Greenbaum LE, Peng Y. Transcriptional regulatory signals define cytokine-dependent and -independent pathways in liver regeneration. Semin Liver Dis 1999;19:117–27. [DOI] [PubMed] [Google Scholar]
- 28. Aydemir TB, Sitren HS, Cousins RJ. The zinc transporter Zip14 influences c-Met phosphorylation and hepatocyte proliferation during liver regeneration in mice. Gastroenterology 2012;142:1536-46e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borowiak M, Garratt AN, Wüstefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci USA 2004;101:10608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bellomo E, Birla Singh K, Massarotti A, Hogstrand C, Maret W. The metal face of protein tyrosine phosphatase 1B. Coord Chem Rev 2016;327–28:70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang T, Li L, Tang J, Li Y, Lin WY, Martin F, Grant D, Solloway M, Parker L, Ye W et al. . A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol 2010;28:749–55. [DOI] [PubMed] [Google Scholar]
- 32. Aydemir TB, Chang S-M, Guthrie GJ, Maki AB, Ryu MS, Karabiyik A, Cousins RJ. Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (endotoxemia). PLoS One 2012;7:e48679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wessels I, Cousins RJ. Zinc dyshomeostasis during polymicrobial sepsis in mice involves zinc transporter Zip14 and can be overcome by zinc supplementation. Am J Physiol Gastrointest Liver Physiol 2015;309:G768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aydemir TB, Troche C, Kim M-H, Cousins RJ. Hepatic ZIP14-mediated zinc transport contributes to endosomal insulin receptor trafficking and glucose metabolism. J Biol Chem 2016;291:23939–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tejwani GA, Pedrosa FO, Pontremoli S, Horecker BL. Dual role of Zn2+ as inhibitor and activator of fructose 1,6-bisphosphatase of rat liver. Proc Natl Acad Sci USA 1976;73:2692–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quarterman J, Florence E. Observations on glucose tolerance and plasma levels of free fatty acids and insulin in the zinc-deficient rat. Br J Nutr 1972;28:75–9. [DOI] [PubMed] [Google Scholar]
- 37. Brand IA, Kleineke J. Intracellular zinc movement and its effect on the carbohydrate metabolism of isolated rat hepatocytes. J Biol Chem 1996;271:1941–9. [DOI] [PubMed] [Google Scholar]
- 38. Ellis CD, Wang F, MacDiarmid CW, Clark S, Lyons T, Eide DJ. Zinc and the Msc2 zinc transporter protein are required for endoplasmic reticulum function. J Cell Biol 2004;166325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Homma K, Fujisawa T, Tsuburaya N, Yamaguchi N, Kadowaki H, Takeda K, Nishitoh H, Matsuzawa A, Naguro I, Ichijo H. SOD1 as a molecular switch for initiating the homeostatic ER stress response under zinc deficiency. Mol Cell 2013;52:75–86. [DOI] [PubMed] [Google Scholar]
- 40. Kim M-H, Aydemir TB, Cousins RJ. Dietary zinc regulates apoptosis through the phosphorylated eukaryotic initiation factor 2 /activating transcription factor-4/C/EBP-homologous protein pathway during pharmacologically induced endoplasmic reticulum stress in livers of mice. J Nutr 2016;146:2180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim M-H, Aydemir TB, Kim J, Cousins RJ. Hepatic ZIP14-mediated zinc transport is required for adaptation to endoplasmic reticulum stress. Proc Natl Acad Sci USA 2017;114:E5805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gu F, Nguyên DT, Stuible M, Dubé N, Tremblay ML, Chevet E. Protein-tyrosine phosphatase 1B potentiates IRE1 signaling during endoplasmic reticulum stress. J Biol Chem 2004;279:49689–93. [DOI] [PubMed] [Google Scholar]
- 43. Delibegovic M, Zimmer D, Kauffman C, Rak K, Hong EG, Cho YR, Kim JK, Kahn BB, Neel BG, Bence KK. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes 2009;58:590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Agouni A, Mody N, Owen C, Czopek A, Zimmer D, Bentires-Alj M, Bence KK, Delibegović M. Liver-specific deletion of protein tyrosine phosphatase (PTP) 1B improves obesity- and pharmacologically induced endoplasmic reticulum stress. Biochem J 2011;438:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guthrie GJ, Aydemir TB, Troche C, Martin AB, Chang S-M, Cousins RJ. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am J Physiol Gastrointest Liver Physiol 2015;308:G171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zackular JP, Moore JL, Jordan AT, Juttukonda LJ, Noto MJ, Nicholson MR, Crews JD, Semler MW. Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat Med 2016;22:1330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ranaldi G, Ferruzza S, Canali R, Leoni G, Zalewski PD, Sambuy Y, Perozzi G, Murgia C. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFα. J Nutr Biochem 2013;24:967–76. [DOI] [PubMed] [Google Scholar]
- 48. Farhadi A, Keshavarzian A, Ranjbaran Z, Fields JZ, Banan A. The role of protein kinase C isoforms in modulating injury and repair of the intestinal barrier. J Pharmacol Exp Ther 2006;316:1–7. [DOI] [PubMed] [Google Scholar]
- 49. Inai T, Kobayashi J, Shibata Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur J Cell Biol 1999;78:849–55. [DOI] [PubMed] [Google Scholar]
- 50. Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 2002;115:4969–76. [DOI] [PubMed] [Google Scholar]
- 51. Tominaga K, Kagata T, Johmura Y, Hishida T, Nishizuka M, Imagawa M. SLC39A14, a LZT protein, is induced in adipogenesis and transports zinc. FEBS J 2005;272:1590–9. [DOI] [PubMed] [Google Scholar]
- 52. Maxel T, Smidt K, Larsen A, Bennetzen M, Cullberg K, Fjeldborg K, Lund S, Pedersen SB, Rungby J. Gene expression of the zinc transporter ZIP14 (SLC39a14) is affected by weight loss and metabolic status and associates with PPARγ in human adipose tissue and 3T3-L1 pre-adipocytes. BMC Obes 2015;2:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. MacDonald RS. The role of zinc in growth and cell proliferation. J Nutr 2000;130:1500S–8S. [DOI] [PubMed] [Google Scholar]
- 54. Hojyo S, Fukada T, Shimoda S, Ohashi W, Bin BH, Koseki H, Hirano T. The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth. PLoS One 2011;6:e18059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aydemir TB, Troche C, Kim J, Kim MH, Teran OY, Leeuwenburgh C, Cousins RJ. Aging amplifies multiple phenotypic defects in mice with zinc transporter Zip14 (Slc39a14) deletion. Exp Gerontol 2016;85:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kronenberg HM. Developmental regulation of the growth plate. Nature 2003;423:332–6. [DOI] [PubMed] [Google Scholar]
- 57. Mayo KE, Godfrey PA, Suhr ST, Kulik DJ, Rahal JO. Growth hormone-releasing hormone: synthesis and signaling. Recent Prog Horm Res 1995;50:35–73. [DOI] [PubMed] [Google Scholar]
- 58. Lang C, Murgia C, Leong M, Tan LW, Perozzi G, Knight D, Ruffin R, Zalewski P. Anti-inflammatory effects of zinc and alterations in zinc transporter mRNA in mouse models of allergic inflammation. AJP Lung Cell Mol Physiol 2006;292:L577–84. [DOI] [PubMed] [Google Scholar]
- 59. Chen H, Li C, Fang M, Zhu M, Li X, Zhou R, Li K, Zhao S. Understanding Haemophilus parasuis infection in porcine spleen through a transcriptomics approach. BMC Genomics 2009;10:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thambiayya K, Wasserloos K, Kagan VE, Stoyanovsky D, Pitt BR. A critical role for increased labile zinc in reducing sensitivity of cultured sheep pulmonary artery endothelial cells to LPS-induced apoptosis. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sayadi A, Nguyen A-T, Bard FA, Bard-Chapeau EA. Zip14 expression induced by lipopolysaccharides in macrophages attenuates inflammatory response. Inflamm Res 2013;62:133–43. [DOI] [PubMed] [Google Scholar]
- 62. Wex T, Grungreiff K, Schutte K, Stengritt M, Reinhold D. Expression analysis of zinc transporters in resting and stimulated human peripheral blood mononuclear cells. Biomed Rep 2014;2:217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA 1996;93:5624–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vert G, Briat JF, Curie C. Arabidopsis IRT2 gene encodes a root-periphery iron transporter. Plant J 2001;26:181–9. [DOI] [PubMed] [Google Scholar]
- 65. Grass G, Franke S, Taudte N, Nies DH, Kucharski LM, Maguire ME, Rensing C. The metal permease ZupT from Escherichia coli is a transporter with a broad substrate spectrum. J Bacteriol 2005;187:1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gao Y, Xu Y, Wu D, Yu F, Yang L, Yao Y, Liang Z, Lau ATY. Progressive silencing of the zinc transporter Zip8 (Slc39a8) in chronic cadmium-exposed lung epithelial cells. Acta Biochim Biophys Sin (Shanghai) 2017;49:444–9. [DOI] [PubMed] [Google Scholar]
- 67. Zhao N, Gao J, Enns CA, Knutson MD. ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. J Biol Chem 2010;285:32141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jenkitkasemwong S, Wang C-Y, Coffey R, Zhang W, Chan A, Biel T, Kim JS, Hojyo S, Fukada T, Knutson MD. SLC39A14 is required for the development of hepatocellular iron overload in murine models of hereditary hemochromatosis. Cell Metab 2015;22:138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nam H, Wang C-Y, Zhang L, Zhang W, Hojyo S, Fukada T, Knutson MD. ZIP14 and DMT1 in the liver, pancreas, and heart are differentially regulated by iron deficiency and overload: implications for tissue iron uptake in iron-related disorders. Haematologica 2013;98:1049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhao N, Zhang A-S, Worthen C, Knutson MD, Enns CA. An iron-regulated and glycosylation-dependent proteasomal degradation pathway for the plasma membrane metal transporter ZIP14. Proc Natl Acad Sci USA 2014;111:9175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Coffey R, Knutson MD. The plasma membrane metal-ion transporter ZIP14 contributes to nontransferrin-bound iron uptake by human β-cells. Am J Physiol Cell Physiol 2017;312:C169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hansen SL, Trakooljul N, Liu H-C, Moeser AJ, Spears JW. Iron transporters are differentially regulated by dietary iron, and modifications are associated with changes in manganese metabolism in young pigs. J Nutr 2009;139:1474–9. [DOI] [PubMed] [Google Scholar]
- 73. Hansen SL, Trakooljul N, Spears JW, Liu H-C. Age and dietary iron affect expression of genes involved in iron acquisition and homeostasis in young pigs. J Nutr 2010;140:271–7. [DOI] [PubMed] [Google Scholar]
- 74. Iyengar V, Pullakhandam R, Nair KM. Coordinate expression and localization of iron and zinc transporters explain iron-zinc interactions during uptake in Caco-2 cells: implications for iron uptake at the enterocyte. J Nutr Biochem 2012;23:1146–54. [DOI] [PubMed] [Google Scholar]
- 75. Xu J, Hwang JCY, Lees HA, Wohlgemuth SE, Knutson MD, Judge AR, Dupont-Versteegden EE, Marzetti E, Leeuwenburgh C. Long-term perturbation of muscle iron homeostasis following hindlimb suspension in old rats is associated with high levels of oxidative stress and impaired recovery from atrophy. Exp Gerontol 2012;47:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ding D, Salvi R, Roth JA. Cellular localization and developmental changes of Zip8, Zip14 and transferrin receptor 1 in the inner ear of rats. BioMetals 2014;27:731–44. [DOI] [PubMed] [Google Scholar]
- 77. Giorgi G, D'Anna MC, Roque ME. Iron homeostasis and its disruption in mouse lung in iron deficiency and overload. Exp Physiol 2015;100:1199–216. [DOI] [PubMed] [Google Scholar]
- 78. Sterling J, Guttha S, Song Y, Song D, Hadziahmetovic M, Dunaief JL. Iron importers Zip8 and Zip14 are expressed in retina and regulated by retinal iron levels. Exp Eye Res 2017;155:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fujishiro H, Yano Y, Takada Y, Tanihara M, Himeno S. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics 2012;4:700–8. [DOI] [PubMed] [Google Scholar]
- 80. Fujishiro H, Doi M, Enomoto S, Himeno S. High sensitivity of RBL-2H3 cells to cadmium and manganese: an implication of the role of ZIP8w. Metallomics 2011;3:710–8. [DOI] [PubMed] [Google Scholar]
- 81. Ma C, Schneider SN, Miller M, Nebert DW, Lind C, Roda SM, Afton SE, Caruso JA, Genter MB. Manganese accumulation in the mouse ear following systemic exposure. J Biochem Mol Toxicol 2008;22:305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fujishiro H, Yoshida M, Nakano Y, Himeno S. Interleukin-6 enhances manganese accumulation in SH-SY5Y cells: implications of the up-regulation of ZIP14 and the down-regulation of ZnT10. Metallomics 2014;6:944–9. [DOI] [PubMed] [Google Scholar]
- 83. Tuschl K, Meyer E, Valdivia LE, Zhao N, Dadswell C, Abdul-Sada A, Hung CY, Simpson MA, Chong WK, Jacques TS et al. . Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism and dystonia. Nat Commun 2016;7:11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Aydemir TB, Kim M-H, Kim J, Colon-Perez LM, Banan G, Mareci TH, Febo M, Cousins RJ. Metal transporter Zip14 (Slc39a14) deletion in mice increases manganese deposition and produces neurotoxic signatures and diminished motor activity. J Neurosci 2017;37:5996–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol 2003;157:1015–22. [DOI] [PubMed] [Google Scholar]
- 86. Bertinchamps AJ, Miller ST, Cotzias GC. Interdependence of routes excreting manganese. Am J Physiol 1966;211:217–24. [DOI] [PubMed] [Google Scholar]
- 87. Schramm VL, Brandt M. The manganese(II) economy of rat hepatocytes. Fed Proc 1986;45:2817–20. [PubMed] [Google Scholar]
- 88. Xin Y, Gao H, Wang J, Qiang Y, Imam MU, Li Y, Wang J, Zhang R, Zhang H, Yu Y et al. . Manganese transporter Slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice. Cell Discov 2017;3:17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M. Manganese is essential for neuronal health. Annu Rev Nutr 2015;35:71–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Anazi S, Maddirevula S, Faqeih E, Alsedairy H, Alzahrani F, Shamseldin HE, Patel N, Hashem M, Ibrahim N, Abdulwahab F et al. . Clinical genomics expands the morbid genome of intellectual disability and offers a high diagnostic yield. Mol Psychiatry 2017;22:615–24. [DOI] [PubMed] [Google Scholar]
- 91. Nakamura Y, Ohba K-I, Ohta H. Participation of metal transporters in cadmium transport from mother rat to fetus. J Toxicol Sci 2012;37:1035–44. [DOI] [PubMed] [Google Scholar]
- 92. Min K-S, Takano M, Amako K, Ueda H, Tanaka K. Involvement of the essential metal transporter Zip14 in hepatic Cd accumulation during inflammation. Toxicol Lett 2013;218:91–6. [DOI] [PubMed] [Google Scholar]
- 93. Jorge-Nebert LF, Gálvez-Peralta M, Landero Figueroa J, Somarathna M, Hojyo S, Fukada T, Nebert DW. Comparing gene expression during cadmium uptake and distribution: untreated versus oral Cd-treated wild-type and ZIP14 knockout mice. Toxicol Sci 2015;143:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rentschler G, Kippler M, Axmon A, Raqib R, Skerfving S, Vahter M, Broberg K. Cadmium concentrations in human blood and urine are associated with polymorphisms in zinc transporter genes. Metallomics 2014;6:885–91. [DOI] [PubMed] [Google Scholar]
- 95. Liu Y, Zhu X, Zhu J, Liao S, Tang Q, Liu K, Guan X, Zhang J, Feng Z. Identification of differential expression of genes in hepatocellular carcinoma by suppression subtractive hybridization combined cDNA microarray. Oncol Rep 2007;18:943–51. [PubMed] [Google Scholar]
- 96. Danielsen A, Steinnes E. A study of some selected trace elements in normal and cancerous tissue by neutron activation analysis. J Nucl Med 1970;11:260–4. [PubMed] [Google Scholar]
- 97. Al-Ebraheem A, Farquharson MJ, Ryan E. The evaluation of biologically important trace metals in liver, kidney and breast tissue. Appl Radiat Isot 2009;67:470–4. [DOI] [PubMed] [Google Scholar]
- 98. Franklin RB, Levy BA, Zou J, Hanna N, Desouki MM, Bagasra O, Johnson LA, Costello LC. ZIP14 zinc transporter downregulation and zinc depletion in the development and progression of hepatocellular cancer. J Gastrointest Cancer 2012;43:249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang L, Liu X, Zhang X, Chen R. Identification of important long non-coding RNAs and highly recurrent aberrant alternative splicing events in hepatocellular carcinoma through integrative analysis of multiple RNA-Seq datasets. Mol Genet Genomics 2016;291:1035–51. [DOI] [PubMed] [Google Scholar]
- 100. Thorsen K, Mansilla F, Schepeler T, Øster B, Rasmussen MH, Dyrskjøt L, Karni R, Akerman M, Krainer AR, Laurberg S et al. . Alternative splicing of SLC39A14 in colorectal cancer is regulated by the Wnt pathway. Mol Cell Proteomics 2011;10:M110.002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sveen A, Bakken AC, Ågesen TH, Lind GE, Nesbakken A, Nordgård O, Brackmann S, Rognum TO, Lothe RA, Skotheim RI. The exon-level biomarker SLC39A14 has organ-confined cancer-specificity in colorectal cancer. Int J Cancer 2012;131:1479–85. [DOI] [PubMed] [Google Scholar]
- 102. Xu X-M, Wang C-G, Zhu Y-D, Chen WH, Shao SL, Jiang FN, Liao QD. Decreased expression of SLC 39A14 is associated with tumor aggressiveness and biochemical recurrence of human prostate cancer. Onco Targets Ther 2016;9:4197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. King JC, Cousins RJ. Zinc. In: Ross AC, editor. Modern nutrition in health and disease. 11th ed Philadelphia: Wolters Kluwer Health/Lippincott, Williams & Wilkins;2014. p. 189–205. [Google Scholar]
- 104. Lee D-H, Liu DY, Jacobs DR, Shin HR, Song K, Lee IK, Kim B, Hider RC. Common presence of non-transferrin-bound iron among patients with type 2 diabetes. Diabetes Care 2006;29:1090–5. [DOI] [PubMed] [Google Scholar]
- 105. Brittenham GM, Andersson M, Egli I, Foman JT, Zeder C, Westerman ME, Hurrell RF. Circulating non-transferrin-bound iron after oral administration of supplemental and fortification doses of iron to healthy women: a randomized study. Am J Clin Nutr 2014;100:813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Buchman AA. Manganese. In: Ross AC, editor. Modern nutrition in health and disease. 11th ed.Philadelphia: Wolters Kluwer Health/Lippincott, Williams & Wilkins;2014. p. 238–44. [Google Scholar]
- 107. Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S et al. . Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 2010;115:4284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, Jhang J, Schwartz J, Sharma S, Sheth S et al. . Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood 2011;118:6675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]