ABSTRACT
Background
Hypophosphatemia (HYP) is common among calcium stone formers (SFs) and in rare cases is associated with mutations in sodium-phosphate cotransporters or in Na+/H+ exchanger regulatory factor 1 (NHERF1), but the majority of cases are unexplained. We hypothesized that reduced sodium-phosphate cotransporter activity mediated via NHERF1 or a similar PDZ domain–containing protein, causes HYP. If so, other transport activities controlled by NHERF1, such as NHE3 and URAT1, might be reduced in HYP.
Methods
To test this idea, we analyzed two large but separate sets of 24-h urine samples and paired serums of 2700 SFs from the University of Chicago and 11 073 SFs from Litholink, a national laboratory. Patients were divided into quintiles based on serum phosphate.
Results
Males were more common in the lowest phosphate tiles in both datasets. Phosphate excretion did not vary across the quintiles, excluding diet as a cause of HYP. Tubule maximum (Tm) phosphate per unit glomerular filtration rate decreased and fractional excretion increased with decreasing phosphate quintiles, indicating reduced tubule phosphate reabsorption was responsible for HYP. Urine pH and serum chloride increased with decreasing serum phosphate, suggesting a coordinate change in NHE3 activity. Serum uric acid and Tm uric acid decreased significantly with decreasing serum phosphate, while uric acid excretion did not vary.
Conclusion. HYP in SFs results from decreased tubule phosphate reabsorption and, being associated with related changes in other proximal tubule transporters, may arise from alterations in or signaling to PDZ-containing proteins.
Keywords: calcium, nephrolithiasis, NHERF1, phosphate, uric acid
INTRODUCTION
Patients who form calcium renal stones [stone formers (SFs)] [1], most specifically the large subset of SFs with unexplained, normocalcemic, ‘idiopathic’ hypercalciuria (IH) [2], frequently exhibit hypophosphatemia (HYP). Renal tubule phosphate reabsorption gauged by tubule maximum (Tm) phosphate transport per unit glomerular filtration rate (GFR) is reduced but the mechanism is not fully known. Because reduced phosphate intake increased, and 2 months of oral phosphate loading decreased serum phosphate and Tm phosphate, Barilla et al. [3] considered HYP in SFs as possibly a renal adaptation to a high phosphate diet. Prié et al. [2] documented HYP in male SFs and, like Barilla et al., dissociated it from elevated serum parathyroid hormone (PTH), calcitriol or calcium level. Even so, urine calcium excretion was higher in patients with HYP and reduced Tm phosphate. In SFs, Steiniche et al. [4] found a higher than normal urine phosphorus:creatinine ratio and suggested a renal phosphate leak contributed to HYP and reduced bone mineral content. Others have also found reduced Tm phosphate and increased fractional excretion of phosphate (FEP) in hypophosphatemic SFs [5, 6].
Abnormality of the sodium phosphate cotransporters Npt2a and Npt2c may cause HYP in SFs. Prié et al. [7] reported two patients with nephrolithiasis and persistent HYP (serum phosphate level <2.48 mg/dL, 0.8 mmol/L) who were found to be heterozygous for separate mutations in SLC34A1, the gene encoding Npt2a. Magen et al. [8] identified two siblings with bone disease and Fanconi’s syndrome who had homozygous mutations of SLC34A1 that resulted in phosphaturia and HYP. Functional studies of the mutant Npt2a in Xenopuslaevis oocytes revealed a defect in localization of the protein to the plasma membrane, suggesting that intracellular accumulation of the mutant protein was responsible not only for impaired renal phosphate reabsorption, but possibly a more generalized proximal tubule dysfunction. More recently, a genome-wide association study performed in Iceland found nine common DNA sequence variants located in or near SLC34A1 to be significantly associated with kidney stones [9]. The strongest marker was associated with both stones and decreased serum phosphate, as well as reduced serum PTH. Mutations in SLC34A1 have also been found to cause idiopathic infantile hypercalcemia with associated phosphate wasting, hypercalciuria and nephrocalcinosis [10]. Mutations of SLC34A3, the gene that encodes Npt2c, cause hereditary hypophosphatemic rickets with hypercalciuria, and one person, a compound heterozygote with two mutations in this gene, presented with renal stones as well [11]. Such mutations of this gene cannot be responsible for the HYP of common SFs because rickets is rare.
Defects in SLC9A3R1, encoding Na+/H+ exchanger regulatory factor 1 (NHERF1) also cause abnormalities in renal phosphate handling. NHERF1 null mice have increased phosphaturia and lower serum phosphate concentrations compared with wild-type mice [12]. In a study of patients with HYP and renal stones, bone mineral reduction, or both, 4 of 92 patients had mutations of NHERF1 [13]. NHERF1 binds to both NPT2a and the PTH receptor, facilitating hormonal control of phosphate reabsorption. The mutant NHERF1 genes produced more cyclic adenosine monophosphate in response to PTH than the wild type, leading to phosphaturia and HYP [13]. Another study identified a patient with calcium lithiasis and phosphate wasting who was found to have a mutation in the PDZ1 domain of NHERF1 [14]. NHERF1 is a scaffolding protein that links to other transporters and receptors via its two PDZ domains; the PDZ1 domain is critical for NHERF1–NPT2a interaction in humans and for localization of NPT2a to the plasma membrane.
NHERF1 belongs to a family of four structurally related PDZ domain–containing proteins (NHERF1–4) that regulate the apical membrane targeting of multiple transmembrane proteins and anchoring of these proteins to the actin cytoskeleton [15]. NHERF proteins can form homo- and heterodimers or undergo oligomerization, forming large scaffolding complexes of multiple NHERF isoforms. In the proximal tubule there is evidence that NHERF1 and NHERF3 (PDZK1), alone or in combination, are especially important in regulating the membrane abundance, localization and function of many transporter proteins in addition to NPT2a [16–19]. Indeed, PDZK1 regulates the activity of URAT1 [20], the major luminal urate reabsorption transporter; a variant of PDZK1 has been found to be associated with serum uric acid levels and gout susceptibility [21].
Although mutations of NHERF1 seem too uncommon to explain it, the HYP of ordinary calcium SFs could be mediated via NHERF1 or another component of the scaffolding complex as a final effector of transport function. If NHERF1 were indeed part of such a common pathway, HYP should not arise from diet phosphate loading and should coordinate with changes in other transport functions regulated through NHERF1. Several predictions would follow: (i) HYP must be due to reduced tubule phosphate reabsorption without an increase in urine phosphate excretion; this is a requirement if HYP is not an adaptation to high phosphate intake. (ii) Since NHERF1 regulates the sodium–hydrogen exchanger NHE3 [22, 23], and NHERF1 effects on NHE3 are inhibitory [24], signaling through NHERF1 that reduces phosphate transport [13] may also reduce proximal tubule bicarbonate transport. Therefore one might expect increased urine pH with HYP, due to increased distal bicarbonate delivery, and possibly an increase in serum chloride or a decrease in serum total CO2 content. (iii) Since NHERF1 can regulate the major urate transporter URAT1 in mice [25], and URAT1 is also the apical membrane transporter for urate in humans [26, 27] and can associate with NHERF1 via its PDZ domain, one might expect that serum urate levels and tubule urate reabsorption would decrease with serum phosphate. We have used large numbers of SFs from two independent sources to test the above three predictions of integrated regulation of tubular transport through a common pathway that includes NHERF1.
MATERIALS AND METHODS
Data sources
We obtained our data from the University of Chicago Kidney Stone Program (UC) and Litholink Corporation (LL). The former is a patient care program with its own clinical laboratory that has been in existence since 1969; the latter is a national kidney stone testing service. We excluded patients with bowel disease, primary hyperparathyroidism, renal tubular acidosis and cystinuria. At UC, being the physicians for the patients, we could also exclude vitamin D excess, sarcoidosis, drug-induced stones or stones composed of or containing any amount of struvite, uric acid or cystine; at LL we determined exclusions via intake telephone interviews.
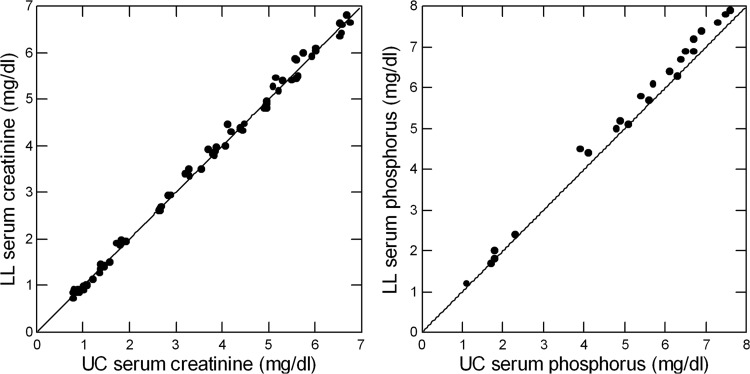
The laboratories have exchanged >300 urine crossover samples since the founding of LL in 1995. Regressing LL on UC data gives slopes of 1.02 ± 0.01, 1.04 ± 0.01, 1.01 ± 0.01 and 0.99 ± 0.01 for urine creatinine, phosphorus, uric acid and chloride concentrations, respectively (r2 = 0.99, P < 0.001 for all). Crossover of serum samples has not occurred. Using serum proficiency testing data from the College of American Pathologists from 2004 to 2008, serum creatinine values (Figure 1, left panel) for the two laboratories lie along the line of identity (slope = 1.01 ± 0.01, r2 = 0.99). Serum phosphorus values (Figure 1, right panel) are slightly higher at LL compared with UC (slope = 1.04 ± 0.02, r2 = 0.99). Regression slopes for serum uric acid and chloride (not illustrated graphically) were 0.98 ± 0.01 and 0.99 ± 0.02, respectively (r2 = 0.99, P < 0.001 for all four values). Intercepts for all regressions (data not shown) do not differ from zero except urine uric acid (0.78 ± 0.37, P = 0.04). Our hypothesis testing does not require that we compare results from UC to LL. We comment on the differences we encountered without further analysis.
FIGURE 1.
Comparison of serum creatinine (left panel) and phosphorus (right panel) measurements from College of American Pathologists proficiency testing for UC and LL. The diagonal line in each graph is the line of identity; regression values are provided in the ‘Materials and methods’ section.
At UC, all patients were studied before treatment with three 24-h urine samples, each with a corresponding blood sample drawn in the 14-h postabsorptive state between 7 and 9 a.m. At the time of their collections, UC patients were not taking any medications that could affect stones or mineral metabolism, such as thiazides and other diuretic agents, vitamin D supplements or alkali supplements. LL patients were instructed to refrain from taking these medications during the collection periods but compliance was not determined. Blood samples were paired with their urine samples and planned for the fasting period, before treatment, at LL as well, but ascertainment relied on patient reporting. Patients at LL collected one or two 24-h urine samples before treatment, depending on the choice of their physician. Methods for urine collection at UC and LL are detailed elsewhere [28–30].
Serum and urine measurements
For each blood sample we measured serum calcium, phosphorus, magnesium, sodium, potassium, total CO2 content, chloride, uric acid and creatinine. In each urine sample we measured volume, pH, calcium, phosphorus, magnesium, sodium, potassium, uric acid, creatinine, oxalate, citrate, chloride, ammonium and sulfate. From these we calculated supersaturation (SS) with respect to calcium oxalate, calcium phosphate and uric acid. Measurements were made at UC and LL using methods detailed elsewhere [31, 32]. SS was calculated using EQUIL 2 [33].
Initial outlier screening
Blood and urine samples were averaged so each SFs is represented by one row of data. Although we sought to exclude systemic diseases, we found some outlier urine results and abnormal serum data. The former were a mixture of values so extreme as to suspect laboratory or collection errors or physiological extremes of sodium, potassium or mineral depletions that would be expected in patients with bowel disease or eating disorders. The latter suggested systemic diseases that we did not successfully exclude.
We used the following cut points to eliminate rows (SFs) (suspected causes are in parentheses) of 24-h urine values: citrate <50 mg/day (potassium depletion, renal tubular acidosis), chloride <20 mmol/day (sodium depletion, vomiting), creatinine <100 or >3000 mg/day (collection artifacts), potassium <10 mmol/day (potassium depletion, vomiting, prior diuretics), magnesium <20 mg/day (gastrointestinal disease, eating disorders, prior diuretic-induced magnesium depletion), sodium <20 mmol/day (sodium depletion), ammonium ion <10 (use of alkali or abnormal diet) or >100 mmol/day (infection with bacteria that express urease), oxalate >90 mg/day (primary or enteric hyperoxaluria) and pH <4.5 or >7.9 (errors, infection). For serum measurements we need not list possible causes, as they are apparent: calcium <8.5 or >10.8 mg/dL, chloride <80 or >112 mmol/L, total CO2 content <20 or >33 mmol/L, creatinine <0.4 or >2 mg/dL, potassium <3 or >5.5 mmol/L, magnesium <1.2 or >2.5 mg/dL, sodium <135 or >145 mmol/L, phosphate <1 or >5 mg/dL, uric acid >12 mg/dL. Finally, we excluded subjects with <20 or >200 kg body weight.
Patients
From the UC registry of 4187 patients (1509 females) through 2008, we initially selected 3242 patients (1112 females) without known systemic diseases; of these, 83% (2700) of patients (923 females) remained after outlier exclusions. Of these, 1108 formed calcium oxalate stones containing <50% calcium phosphate, 137 formed stones containing >50% calcium phosphate and the rest—1455—were classified as having calcium stones of unspecified crystallographic composition. From an initial 12 839 patients (5586 females) at LL who had both blood and urine data, 86% (11 073) of cases (4798 females) remained after screening for outlier values. Stone analysis results are not available at LL in sufficient numbers to warrant further evaluation.
Analysis
Formation of serum phosphate subgroups
In deference to US clinical usage, we present mg/dL rather than mmol/L and refer to serum ‘phosphate’ even though we measure phosphorus. Using the screened populations from UC and LL, sexes combined, we divided patients into five tiles of serum phosphate via four cut points: UC: 2.687, 2.953, 3.197, 3.470; LL: 2.776, 3.090, 3.360, 3.700 (all values are mg/dL). Tiles are calculated to contain approximately equal numbers of patients. For comparison, among 217 normal subjects with values measured at UC, not otherwise presented here, corresponding values were 2.950, 3.270, 3.552 and 3.850.
Values central to hypothesis tests
Key values for this analysis were serum and urine creatinine, phosphate and uric acid for calculation of Tm per unit GFR and fractional excretion (FE) for phosphate (TmP and FEP) and uric acid (TmUa and FEUa), as well as urine pH, and serum total CO2 content and chloride concentration. Secondary values included urine citrate and potassium excretion, considered as reflecting possible increased distal bicarbonate delivery.
Values were analyzed by sex and phosphate tiles by analysis of variance (ANOVA). Values were further analyzed with adjustment for age, body weight and, as applicable, urine creatinine and sodium excretion, using general linear modeling. We recognize that bicarbonate handling could also have been calculated to our advantage, but our measurement set lacks urine bicarbonate. Because we hypothesize that key variables should change directionally with serum phosphate, we analyzed unadjusted and adjusted values for trend using polynomial contrast of order 1 (linear trending) within either the simple model by sex and tile, or the more complex fully adjusted model. We did not analyze men and women separately, as our hypothesis testing does not require it.
Other variables of special interest
Serum and urine calcium and magnesium are unrelated to the central hypothesis but have intrinsic interest in SFs. Urine calcium and magnesium levels, and FE of magnesium (FEMg) were analyzed and appear in the final tables. FE of calcium (FECa) was not significantly variable between tiles at either site and is not presented further. Urine citrate and potassium differed with phosphate tiles at UC and LL and are presented in the Results section.
Calculations
Tm/GFR for phosphate and urate was calculated as [34, 35]:
| (1) |
where U(x) is urine and S(x) is serum concentration of x and Cr is creatinine. This expression is the difference between serum concentration and the tubule concentration one calculates from urine concentration corrected for the urine/serum creatinine ratio, in other words tubule concentration corrected back for water extraction using creatinine as a marker. Tm(x) is also absolute reabsorption of x (R(x)) per unit of glomerular filtration rate (g), estimated here by creatinine clearance, since by mass balance the filtered load is the sum of urine excretion and absolute reabsorption:
| (2) |
where V is urine volume flow. Dividing Equation (2) through by g will yield Equation (1). Put another way, the change in concentration in Equation (1) (Tm(x)), multiplied by g, is R, and one could calculate Tm just that way. Looked at a third way, Tm(x) divided through by S(x) will give fractional reabsorption, R, divided by filtered load:
| (3) |
the right-hand term is the definition of fractional reabsorption in which urine volume has been cancelled out. The term in brackets is FE(x), urine excretion of x divided by filtered load:
| (4) |
In our analysis we considered both Tm(x) and FE(x) for phosphate and urate, as they interact differently with S(x) (a difference and a ratio) and have somewhat different physiological interpretations.
Statistical methods
The formation of tiles, ANOVA, general linear modeling, contrasts (for trend analysis) and table analyses were performed using standard software (Systat, San Jose, CA, USA).
RESULTS
Patient characteristics
Gender, age and body weight of subjects
The fraction of females varies among the five tiles at both sites, rising progressively for tiles 1–5, successively: UC 20, 29, 33, 40 and 49% females; LL 27, 36, 44, 51 and 59%, χ2 =115 and 572, respectively, P < 0.001 for both sites (Tables 1 and 2). At UC, age decreased across the tiles (Tables 1 and 2; effects of tiles and sex, P < 0.001; cross product of tile and sex, P < 0.001). At LL, age dependence on tiles was borderline (P = 0.049); as is obvious, age was lower in females. Body weight varied at UC and LL by tile and sex and the cross product of sex by tile was significant (P < 0.001) at UC and not significant at LL. Because of the marked sex imbalance, males predominant in the lowest phosphate tiles, the sexes are presented separately; our regression models use both sexes together with sex as a factor. Age and body weight are incorporated in our models to adjust for the variable relationship they have to phosphate tile at the two sites.
Table 1.
Selected unadjusted values by phosphate tile and sex (UC)
| Men |
Women |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UC tile (n) | 1 (432) | 2 (381) | 3 (364) | 4 (326) | 5 (274) | 1 (109) | 2 (154) | 3 (181) | 4 (213) | 5 (266) |
| Age (year)a | 46 ± 1 | 47 ± 1 | 45 ± 1 | 45 ± 1 | 40 ± 1 | 41 ± 1 | 43 ± 1 | 42 ± 1 | 42 ± 1 | 40 ± 1 |
| Weight (kg)a | 88 ± 1 | 86 ± 1 | 84 ± 1 | 84 ± 1 | 81 ± 1 | 75 ± 1 | 75 ± 1 | 69 ± 1 | 72 ± 1 | 68 ± 1 |
| S Phos (mg/dL)a | 2.4 ± 0.01 | 2.8 ± 0.01 | 3.1 ± 0.01 | 3.3 ± 0.01 | 3.8 ± 0.01 | 2.5 ± 0.02 | 2.8 ± 0.01 | 3.1 ± 0.01 | 3.3 ± 0.01 | 3.8 ± 0.01 |
| U Phos (mg/day) | 1041 ± 12 | 1003 ± 13 | 1015 ± 13 | 1032 ± 14 | 988 ± 15 | 748 ± 25 | 783 ± 21 | 740 ± 19 | 763 ± 18 | 735 ± 16 |
| CCr (L/day)b | 179 ± 2 | 177 ± 2 | 177 ± 2 | 177 ± 2 | 177 ± 2 | 151 ± 3 | 149 ± 3 | 146 ± 3 | 142 ± 3 | 138 ± 2 |
| FEP (%)a | 25 ± 0.2 | 20 ± 0.2 | 19 ± 0.3 | 18 ± 0.3 | 15 ± 0.3 | 20 ± 0.5 | 19 ± 0.4 | 17 ± 0.4 | 16 ± 0.3 | 14 ± 0.3 |
| TmP (mg/dL)a | 1.82 ± 0.01 | 2.25 ± 0.01 | 2.49 ± 0.01 | 2.72 ± 0.01 | 3.2 ± 0.01 | 2 ± 0.02 | 2.3 ± 0.02 | 2.56 ± 0.02 | 2.79 ± 0.02 | 3.26 ± 0.01 |
| UpH | 6.04 ± 0.02 | 6.03 ± 0.02 | 6.0 ± 0.02 | 5.98 ± 0.02 | 6.01 ± 0.03 | 6.11 ± 0.04 | 6.06 ± 0.03 | 6.17 ± 0.03 | 6.07 ± 0.03 | 6.09 ± 0.03 |
| S Cl (mEq/L)a | 106.6 ± 0.2 | 106.7 ± 0.2 | 106.3 ± 0.3 | 105.3 ± 0.3 | 105.6 ± 0.3 | 107.6 ± 0.3 | 107.2 ± 0.3 | 106.3 ± 0.3 | 106.9 ± 0.2 | 106.4 ± 0.2 |
| S Ua (mg/dL) | 5.9 ± 0.1 | 5.9 ± 0.1 | 5.9 ± 0.1 | 6.0 ± 0.1 | 5.9 ± 0.1 | 4.4 ± 0.1 | 4.4 ± 0.1 | 4.4 ± 0.1 | 4.6 ± 0.1 | 4.5 ± 0.1 |
| UUa (mg/day)b | 709 ± 8 | 701 ± 9 | 690 ± 9 | 690 ± 9 | 689 ± 10 | 556 ± 16 | 552 ± 13 | 534 ± 12 | 549 ± 11 | 509 ± 10 |
| TmUa (mg/dL) | 5.5 ± 0.06 | 5.54 ± 0.06 | 5.52 ± 0.06 | 5.61 ± 0.07 | 5.55 ± 0.07 | 4.03 ± 0.12 | 4.1 ± 0.1 | 4 ± 0.1 | 4.2 ± 0.08 | 4.13 ± 0.07 |
| FEUa (%) | 7.1 ± 0.1 | 7 ± 0.1 | 6.9 ± 0.1 | 6.8 ± 0.1 | 6.8 ± 0.1 | 8.5 ± 0.2 | 8.8 ± 0.1 | 9.1 ± 0.2 | 9 ± 0.2 | 8.7 ± 0.1 |
| UCa (mg/day)c | 257 ± 5 | 239 ± 5 | 232 ± 5 | 237 ± 5 | 235 ± 6 | 204 ± 10 | 201 ± 8 | 196 ± 7 | 192 ± 7 | 175 ± 6 |
| UMg (mg/day) | 112 ± 2 | 107 ± 2 | 107 ± 2 | 110 ± 2 | 113 ± 2 | 84 ± 3 | 87 ± 3 | 88 ± 2 | 87 ± 2 | 87 ± 2 |
S, serum; Phos, phosphate; FEP, fractional excretion of phosphorus; FEUa, fractional excretion of uric acid; CCr, creatinine clearance; Ua, uric acid; U, urine; TmP, tubular maximum for phosphate; TmUa, tubular maximum for urate; Ca, calcium; Mg, magnesium; Cl, chloride. aDiffers across tiles within sex (P < 0.001; bP < 0.05; cP < 0.01). All values differ by sex, (P < 0.05). All variables significant by tile are significant by trend analysis (P < 0.05).
Table 2.
Selected unadjusted values by phosphate tile and sex (LL)
| Men |
Women |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LL tile (n) | 1 (1613) | 2 (1429) | 3 (1258) | 4 (1070) | 5 (905) | 1 (601) | 2 (800) | 3 (978) | 4 (1111) | 5 (1308) |
| Age (year)a | 52 ± 0.3 | 52 ± 0.3 | 52 ± 0.3 | 51 ± 0.4 | 48 ± 0.4 | 42 ± 0.5 | 44 ± 0.5 | 45 ± 0.4 | 45 ± 0.4 | 47 ± 0.4 |
| Weight (kg)b | 91 ± 1 | 91 ± 1 | 91 ± 1 | 92 ± 1 | 92 ± 1 | 78 ± 1 | 76 ± 1 | 75 ± 1 | 74 ± 1 | 75 ± 1 |
| S Phos (mg/dL)c | 2.5 ± 0.01 | 2.9 ± 0.01 | 3.2 ± 0.01 | 3.5 ± 0.01 | 4 ± 0.01 | 2.5 ± 0.01 | 2.9 ± 0.01 | 3.2 ± 0.01 | 3.5 ± 0.01 | 4 ± 0.01 |
| U Phos (mg/day) | 1130 ± 8 | 1143 ± 9 | 1164 ± 9 | 1126 ± 10 | 1139 ± 11 | 849 ± 13 | 851 ± 12 | 852 ± 11 | 850 ± 10 | 832 ± 9 |
| CCr (L/d)c | 167 ± 1 | 166 ± 1 | 168 ± 1 | 163 ± 2 | 165 ± 2 | 146 ± 2 | 141 ± 1 | 139 ± 1 | 136 ± 1 | 131 ± 1 |
| FEP (%)c | 28 ± 0.1 | 24 ± 0.2 | 22 ± 0.2 | 20 ± 0.2 | 18 ± 0.2 | 24 ± 0.2 | 21 ± 0.2 | 19 ± 0.2 | 18 ± 0.2 | 16 ± 0.2 |
| TmP (mg/dL)c | 1.78 ± 0.01 | 2.24 ± 0.01 | 2.52 ± 0.01 | 2.81 ± 0.01 | 3.3 ± 0.01 | 1.91 ± 0.01 | 2.34 ± 0.01 | 2.61 ± 0.01 | 2.89 ± 0.01 | 3.39 ± 0.01 |
| UpHa | 6 ± 0.01 | 5.98 ± 0.01 | 5.97 ± 0.01 | 5.96 ± 0.01 | 5.97 ± 0.01 | 6.16 ± 0.02 | 6.12 ± 0.02 | 6.11 ± 0.02 | 6.11 ± 0.01 | 6.09 ± 0.01 |
| S Cl (mEq/L)c | 106.4 ± 0.1 | 106 ± 0.1 | 105.8 ± .1 | 105.6 ± 0.1 | 105.2 ± .1 | 106.8 ± 0.1 | 106.5 ± 0.1 | 106.2 ± 0.1 | 106.1 ± 0.1 | 105.7 ± .1 |
| S Ua (mg/dL)c | 6.14 ± 0.03 | 6.19 ± 0.03 | 6.21 ± 0.04 | 6.23 ± 0.04 | 6.24 ± 0.04 | 4.78 ± 0.05 | 4.83 ± 0.04 | 4.84 ± 0.04 | 4.81 ± 0.04 | 5.02 ± 0.03 |
| UUa (mg/day) | 750 ± 5 | 753 ± 5 | 762 ± 6 | 747 ± 6 | 765 ± 7 | 611 ± 8 | 599 ± 7 | 596 ± 7 | 593 ± 6 | 586 ± 6 |
| TmUa (mg/dL)b | 5.68 ± 0.03 | 5.73 ± 0.03 | 5.74 ± 0.03 | 5.77 ± 0.04 | 5.77 ± 0.04 | 4.36 ± 0.05 | 4.39 ± 0.04 | 4.4 ± 0.04 | 4.36 ± 0.04 | 4.56 ± 0.03 |
| FEUa (%) | 7.86 ± 0.08 | 7.73 ± 0.09 | 7.68 ± 0.09 | 7.73 ± 0.1 | 7.88 ± 0.11 | 9.25 ± 0.1 | 9.37 ± 0.12 | 9.46 ± 0.11 | 9.68 ± 0.1 | 9.6 ± 0.09 |
| UCa (mg/day) | 244 ± 3 | 244 ± 3 | 241 ± 3 | 240 ± 3 | 245 ± 4 | 222 ± 5 | 212 ± 4 | 210 ± 4 | 210 ± 3 | 205 ± 3 |
| UMg (mg/day)c | 113 ± 1 | 115 ± 1 | 115 ± 1 | 112 ± 1 | 119 ± 1 | 87 ± 2 | 89 ± 1 | 90 ± 1 | 94 ± 1 | 93 ± 1 |
S, serum; Phos, phosphate; FEP, fractional excretion of phosphorus; FEUa, fractional excretion of uric acid; CCr, creatinine clearance; Ua, uric acid; U, urine; TmP, tubular maximum for phosphate; TmUa, tubular maximum for urate; Ca, calcium; Mg, magnesium; Cl, chloride. adiffers across tiles within sex, P < 0.05; bP < 0.01; cP < 0.001. All values differ by sex P < 0.05. All variables significant by tile are significant by trend analysis (P < 0.05) except age and weight which are not significant for trend. FEUa and urine calcium excretion were significant for trend, not by tile (P < 0.05).
Clinical findings at UC
Clinical data concerning stones and stone-related morbidities were adjusted for age and sex and for the interval from first stone to entry into our program; stone-related morbidities were further adjusted for numbers of stones. None of the following showed any variation by tile: number of stones, procedures, emergency room visits, infections, hospitalizations and systolic and diastolic blood pressure. These negative clinical findings are not presented further. At UC we have enough data to assess whether the percent of calcium phosphate in stones varied with tiles; it did not and this is not presented further. LL could not provide sufficient clinical data for a corresponding analysis.
Serum and urine phosphate
Unadjusted values by sex and phosphate tile
By design, unadjusted serum phosphate levels rose at UC and LL progressively through the five phosphate tiles of each sex (Tables 1 and 2). The 24-h urine phosphate excretion did not vary among tiles at UC or LL. Creatinine clearance values varied among tiles at both sites and decreased with increasing tile number—e.g. with higher serum phosphate levels. TmP fell and FEP rose progressively with decreasing serum phosphate at both sites and in both sexes (Tables 1 and 2). In other words, low serum phosphate values reflect reduced TmP and increased FEP.
Values adjusted for sex, age, body weight, urine creatinine and urine sodium
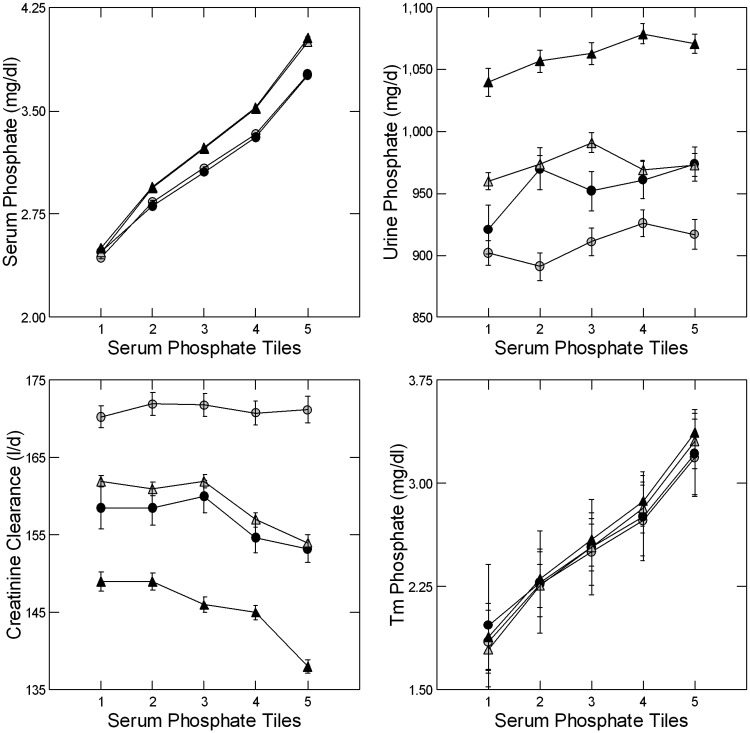
Like the unadjusted values (Tables 1 and 2), adjusted serum phosphate (Figure 2, upper left panel) varied with tiles (Table 3). Although male and female values overlap (Figure 2, upper left panel), the interaction terms of sex by tile were marginally significant (Table 3). Urine phosphate excretion did not vary among the tiles at UC but did at LL, and trended downward (Table 3) with decreasing serum phosphate levels at both sites (Figure 2, upper right panel). Adjusted creatinine clearance (Figure 2, lower left panel) did not vary between tiles at UC but did at LL (Table 3). TmP trended downward (Figure 2, lower right panel, Table 3) and FEP trended upward with decreasing phosphate tiles at both sites (Table 4). Overall, decreasing serum phosphate levels reflected a decrease in TmP and an increase in FEP that cannot be ascribed to an increase of urine phosphate excretion.
FIGURE 2.
Adjusted serum and urine phosphate and tubule phosphate reabsorption. Serum phosphate decreases with tile number (upper left panel) by design in male (gray) and female (black) subjects from UC (circles) and LL (triangles). Urine phosphate excretion (upper right panel) does not vary with tile at UC but does at LL (Table 3), with a trend towards decresing values with lower serum phosphate at both sites. Creatinine clearance (lower left panel) varies with phosphate tile at LL but not UC; values trend upward with decreasing serum phosphate at LL but not UC. TmP (lower right panel) decreases with decreasing serum phosphate at UC and LL (Table 3). Values for both sexes and sites are tightly bundled. All graphed values are ±SEM.
Table 3.
Significances of covariates for graphed adjusted values
| Covariate | Age | Body weight | Sex | UCreat (mg/day) | UNa (mEq/day) | Trend | Tile | Sex × Tile |
|---|---|---|---|---|---|---|---|---|
| S Phos (mg/dL) | 0.001/NS | NS/0.029 | NS/0.001 | 0.001/NS | NS | 0.001 | 0.001 | 0.025/0.044 |
| U Phos (mg/day) | 0.001 | 0.001 | 0.001 | 0.001 | NA | 0.01 | NS/0.011 | NS |
| CCr (L/day) | 0.001 | 0.001 | 0.001 | NA | 0.001 | NS/0.001 | NS/0.001 | NS |
| TmP (mg/dL) | 0.001 | NS | 0.001 | NA | 0.001 | 0.001 | 0.001 | 0.01/NS |
| UpH | 0.001 | 0.001 | 0.018/0.001 | NA | 0.001 | 0.001 | 0.011/0.001 | NS |
| S Cl (mEq/L) | 0.035 | NS/0.001 | 0.001 | NS/0.001 | NS/0.001 | 0.001 | 0.001 | 0.034/NS |
| S Urate (mg/dL) | 0.001 | 0.001 | 0.001 | NA | 0.001 | 0.001 | 0.001 | NS |
| UUa (mg/day) | 0.001 | NS/0.001 | 0.001 | 0.001 | 0.001 | NS | NS | NS |
| TmUa (mg/dL) | 0.003/0.001 | 0.001 | 0.001 | NA | 0.001 | 0.001 | 0.001 | NS |
| U Mg (mg/day) | 0.033/0.001 | 0.012/0.001 | NS | 0.001 | 0.001 | 0.006/0.001 | 0.028/0.001 | NS/0.002 |
| U Ca (mg/day) | NS/0.001 | NS/0.001 | 0.001 | 0.001 | 0.001 | 0.02/0.01 | NS | NS |
Single P-values apply to both UC and LL; when the two sites differ, values are presented as UC/LL and are in bold. NS, P > 0.05; NA, not used as a covariate. Of these variables, all are presented in the figures. Trend P-values from contrast analysis at UC and LL, respectively. Directionality is observed from the figures.
Table 4.
Adjusted values significant for tiles at UC and LL but not graphed
| Men |
Women |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| UC tile | ||||||||||
| FEP (%) | 24 ± 0.2 | 20 ± 0.2 | 18.6 ± 0.2 | 17.6 ± 0.3 | 15.2 ± 0.3 | 21 ± 0.4 | 19.3 ± 0.4 | 17.4 ± 0.3 | 17.1 ± 0.3 | 15.2 ± 0.3 |
| SS Capb | 1.69 ± 0.06 | 1.72 ± 0.06 | 1.49 ± 0.06 | 1.46 ± 0.07 | 1.42 ± 0.08 | 1.53 ± 0.10 | 1.48 ± 0.09 | 1.51 ± 0.08 | 1.40 ± 0.08 | 1.30 ± 0.08 |
| UCit (mg/day)b | 476 ± 15 | 487 ± 17 | 505 ± 18 | 489 ± 20 | 506 ± 22 | 528 ± 28 | 641 ± 24 | 629 ± 23 | 614 ± 22 | 623 ± 22 |
| S Ca (mg/dL)a | 9.62 ± 0.02 | 9.54 ± 0.02 | 9.59 ± 0.02 | 9.58 ± 0.02 | 9.59 ± 0.02 | 9.42 ± 0.03 | 9.44 ± 0.03 | 9.44 ± 0.02 | 9.50 ± 0.02 | 9.56 ± 0.02 |
| UK (mEq/day)a | 55 ± 1 | 57 ± 1 | 57 ± 1 | 58 ± 1 | 59 ± 1 | 55 ± 2 | 59 ± 1 | 60 ± 1 | 60 ± 1 | 62 ± 1 |
| FEMg (%)b | 3.14 ± 0.05 | 3.05 ± 0.06 | 3.06 ± 0.06 | 3.15 ± 0.06 | 3.33 ± 0.07 | 2.94 ± 0.1 | 3.01 ± 0.09 | 3.09 ± 0.08 | 3.12 ± 0.07 | 3.21 ± 0.07 |
| LL tile | ||||||||||
| FEP (%)a | 27.7 ± 0.2 | 23.2 ± 0.2 | 21.3 ± 0.2 | 19.6 ± 0.2 | 17.3 ± 0.2 | 24.8 ± 0.2 | 21.6 ± 0.2 | 19.9 ± 0.2 | 18.6 ± 0.2 | 16.7 ± 0.2 |
| SS Capa | 1.61 ± 0.03 | 1.52 ± 0.03 | 1.45 ± 0.03 | 1.34 ± 0.03 | 1.36 ± 0.04 | 1.61 ± 0.04 | 1.48 ± 0.04 | 1.38 ± 0.04 | 1.36 ± 0.03 | 1.27 ± 0.03 |
| UCit (mg/day)c | 543 ± 8 | 547 ± 8 | 550 ± 9 | 554 ± 9 | 563 ± 10 | 678 ± 12 | 718 ± 11 | 708 ± 10 | 727 ± 10 | 705 ± 9 |
| S Ca (mg/dL)a | 9.64 ± 0.01 | 9.65 ± 0.01 | 9.66 ± 0.01 | 9.68 ± 0.01 | 9.68 ± 0.01 | 9.57 ± 0.02 | 9.56 ± 0.01 | 9.58 ± 0.01 | 9.60 ± 0.01 | 9.67 ± 0.01 |
| UK (mEq/day)a | 57 ± 1 | 58 ± 1 | 60 ± 1 | 60 ± 1 | 62 ± 1 | 60 ± 1 | 61 ± 1 | 63 ± 1 | 63 ± 1 | 64 ± 1 |
| FEMg (%)a | 3.33 ± 0.03 | 3.34 ± 0.03 | 3.32 ± 0.04 | 3.37 ± 0.04 | 3.55 ± 0.04 | 3.13 ± 0.05 | 3.27 ± 0.05 | 3.30 ± 0.04 | 3.46 ± 0.04 | 3.54 ± 0.04 |
Values in the cells are adjusted for age, sex, urine sodium, urine creatinine excretion (except for FEP and creatinine excretion itself) and body weight.
aVaries across tiles, P < 0.001, bP < 0.01, cP < 0.05. All variables significant by tile are significant by trend analysis (P < 0.05).
Urine pH and serum chloride
Values by sex and phosphate tile (Tables 1 and 2)
Urine pH did not vary among the tiles at UC but did at LL. Serum chloride varied across the tiles, at both sites, increasing as phosphate levels decreased.
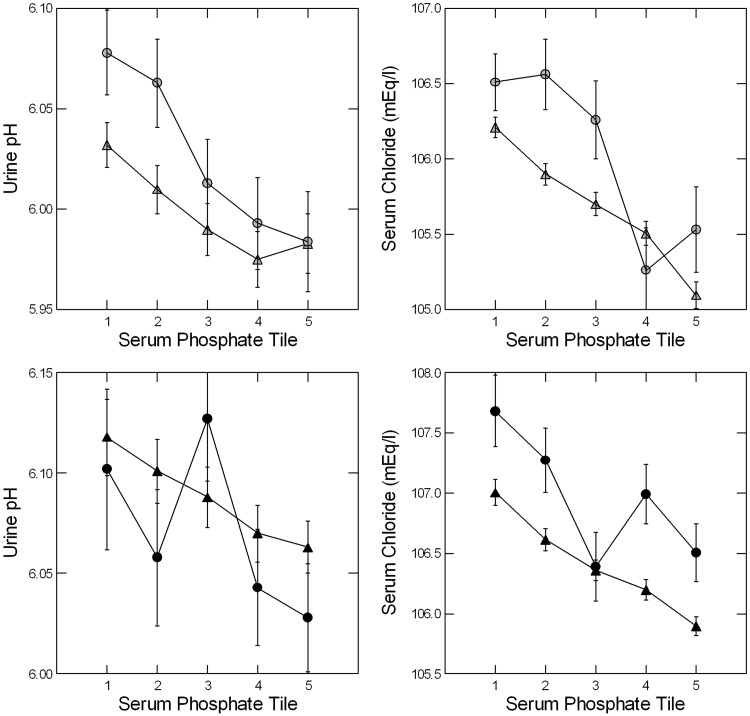
Values additionally adjusted for sex, age, body weight, urine creatinine and urine sodium
Urine pH varied among the tiles and increased with decreasing serum phosphate (Figure 3, left panels; Table 3). Serum chloride concentration increased progressively with decreasing serum phosphate (Figure 3, right panels; Table 3). Trends were significant for both (Table 3). Serum bicarbonate and serum sodium values did not vary between the five phosphate tiles, either fully adjusted or by sex and phosphate tile (values not shown). Altogether, with adjustments, a decreasing serum phosphate was accompanied by increasing urine pH and serum chloride at both sites.
FIGURE 3.
Adjusted urine pH and serum chloride values by tile. At LL and UC, urine pH and serum chloride increased with decreasing serum phosphate (Table 3). Symbols as in Figure 2. Males are in the upper panels, females in the lower. Upward trends for both with decreasing serum phosphate were highly significant (Table 3).
Uric acid
Values by sex and phosphate tile
Unadjusted values of serum uric acid and TmUa decreased with the phosphate tile at LL but not UC (Tables 1 and 2). The 24-h uric acid excretion did not vary among the tiles at LL (although the trend by tile was significant) but did so at UC, with the highest values at the lowest serum phosphate levels (Tables 1 and 2).
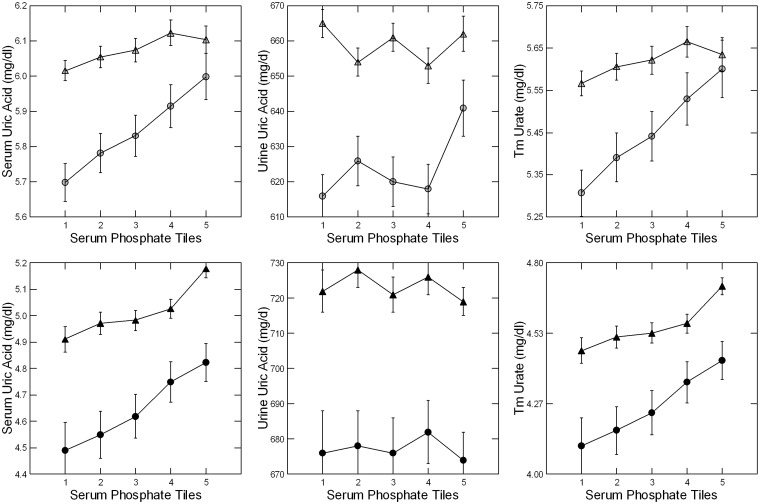
Values additionally adjusted for sex, age, body weight, serum and urine creatinine and urine sodium
Adjusted serum uric acid and TmUa decreased significantly with decreasing serum phosphate (Figure 4, left and right panels) at both UC and LL; trends were significant at both sites (Table 3). Urine uric acid excretion did not vary significantly among the five tiles at either site (Figure 4, middle panels; Table 3). FEUa (data not shown) did not change with the phosphate tile, perhaps because of changes in creatinine clearance already mentioned. Overall, the decrease in serum uric acid with decreasing serum phosphate reflects a reduction of TmUa, an estimate of tubule reabsorption, at UC and LL.
FIGURE 4.
Uric acid excretion, serum levels and Tm values. Adjusted serum urate values (left hand panels) decreased with phosphate tile at both LL and UC. Urine uric acid excretion (middle panels) did not vary with phosphate tile. TmUa (right hand panels) decreased with decreasing serum phosphate at both sites. The downward trend for serum uric acid and TmUa with decreasing serum phosphate were highly significant (Table 3). Symbols as in Figure 2; panels by sex as in Figure 3.
Urine calcium and magnesium and FEMg
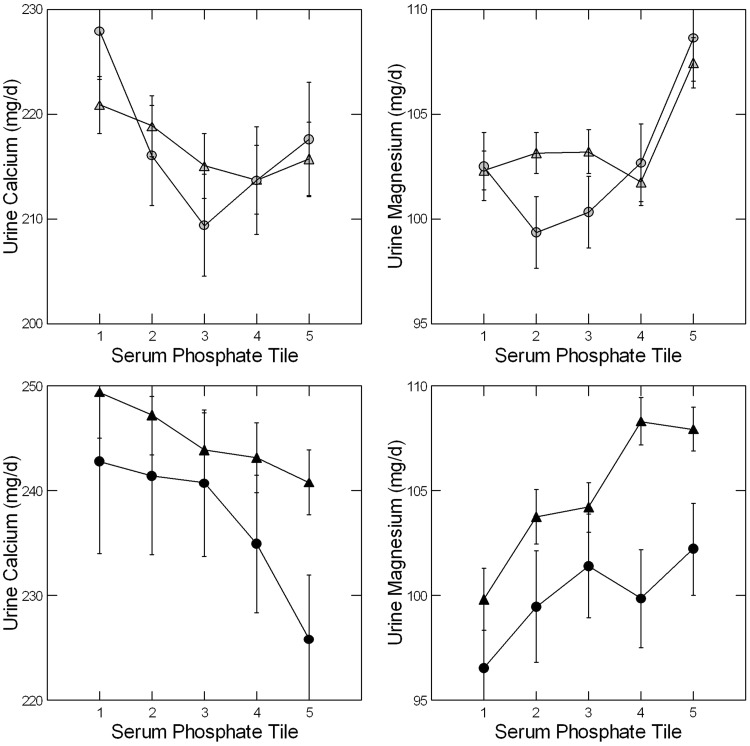
Unadjusted urine calcium increased with decreasing serum phosphate at UC but not LL (Tables 1 and 2). Adjusted urine calcium did not vary among the tiles (Figure 5, left panels) at either site, but there was a significant trend towards higher values at lower serum phosphate levels (Table 3). Urine magnesium excretion varied between tiles unadjusted (Tables 1 and 2) at LL and adjusted at both sites (Figure 5, right panels; Table 3) mainly because the highest phosphate tiles had higher excretions in both sexes at both sites than the remaining tiles. FECa (data not shown) did not vary among the tiles, but FEMg did, with lower values in the lowest phosphate tiles (Table 4).
FIGURE 5.
Adjusted urine calcium and magnesium excretions. Urine calcium (left panels) did not vary with phosphate tile but trended upward with decreasing serum phosphate at UC and LL (Table 3). Urine magnesium excretion (right panels) decreased significantly with phosphate tile at UC and LL. Symbols as in Figure 2; panels by sex as in Figure 3; statistical analysis in Table 3.
Other measurements
SS for calcium phosphate varied among the tiles (Table 4) and was highest in the two lowest phosphate groups for both sexes and at both sites. This reflects the slightly higher pH and calcium excretions and lower citrate excretions (Table 4) in those groups. At UC, where we have stone composition data, we did not find a corresponding variation of percentage of calcium phosphate in stones (data not shown). Serum calcium varied among the tiles (Table 4), with a slight downward trend as serum phosphate decreased. Urine excretions of potassium and citrate varied among the tiles at both sites, with the lowest values in the lowest phosphate tiles.
DISCUSSION
We have used large datasets to test the hypothesis that the common HYP of SFs is mediated via NHERF1 or related PDZ domain–containing proteins. Our main findings support the idea. We observed that TmP trended downward and FEP trended upward with decreasing serum phosphate levels. Urine phosphate excretion was not higher in the lowest serum phosphate tile, and in fact showed a downward trend in parallel with decreasing serum phosphate. Thus HYP arises from decreased TmP and increased FEP that cannot be ascribed to an increase of urine phosphate excretion. Urine pH and serum chloride levels increased and serum urate levels and TmUa decreased with the phosphate tile, as might be expected from a general increase in signaling pathways that reduce phosphate, urate and bicarbonate reabsorption. Although changes in serum chloride and urate and in urine pH are beyond the detection limits of a smaller study, they are sufficient for the hypothesis testing proposed here.
TmUa was reduced along with TmP, but FEUa was not. Because FEUa is a ratio of urine excretion to filtered load, it depends critically on glomerular filtration that was somewhat variable from tile to tile and between sites. TmUa is a concentration difference, serum urate minus urine urate corrected for water reabsorption using the urine/serum creatinine ratio. As serum phosphate decreases, this difference decreases, meaning, presumably, proximal tubules create a lesser reabsorptive step across their epithelia, as one would expect from downregulation of its transporters.
NHERF1 and PDZK1 in proximal tubule regulation
The activity of Npt2a, NHE3 and URAT1, the main apical membrane transporters for sodium phosphate cotransport, proton–sodium exchange, and urate reabsorption, are all regulated via NHERF1 [36]. PDZK1 (NHERF3), another major scaffolding molecule, interacts with all three transporters [37] as well as with NHERF1 [38] and may also regulate them in a coordinated manner. PDZK1 knockout mice have reduced stabilization of Npt2c in the apical membrane [39] as well as increased FEP [40]. PDZK1 is also known to be a genetic determinant of serum uric acid levels via its interaction with multiple proximal tubule transporters that govern urate uptake and secretion; common PDZK1 gene variants are associated with gout susceptibility [21, 41].
Regulation via a common pathway, likely including NHERF1, presumably accounts for the well-known fact that PTH inhibits proximal tubule bicarbonate reabsorption [42] as well as phosphate reabsorption [43, 44]. NHERF1 knockout mice excrete more uric acid than wild-type mice and are, at least for the first 24 weeks of life, phosphaturic [22]. In other words, although the scaffolding proteins mediate actions of specific regulating hormones like PTH, there is evidence for what we can refer to as ‘crosstalk’, which is what we sought for here.
Role of diet, PTH and FGF23
Diet and PTH
The common HYP of SFs cannot be ascribed to diet phosphate loading or increased intestinal phosphate absorption with a corresponding increase in renal phosphate excretion; such an increase of excretion is not present. Increased PTH secretion also is not a plausible cause, as studies of patients like ours have revealed normal PTH levels [2]. We have documented daylong HYP and increased FEP in SFs versus normal control subjects eating the same fixed diets in a clinical research center setting. Serum PTH levels were not higher in patients than controls despite having 20 measurements in each person over the course of the day [45]. Of note, FEP was constant throughout the day, so our estimates here, based on 24-h urine samples and fasting blood, are valid estimates of what one would obtain from a daylong study. Altogether, HYP in SFs is not due to diet or high serum PTH.
FGF23
A well-known inhibitor of renal phosphate reabsorption, FGF23 was elevated in the serum of SFs [46] and degree of HYP varied with serum FGF23 levels. Overexpression of FGF23 in mice decreases gene expression for Klotho and PDZK1 as well as NPT2a [47], so abnormal FGF23 regulation could conceivably reduce Tm for urate and phosphate, as well as NHE3 activity, via its effects on PDZK1 expression [37]. In fact, Sneddon et al. [48] found that FGF23 and PTH both inhibit phosphate uptake in human proximal tubule cells but act through distinct mechanisms that converge on a final common effector, NHERF1, to downregulate Npt2a. Additional studies are needed to resolve the effects of FGF23 on tubular transport in SFs and to discern the relative contributions of PDZK1 and NHERF1.
Sodium hydrogen exchange
Our evidence for reduced NHE3 activity with decreasing serum phosphate is indirect, but the changes we observed in urine pH and serum chloride are compatible with reduced NHE3 activity that has subtly altered the systemic acid–base balance by increasing bicarbonate delivery to distal nephron segments. No data are published on urine pH in the NHERF1 knockout mouse, nor for the SFs with NHERF1 mutations described thus far by others. Serum chloride levels in the NHERF1 knockout mice are 2 mEq/L above the wild type [22], similar to the difference between the lowest and highest serum phosphate tiles here. We have power to detect such small variations; for the mice, the difference is not significant.
We recognize that increasing serum chloride could reflect the charge balance: loss of phosphate equivalents is balanced by an increase of another anion. The change in serum phosphate molarity from the highest to lowest tiles is ∼0.6 mEq/L; the increase in serum chloride is ∼1 mEq/L, roughly comparable. Accordingly, the changes in chloride we found support our hypothesis but are also subject to an alternative interpretation. If we could have documented a decrease in serum bicarbonate this caveat would be unnecessary. Additional research is needed to clarify this point.
Urate reabsorption
The main urate reabsorptive transporter in the proximal tubule, URAT1, associates with and is regulated by PDZK1 and NHERF1 [25, 27]. The NHERF1 knockout mouse is hyperuricosuric [22], presumably because of reduced urate reabsorption; serum urate levels are not provided. In our patients, serum urate levels decreased with the serum phosphate tile and urine urate excretion rates are constant. TmUa decreased with decreasing serum phosphate, consistent with reduced tubule urate reabsorption. Altogether we have reasonable evidence that urate transport is reduced in SFs with versus those without HYP. Our findings agree with coordinated downregulation of URAT1, NHE3 and phosphate transport.
Urine calcium and magnesium
Urine magnesium and calcium excretions by NHERF1 knockout mice, heterozygote mice included [12], exceed wild type, probably because of increased serum calcitriol [22]. The few patients reported to date with NHERF1 gene mutations were hypercalciuric and had elevated serum calcitriol levels [13]. We do not have calcitriol levels, but Prié et al. [2] found serum calcitriol levels in SFs with and without HYP did not differ; all SFs values, on average, exceeded normal. We found that calcium excretion trended upward and serum calcium trended downward with HYP. Being SFs, our patients were generally hypercalciuric compared with normal people. Urine magnesium excretion and FEMg, but not serum magnesium, decreased with serum phosphate levels. We have no explanation for these changes.
Stone formation risk
Urine SS with respect to calcium phosphate (CaP) was higher in the lowest phosphate tiles because of combined higher urine calcium and pH and lower urine citrate. Increased CaP SS is clinically relevant because CaP crystals (as apatite) initiate calcium oxalate stone overgrowths on papillary interstitial plaque [49] and can be the predominant mass of some calcium stones. The NHERF1 knockout mouse is unique among animal models of stone disease in forming interstitial apatite plaque [22] of the kind regularly documented in biopsies from humans with calcium renal stones [50].
Conclusions
Coordinate variations in three sets of measurements that each reflect different proximal tubule transport functions point to a common and widespread variability within some large-scale transport regulators like NHERF1 and PDZK1. Observational research like ours cannot prove such a mechanism but does suggest a need for new research of a more experimental nature. Even so, whatever causes variable serum phosphate via variation of phosphate reabsorption in the main bulk of SFs seems to be exerting an effect, perhaps via the many regulatory steps between signalers like PTH and integrative molecules like NHERF1 and PDZK1, on at least Npt2a or 2c, NHE3 and URAT1.
FUNDING
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. This work was supported by grant P0156788 from the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health.
CONFLICT OF INTEREST STATEMENT
F.L.C. and E.M.W. are consultants for Litholink and Labcorp. The results presented in this article have not been published previously in whole or part, except in abstract format
REFERENCES
- 1. Prié D, Beck L, Silve C. et al. Hypophosphatemia and calcium nephrolithiasis. Nephron Exp Nephrol 2004; 98: e50–e54 [DOI] [PubMed] [Google Scholar]
- 2. Prié D, Ravery V, Boccon-Gibod L. et al. Frequency of renal phosphate leak among patients with calcium nephrolithiasis. Kidney Int 2001; 60: 272–276 [DOI] [PubMed] [Google Scholar]
- 3. Barilla DE, Zerwekh JE, Pak CYC.. A critical evaluation of the role of phosphate in the pathogenesis of absorptive hypercalciuria. Miner Electrolyte Metabol 1979; 2: 302–309 [Google Scholar]
- 4. Steiniche T, Mosekilde L, Christensen MS. et al. A histomorphometric determination of iliac bone remodeling in patients with recurrent renal stone formation and idiopathic hypercalciuria. APMIS 1989; 97: 309–316 [DOI] [PubMed] [Google Scholar]
- 5. Coe FL, Favus MJ, Crockett T. et al. Effects of low-calcium diet on urine calcium excretion, parathyroid function and serum 1,25(OH)2D3 levels in patients with idiopathic hypercalciuria and in normal subjects. Am J Med 1982; 72: 25–32 [DOI] [PubMed] [Google Scholar]
- 6. Lemann J Jr, Dominguez JH, Gray RW.. Idiopathic hypercalciuria: a defect in phosphate and vitamin d metabolism? In: Finlayson B, Thomas WC Jr (eds). Colloquium on Renal Lithiasis. Gainesville: University Press of Florida, 1976, 276–290 [Google Scholar]
- 7. Prié D, Huart V, Bakouh N. et al. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med 2002; 347: 983–991 [DOI] [PubMed] [Google Scholar]
- 8. Magen D, Berger L, Coady MJ. et al. A loss-of-function mutation in NaPi-IIa and renal Fanconi's syndrome. N Engl J Med 2010; 362: 1102–1109 [DOI] [PubMed] [Google Scholar]
- 9. Oddsson A, Sulem P, Helgason H. et al. Common and rare variants associated with kidney stones and biochemical traits. Nat Commun 2015; 6: 7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schlingmann KP, Ruminska J, Kaufmann M. et al. Autosomal-recessive mutations in SLC34A1 encoding sodium-phosphate cotransporter 2A cause idiopathic infantile hypercalcemia. J Am Soc Nephrol 2016; 27: 604–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Page K, Bergwitz C, Jaureguiberry G. et al. A patient with hypophosphatemia, a femoral fracture, and recurrent kidney stones: report of a novel mutation in SLC34A3. Endocr Pract 2008; 14: 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shenolikar S, Voltz JW, Minkoff CM. et al. Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci USA 2002; 99: 11470–11475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karim Z, Gerard B, Bakouh N. et al. NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med 2008; 359: 1128–1135 [DOI] [PubMed] [Google Scholar]
- 14. Courbebaisse M, Leroy C, Bakouh N. et al. A new human NHERF1 mutation decreases renal phosphate transporter NPT2a expression by a PTH-independent mechanism. PLoS One 2012; 7: e34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walsh DR, Nolin TD, Friedman PA.. Drug transporters and Na+/H+ exchange regulatory factor PSD-95/drosophila discs large/ZO-1 proteins. Pharmacol Rev 2015; 67: 656–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miyazaki H, Anzai N, Ekaratanawong S. et al. Modulation of renal apical organic anion transporter 4 function by two PDZ domain-containing proteins. J Am Soc Nephrol 2005; 16: 3498–3506 [DOI] [PubMed] [Google Scholar]
- 17. Wang WJ, Murray JW, Wolkoff AW.. Oatp1a1 requires PDZK1 to traffic to the plasma membrane by selective recruitment of microtubule-based motor proteins. Drug Metab Dispos 2014; 42: 62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park J, Kwak JO, Riederer B. et al. Na+/H+ exchanger regulatory factor 3 is critical for multidrug resistance protein 4-mediated drug efflux in the kidney. J Am Soc Nephrol 2014; 25: 726–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng J, Chan T, Cheung FS. et al. PDZK1 and NHERF1 regulate the function of human organic anion transporting polypeptide 1A2 (OATP1A2) by modulating its subcellular trafficking and stability. PLoS One 2014; 9: e94712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anzai N, Miyazaki H, Noshiro R. et al. The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C terminus. J Biol Chem 2004; 279: 45942–45950 [DOI] [PubMed] [Google Scholar]
- 21. Higashino T, Matsuo H, Sakiyama M. et al. Common variant of PDZ domain containing 1 (PDZK1) gene is associated with gout susceptibility: a replication study and meta-analysis in Japanese population. Drug Metab Pharmacokinet 2016; 31: 464–466 [DOI] [PubMed] [Google Scholar]
- 22. Weinman EJ, Mohanlal V, Stoycheff N. et al. Longitudinal study of urinary excretion of phosphate, calcium, and uric acid in mutant NHERF-1 null mice. Am J Physiol Renal Physiol 2006; 290: F838–F843 [DOI] [PubMed] [Google Scholar]
- 23. Weinman EJ, Cunningham R, Wade JB. et al. The role of NHERF-1 in the regulation of renal proximal tubule sodium-hydrogen exchanger 3 and sodium-dependent phosphate cotransporter 2a. J Physiol 2005; 567: 27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Donowitz M, Cha B, Zachos NC. et al. NHERF family and NHE3 regulation. J Physiol (Lond) 2005; 567: 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cunningham R, Brazie M, Kanumuru S. et al. Sodium-hydrogen exchanger regulatory factor-1 interacts with mouse urate transporter 1 to regulate renal proximal tubule uric acid transport. J Am Soc Nephrol 2007; 18: 1419–1425 [DOI] [PubMed] [Google Scholar]
- 26. Anzai N, Ichida K, Jutabha P. et al. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem 2008; 283: 26834–26838 [DOI] [PubMed] [Google Scholar]
- 27. Endou H, Anzai N.. Urate transport across the apical membrane of renal proximal tubules. Nucleos Nucleot Nucl 2008; 27: 578–584 [DOI] [PubMed] [Google Scholar]
- 28. Lingeman J, Mardis H, Kahnoski R. et al. Medical reduction of stone risk in a network of treatment centers compared to a research clinic. J Urol 1998; 160: 1629–1634 [PubMed] [Google Scholar]
- 29. Asplin J, Parks J, Lingeman J. et al. Supersaturation and stone composition in a network of dispersed treatment sites. J Urol 1998; 159: 1821–1825 [DOI] [PubMed] [Google Scholar]
- 30. Asplin JR, Lingeman J, Kahnoski R. et al. Metabolic urinary correlates of calcium oxalate dihydrate in renal stones. J Urol 1998; 159: 664–668 [PubMed] [Google Scholar]
- 31. Parks JH, Goldfischer ER, Coe FL.. Changes in urine volume accomplished by physicians treating nephrolithiasis. J Urol 2003; 169: 863–866 [DOI] [PubMed] [Google Scholar]
- 32. Parks JH, Coward M, Coe FL.. Correspondence between stone composition and urine supersaturation in nephrolithiasis. Kidney Int 1997; 51: 894–900 [DOI] [PubMed] [Google Scholar]
- 33. Werness PG, Brown CM, Smith LH. et al. EQUIL 2: a basic computer program for the calculation of urinary saturation. J Urol 1985; 134: 1242–1244 [DOI] [PubMed] [Google Scholar]
- 34. Bijvoet OL, Morgan DB, Fourman P.. The assessment of phosphate reabsorption. Clin Chim Acta 1969; 26: 15–24 [DOI] [PubMed] [Google Scholar]
- 35. Stark H, Eisenstein B, Tieder M. et al. Direct measurement of TP/GFR: a simple and reliable parameter of renal phosphate handling. Nephron 1986; 44: 125–128 [DOI] [PubMed] [Google Scholar]
- 36. Mahon MJ, Cole JA, Lederer ED. et al. Na+/H+ exchanger-regulatory factor 1 mediates inhibition of phosphate transport by parathyroid hormone and second messengers by acting at multiple sites in opossum kidney cells. Mol Endocrinol 2003; 17: 2355–2364 [DOI] [PubMed] [Google Scholar]
- 37. Gisler SM, Pribanic S, Bacic D. et al. PDZK1: I. a major scaffolder in brush borders of proximal tubular cells. Kidney Int 2003; 64: 1733–1745 [DOI] [PubMed] [Google Scholar]
- 38. Biber J, Gisler SM, Hernando N. et al. PDZ interactions and proximal tubular phosphate reabsorption. Am J Physiol Renal Physiol 2004; 287: F871–F875 [DOI] [PubMed] [Google Scholar]
- 39. Giral H, Lanzano L, Caldas Y. et al. Role of PDZK1 protein in apical membrane expression of renal sodium-coupled phosphate transporters. J Biol Chem 2011; 286: 15032–15042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Capuano P, Bacic D, Stange G. et al. Expression and regulation of the renal Na/phosphate cotransporter NaPi-IIa in a mouse model deficient for the PDZ protein PDZK1. Pflugers Arch 2005; 449: 392–402 [DOI] [PubMed] [Google Scholar]
- 41. Yang Q, Kottgen A, Dehghan A. et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet 2010; 3: 523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bank N, Aynediian HS.. A micropuncture study of the effect of parathyroid hormone on renal bicarbonate reabsorption. J Clin Invest 1976; 58: 336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deliot N, Hernando N, Horst-Liu Z. et al. Parathyroid hormone treatment induces dissociation of type IIa Na+-P(i) cotransporter-Na+/H+ exchanger regulatory factor-1 complexes. Am J Physiol Cell Physiol 2005; 289: C159–C167. [DOI] [PubMed] [Google Scholar]
- 44. Weinman EJ, Biswas RS, Peng G. et al. Parathyroid hormone inhibits renal phosphate transport by phosphorylation of serine 77 of sodium-hydrogen exchanger regulatory factor-1. J Clin Invest 2007; 117: 3412–3420 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45. Worcester EM, Gillen DL, Evan AP. et al. Evidence that postprandial reduction of renal calcium reabsorption mediates hypercalciuria of patients with calcium nephrolithiasis. Am J Physiol Renal Physiol 2007; 292: F66–F75 [DOI] [PubMed] [Google Scholar]
- 46. Rendina D, Mossetti G, De FG. et al. Fibroblast growth factor 23 is increased in calcium nephrolithiasis with hypophosphatemia and renal phosphate leak. J Clin Endocrinol Metab 2006; 91: 959–963 [DOI] [PubMed] [Google Scholar]
- 47. Marsell R, Krajisnik T, Goransson H. et al. Gene expression analysis of kidneys from transgenic mice expressing fibroblast growth factor-23. Nephrol Dial Transplant 2008; 23: 827–833 [DOI] [PubMed] [Google Scholar]
- 48. Sneddon WB, Ruiz GW, Gallo LI. et al. Convergent signaling pathways regulate parathyroid hormone and fibroblast growth factor-23 action on NPT2A-mediated phosphate transport. J Biol Chem 2016; 291: 18632–18642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Evan AP, Coe FL, Lingeman JE. et al. Mechanism of formation of human calcium oxalate renal stones on Randall's plaque. Anat Rec (Hoboken) 2007; 290: 1315–1323 [DOI] [PubMed] [Google Scholar]
- 50. Evan AP, Lingeman JE, Coe FL. et al. Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 2003; 111: 607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]