Abstract
Objective
To characterize the use and safety of newer disease-modifying therapies (DMTs) in children with multiple sclerosis (MS) and clinically isolated syndrome (CIS) treated under 18 years of age.
Methods
This is a cohort study including children with MS or CIS followed at 12 outpatient practices participating in the US Network of Pediatric MS Centers. DMT use, including duration, dose, and side effects, was analyzed. Newer DMTs were defined as agents receiving Food and Drug Administration approval or with increased use in adult MS after 2005.
Results
As of July 2017, 1,019 pediatric patients with MS (n = 748) or CIS (n = 271) were enrolled (65% female, mean onset 13.0 ± 3.9 years, mean follow-up 3.5 ± 3.1 years, median 1.6 visits per year). Of these, 78% (n = 587) with MS and 11% (n = 31) with CIS received DMT before 18 years of age. This consisted of at least one newer DMT in 42%, including dimethyl fumarate (n = 102), natalizumab (n = 101), rituximab (n = 57), fingolimod (n = 37), daclizumab (n = 5), and teriflunomide (n = 3). Among 17%, the initial DMT prescribed was a newer agent (36 dimethyl fumarate, 30 natalizumab, 22 rituximab, 14 fingolimod, 2 teriflunomide). Over the last 10 years, the use of newer agents has increased, particularly in those ≥12 years and to lesser extent in those <12 years. The short-term side effect profiles of newer DMTs did not differ from those reported in adults.
Conclusion
Newer DMTs are often used in pediatric MS, and have similar short-term safety, tolerability, and side effect profiles as in adults. These findings may help inform pediatric MS management.
About 3%–5% of individuals with multiple sclerosis (MS) develop symptoms before 18 years of age.1,2 Treatment of pediatric MS is challenging given higher relapse rates3,4 and higher accumulation of new MRI lesions compared to adults,5 as well as the lack of safety and efficacy data for disease-modifying therapies (DMTs) in children.
Conventionally, first-line treatments for pediatric MS include interferon-β6–12 and glatiramer acetate,13,14 supported by observational data and commonly implemented in clinical practice, especially in locations where treatment algorithms dictate medication availability such as in the European Union. However, these may be poorly tolerated and often fail to control the disease, requiring escalation to more potent agents.15–17 There are limited case series data regarding the effectiveness and safety of newer DMTs in children, including natalizumab,18–22 rituximab,23,24 dimethyl fumarate,25 fingolimod,26 and daclizumab.27 Although one randomized trial was recently completed28 and several other trials are ongoing for some of the newer DMTs in pediatric MS, large observational studies are critical for characterizing the real-world use of these therapies and potentially provide longer follow-up relative to clinical trials.
We aimed to characterize the patterns of use of newer DMTs in children with MS and clinically isolated syndrome (CIS) treated under 18 years of age in a large US pediatric MS cohort, as well as explore the safety and tolerability profiles of newer DMTs in children.
Methods
Study design
This is a multicenter observational cohort study involving 12 regional pediatric MS referral centers from across the United States participating in the US Network of Pediatric MS Centers.29 The sites include Boston Children's Hospital, Cleveland Clinic, Loma Linda University, Massachusetts General Hospital, Mayo Clinic, New York University Langone Medical Center, State University of New York at Buffalo, Texas Children's Hospital, University of Alabama at Birmingham, University of California San Francisco, University of Colorado, and Washington University in Saint Louis.
There was prospective collection of clinical data in an online curated database including treatment information using standardized case report forms from May 2011 through July 2017. Clinical information prior to 2011 was retrospectively entered from medical records. The data are stored and managed with quality control at the Data Coordinating and Analysis Center at the University of Utah.
Study population
Patients were identified from the US Network of Pediatric MS Centers database who had a diagnosis of MS or CIS prior to 18 years of age at their most recent visit date.30 Patients who entered a clinical trial with a DMT were excluded.
Measurements
The patient's age at DMT start, year the DMT was prescribed, duration of use, dose, and reasons for discontinuation were derived. DMTs included therapies Food and Drug Administration (FDA)–approved in adult MS as well as rituximab, which although not FDA-approved in MS is supported by a phase 2 study31 and is used commonly in adult MS. Based on clinical assessments, up to 3 adverse events were recorded for each DMT each time a patient received a given therapy. Side effects collected in the database include amenorrhea, anxiety, arrhythmia, cardiomyopathy, cataracts, depression, flu-like symptoms, gastrointestinal symptoms (nausea, vomiting, abdominal pain), hair loss, headache, hematuria, hyperglycemia, hypertension, hypotension, infections (increased or severe), injection site reactions (necrosis, cellulitis, lipoatrophy), liver dysfunction, osteopenia, rash, renal dysfunction, thyroid dysfunction, and weight gain, with the option of specifying “other” or “unknown.” Number of side effects per 100 person-years of exposure were calculated for each DMT of interest. Number and proportion of patients who discontinued each DMT were also derived. Reasons for discontinuation collected in the database include ineffectiveness, side effects, financial considerations, and personal choice, with the option of specifying other or unknown. In those who discontinued DMT, the occurrence of a relapse or new/enlarging T2 or gadolinium-enhancing lesion during up to 6 months prior to treatment discontinuation were also evaluated, as well as the presence of JC virus (JCV) antibodies in those on natalizumab.
Newer DMTs were defined as agents receiving FDA approval or with increased use in adult MS after 2005. DMTs classified as newer include dimethyl fumarate, fingolimod, teriflunomide, natalizumab, rituximab, alemtuzumab, and daclizumab. DMTs were also classified as injectable (glatiramer acetate, β-interferons), oral (dimethyl fumarate, fingolimod, teriflunomide), or IV (natalizumab, rituximab, alemtuzumab).
Baseline characteristics collected to assess predictors of ever using a newer DMT before 18 years included age at MS onset, sex, diagnosis, year of disease onset, severity of the first relapse, number of relapses in the first 2 years of disease, Expanded Disability Status Scale (EDSS) score at first visit, and type of insurance at baseline.
Statistical analysis
This is a descriptive study examining patterns of newer DMT use and side effects. Mean, SD, median, and interquartile range were reported as appropriate. Characteristics of those starting first-line on a newer DMT compared to an injectable DMT were compared using χ2 test for categorical variables and Wilcoxon rank sum test for numerical variables. χ2 analyses were used to examine patterns of DMT use over the last 10 years.
Multivariable logistic regression was used to assess prespecified predictors of ever using a newer DMT before 18 years in those treated with at least one DMT before 18 years with complete data. These included clinically relevant potential predictors including age at MS onset, sex, diagnosis (MS vs CIS), year of disease onset (≤2005, 2006–2009, 2010–2013, 2014–2017), severity of the first relapse (mild, moderate, or severe), number of relapses in the first 2 years of disease, EDSS score at first event, and type of insurance at baseline (private insurance, government insurance, other). Overall significance of categorical variables was calculated with likelihood ratio tests, and a test for linear trend was used to evaluate for a linear trend in the year of disease onset. We also assessed the effect of study site. All tests were 2-sided with α of 0.05. Analyses were performed using STATA 15.
Standard protocol approvals, registrations, and patient consents
The ethics committees of participating institutions approved this study. Parents and participants signed consent forms and assent forms, when required by each center's institutional review board, prior to enrollment.
Data availability
Data are available to qualified investigators on request for the purposes of replicating procedures or results by contacting the corresponding author.
Results
Patient characteristics
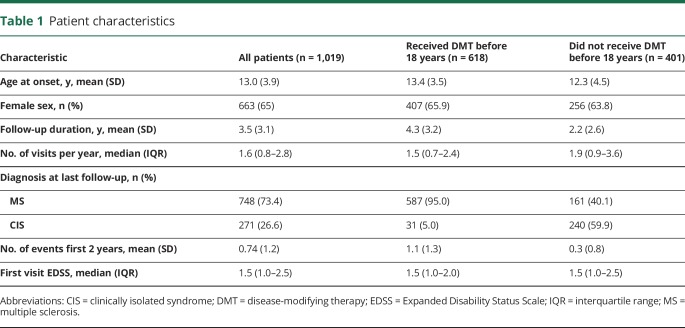
As of July 2017, our database included 1,019 patients with pediatric-onset MS (n = 748) or CIS (n = 271). Overall, 60.7% (618) received DMT before 18 years, with 78.5% with MS and 11.4% with CIS receiving DMT before 18 years. Our cohort included a broad distribution of onset age, with mean onset age 13.0 ± 3.9 years, with mean follow-up 3.5 ± 3.1 years. Patients were seen a median of 1.6 times per year (table 1).
Table 1.
Patient characteristics
Patterns of DMT use
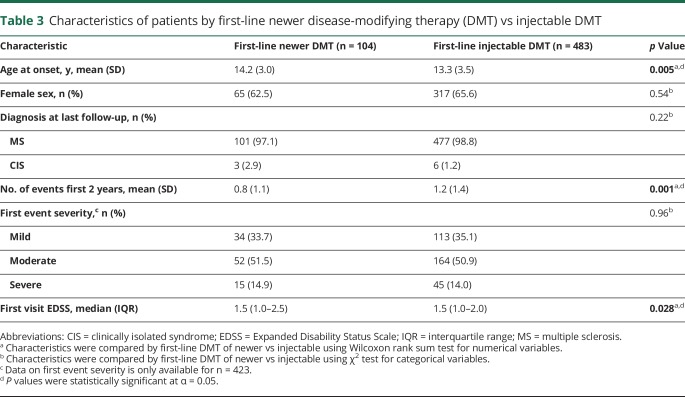
Of those who received DMT before 18 years (n = 618), at least one newer DMT was used before 18 years in 41.9%, and 16.8% received a newer DMT as first-line therapy before 18 years (table 2). Before 18 years, 102 received dimethyl fumarate, 101 natalizumab, 57 rituximab, 37 fingolimod, 5 daclizumab, and 3 teriflunomide. First-line treatment with newer DMTs before 18 years included 36 dimethyl fumarate, 30 natalizumab, 22 rituximab, 14 fingolimod, and 2 teriflunomide. Of the 483 whose first-line treatment was an injectable therapy, 147 (30.4%) switched to a newer therapy before 18 years. Those started on first-line newer DMT compared to injectable DMT were older (p = 0.005) and had higher EDSS at the first visit (p = 0.028), but had fewer events in the first 2 years of disease (p = 0.001), likely as a result of the DMT (table 3).
Table 2.
Newer disease-modifying therapies (DMTs) used before 18 years of age in those who received at least one DMT before 18 years
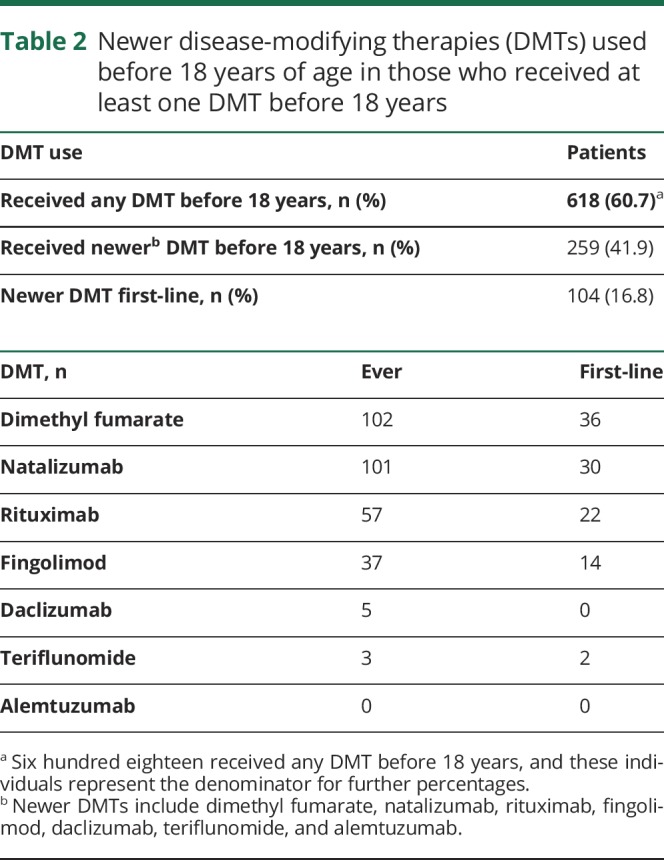
Table 3.
Characteristics of patients by first-line newer disease-modifying therapy (DMT) vs injectable DMT
There were 315 patients with MS/CIS onset before 12 years of age. Of the 179 with MS onset before 12 years, 56.4% had received a DMT before 12 years and 82.1% before 18 years. Of the 136 with CIS onset before 12 years, 5.9% had received DMT before 12 years and 6.6% before 18 years. Of the 109 children who received DMT before 12 years, 24 (22%) received treatment with a newer DMT before 12 years, including 11 natalizumab, 8 dimethyl fumarate, 6 rituximab, and 2 fingolimod.
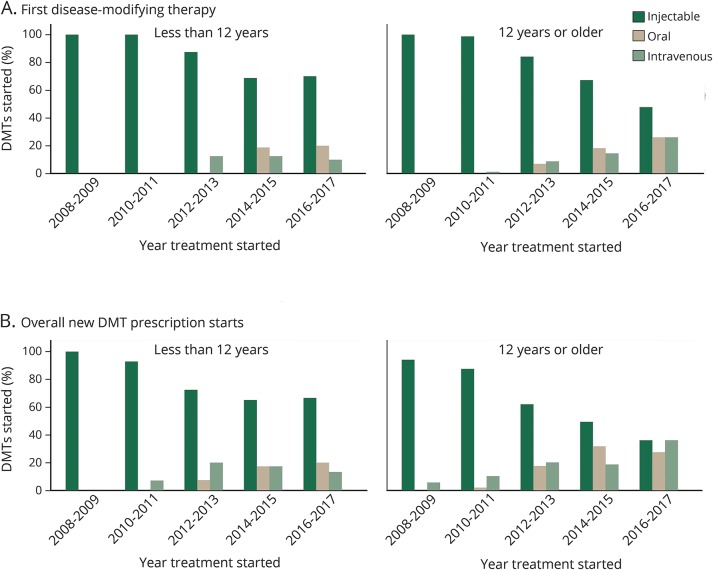
As shown in the figure, there was increasing use of both oral and IV newer agents in the last several years in both those younger than 12 and 12 years or older, and these newer agents were increasingly used as first-line agents in addition to overall (p < 0.001 for both overall and first-line for all ages combined and those 12 years and older; p = 0.016 for overall use in those younger than 12 years and p = 0.017 for first-line use in those younger than 12 years). In 2008–2009, 100% were started on an injectable first-line, while in 2016–2017, only 48% of those ≥12 years and 70% of those <12 years started an injectable first-line (figure).
Figure. Patterns of disease-modifying therapy (DMT) use including first and overall DMT use by treatment initiation age.
DMT use over the last 10 years, separated into categories of injectable, oral, and IV therapies in those treated less than 12 years and 12 years or older. (A) First DMT prescribed. (B) Overall new prescription starts for DMTs. Proportions are displayed per drug started. χ2 testing showed there was a relationship between year of treatment and proportion on each category of DMT both overall and as first-line in the entire cohort and for those 12 years and older at treatment initiation (p < 0.001). There was also a relationship between year of treatment and proportion on each category of DMT overall (p = 0.016) and first-line (p = 0.017) in those treated at less than 12 years of age.
Side effects of newer DMTs
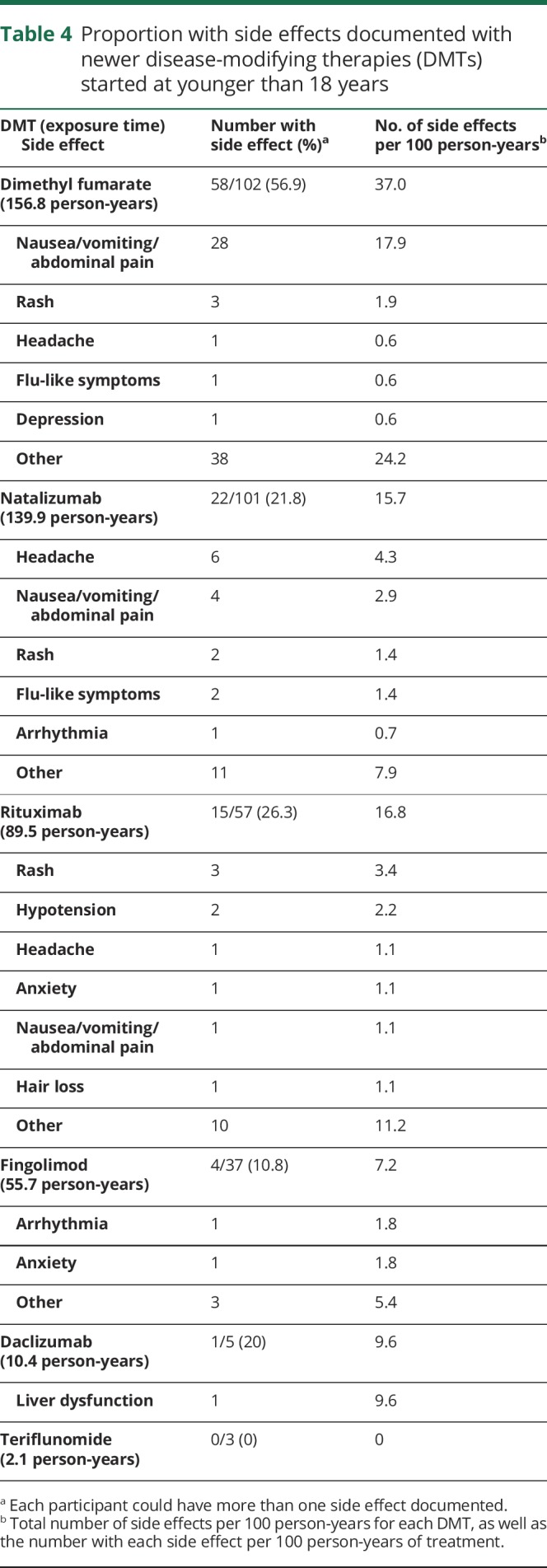
Short-term side effects of newer DMTs were not different than reported in adults in pivotal trials,31–34 with no new side effects identified (table 4). The longest patient exposure time available was for dimethyl fumarate (156.8 person-years), followed by natalizumab (139.9 person-years), with shorter exposure time for other newer DMTs. Overall, the most side effects were seen with dimethyl fumarate, which most commonly included gastrointestinal side effects and rash. It should be noted that flushing is not an option in the side effect field, so it is possible some documented rashes may represent flushing. Most common side effects with natalizumab included headache and gastrointestinal symptoms. Side effects most commonly reported on rituximab included rash and hypotension. During fingolimod exposure, there was 1 case of arrhythmia. There was 1 case of liver dysfunction during IV daclizumab exposure.
Table 4.
Proportion with side effects documented with newer disease-modifying therapies (DMTs) started at younger than 18 years
Details of newer therapy use
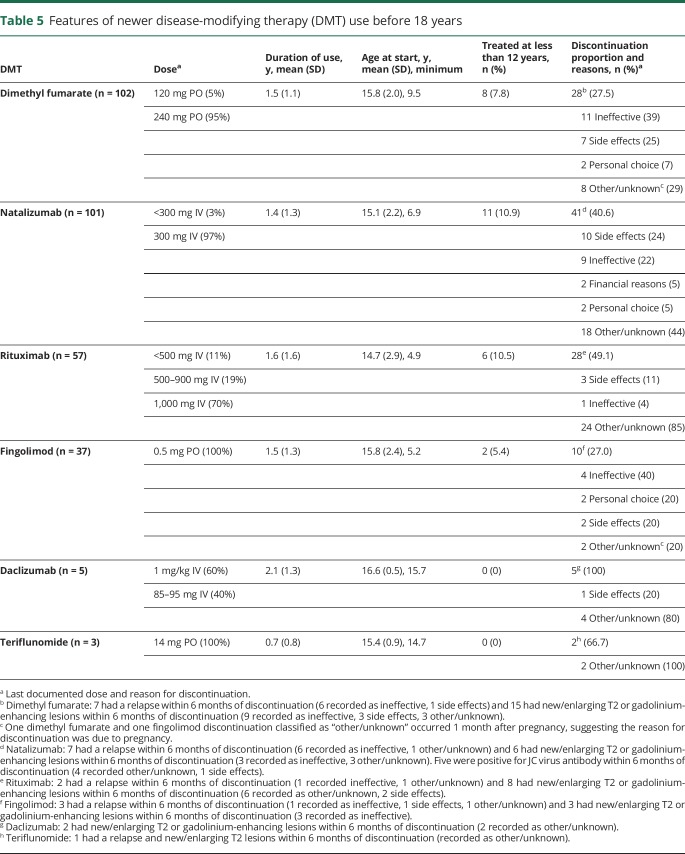
We describe the use of newer DMTs in table 5. The most common dose of natalizumab was 300 mg IV, with a mean treatment duration of 1.4 (SD 1.3) years. Of those who started natalizumab, 8% were positive for the JCV antibody before starting therapy, while 22% who were initially negative for JCV seroconverted during natalizumab treatment.
Table 5.
Features of newer disease-modifying therapy (DMT) use before 18 years
Dimethyl fumarate was used for a mean of 1.5 (SD 1.1) years, most commonly at the standard dose of 240 mg BID, but occasionally at 120 mg BID. Rituximab was used mainly at doses from 500 to 1,000 mg IV, with a mean treatment duration of 1.6 (SD 1.6) years. Fingolimod was always used at 0.5 mg PO, with a mean treatment duration of 1.5 (SD 1.3) years. Daclizumab was used as an escalation therapy only and was given IV as done in a prior study in pediatric MS before being approved and subsequently withdrawn as a subcutaneous injection in adults,27 with a mean duration of 2.1 (SD 1.3) years of treatment. Teriflunomide 14 mg was used for a mean duration of 0.7 (SD 0.8) years.
The mean age at start of newer DMTs ranged from 14.7 years for rituximab to 16.6 years for daclizumab. Natalizumab, dimethyl fumarate, rituximab, and fingolimod were used under 12 years of age, while daclizumab and teriflunomide were only used at older ages.
A relatively high proportion of patients discontinued newer DMTs, ranging from 27% discontinuing fingolimod to 100% discontinuing daclizumab. Reasons for discontinuation when available are displayed in table 5.
There were 2 pregnancies recorded in individuals under 18 years during treatment with newer DMTs. One occurred on fingolimod with the pregnancy outcome unknown. One pregnancy occurred on dimethyl fumarate and resulted in an induced abortion. Both patients discontinued DMT about 1 month after pregnancy began. Further details of these pregnancies are unavailable.
Predictors of newer DMT use
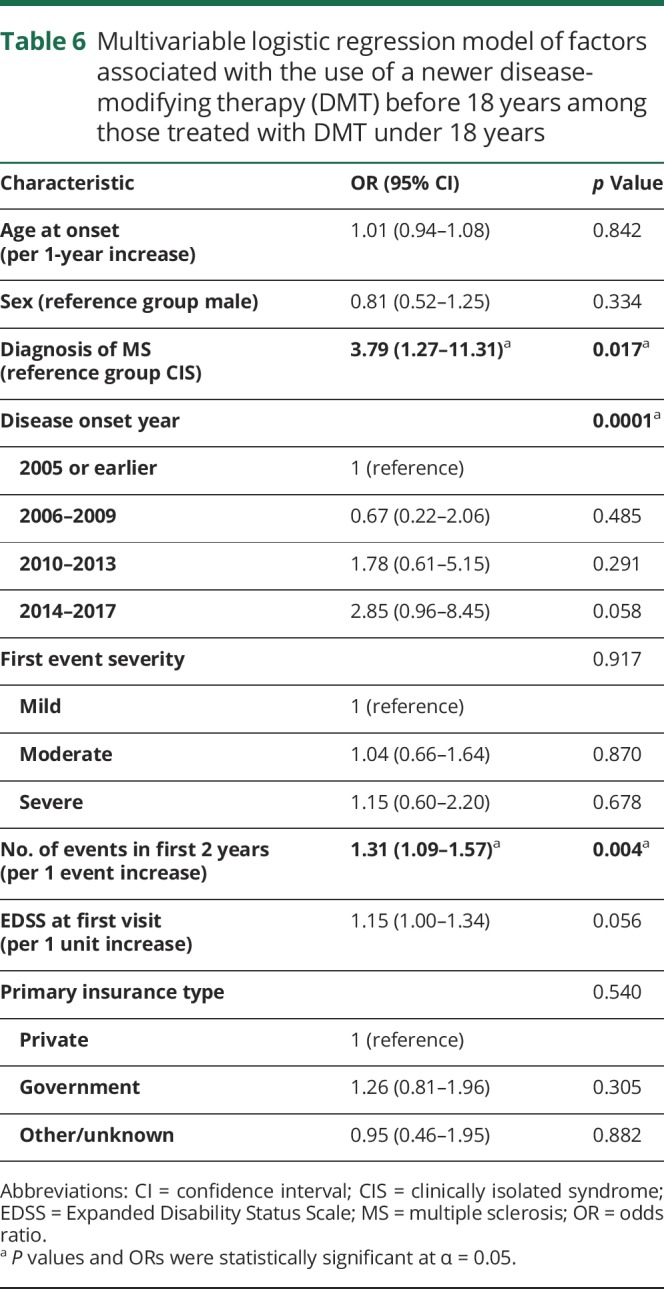
We employed a multivariable logistic regression model to assess which factors were independently associated with whether individuals received a newer DMT before 18 years among those treated with DMT under 18 years with complete data (n = 422) (table 6). Those with MS had higher odds of receiving a newer DMT before 18 years than those with CIS (odds ratio [OR] 3.8 [95% confidence interval (CI) 1.3–11.3], p = 0.017). Those who had more recent disease onset had higher odds of receiving a newer DMT before 18 years compared to more remote onset (OR 2.9 [95% CI 1.0–8.5] for 2014–2017 compared to 2005 and earlier, p = 0.0001 overall; test for linear trend p = 0.016). For every increase in number of relapses in the first 2 years of disease by 1, there was 1.3 times the odds of receiving a newer DMT before 18 years (OR 1.3 [95% CI 1.1–1.6], p = 0.004). Other variables that are listed in Methods were not statistically significant predictors of receiving a newer DMT before 18 years, and the overall model was statistically significant (p < 0.001) but only explained 7.9% of the variability in whether one received a newer DMT before 18 years. When age at onset was dichotomized to <12 vs ≥12 years, results did not differ and age at onset was not a statistically significant predictor of use of a newer DMT before 18 years. When we added study site to the logistic regression model, this was a significant predictor of newer DMT use before 18 years of age, and additional variability of 8.4% was explained.
Table 6.
Multivariable logistic regression model of factors associated with the use of a newer disease-modifying therapy (DMT) before 18 years among those treated with DMT under 18 years
Discussion
In this large prospective cohort of pediatric MS/CIS, there is increasing adoption of newer medications despite limited information on safety and efficacy in the pediatric age range. In those treated under 18 years of age, newer DMTs were started first-line in almost 20% and were used in over 40% of patients during follow-up. Those started on newer DMTs first-line were more likely to be older and with higher baseline disability than those started on injectable agents first-line, while those started on newer DMT had lower relapse rate in the first 2 years of disease, likely as a result of newer therapy. As expected, a diagnosis of MS rather than CIS, more recent disease onset, and higher number of relapses in the first 2 years were associated with use of a newer DMT before 18 years. The most commonly used newer DMTs included dimethyl fumarate and natalizumab.
Over the last 10 years, both oral and IV newer DMTs were started more frequently over time, including as first-line therapy. There was a stronger trend for adopting newer medications in adolescents than young children (younger than 12 years), suggesting there is more concern about the use of newer DMTs in the youngest with pediatric MS. In those 12 years and older, fewer than half started initial treatment with an injectable in 2016–2017, with similar proportions starting oral and IV DMTs first-line. It is unknown whether this is driven by patient or physician preference. Only 8.4% of the variability in the use of newer DMT before 18 years was explained by study site.
This cohort importantly includes 109 young children with MS treated with DMT before 12 years, since those under 10 years are excluded from pediatric trials and those under 12 are underrepresented. Those with CIS onset before 12 years were uncommonly treated compared to those diagnosed with MS, which may be due to diagnostic uncertainty. In our cohort, 24 children with MS received newer DMTs before 12 years. Only 3% on natalizumab and 5% on dimethyl fumarate received lower than adult doses, typically in these younger children.
There were no new side effect concerns identified compared to those observed in adults. Rates of side effects are consistent with those observed in adult studies, with lower rates in our cohort compared to randomized studies in adults, likely due to the observational design.31–34 There were no cases of progressive multifocal leukoencephalopathy, although the duration of treatment and follow-up is relatively short.
A relatively high proportion of individuals discontinued certain newer DMTs including natalizumab, rituximab, teriflunomide, and daclizumab. Reasons included ongoing clinical or radiologic disease activity as well as side effects and potentially JCV antibody positivity for natalizumab. There were many cases with an unclear reason for discontinuation, although a portion of these cases had recent evidence of disease activity.
The need for treatment escalation in refractory and highly active cases of pediatric MS has been described previously.15,16 We found that 30% of those started initially on injectable therapy switched to a newer agent before 18 years of age in our cohort. There are several observational studies demonstrating the effectiveness of natalizumab in pediatric MS, mainly in those who failed an injectable therapy,16,18–22 while there are more limited and small retrospective studies of other newer agents including rituximab,23,24 dimethyl fumarate,25 fingolimod,16,26 and daclizumab,27 with no studies of teriflunomide. The first randomized trial of DMT in pediatric MS demonstrated superiority of fingolimod over intramuscular interferon-β-1a.28 Randomized trials of other newer DMTs are ongoing, including of dimethyl fumarate (ClinicalTrials.gov: NCT02283853) and teriflunomide (ClinicalTrials.gov: NCT02201108). As results become available, it is likely that patterns of DMT use in children will evolve. Taken together, our data along with findings from clinical trials are reassuring regarding the safety of these agents in the pediatric age group, although longer follow-up is required.
Limitations of this study include the potential for underestimation of side effects due to a limited number of specific side effects captured in the database. We also do not capture laboratory safety monitoring or reliable vaccination status in the database. Despite this, the distribution of side effects observed in our large number of children treated with newer DMTs is informative. We also lack long-term safety data for newer DMTs due to the limited time these agents have been available. Reasons for discontinuation of newer DMTs were not available in many cases, and thus reasons for the relatively high proportion discontinuing newer DMTs is not available. Finally, information regarding lesion load on MRI at baseline and severity of relapses other than the first event were not available to assess as predictors of newer DMT use given that these are not available in the database. Despite these limitations, our findings are generalizable to a broad range of patients with pediatric MS/CIS in the United States given that centers across diverse geographic areas of the United States are included, involving more than one provider at some sites, and many individuals with pediatric MS are seen in tertiary centers. Our findings may differ from locations such as Canada and the European Union, as access to certain newer DMTs may be more limited due to treatment algorithms required by public drug coverage programs. Practice preferences may also vary by providers in different countries.
Our findings documenting the use, short-term safety, tolerability, and side effects of newer DMTs in this cohort are informative for clinical management of patients with pediatric MS/CIS. Strengths of our study include multicenter data from across the United States captured in the largest available database of pediatric MS/CIS with prospective data collection and quality control. We also examine a diverse range of DMTs over the last 10 years, allowing for a complete overview of the increasing use of newer oral and IV DMTs over time in pediatric MS/CIS.
Development of shared treatment strategies across multiple centers may allow systematic evaluation of the risks and benefits of newer DMTs in children in a real-word setting. However, this would likely be challenging to implement given differing practice styles and medication coverage by country and insurer.
Observational studies have an important role in providing real-world data and guiding clinical practice, although randomized clinical trials provide gold standard evidence for dosing, efficacy, and safety. Follow-up is ongoing to evaluate the individual effectiveness of the commonly used newer DMTs on relapse rate in this observational cohort. This will complement clinical trial data with real-world experience using newer DMTs in children. Additional study of long-term side effects and safety of these agents in children during development are needed and will be possible as longer follow-up becomes available.
Acknowledgment
The authors thank the research coordinators and the patients and their families who participated in the study.
Glossary
- CI
confidence interval
- CIS
clinically isolated syndrome
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- FDA
Food and Drug Administration
- JCV
JC virus
- MS
multiple sclerosis
- OR
odds ratio
Footnotes
Podcast: NPub.org/jpklk4
CME Course: NPub.org/cmelist
Contributor Information
Collaborators: US Network of Pediatric MS Centers, Kristen M Krysko, Jennifer Graves, Mary Rensel, Bianca Weinstock-Guttman, Gregory Aaen, Anita Belman, Leslie Benson, Tanuja Chitnis, Mark Gorman, Manu Goyal, Yolanda Harris, Lauren Krupp, Timothy Lotze, Soe Mar, Manikum Moodley, Jayne Ness, Moses Rodriguez, John Rose, Teri Schreiner, Jan-Mendelt Tillema, Michael Waltz, T. Charles Casper, and Emmanuelle Waubant
Author contributions
Kristen M. Krysko: study concept and design, acquisition of data, analysis and interpretation of data, drafting of manuscript. Jennifer Graves: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of manuscript for intellectual content. Mary Rensel: acquisition of data, critical revision of manuscript for intellectual content. Bianca Weinstock-Guttman: acquisition of data, critical revision of manuscript for intellectual content. Gregory Aaen: acquisition of data, critical revision of manuscript for intellectual content. Leslie Benson: acquisition of data, critical revision of manuscript for intellectual content. Tanuja Chitnis: acquisition of data, critical revision of manuscript for intellectual content. Mark Gorman: acquisition of data, critical revision of manuscript for intellectual content. Manu Goyal: acquisition of data, critical revision of manuscript for intellectual content. Lauren Krupp: acquisition of data, critical revision of manuscript for intellectual content. Timothy Lotze: acquisition of data, critical revision of manuscript for intellectual content. Soe Mar: acquisition of data, critical revision of manuscript for intellectual content. Moses Rodriguez: acquisition of data, critical revision of manuscript for intellectual content. John Rose: acquisition of data, critical revision of manuscript for intellectual content. Michael Waltz: study concept and design, analysis and interpretation of data, critical revision of manuscript for intellectual content. T. Charles Casper: study concept and design, analysis and interpretation of data, critical revision of manuscript for intellectual content. Emmanuelle Waubant: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of manuscript for intellectual content.
Study funding
Study funded by the National Multiple Sclerosis Society (HC-1509-06233 [Dr. Casper]). Dr. Krysko is funded by a Sylvia Lawry Physician Fellowship from the National Multiple Sclerosis Society (FP-1605-08753). The funders did not have a role in the design or analysis of this study.
Disclosure
K. Krysko: fellowship support from the National Multiple Sclerosis Society. J. Graves: nonpromotional, unbranded educational seminar speaking for Biogen and Genzyme; research support from Biogen and Genentech. M. Rensel: consultant/speaker for Biogen, Teva, Genzyme, and Novartis; research support from Medimmune. B. Weinstock-Guttman: consultant/speaker for Biogen, Teva, Novartis, Genzyme, Genentech, and EMD Serono; research support from Biogen, Teva, Novartis, Genentech, and EMD Serono. G. Aaen has participated in clinical trials funded by Biogen. L. Benson reports no disclosures relevant to the manuscript. T. Chitnis: advisory board member for Bayer, Biogen, Novartis, and Sanofi-Genzyme; research support from Verily, Octave, Serono, and Biogen; has participated in clinical trials sponsored by Sanofi-Genzyme and Novartis. M. Gorman has participated in clinical trials funded by Novartis and Biogen. M. Goyal has received fees for providing consultations on medicolegal cases related to neuroradiology and has IBM Stock. L. Krupp: consultant/speaker for Biogen, Novartis, RedHill Biopharma, Everyday Health, Shire, and Gerson Lehman; compensation was received from Biogen, Reata Pharma USA, AbbVie, Amicus Therapeutics, SA Inventions, Finkhar, Janssen Pharmaceuticals Eisai, IPSOS, Octapharma, Atara Biotherapeutics, ERT Inc., and Research Tech. T. Lotze: consultant/speaker for Biogen. S. Mar and M. Rodriguez report no disclosures relevant to the manuscript. J. Rose: research support from Teva Neuroscience and Biogen. M. Waltz and T. Casper report no disclosures relevant to the manuscript. E. Waubant has not received any pharmaceutical company honorarium; is site PI for a Novartis and Roche trial; has volunteered on an advisory board for a Novartis trial; is a nonrenumerated advisor for clinical trial design to Novartis, Biogen-Idec, Sanofi, Genentech, Serono, and Celgene; has funding from the NMSS, PCORI, and the Race to Erase MS; and is the section editor for Annals of Clinical and Translational Neurology and co-Chief editor for MSARD. Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology March 12, 2018. Accepted in final form July 25, 2018.
References
- 1.Banwell B, Ghezzi A, Bar-Or A, Mikaeloff Y, Tardieu M. Multiple sclerosis in children: clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurol 2007;6:887–902. [DOI] [PubMed] [Google Scholar]
- 2.Waldman A, Ness J, Pohl D, et al. Pediatric multiple sclerosis: clinical features and outcome. Neurology 2016;87(suppl 2):S74–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorman MP, Healy BC, Polgar-Turcsanyi M, Chitnis T. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol 2009;66:54–59. [DOI] [PubMed] [Google Scholar]
- 4.Benson LA, Healy BC, Gorman MP, et al. Elevated relapse rates in pediatric compared to adult MS persist for at least 6 years. Mult Scler Relat Disord 2014;3:186–193. [DOI] [PubMed] [Google Scholar]
- 5.Yeh EA, Weinstock-Guttman B, Ramanathan M, et al. Magnetic resonance imaging characteristics of children and adults with paediatric-onset multiple sclerosis. Brain 2009;132:3392–3400. [DOI] [PubMed] [Google Scholar]
- 6.Waubant E, Hietpas J, Stewart T, et al. Interferon beta-1a in children with multiple sclerosis is well tolerated. Neuropediatrics 2001;32:211–213. [DOI] [PubMed] [Google Scholar]
- 7.Mikaeloff Y, Moreau T, Debouverie M, et al. Interferon-beta treatment in patients with childhood-onset multiple sclerosis. J Pediatr 2001;139:443–446. [DOI] [PubMed] [Google Scholar]
- 8.Ghezzi A, Amato MP, Capobianco M, et al. Disease-modifying drugs in childhood-juvenile multiple sclerosis: results of an Italian co-operative study. Mult Scler 2005;11:420–424. [DOI] [PubMed] [Google Scholar]
- 9.Ghezzi A, Amato MP, Capobianco M, et al. Treatment of early-onset multiple sclerosis with intramuscular interferonbeta-1a: long-term results. Neurol Sci 2007;28:127–132. [DOI] [PubMed] [Google Scholar]
- 10.Pohl D, Rostasy K, Gärtner J, Hanefeld F. Treatment of early onset multiple sclerosis with subcutaneous interferon beta-1a. Neurology 2005;64:888–890. [DOI] [PubMed] [Google Scholar]
- 11.Mikaeloff Y, Caridade G, Tardieu M, Suissa S; KIDSEP study group of the French Neuropediatric Society. Effectiveness of early beta interferon on the first attack after confirmed multiple sclerosis: a comparative cohort study. Eur J Paediatr Neurol 2008;12:205–209. [DOI] [PubMed] [Google Scholar]
- 12.Pakdaman H, Fallah A, Sahraian MA, Pakdaman R, Meysamie A. Treatment of early onset multiple sclerosis with suboptimal dose of interferon beta-1a. Neuropediatrics 2006;37:257–260. [DOI] [PubMed] [Google Scholar]
- 13.Kornek B, Bernert G, Balassy C, Geldner J, Prayer D, Feucht M. Glatiramer acetate treatment in patients with childhood and juvenile onset multiple sclerosis. Neuropediatrics 2003;34:120–126. [DOI] [PubMed] [Google Scholar]
- 14.Ghezzi A, Amato MP, Annovazzi P, et al. Long-term results of immunomodulatory treatment in children and adolescents with multiple sclerosis: the Italian experience. Neurol Sci 2009;30:193–199. [DOI] [PubMed] [Google Scholar]
- 15.Yeh EA, Waubant E, Krupp LB, et al. Multiple sclerosis therapies in pediatric patients with refractory multiple sclerosis. Arch Neurol 2011;68:437–444. [DOI] [PubMed] [Google Scholar]
- 16.Huppke P, Huppke B, Ellenberger D, et al. Therapy of highly active pediatric multiple sclerosis. Mult Scler Epub 2017 Sep 1. [DOI] [PubMed] [Google Scholar]
- 17.Baroncini D, Zaffaroni M, Moiola L, et al. Long-term follow-up of pediatric MS patients starting treatment with injectable first-line agents: a multicentre, Italian, retrospective, observational study. Mult Scler Epub 2018 Jan 1. [DOI] [PubMed] [Google Scholar]
- 18.Arnal-Garcia C, García-Montero MR, Málaga I, et al. Natalizumab use in pediatric patients with relapsing-remitting multiple sclerosis. Eur J Paediatr Neurol 2013;17:50–54. [DOI] [PubMed] [Google Scholar]
- 19.Kornek B, Aboul-Enein F, Rostasy K, et al. Natalizumab therapy for highly active pediatric multiple sclerosis. JAMA Neurol 2013;70:469–475. [DOI] [PubMed] [Google Scholar]
- 20.Ghezzi A, Moiola L, Pozzilli C, et al. Natalizumab in the pediatric MS population: results of the Italian registry. BMC Neurol 2015;15:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghezzi A, Pozzilli C, Grimaldi LME, et al. Natalizumab in pediatric multiple sclerosis: results of a cohort of 55 cases. Mult Scler 2013;19:1106–1112. [DOI] [PubMed] [Google Scholar]
- 22.Alroughani R, Ahmed SF, Behbehani R, Al-Hashel J. The use of natalizumab in pediatric patients with active relapsing multiple sclerosis: a prospective study. Pediatr Neurol 2017;70:56–60. [DOI] [PubMed] [Google Scholar]
- 23.Beres SJ, Graves J, Waubant E. Rituximab use in pediatric central demyelinating disease. Pediatr Neurol 2014;51:114–118. [DOI] [PubMed] [Google Scholar]
- 24.Salzer J, Lycke J, Wickström R, Naver H, Piehl F, Svenningsson A. Rituximab in paediatric onset multiple sclerosis: a case series. J Neurol 2016;263:322–326. [DOI] [PubMed] [Google Scholar]
- 25.Makhani N, Schreiner T. Oral dimethyl fumarate in children with multiple sclerosis: a dual-center study. Pediatr Neurol 2016;57:101–104. [DOI] [PubMed] [Google Scholar]
- 26.Fragoso YD, Alves-Leon SV, Barreira AA, et al. Fingolimod prescribed for the treatment of multiple sclerosis in patients younger than age 18 years. Pediatr Neurol 2015;53:166–168. [DOI] [PubMed] [Google Scholar]
- 27.Gorman MP, Tillema JM, Ciliax AM, Guttmann CRG, Chitnis T. Daclizumab use in patients with pediatric multiple sclerosis. Arch Neurol 2012;69:78–81. [DOI] [PubMed] [Google Scholar]
- 28.Chitnis T, Arnold DL, Banwell B, et al. PARADIGMS: a randomised double-blind study of fingolimod versus interferon β-1a in paediatric multiple sclerosis. Mult Scler J 2017;23(3 suppl):976–1023. [Google Scholar]
- 29.Casper TC, Rose JW, Roalstad S, et al. The US Network of pediatric multiple sclerosis centers: development, progress, and next steps. J Child Neurol 2015;30:1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krupp LB, Tardieu M, Amato MP, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler 2013;19:1261–1267. [DOI] [PubMed] [Google Scholar]
- 31.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008;358:676–688. [DOI] [PubMed] [Google Scholar]
- 32.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. [DOI] [PubMed] [Google Scholar]
- 33.Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387–401. [DOI] [PubMed] [Google Scholar]
- 34.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098–1107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available to qualified investigators on request for the purposes of replicating procedures or results by contacting the corresponding author.