Abstract
Objectives. Advances in diagnostic techniques have led to better distinction between types of vasculitis, potentially affecting the utility of the 1990 ACR classification criteria for vasculitis. This study tested the performance of these criteria in a contemporary vasculitis cohort.
Methods. The Diagnosis and Classification in Vasculitis Study provided detailed clinical, serological, pathological and radiological data from patients with primary systemic vasculitis and clinical context-specific comparator conditions. Fulfilment of six ACR criteria sets and their diagnostic performance was evaluated in patients with a given type of vasculitis and its comparator conditions.
Results. Data from 1095 patients with primary systemic vasculitis and 415 with comparator conditions were available. For classification, sensitivities and specificities for ACR classification criteria were, respectively, 81.1% and 94.9% for GCA; 73.6% and 98.3% for Takayasu’s arteritis; 65.6% and 88.7% for granulomatosis with polyangiitis; 57.0% and 99.8% for eosinophilic granulomatosis with polyangiitis; 40.6% and 87.8% for polyarteritis nodosa; 28.9% and 88.5% for microscopic polyangiitis; and 72.7% and 96.3% for IgA-vasculitis. Overall sensitivity was 67.1%. Of cases identified by their respective criteria, 16.9% also met criteria for other vasculitides. Diagnostic specificity ranged from 64.2 to 98.9%; overall, 113/415 comparators (27.2%) fulfilled at least one of the ACR classification criteria sets.
Conclusion. Since publication of the ACR criteria for vasculitis, the sensitivity for each type of vasculitis, except GCA, has diminished, although the specificities have remained high, highlighting the need for updated classification criteria.
Keywords: vasculitis, Churg–Strauss syndrome, giant cell arteritis, polyarteritis nodosa, anti-neutrophil cytoplasm antibody, Takayasu’s disease, microscopic polyangiitis
Rheumatology key messages
At diagnosis, the sensitivity of the 1990 ACR Criteria for the vasculitides was low (67.1%).
Diagnostic criteria for vasculitis are needed for use in clinical practice.
New classification and diagnostic criteria for vasculitis should incorporate microscopic polyangiitis and more novel widely available tests.
Introduction
In 1990, the ACR published criteria for the classification of seven types of systemic vasculitis: GCA, Takayasu’s arteritis (TAK), eosinophilic granulomatosis with polyangiitis (Churg–Strauss, EGPA), granulomatosis with polyangiitis (GPA), polyarteritis nodosa (PAN), IgA vasculitis (Henoch–Schönlein, IgAV) and hypersensitivity vasculitis [1].
Although the 1990 ACR Classification Criteria have been widely applied in clinical studies and facilitated research in vasculitis, they also have important limitations [2]. Firstly, microscopic polyangiitis (MPA) was not one of the named conditions because it was not a widely recognized condition in the 1980s. Secondly, the criteria were developed before the widespread use of testing for ANCA which has since become a fundamental aspect in the diagnosis and classification of ANCA-associated vasculitis (AAV) [3]. Thirdly, introduction and widespread use of new diagnostic techniques (e.g. computerized tomography and MRI) have contributed to a better distinction between different types of vasculitis [4, 5]. Although their suboptimal performance in classification of vasculitides has been previously documented in several studies [6–9], they are still used in clinical research. Furthermore, many clinicians apply the ACR criteria in clinical practice for diagnosis, although these criteria were not designed for this purpose and are inadequate as diagnostic tools [10]. We aimed to test whether the 1990 ACR classification criteria would perform similarly in a large international and more heterogeneous cohort of vasculitis patients recruited to the Diagnosis and Classification of Vasculitis Study (DCVAS), a major international research initiative to develop a revised single classification system and a validated set of diagnostic criteria for the vasculitides.
This analysis tested the performance of six of the 1990 ACR classification criteria for vasculitis for use in both classification (original intent) and diagnosis of patients with vasculitis and comparator conditions enrolled in the DCVAS.
Methods
Patients and inclusion criteria
The data source was the DCVAS project, a prospective multicentre study to develop diagnostic and classification criteria in vasculitis [10], and included all patients recruited between September 2010 and June 2014. The dataset has detailed clinical, serological, pathological and radiological data from patients with primary systemic vasculitis (PSV) and patients with clinical context-specific comparator conditions. The detailed methodology of the DCVAS study has been described elsewhere [11]. The physicians submitting cases were asked to confirm their opinion on the diagnosis and their level of diagnostic certainty (very certain: ⩾75%; moderately certain: 50–74%; uncertain: 25–49%; very uncertain: <25%) for each patient. Included in this analysis were data from patients with a baseline diagnosis of any PSV with a recorded confidence in diagnosis of ⩾75% by the submitting clinician, as well as patients with conditions considered to be comparators for GCA, TAK, AAV, PAN and IgAV (definitions shown in supplementary Table S1, available at Rheumatology Online). Patients who had a change of diagnosis at the 6 months’ follow-up, and patients with GPA or MPA without either a positive ANCA test or a biopsy were excluded from the analysis, because certainty of the initial diagnosis was deemed insufficient. This study was approved by the Berkshire Research Ethics Committee (no. 10/H0505/19, dated 7 May 2010), written informed consent was obtained from all patients prior to enrolment and the study was conducted in accordance with good clinical practice and the principles of the Declaration of Helsinki.
Analysis
The presence of each individual ACR criterion was evaluated in each case of PSV. The 1990 ACR classification criteria were considered fulfilled if the specified number of features for each criteria set was met (e.g. three out of five for GCA). The definitions for each criterion are shown in supplementary Table S2, available at Rheumatology Online. Criteria involving imaging data were considered present based on either the originally described methods (e.g. conventional angiography) or by using current comparable methods (magnetic resonance angiography or computerized tomography angiography). Missing information was considered absent.
Each of the six sets of the 1990 ACR criteria was tested against all patients with PSV in the DCVAS cohort (including patients with other types of vasculitis not covered by the ACR criteria) to assess the sensitivity and specificity of the criteria for classification of GCA, TAK, GPA, EGPA, PAN and IgAV. The ACR criteria for hypersensitivity vasculitis were not assessed due to low patient numbers (n = 9); these patients were included as patients with other forms of PSV. Diagnostic specificity was evaluated by applying the respective criteria sets to patients with each given type of vasculitis and their comparators (e.g. all patients with GCA plus GCA-comparators). Physician-submitted diagnosis was considered to be the gold standard. Because there was no distinction between PAN and MPA in the original ACR criteria, the ACR PAN criteria were used for both PAN and MPA in the DCVAS cohort, although we fully appreciate that this is a somewhat academic exercise.
Results
Patients and diagnostic workup
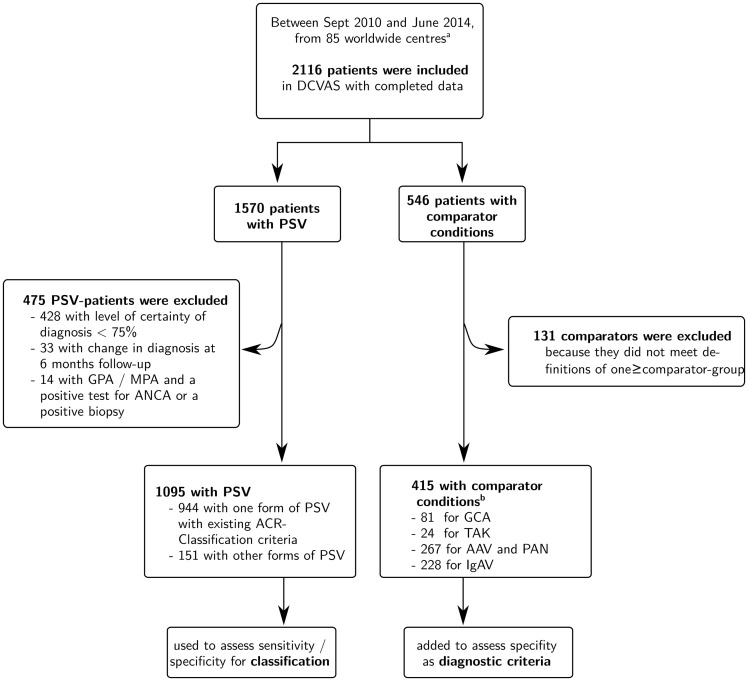
Data from 2116 patients (1570 with PSV; 564 with comparator conditions) from 85 centres worldwide were available (Fig. 1). Six hundred and six patients (475 with PSV; 131 with comparator conditions) were excluded. In total, 1095 patients with a physician-submitted diagnosis of PSV were included in the analysis; 944 of these patients had one of the diagnoses for which ACR classification criteria are available, and 151 had other types of PSV (Table 1). These 1095 patients were used to assess the criteria performance for classification. Four hundred and fifteen patients who did not have vasculitis were included in the analysis as comparators to assess diagnostic performance of the criteria; 38.3% of the comparator cases were included in more than one comparator group. An overview of clinical conditions in the comparator patients is shown in supplementary Table S3, available at Rheumatology Online.
Fig. 1.
Study population
aThe study was promoted at international conferences; all centres willing to participate were invited to take part in the study. At the time of this analysis data had been collected in rheumatology, renal, internal medicine, immunology and neurology centres in a total of 31 countries in Asia, Australasia, Europe, North America and South America. bSome comparator patients were used for more than one comparator group. AAV: ANCA-associated vasculitis; DCVAS: Diagnostic and Classification in Vasculitis Study; GPA: granulomatosis with polyangiitis; IgAV: IgA-vasculitis; MPA: microscopic polyangiitis; PSV: primary systemic vasculitis; TAK: Takayasu’s arteritis.
Table 1.
Sensitivity and specificity of the ACR criteria to classify and diagnose patients with and without vasculitis
| Patient group | 1990 ACR Criteria tested in the DCVAS population | 1990 ACR Criteria performance characteristics in original cohort | ||||
|---|---|---|---|---|---|---|
| Classificationb | Diagnosticc | Classification | ||||
| Vasculitis | n | Sensitivitya (95% CI), % | Specificity (95% CI), % | Specificity (95% CI), % | Sensitivity, % | Specificity, % |
| GCA | 345 | 81.1 (76.6, 85.1) | 94.9 (93.1, 96.3) | 64.2 (52.8, 74.6) | 93.5 | 91.2 |
| TAK | 53 | 73.6 (59.7, 84.7) | 98.3 (97.3, 99.0) | 87.5 (67.6, 97.3) | 90.5 | 97.8 |
| GPA | 275 | 65.6 (59.9, 71.4) | 88.7 (86.3, 90.7) | 88.0 (83.5, 91.7) | 88.2 | 92.0 |
| EGPA | 79 | 57.0 (45.3, 68.1) | 99.8 (99.3, 100) | 98.9 (96.8, 99.8) | 85.0 | 99.7 |
| PAN | 32 | 40.6 (23.7, 59.4) | 87.8 (85.7, 89.7) | 92.5 (88.7, 95.7) | 82.2 | 86.6 |
| MPA | 94 | 28.9 (20.1, 39.0) | 88.5 (86.4, 90.4) | 92.5 (88.7, 95.3) | NS | NS |
| IgAV | 66 | 72.7 (60.4, 83.0) | 96.3 (94.9, 97.3) | 90.4 (87.6, 93.9) | 87.1 | 87.7 |
Sensitivity is the same for diagnosis and classification because the same vasculitis patients were used for both analyses.
Individual ACR criteria were applied to all patients with PSV (n = 1095) including 151 patients with other forms of vasculitis without existing ACR criteria [aortitis (n = 7), other large vessel vasculitis (n = 15), single organ vasculitis (n = 25), undefined small vessel vasculitis (n = 51), Behçet’s disease (n = 30), other undefined primary vasculitis with no specific vessel size (n = 2), cryoglobulinaemic vasculitis (n = 12) and CNS vasculitis (n = 9)].
Individual ACR criteria were applied to vasculitis-specific comparators (GCA comparators: 81; AAV/PAN comparators: 267; IgAV comparators: 228; TAK comparators: 24); some of the comparators served for more than one form of vasculitis. DCVAS: Diagnostic and Classification Criteria in Vasculitis Study; EGPA: eosinophilic granulomatosis with polyangiitis; GPA: granulomatosis with polyangiitis; IgAV: IgA-vasculitis; MPA: microscopic polyangiitis; PAN: polyarteritis nodosa; TAK: Takayasu’s arteritis.
The number of imaging procedures, biopsies and tests for ANCA, with the average number of investigations performed by contributing centres in the main vasculitis categories, are presented in Table 2. In 60 (5.5%) patients with PSV and 22 (5.3%) comparators, neither an imaging study nor a biopsy had been performed; in 21 (1.1%) and 5 (1.2%), respectively, data were missing in these categories.
Table 2.
Diagnostic workup by contributing centres
| Diagnostic tests | No. of patients (%) | Median by centre (IQR) |
|---|---|---|
| GCA | n = 345 (45 centres) | 3 (1–8) |
| TA Biopsy, n (%) | 289 (83.8) | 100 (80–100) |
| US, n (%) | 120 (34.8) | 0 (0–68.8)a |
| CTA/MRA, n (%) | 50 (14.5) | 0 (0–38) |
| PET, n (%) | 61 (17.8) | 0 (0–10)b |
| Takayasu’s arteritis | n = 53 (23 centres) | 2 (1–3) |
| Biopsy, n (%) | 12 (22.6) | 0 (0–50) |
| CTA/MRA/US, n (%) | 43 (81.1) | 100 (71–100) |
| PET, n (%) | 13 (24.5) | 0 (0–45) |
| AAV/PAN | n = 546 (70 centres) | 4 (2–10) |
| ANCA, n (%) | 545 (99.8) | 100 (100–100) |
| Biopsy, n (%) | 416 (76.2) | 88.2 (62.5–100) |
Majority of US scans were performed by the major GCA-recruiting centres: one Slovenian, one Swiss, one German (all with performance rate >70%) and four UK centres (performance rate 9–33%). The centres that recruited <10 GCA patients infrequently performed US as a part of regular diagnostic workup.
PET scans were performed in 12 centres in 9–100% of GCA patients/centre. AAV: ANCA-associated vasculitis; CTA: computerized tomography angiogram; MRA: magnetic resonance imaging—angiography; PAN: polyarteritis nodosa; TA: temporal artery.
Performance of the criteria for classification
The performance of the individual 1990 ACR classification criteria for sensitivity and specificity within the DCVAS cohort is shown in Table 1. For classification, the sensitivity of the 1990 ACR criteria ranged from 81.1% for GCA to 28.9% for MPA and the specificity ranged from 99.8% for EGPA to 88.5% in MPA (using the PAN criteria).
The sensitivity of the criteria for GPA and EGPA improved when a positive ANCA was considered as a surrogate for a positive biopsy: GPA: 90.5 (95% CI: 86.5, 93.7%); EGPA: 68.4 (95% CI: 56.9, 78.4%). Specificity remained high in EGPA [98.3 (95% CI: 97.3, 99.0%)], but was reduced in GPA [68.4 (95% CI: 56.9, 78.4%)]. Applying solely positive PR3-ANCA as biopsy surrogate for GPA yielded a sensitivity of 89.1% (95% CI: 84.8, 92.5%) and a specificity of 85.1% (95% CI: 82.5, 87.5%).
There was substantial heterogeneity in sensitivity and specificity across centres for most of the diagnoses; however, the number of cases with certain PSV was quite low in some centres (supplementary Table S4, available at Rheumatology Online).
Overall performance of the criteria
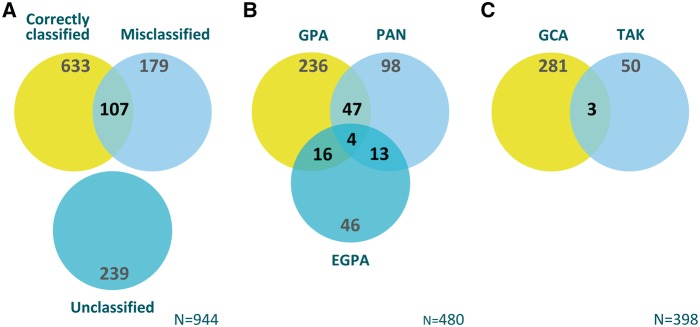
Overall, 633 of 944 patients with one of the forms of vasculitis covered by the ACR criteria were captured by the ACR classification criteria (sensitivity 67.1%). Of all 1095 patients with PSV, 267 (24.4%) fulfilled criteria for at least one condition other than their physician-submitted diagnosis, including 107 of 633 patients (16.9%) who were correctly captured by ACR criteria. Overall accuracy and overlap of the application of the 1990 ACR criteria is illustrated by Fig. 2.
Fig. 2.
Accuracy and overlap of the 1990 ACR classification criteria in various forms of vasculitis
(A) Patients in DCVAS with one of the forms of primary systemic vasculitis with existing 1990 ACR Criteria (n = 944) that were: classified in accordance with physician’s submitted diagnosis (Correctly Classified), not classified in accordance with physician’s submitted diagnosis (Misclassified), or not classified by any of the ACR criteria sets (Unclassified); overlap between Correctly Classified and Misclassified includes patients who were classified by ACR criteria as having more than one diagnosis (one concordant and another non-concordant with physician’s submitted diagnosis. (B) The number of overlapping diagnoses when ACR 1990 criteria were applied to patients determined by the submitting physician as having a form of small-vessel vasculitis. (C) The number of overlapping diagnoses when ACR 1990 criteria were applied to patients determined by the submitting physician as having a form of large-vessel vasculitis. DCVAS: Diagnostic and Classification Criteria in Vasculitis Study; EGPA: eosinophilic granulomatosis with polyangiitis; GPA: granulomatosis with polyangiitis; PAN: polyarteritis nodosa; TAK: Takayasu’s arteritis.
Diagnostic performance of the criteria
When applied as diagnostic criteria, that is, to patients with a given type of vasculitis and its disease context comparators, the specificity of the ACR criteria ranged from 64.2% in GCA to 98.9% in EGPA (Table 1); overall 113 of 415 (27.2%) patients with vasculitis comparator conditions fulfilled one of the ACR classification criteria sets.
Differences between patients captured and patients not captured by the criteria
Table 3 and supplementary Table S5, available at Rheumatology Online, compare the demographic characteristics, disease manifestations and fulfilment of individual ACR criteria in patients with physician-submitted diagnosis concordant with 1990 ACR criteria or not (correctly vs not correctly classified). Compared with patients correctly classified per ACR criteria (true positives), patients who were not correctly classified (false negatives) had fewer of each ACR criteria (supplementary Table S5, available at Rheumatology Online). However, in terms of non-criteria characteristics (Table 2), the percentage of positive ANCA tests in both groups of patients with AAV were comparably high (87.4% vs 87.8%; P = 0.984). Similarly, the groups did not differ in terms of positive biopsy results (70.5% vs 71.2%; P = 0.881), when less stringent than ACR biopsy definitions were applied (biopsy consistent with vasculitis but not definite or definite vasculitis). In contrast, only 5.5% of patients with GPA or EGPA not captured by the criteria met the corresponding original ACR biopsy definitions. Patients with large vessel vasculitis (GCA or TAK) who were not correctly classified were more likely to have abnormal findings on angiography and PET scans.
Table 3.
Demographic data and non-criteria characteristics of patients with primary systemic vasculitis with available ACR criteria
| Characteristic | Correctly classified by the 1990 ACR criteria | P-valuea | |
|---|---|---|---|
| Yes | Nob | ||
| All patients (n = 944) | 633 (67.1) | 311 (32.9) | |
| Age, mean (s.d.), years | 60.9 (17.6) | 58.1 (17.5) | 0.022 |
| Female, n (%) | 360 (56.9) | 178 (56.6) | 0.944 |
| Large-vessel vasculitis (n = 398) | 319 (80.2) | 79 (19.8) | |
| Age, mean (s.d.), years | 68.5 (15.4) | 63.3 (15.7) | 0.010 |
| LV-GCAc | 5 (1.6) | 26 (32.9) | <0.001 |
| Vascular US performed | 107 (33.5) | 28 (35.4) | 0.749 |
| Positive vascular US | 84 (26.3) | 22 (27.8) | 0.785 |
| PET scan performed | 37 (11.6) | 37 (46.8) | <0.001 |
| Positive PET scan | 21 (6.6) | 31 (39.2) | <0.001 |
| MRA/CTA performed | 63 (19.8) | 26 (32.9) | 0.012 |
| Positive MRA/CTA | 49 (77.8) | 23 (88.5) | 0.005 |
| ANCA-associated vasculitis (n = 448) | 253 (56.5) | 195 (43.5) | |
| Age, mean (s.d.), years | 54.3 (15.2) | 58.2 (16.5) | 0.009 |
| ANCA positive | 221 (87.4) | 171 (87.8) | 0.914 |
| PR3/MPO positive | 218 (86.2) | 161 (82.6) | 0.295 |
| Limited diseased (only GPA and EGPA) | 43 (19.0) | 33 (25.8) | 0.137 |
| Biopsy performed | 193 (76.3) | 139 (71.3) | 0.231 |
| Consistent with vasculitise | 136 (70.5) | 99 (71.2) | 0.881 |
Figures refer to number of patients with characteristic and percentage of group, unless stated otherwise.
Demographics and characteristics between patients with regards to classification by the ACR criteria were analysed using Pearson’s χ2 test or t test for equality of means, as appropriate. All P-values are two-tailed and were considered significant if < 0.05.
Not correctly classified by 1990 ACR criteria means that either they did not meet the criteria or were classified with a vasculitis not concordant with the one submitted by the investigator.
Defined as GCA with clinical or radiologic evidence of large-vessel involvement but without clinical evidence of cranial involvement.
Defined as GPA/EGPA with upper/lower respiratory tract disease without any other systemic involvement or constitutional symptoms.
Based on DCVAS items: biopsy consistent with vasculitis but not definite or definite vasculitis (not necessarily reflecting the ACR-biopsy definitions). CTA: CT-angiography; DCVAS: Diagnostic and Classification Criteria in Vasculitis Study; EGPA: eosinophilic granulomatosis with polyangiitis; GPA: granulomatosis with polyangiiti; IgAV: IgA-vasculitiss; MPA: microscopic polyangiitis; MPO: myeloperoxidase; MRA: MR-angiography; PAN: polyarteritis nodosa; TAK: Takayasu’s arteritis.
Discussion
This analysis demonstrates that the sensitivity of the 1990 ACR classification criteria has declined substantially over the last two decades. Overall, one-third of patients who had a physician-submitted diagnosis consistent with one of the types of vasculitis covered by the ACR criteria were not correctly classified by using the criteria. This was most striking with GPA and EGPA and may reflect improved recognition of a wider spectrum of disease and greater reliance on novel diagnostic tests, especially due to routine testing for ANCA [12]. ANCA testing is especially helpful in the diagnosis of GPA and MPA; it improves the specificity for diagnosis of EGPA; the presence of ANCA helps to rule out PAN [5].
Individual ACR criteria items were less frequently fulfilled in those cases with PSV that were not classified in agreement with the physician’s diagnosis than in cases that were correctly classified. While this is an expected finding, it likely reflects the greater reliance on diagnostic tools not covered by the ACR criteria. Furthermore, results of ANCA tests, modern imaging modalities and biopsies with less stringent definitions were positive in the majority of patients not captured by the ACR criteria. Thus, the stringency of the definitions for biopsy positivity and lack of inclusion of modern imaging tools and ANCA in the criteria may have had a large impact on the sensitivity of the ACR criteria. Indeed, sensitivity of the criteria for GPA improved when PR-3 ANCA was used as a surrogate for the ACR biopsy criterion with almost no loss of specificity. Newer diagnostic tools may have enabled an expansion of the clinical phenotype described within disease subtypes and broadened the appreciation of overlap between diseases, including the spectrum of large vessel disease, and the overlap between classification of patients with small vessel vasculitis and PAN [10, 13, 14]. This expansion of the spectrum of disease can reduce sensitivity of classification criteria as shown in this study.
The sensitivity for PAN of 40.6% was particularly low compared with the originally reported 82.2%. Since the 1990 ACR criteria for PAN were derived from combined cohort of patients with PAN and MPA, we wished to explore how these criteria performed in a cohort of patients with PAN compared with patients with MPA. Results from these analyses highlight that the PAN criteria have poor sensitivity not only for MPA (28.9%), which is perhaps not surprising, but also for PAN (40.6%). The predominance of non-HBV related PAN in our cohort (84.4%) could be one of the reasons for the low sensitivity of the criteria for this entity. However, poor performance of the 1990 ACR PAN criteria was previously reported with a sensitivity of 50.8%, when compared with other vasculitides as controls [9]. It is also interesting that specificity of the 1990 PAN classification criteria is similar for PAN and MPA (87.8% vs 88.5%). These comparative analyses highlight that the 1990 criteria for PAN are not fit for purpose for classifying patients with either MPA or PAN. Furthermore, the lack of MPA recognition by 1990 ACR criteria may have affected not only performance of the PAN criteria, but also the criteria for the other small vessel vasculitides.
The specificity of the ACR criteria for classification of most of the vasculitides within the DCVAS cohort was comparable to the original reports on the performance of the criteria. However, application of the criteria to the whole vasculitis cohort resulted in considerable overlap between types of AAV and PAN (Fig. 2), which may have a negative impact on the criteria’s accuracy in classifying patients enrolled into clinical studies and trials [13]. It is important that the high specificity for classification is also derived from the analogous approach to the original ACR criteria development: each criteria set was applied to all patients with PSV. With a total of 1095 patients in this study, including several with large-vessel disease, the overall specificity for, for example, EPGA is likely to be high. Their unsuitability to distinguish between the more similar forms of PSV is demonstrated by their considerable overlap amongst these diagnoses (Fig. 2).
When the ACR criteria were applied to comparator patients (i.e. those without vasculitis), over a quarter of patients met at least one ACR criteria set, underscoring that the 1990 ACR classification criteria are not well suited for diagnostic use, as demonstrated previously [10]. The individual diagnostic specificity was, however, high for each individual criteria set. Since many of the patients included in the comparator groups did not have multisystem disease and hypereosinophilic disorders were rare, this may account for the particular high specificity, for example, for EGPA.
This study has some limitations to consider. Firstly, DCVAS was collecting data only up to the time of diagnosis. Some potentially relevant data for the criteria such as biopsy results could have been available only after the date of diagnosis. However, we formally asked investigators to report any change in diagnosis that might have occurred as a result of new information becoming available during the 6 months after the initial diagnosis. In contrast, for some patients in the original 1990 ACR cohort, autopsy data were included in the analysis [15]. The lower sensitivity found in the present analysis might thus be anticipated; however, over 70% of patients with AAV had a biopsy performed with the result available at the time of study enrolment. Furthermore, patients who were captured by the criteria did not differ in terms of available biopsy results from patients who were not. This makes a significant impact on sensitivity less likely, although in some cases diagnosis could be made based on clinical presentation and ANCA alone without biopsy results, hence highlighting the need for future incorporation of these tests. Secondly, the use of the clinical diagnosis submitted by the recruiting physician as the gold standard could lead to circularity in attempts to classify patients.
Thirdly, centres may differ in their diagnostic approach, potentially leading to considerable heterogeneity in the criteria’s performance between centres. The validity of these findings, however, is augmented by the testing of the criteria in the largest and most heterogeneous cohort of patients with vasculitis recruited and the large number of specialized vasculitis centres worldwide participating in the study, the setting for which the 1990 ACR classification criteria were designed to be used.
In conclusion, the results of this study emphasize the need for updated classification and diagnostic criteria for the systemic vasculitides that incorporate newer diagnostic modalities and potentially redefine the boundaries between the individual diseases.
Funding: This work was supported by the EULAR; the ACR; and the Vasculitis Foundation [grant number EULAR: 15855].
Disclosure statement: R.A.L. reports departmental financial support from GlaxoSmithKline (GSK) and Chemocentryx, personal fees from Roche, personal fees from Janssen, personal fees from Union Chimique Belge; all outside of the submitted work. P.A.M. reports research support from Actelion, Bristol Myers Squibb, Celgene, ChemoCentryx, Genentech/Roche, GSK and consulting fees from Alexion, Actelion, ChemoCentryx, Genentech/Roche, Sanofi; all outside of the submitted work. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
Supplementary Material
Acknowledgements
We acknowledge the patients and clinicians involved in the DCVAS project (a full list of collaborators involved in this study can be found as supplementary data, available at Rheumatology Online).
References
- 1. Fries JF, Hunder GG, Bloch DA. et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Summary. Arthritis Rheum 1990;33:1135–6. [DOI] [PubMed] [Google Scholar]
- 2. Basu N, Watts R, Bajema I. et al. EULAR points to consider in the development of classification and diagnostic criteria in systemic vasculitis. Ann Rheum Dis 2010;69:1744–50. [DOI] [PubMed] [Google Scholar]
- 3. van der Woude FJ, Rasmussen N, Lobatto S. et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet 1985;1:425–9. [DOI] [PubMed] [Google Scholar]
- 4. Watts RA, Suppiah R, Merkel PA, Luqmani R.. Systemic vasculitis—is it time to reclassify? Rheumatology 2011;50:643–5. [DOI] [PubMed] [Google Scholar]
- 5. Jennette JC, Falk RJ, Bacon PA. et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 6. Lane SE, Watts RA, Barker THW, Scott DGI.. Evaluation of the Sørensen diagnostic criteria in the classification of systemic vasculitis. Rheumatology 2002;41:1138–41. [DOI] [PubMed] [Google Scholar]
- 7. Bruce IN, Bell AL.. A comparison of two nomenclature systems for primary systemic vasculitis. Br J Rheumatol 1997;36:453–8. [DOI] [PubMed] [Google Scholar]
- 8. Reid AJ, Harrison BD, Watts RA. et al. Churg-Strauss syndrome in a district hospital. QJM 1998;91:219–29. [DOI] [PubMed] [Google Scholar]
- 9. Henegar C, Pagnoux C, Puéchal X. et al. A paradigm of diagnostic criteria for polyarteritis nodosa: analysis of a series of 949 patients with vasculitides. Arthritis Rheum 2008;58:1528–38. [DOI] [PubMed] [Google Scholar]
- 10. Rao JK, Allen NB, Pincus T.. Limitations of the 1990 American College of Rheumatology classification criteria in the diagnosis of vasculitis. Ann Intern Med 1998;129:345–52. [DOI] [PubMed] [Google Scholar]
- 11. Craven A, Robson J, Ponte C. et al. ACR/EULAR-endorsed study to develop Diagnostic and Classification Criteria for Vasculitis (DCVAS). Clin Exp Nephrol 2013;17:619–21. [DOI] [PubMed] [Google Scholar]
- 12. Guillevin L, Lhote F, Amouroux J. et al. Antineutrophil cytoplasmic antibodies, abnormal angiograms and pathological findings in polyarteritis nodosa and Churg-Strauss syndrome: indications for the classification of vasculitides of the polyarteritis Nodosa Group. Br J Rheumatol 1996;35:958–64. [DOI] [PubMed] [Google Scholar]
- 13. Watts R, Lane S, Hanslik T. et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2007;66:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jennette JC, Falk RJ, Andrassy K. et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 1994;37:187–92. [DOI] [PubMed] [Google Scholar]
- 15. Bloch DA, Michel BA, Hunder GG. et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Patients and methods. Arthritis Rheum 1990;33:1068–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.