Abstract
Background: Absence of detectable viraemia after treatment cessation in some vertically HIV-infected (VHIV) children suggests that early initiation of HAART could lead to functional cure.
Objectives: We described the factors associated with HIV antibody levels and the viral reservoir size in HAART-treated VHIV children.
Methods: Study included 97 VHIV children with virological suppression, in Bamako, Mali. The anti-gp41 antibody activities and HIV serostatus were assessed. The viral reservoir size was measured by quantifying total cell-associated HIV DNA.
Results: Among the children studied, the median total HIV DNA level was 445 copies/106 cells (IQR = 187–914) and the median anti-gp41 antibody activity was 0.29 OD (IQR = 0.18–0.75). Low activity of anti-gp41 antibodies was associated with a younger age of HAART initiation (P = 0.01). Overall, eight HIV-1 seroreversions were identified.
Conclusions: Study identified potential candidates with low viral reservoir and low antibody levels or activities for future trials aiming to reduce HIV-1 reservoir to limit HAART duration.
Introduction
Paediatric HIV infection remains a major public health issue despite large implementation of prevention of mother-to-child transmission programmes.1 It requires early and lifelong HAART to control viral replication with the risk of accumulating toxicity and viral drug resistance. International guidelines are now recommending initiation of HAART in all vertically HIV-1-infected (VHIV) children regardless of clinical and immunological conditions,2 as early treatment reduces mortality and improves immune recovery.3
Proviral DNA HIV-1 reservoirs are established early during infection and represent a barrier to functional cure.4 A low HIV viral reservoir is associated with a lower risk of disease progression. Despite prolonged HAART, HIV-1 persists as transcriptionally inactive pro-viruses in long half-life memory resting CD4 T cells.5 The lowest reservoir has been described in elite controllers in whom HIV-1 replication is controlled without treatment.6 However, in post-treatment controllers, HIV-1 remission and low reservoir are observed after HAART interruption mainly when treatment was initiated during primary HIV infection.7 The relation between the size of the viral reservoir, and the precocity and duration of HAART has been previously described in children.8–10 However, more studies are required to identify simple predictors of the reservoir size in VHIV children, in real world conditions within high HIV prevalence settings, particularly in VHIV children.
The absence of detectable viraemia 12 years after treatment cessation in one VHIV child has suggested that early HAART initiation could lead to functional cure.11 HIV-infected people first develop anti-gp41 antibodies and only several weeks later, anti-gp120 antibodies.12 The Berlin patient, who was cured of HIV following a stem cell transplant, displayed a complete loss of anti-p24 antibodies and a low but still detectable response to gp41.13 Only few elite controller subpopulations show this HIV antibody profile. Thus, monitoring the response to p24 and gp41 may be useful in HIV cure studies.
Several HIV-1 seroreversions have been observed in VHIV children who initiated HAART within the first months of life.14,15 By blocking viral replication, the early virostatic treatment might prevent the development of the HIV-1 specific antibody response, either quantitatively (antibody level) or qualitatively (antibody activity), thus leading to HIV seroreversion or substantially lower anti-HIV antibody levels. One analysis demonstrated that age of HAART initiation and plasma viral load were strong predictors of serostatus, and both were independently associated.16 Kuhn et al.17 showed that the absence of HIV antibody response indicated a smaller HIV-1 viral reservoir, and HAART initiated at 3 months of age was the upper limit to see the benefits of early HAART. Only one study has demonstrated the utility of the HIV serostatus as a surrogate marker of the reservoir size.8
Here, we describe the factors associated with HIV antibody activity or level and the viral reservoir size in HAART-treated VHIV children with heterogeneity of age, time of therapy and duration of virological suppression.
Methods
This cross-sectional study conducted within a prospective cohort included HAART-treated VHIV children followed at the Gabriel Touré Hospital (Bamako, Mali) with sustained virological suppression (HIV-1 RNA plasma ≤ 50 copies/mL). All participants were known as virologically suppressed at their previous visit (6 months) and were confirmed during the study. Participants > 20 years old, those with HIV-2 infection, treated for <3 months, or without any data recorded were excluded from the study. After obtaining the parent’s written or oral informed consent from all participants, 5 mL of extra blood samples were collected in EDTA tubes during the routine follow-up visit. Assent was obtained from child participants according to local institutional review board guidelines. The study was approved by the National AIDS programme at the Ministry of Health in Mali (CSLS/MS) in collaboration with the Malian Institutional Ethics Committee at the Faculty of Medicine, Pharmacy and Odontostomatology of health and life sciences in Bamako under reference number N°10-05-FMPOS.
To evaluate the size of the HIV-1 viral reservoir, total DNA was extracted from PBMCs derived from whole blood using an automated technique (MagNA Pure, Roche, Manheim, Germany). The cell-associated HIV-1 DNA level was quantified using a real-time PCR method, which amplified a region in the LTR gene, as previously described.18 Proviral burden was expressed as HIV DNA copies per 1 million cells (quantification limit: 10 copies/PCR, i.e. 66 copies/106 cells considered as undetectable).
Dried serum spots were used to evaluate the HIV-specific antibody response. The level of antibodies targeting the gp41 immunodominant epitope was measured following a previously described protocol.19 An equimolar mixture of two oligopeptides of 30 amino acids was used, representing the immunodominant epitope consensus sequences of HIV-1 group M and subtype D, respectively. A low mixture concentration allowed the binding of late antibodies that had acquired sufficient avidity and then semi-quantitative detection by spectrophotometry. The result was expressed as an OD. The activity of anti-gp41 antibodies was systematically tested in quadruplicate.
The fourth-generation ARCHITECT HIV Ag/Ab Combo assay (Abbott Laboratories, Wiesbaden, Germany) was performed as previously described to quantify the humoral response in dried serum spots. The result was defined as relative light units, then compared with a cut-off signal. Samples with signal-to-cut-off (S/CO) values ≥1.00 were considered reactive and those <1.00 non-reactive.
We used univariate association between HIV DNA levels, anti-gp41 antibody activities or HIV-antibody levels and variables defining current and past HIV disease (age, sex, WHO stage at study, HAART type at study, CD4 cell count at study, HAART duration, maternal prophylaxis type, age at HAART initiation), with the Spearman rank correlation coefficient for continuous variables and the Fisher’s exact test for categorical variables.
Results
From August 2013 to April 2014, 97 VHIV children with virological suppression were enrolled. Their median age was 9.8 years old at the time of inclusion (IQR = 7–13.1), they started HAART at a median age of 3.3 years (IQR = 1.9–7), and were receiving HAART for a median 5.4 years (IQR = 3.5–7). Table 1 summarizes the demographic, immunological and virological characteristics of the children.
Table 1.
Descriptive characteristics of HAART-treated VHIV children with virological suppression
| Characteristic | n (%) | Median (IQR) | Global range |
|---|---|---|---|
| Age (years) | — | 9 .8 (7–13 .1) | 2 .8–19 |
| Sex | |||
| female | 38 (39) | — | — |
| male | 59 (61) | — | — |
| cART maternal prophylaxis type | |||
| 2 NRTIs + 1 NNRTI | 60 (62) | — | — |
| 2 NRTIs + 1 PI | 37 (38) | — | — |
| Age at HAART initiation (years) | — | 3 .3 (1 .9–7) | 0 .41–16 |
| Duration of HAART (years) | — | 5 .4 (3 .5–7) | 0 .33–12 .1 |
| WHO stage at HAART initiation | |||
| 1 or 2 | 36 (39) | — | — |
| 3 or 4 | 52 (61) | — | — |
| missing data: 9 | |||
| HAART type at study | |||
| 2 NRTIs + 1 NNRTI | 61 (63) | — | — |
| 2 NRTIs + 1 PI | 36 (37) | — | — |
| CD4 cell count at study (cells/mm3) | — | 820 (605–1120) | 46 –2000 |
| missing data: 1 | |||
| HIV DNA level at study (copies/106 cells) | — | 445 (87–902) | 0 –7378 |
| <66 copies/106 cells (i .e . undetectable) | 12 (13) | — | — |
| missing data: 2 | |||
| Anti-gp41 antibodies activity at study (OD) | — | 0 .29 (0 .18–0 .75) | 0 .09–2 .23 |
| missing data: 9 | |||
| Anti-HIV antibodies level at study (S/CO) | — | 14 .1 (4 .1–39 .3) | 0 .31–520 .6 |
| <1 .0 S/CO (i .e . seroconversion) | 8 (9) | — | — |
| missing data: 10 | |||
cART, combination ART .
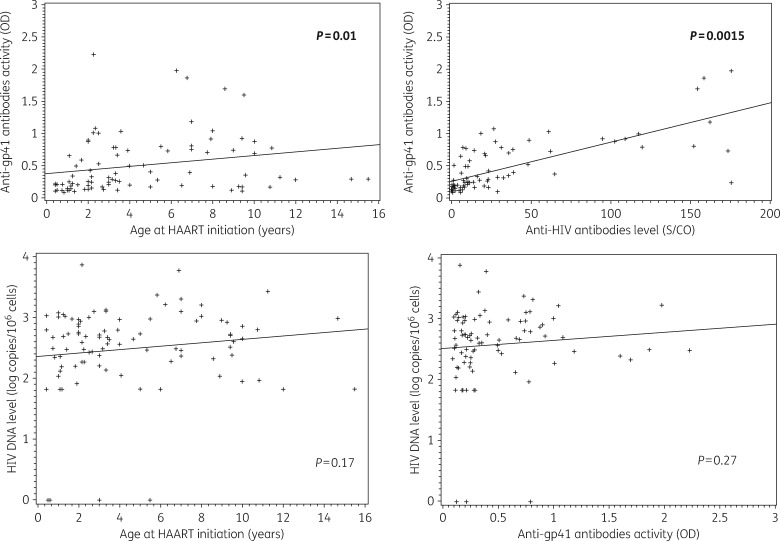
The median anti-gp41 antibodies activity was 0.29 OD (IQR = 0.18–0.75). A low activity of anti-gp41 antibodies was associated with both a younger age at treatment initiation (P = 0.01; Figure 1) and with a lower level of anti-HIV antibodies (P = 0.0015; Figure 1). Overall, eight seroreversions were identified (negative ELISA Architect) in which two children had an HIV DNA under the threshold (one detectable and one undetectable) and a low anti-gp41 antibodies activity. All the seroreverted children started HAART before 2 years of age, at a median age of 1.1 years, and were on HAART for the past 7.3 years in median.
Figure 1.
Distribution of anti-gp41 antibodies activity by age at HAART initiation (a) and by anti-HIV antibodies level (b) using Spearman correlation. Distribution of HIV DNA level by age at HAART initiation (c) and by anti-gp41 antibodies activity (d) using Spearman correlation. Anti-gp41 antibodies activity measured by manual immuno-enzymatic assay. Anti-HIV antibodies level measured by a fourth-generation ARCHITECT HIV Ag/Ab Combo assay.
The median level of total HIV DNA was 445 copies/106 cells (IQR = 187–914). No correlation was found between anti-gp41 antibodies activity or age at treatment initiation and HIV DNA (P = 0.27; Figure 1). The nine children with an HIV DNA level under the threshold tended to have a lower anti-gp41 antibody activity compared with children with an HIV DNA >66 copies/106 cells (P = 0.11).
Discussion
This study indicates that a significant proportion of virologically suppressed VHIV children who initiated HAART before the age of 2 years stopped to produce and/or progressively lost the HIV antibodies. This is consistent with the idea that early HAART halts the antigenic stimulation, which is necessary to sustain an HIV-specific antibody response.8–10,20 In addition, some of these children with seroreversion had a very low HIV reservoir, at least identified in the peripheral blood, and could therefore represent an ideal population for studies investigating novel immunotherapeutic strategies aiming to achieve HAART-free remission.
Although current HAART can effectively control HIV replication to clinically undetectable levels for years, existing strategies do not eradicate HIV-1 reservoirs in VHIV children.11 One of the limitations of our study was that our cohort did not include children who started HAART before 5 months of life, and therefore we were not able to identify more seroreversions. None the less, we found 50% of seroreversion in VHIV children who initiated treatment before 2 years old or 1 year old (8 of 16 and 4 of 8, respectively), consistently with other studies in occidental settings that showed 50% to 94% of seroreversion when treatment was initiated before 3 months of life.14,15,20
Children can acquire HIV-1 in utero, during delivery or breastfeeding.1 In our study, the time of HIV transmission was not known. Nevertheless, we assumed that seroreversion probably occurred in children who had treatment initiation soon after the HIV infection acquisition. Indeed, when using one of the most sensitive assays available, we were able to find several seroreversions. In addition, we found an association between low anti-gp41 antibody activity and a younger age at treatment initiation.
Early effective control of HIV replication has been associated with incomplete development of HIV-specific immune responses in children.14,15,20 It would be of interest to study the initial development of anti-HIV antibodies (such as activity and quantification of anti-gp41 antibodies) in early treated infants to determine whether the primary responses are affected or the influence occurs later on, leading to a decrease of responses over time. The decrease of antibody production and/or their avidity against some epitopes should reflect the absence of circulating antigenic viral particles, showing the absence of residual viral replication.
In conclusion, the results of this study show that HIV-1 seroreversion and low anti-gp41 activity in VHIV children with early HAART initiation happened and should be considered as a proof-of-concept study to evaluate strategies targeting HAART-free remission (i.e. long-term undetectable viraemia for an undefined period, in the absence of HAART).21 This study identified potential candidates with low viral reservoir and low antibody levels or activity. Defining the immunological and virological endpoints after HAART in VHIV children and identifying factors and biomarkers associated with limited proviral reservoir size are essential to define therapeutic strategies, to achieve HIV remission or cure in this population.
Acknowledgements
Global Health Center, Department of Infectious Diseases, Northwestern University, Chicago IL, USA.
Funding
This study was funded by Agence Nationale de Recherche sur le Sida et les Hépatites virales and Early treated Perinatally Infected individuals: Improving Children’s Actual Life Project.
Transparency declarations
None to declare.
References
- 1. UNAIDS. UNAIDS Report on the Global AIDS Epidemic 2013. http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
- 2. Panel on Antiretroviral Therapy and Medical Management of HIV-infected Children. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection 2015 http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf.
- 3. Cotton MF, Violari A, Otwombe K. et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 2013; 382: 1555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ananworanich J, Schuetz A, Vandergeeten C. et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 2012; 7: e33948.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Archin NM, Vaidya NK, Kuruc JD. et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci USA 2012; 109: 9523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lambotte O, Boufassa F, Madec Y. et al. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin Infect Dis 2005; 41: 1053–6. [DOI] [PubMed] [Google Scholar]
- 7. Sáez-Cirión A, Bacchus C, Hocqueloux L. et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013; 9: e1003211.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Persaud D, Patel K, Karalius B. et al. Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr 2014; 168: 1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luzuriaga K, Tabak B, Garber M. et al. HIV type 1 (HIV-1) proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J Infect Dis 2014; 210: 1529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ananworanich J, Puthanakit T, Suntarattiwong P. et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS 2014; 28: 1015–20. [DOI] [PubMed] [Google Scholar]
- 11. Frange P, Faye A, Avettand-Fenoël V. et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV 2016; 3: e49–54. [DOI] [PubMed] [Google Scholar]
- 12. Overbaugh J, Morris L.. The antibody response against HIV-1. Cold Spring Harb Perspect Med 2012; 2: a007039.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burbelo PD, Bayat A, Rhodes CS. et al. HIV antibody characterization as a method to quantify reservoir size during curative interventions. J Infect Dis 2014; 209: 1613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hainaut M, Peltier CA, Gérard M. et al. Effectiveness of antiretroviral therapy initiated before the age of 2 months in infants vertically infected with human immunodeficiency virus type 1. Eur J Pediatr 2000; 159: 778–82. [DOI] [PubMed] [Google Scholar]
- 15. Zanchetta M, Anselmi A, Vendrame D. et al. Early therapy in HIV-1-infected children: effect on HIV-1 dynamics and HIV-1-specific immune response. Antivir Ther (Lond) 2008; 13: 47–55. [PubMed] [Google Scholar]
- 16. Payne H, Mkhize N, Otwombe K. et al. Reactivity of routine HIV antibody tests in children who initiated antiretroviral therapy in early infancy as part of the Children with HIV Early Antiretroviral Therapy (CHER) trial: a retrospective analysis. Lancet Infect Dis 2015; 15: 803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuhn L, Schrall DB, Shiau S. et al. Young age at start of antiretroviral therapy and negative HIV antibody results in HIV-infected children when suppressed. AIDS 2015; 29: 1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Avettand-Fènoël V, Chaix ML, Blanche S. et al. LTR real-time PCR for HIV-1 DNA quantitation in blood cells for early diagnosis in infants born to seropositive mothers treated in HAART area (ANRS CO 01). J Med Virol 2009; 81: 217–23. [DOI] [PubMed] [Google Scholar]
- 19. Barin F, Meyer L, Lancar R. et al. Development and validation of an immunoassay for identification of recent human immunodeficiency virus type 1 infections and its use on dried serum spots. J Clin Microbiol 2005; 43: 4441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luzuriaga K, McManus M, Catalina M. et al. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: control of viral replication and absence of persistent HIV-1-specific immune responses. J Virol 2000; 74: 6984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deeks SG, Lewin SR, Ross AL. et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med 2016; 22: 839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]