Abstract
Background. Prior small studies have suggested an association between low serum albumin and increased risk of venous thromboembolic (VTE) events in patients with nephrotic syndrome (NS).
Methods. From a nationally representative prospective cohort of over 3 million US veterans with baseline estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2, we identified 7037 patients with NS based on ICD-9 codes. Association between serum albumin and risk of incident VTE was assessed using Cox regression analysis with adjustments for age, gender, race, comorbidities, eGFR, body mass index and anticoagulant treatment.
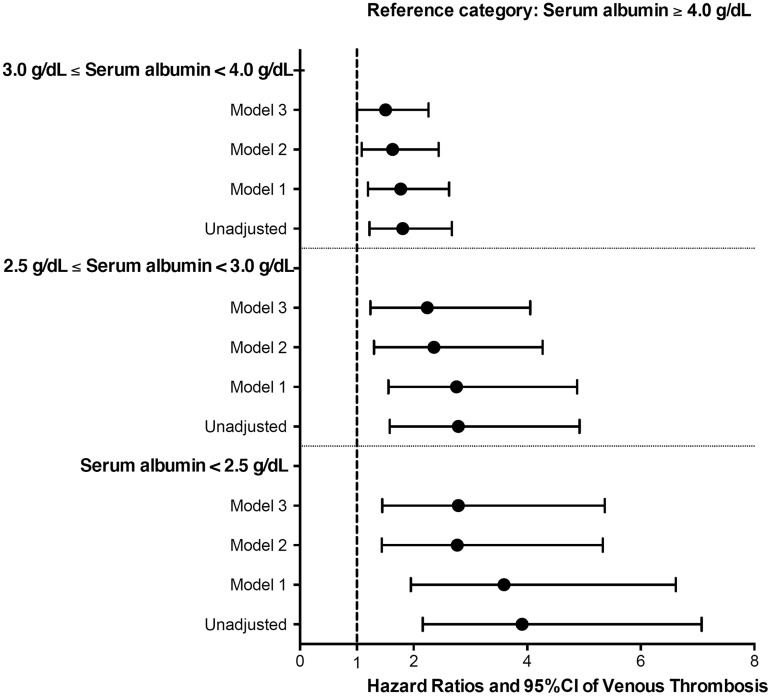
Results. Mean age was 57 ± 11 years, patients were 96% male, 32% African-American and 60% diabetic. There were a total of 158 VTE events over a median follow-up of 8.1 years; 16 events [absolute event rate (AER) 4.1%, event rate 8.5/1000 patient-years (PY)] in patients with albumin <2.5 g/dL, 18 events (AER 3.4%, event rate 5.7/1000 patient-years) in patients with albumin 2.5–2.99 g/dL, 89 events (AER 2.5%, event rate 3.4/1000 patient-years) in patients with albumin 3–3.99 g/dL and 35 events (AER 1.4%, event rate 1.9/1000 patient-years) in patients with albumin ≥4 g/dL. Compared with patients with albumin ≥4 g/dL, those with albumin levels of 3–3.99 g/dL [adjusted hazard ratio (HR): 1.51, 95% confidence interval (CI): 1.01–2.26], 2.5–2.99 g/dL (HR: 2.24, 95% CI: 1.24–4.05) and <2.5 g/dL (HR: 2.79, 95% CI: 1.45–5.37) experienced a linearly higher risk of VTE events.
Conclusions. Lower serum albumin is a strong independent predictor for VTE events in NS. The risk increases proportionately with declining albumin levels. Clinical trials are needed to determine benefit of prophylactic anticoagulation in NS patients with moderately lower serum albumin levels.
Keywords: cohort, nephrotic syndrome, risk factors, serum albumin, venous thromboembolism
INTRODUCTION
Nephrotic syndrome (NS) is associated with a high incidence of venous thromboembolic events (VTE), which are an important cause of morbidity and mortality [1, 2]. However, the reported VTE cumulative incidence largely varies from 5 to 60% in different studies, probably due to methodological issues such as retrospective study design, variation in case ascertainment or severity of the nephrotic state, and the lack of accurate methods to detect subclinical thrombosis [1]. Among the numerous causes of NS, relatively few conditions are consistently associated with a decidedly increased risk for thromboembolism; these include membranous nephropathy (primary and secondary), membranoproliferative glomerulonephritis, minimal change disease and perhaps renal amyloidosis [1, 3].
The pathophysiological mechanisms of VTE in patients with NS have yet to be unraveled. However alteration in plasma levels of proteins involved in coagulation and fibrinolysis, enhanced platelet aggregation, low plasma albumin, hyperviscosity and hyperlipidemia are considered predisposing factors [1, 4–6]. Proteinuria and serum albumin levels are the most studied VTE risk factors in patients with NS [7–9]. In some but not all studies, this risk proportionally increases with the severity of the NS as reflected by higher proteinuria and lower serum albumin levels [7–9]. The reported risks of VTE in patients with NS are based on case reports and modest-sized studies with low numbers of thrombotic events and mostly short-term follow-up and are often limited to certain types of glomerular diseases, and therefore are of limited generalizability.
Data from large cohorts on the absolute risk of VTE in patients with NS of different etiologies are still not available. We conducted a retrospective study to assess the association between serum albumin level and the absolute risk of symptomatic VTE in a large cohort of unselected patients with NS. We hypothesized that lower serum albumin levels are associated with higher risk of VTE in this population.
MATERIALS AND METHODS
Study setting and cohort definition
The institutional review committees at the Memphis and Long Beach Veterans Affairs Medical Centers approved the study. Data were obtained from the Racial and Cardiovascular Risk Anomalies in CKD (RCAV) study, which examines risk factors of incident chronic kidney disease, mortality and other outcomes in US veterans, and which was previously described in detail [10, 11]. Diagnoses of incident NS and VTE were identified from various Veterans Affairs (VA) research databases using ICD-9-CM diagnostic and procedure codes and Current Procedural Terminology (CPT) codes (Supplementary data, Table S1).
The algorithm for cohort definition is shown in Supplementary data, Figure S1. The original RCAV cohort included 3 582 478 patients with baseline estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2. Patients were included in our study if they had a diagnosis of NS, but did not have a diagnosis of VTE at the first encounter when the NS diagnosis was listed. Of 3 582 478 patients, 8394 had a diagnosis of NS, of which 7037 patients had serum albumin levels measured during baseline evaluation.
Exposure and covariates
The main exposure variable was serum albumin measured at baseline. Patients were categorized into four groups based on their serum albumin levels, i.e. <2.5, 2.5–2.99, 3–3.99 and ≥4 g/dL. Socio-demographic characteristics, comorbid conditions and laboratory characteristics were obtained, as previously described [12, 13]. eGFR was calculated from serum creatinine measurements using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [14]. Information about age, gender and race were obtained through the VA Corporate Data Warehouse (CDW) and from Medicare through the VA-Medicare data merge project. Information about comorbidities including causes of NS was collected from the VA Inpatient and Outpatient Medical SAS Datasets using ICD-9-CM diagnostic and procedure codes and CPT codes (Supplementary data, Table S2). Prevalent comorbidities and the presence of anticoagulation were defined as those diagnosed or treated during 1 October 2004 to 30 September 2006.
Outcomes
Incident VTE was defined as an ICD-9-CM code for either renal vein thrombosis, deep venous thrombosis or pulmonary embolism during any inpatient or outpatient encouter any time following cohort entry (Supplementary data, Table S1).
Statistical analysis
Data were summarized using proportions, means ± standard deviation (SD) or medians [interquartile range (IQR)] as appropriate. Continuous variables were compared using Student's t-test and Mann–Whitney U test according to data type. The associations between diagnoses of incident VTE and serum albumin were assessed using the Kaplan–Meier method and the Cox proportional hazard models (for time to event analyses). Proportional hazards assumptions were tested using scaled Schoenfeld residuals, a common and reliable way to check proportionality, which is a key assumption when the Cox proportional hazard model is used.
The start of the follow-up period was the date of the first serum albumin measurement during 1 October 2004 to 30 September 2006. Patients were followed until an incident VTE event or were censored at the date of last healthcare or administrative visit, or on 26 July 2013.
All associations were examined in both unadjusted and adjusted models. Variables entered in the multivariable-adjusted models were selected based on theoretical considerations; we included variables known to be associated both with NS and VTE based on scientific evidence, and which were available in our database. Models were adjusted for the following confounders: Model 1: age, gender, race/ethnicity; Model 2: Model 1 variables, baseline eGFR, body mass index (BMI) and comorbidity burden quantified using the Charlson comorbidity index [15]; Model 3: Model 2 variables and use of anticoagulants. Non-linear associations were assessed using multivariate fractional polynomials and regression splines. Variance influence factors were used to assess collinearity between independent variables. We also performed subgroup analyses in clinically relevant subgroups of patients using our fully adjusted model.
We also examined associations in patients with available measurements for urine protein/albumin (n = 2171), by adjusting for these measures. Missing values were not imputed in primary analyses, but were substituted in our sensitivity analyses with the use of multiple imputation procedures (creating five datasets) using STATA's ‘mi’ set of command in sensitivity analyses. Statistical analyses were performed using Stata MP Version 12 (Stata Corporation, College Station, TX, USA).
RESULTS
Baseline characteristics
Table 1 shows baseline clinical characteristics of the total study population of 7037 patients and of subgroups according to their baseline albumin levels. The mean ± SD overall age was 57 ± 11 years, 96% of patients were male, 64 and 32% of patients were White and African-American, respectively, 60% of the patients were diabetic, 11.4% had cancer and the mean baseline eGFR was 83 ± 17 mL/min/1.73 m2. Patients with serum albumin <2.5 g/dL were more likely to be African-American, and had a higher prevalence of hypertension, cardiovascular disease, congestive heart failure, cerebrovascular disease and peripheral vascular disease. Twenty-six percent of patients had a diagnosis of primary glomerulonephritis (minimal change disease, focal segmental glomerular disease, membranous glomerulonephritis or membranoproliferative glomerulonephritis), 52% had diabetic kidney disease and 22% of patients had unclassified NS.
Table 1.
Characteristics of the study population
| Variable | Total | Alb <2.5 g/dL | Alb 2.5–2.99 g/dL | Alb 3–3.99 g/dL | Alb ≥4 g/dL |
|---|---|---|---|---|---|
| Patients, n (%) | 7037 (100) | 387 (5.5) | 533 (7.6) | 3626 (51.5) | 2491 (5.4) |
| Male, n (%) | 6728 (96) | 375 (97) | 513 (96) | 3456 (95) | 2384 (96) |
| Age, years (mean ± SD) | 57 ± 11 | 57 ± 12 | 57 ± 11 | 58 ± 10 | 58 ± 11 |
| African-American, n (%) | 2177 (32) | 173 (45) | 199 (38) | 1185 (33) | 620 (26) |
| Diabetes, n (%) | 4245 (60) | 220 (57) | 349 (65) | 2314 (64) | 1362 (55) |
| Hypertension, n (%) | 5929 (84) | 337 (87) | 464 (87) | 3126 (86) | 2002 (80) |
| Cardiovascular disease, n (%)a | 1240 (18) | 77 (20) | 114 (21) | 669 (18) | 380 (15) |
| CHF, n (%) | 992 (14) | 125 (32) | 154 (29) | 534 (15) | 179 (7) |
| PVD, n (%) | 960 (14) | 71 (18) | 106 (20) | 516 (14) | 267 (11) |
| CVA, n (%) | 707 (10) | 56 (14) | 69 (13) | 377 (10) | 205 (8) |
| Cause of NS (%) | |||||
| Primary glomerulonephritis | 1810 (26) | 96 (24) | 116 (22) | 849 (24) | 749 (30) |
| Diabetic nephropathy | 3671 (52) | 190 (49) | 315 (59) | 2022 (56) | 1144 (46) |
| Unclassified NS | 1556 (22) | 101 (26) | 102 (19) | 755 (21) | 598 (24) |
| Malignancy, n (%) | 802 (11) | 59 (15) | 71 (13) | 437 (12) | 235 (9) |
| Anticoagulation use, n (%) | 3884 (55) | 173 (45) | 293 (55) | 2159 (60) | 1259 (51) |
| eGFR, mL/min/1.73/m2 (mean ± SD) | 83 ± 17 | 85 ± 17 | 84 ± 17 | 83 ± 17 | 83 ± 16 |
| BMI, kg/m2 (mean ± SD) | 30 ± 6 | 27 ± 6 | 29 ± 6 | 31 ± 6 | 30 ± 5 |
| Charlson comorbidity index (mean ± SD) | 5 ± 3 | 4 ± 3 | 3 ± 2 | 5 ± 3 | 2 ± 2 |
Alb, albumin; SD, standard deviation; CHF, congestive heart failure; PVD, peripheral vascular disease; CVA, cerebrovascular accident; NS, nephrotic syndrome; eGFR, estimated glomerular filtration rate; BMI, body mass index.
aCardiovascular disease was defined as acute myocardial infarction, angina, coronary artery disease, previous coronary artery bypass grafting or percutaneous coronary intervention.
Risk of VTE
The median follow-up time was 8.1 years (IQR: 6.4–8.6 years). There were a total of 158 VTE events [absolute event rate (AER) 2.25%, event rate 3.17 (2.72–3.71)/1000 patient-years], with 16 events [AER 4.1%, event rate 8.46 (5.18–13.80)/1000 patient-years] in patients with albumin <2.5 g/dL, 18 events [AER 3.4%, event rate 5.66 (3.57–8.99)/1000 patient-years] in patients with albumin 2.5–2.99 g/dL, 89 events [AER 2.5%, event rate 3.43 (2.79–4.23)/1000 patient-years] in patients with albumin 3–3.99 g/dL and 35 events [AER 1.4%, event rate 1.86 (1.34–2.59)/1000 patient-years] in patients with albumin ≥4 g/dL.
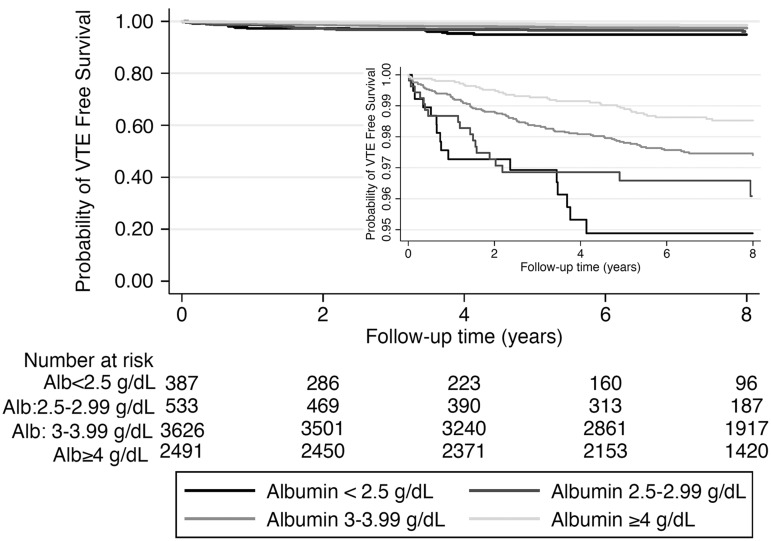
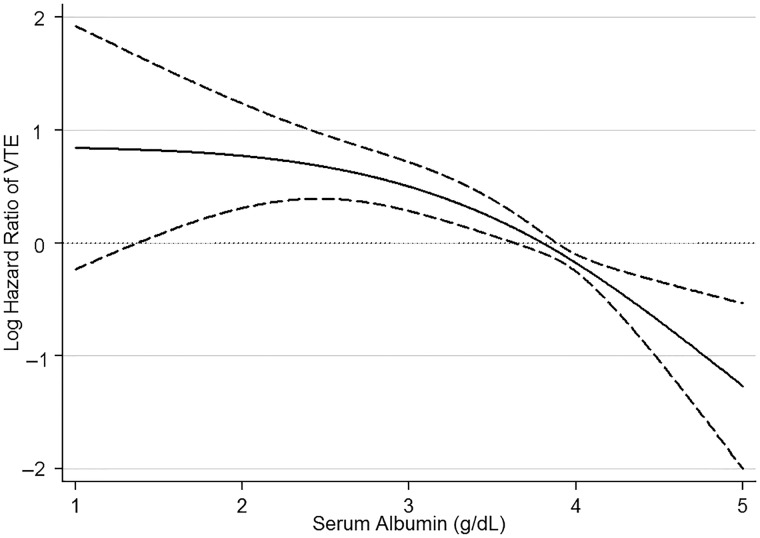
Patients with lower serum albumin had early separation in their event-free survival curves from their counterparts with higher serum albumin level (Figure 1). There was a strong inverse correlation between serum albumin level and the risk of VTE events in unadjusted fractional polynomial analyses (Figure 2). Figure 3 shows the associations between serum albumin levels and incident VTE in unadjusted and adjusted Cox models. Compared with patients with albumin ≥4 g/dL, those with albumin levels of 3–3.99 g/dL had an almost 2-fold unadjusted risk and a 1.5-fold multivariable-adjusted risk of VTE [unadjusted hazard ratio (HR): 1.81, 95% confidence interval (CI): 1.22–2.67 and adjusted HR: 1.51, 95% CI: 1.01–2.26]. Patients with serum albumin 2.5–2.99 g/dL had an almost 3-fold unadjusted and 2-fold adjusted risk (unadjusted HR: 2.79, 95% CI: 1.58–4.92 and adjusted HR: 2.24, 95% CI: 1.24–4.05), whereas those with serum albumin <2.5 g/dL had an almost 4-fold unadjusted and 3-fold adjusted risk (unadjusted HR: 3.91, 95% CI: 2.16–7.07 and adjusted HR: 2.79, 95% CI: 1.45–5.37) of VTE compared with patients with serum albumin ≥4 g/dL (Figure 3). The results were qualitatively similar to a sensitivity analysis excluding patients with known malignancies (data not shown).
FIGURE 1.
Kaplan–Meier curves showing the association between serum albumin (Alb) categories and risk of incident venous thromboembolic events (VTE) for 7037 US Veteran patients with nephrotic syndrome.
FIGURE 2.
Association of serum albumin level with risk of incident venous thromboembolic events (VTE) in unadjusted Cox regression models in 7037 US Veteran patients with nephrotic syndrome.
FIGURE 3.
Association of serum albumin categories with risk of incident venous thromboembolic events in unadjusted and adjusted Cox regression models in 7037 US Veteran patients with nephrotic syndrome. Models were adjusted for the following confounders: Model 1: age, gender, race/ethnicity; Model 2: Model 1 variables, baseline eGFR, body mass index and comorbidities measured with Charlson comorbidity index; Model 3: Model 2 variables and use of anticoagulants. CI, confidence interval.
Figure 4 shows associations between serum albumin levels and incident VTE in patients with different types of NS in multivariable-adjusted Cox models. The association of lower serum albumin level with an increasing trend of VTE was present in both patients with glomerular disease and in patients with diabetic nephropathy. However, in patients with unclassified NS this trend was not observed.
FIGURE 4.
Association of serum albumin categories with risk of incident venous thromboembolic events in fully adjusted Cox regression models in patients with different causes of nephrotic syndrome. Models were adjusted for age, gender, race/ethnicity, baseline estimated glomerular filtration rate, body mass index and comorbidities measured with Charlson comorbidity index and use of anticoagulants. CI, confidence interval; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; MPGN, membranoproliferative glomerulonephritis.
Compared with patients with albumin ≥4 g/dL, patients with albumin levels of 3–3.99 g/dL had similar risk of VTE (HR: 1.31, 95% CI: 0.72–2.38), while patients with albumin levels of 2.5–2.99 g/dL (HR: 2.69, 95% CI: 1.15–6.25) and those with serum albumin <2.5 g/dL (HR: 2.69, 95% CI: 0.93–7.76) had higher risk of VTE after adjustment for the level of urine protein or albumin (n = 2171). Analyses using imputed data also showed qualitatively similar results (data not shown). Supplementary data, Figure S2A–D shows the association between serum albumin categories and risk of incident VTE in clinically relevant subgroups of patients, showing qualitatively similar trends.
DISCUSSION
In a large cohort of the US veterans with baseline eGFR ≥60 mL/min/1.73 m2 and diagnosis of NS at baseline, we examined the association of baseline serum albumin level and risk of incident VTE. Our study formally quantified the increased risk of VTE associated with even a modest reduction in serum albumin level in a large cohort of adult NS patients. Compared with patients with serum albumin ≥4 g/dL, those with albumin levels of 3–3.99 g/dL had a 1.5-fold risk of VTE, whereas those with serum albumin 2.5–2.99 g/dL had an 2-fold higher and those with serum albumin <2.5 g/dL had an almost 3-fold risk of VTE.
Thromboembolic events are a preventable cause of morbidity and mortality in patients with NS [16]. The absolute risk of VTE in our study was 3.2/1000 patient-years, which is almost three times higher compared with what has been reported in the general population (1.07/1000 patient-years) [17]. However, the incidence rate of VTE from the Toronto incident cohort was 17/1000 patient-years [7], a significantly higher event rate than what we observed in our prevalent cohort (3.2/1000 patient-years). Our lower incident rate can be explained by the fact that our cohort was a prevalent NS cohort and the majority of the VTE events most likely occur in the first 6 months after a diagnosis of NS is made [18]; therefore, it is likely that we did not capture VTE events that could have occurred prior to our date of cohort entry. The discrepant VTE event rate can also be explained by the fact that prevalence estimation of VTE in NS is subject to methodological limitations including retrospective study design, small sample sizes, objectivity of thromboembolism definition, inclusion of asymptomatic cases, NS histopathology distribution and imaging methods used to detect thromboembolism [1, 4, 19, 20].
Several previous studies have described an association between hypoalbuminemia and VTE [21]. Bellomo and Atkins [5] showed that serum albumin <2.5 g/dL at presentation was a significant risk factor for recurrence of venous thromboembolism (40 versus 2.7%, P < 0.01). A relatively large retrospective study with 298 patients found an eight times higher absolute risk of VTE in patients with NS, and the ratio of proteinuria to serum albumin, not serum albumin alone, predicted VTE in their cohort of patients with NS of varied causes [18]. Another study by Lionaki et al. [7] reported hypoalbuminemia to be the dominant independent risk factor for VTE, whereas proteinuria was not independently predictive of thrombotic events in patients with membranous nephropathy. In that study 2.8 g/dL was the threshold below which the risk of VTE increased 3.9-fold and when albumin level declined further to <2.2 g/dL the risk of VTE increased 5.8-fold [7]. In our study, compared with patients with serum albumin ≥4 g/dL, even patients with serum albumin levels of 3–3.99 g/dL had a 2-fold higher unadjusted risk and a 1.5-fold higher adjusted risk of VTE, with further increase in relative risk associated with even lower levels of serum albumin. The difference in relative risk associated with lower albumin between our and other studies may be present because VTE has been reported more frequently among patients with a diagnosis of membranous nephropathy compared with other nephrotic diseases [1, 21, 22].
NS is viewed as a thrombophilic or hypercoagulable state although the precise level of this risk is not fully agreed on [16]. A very low serum albumin level seems to be a surrogate measure for abnormalities in antithrombin and fibrinogen that predisposes to thrombosis. In general, mechanisms for thromboembolism fall into two categories: urinary loss of anticoagulant factors and increased synthesis of procoagulant factors. With respect to anticoagulant factors, 40–80% of patients with the NS have reduced circulating levels of antithrombin III [23], owing to urinary losses [24]. Protein C activity and protein S levels also appear to be reduced in patients with the NS [25]. Additionally, abnormalities in factors that promote thrombosis have been shown both among procoagulant proteins and among fibrinolytic proteins. Activation of secondary coagulation in patients with the NS is accompanied by increased levels of factors V and VIII, von Willebrand factor, fibrinogen and α2-macroglobulin, probably owing to increased synthesis [1]. It is thought that the increase in these high-molecular-weight species is a reflection of increased acute-phase synthesis [1]. Hyperfibrinogenemia, in particular, is a hepatic synthetic response to the hypoalbuminemia seen in NS. This increase in fibrinogen promotes platelet aggregation, provides substrate for fibrin formation, increases blood viscosity and promotes erythrocyte aggregation [26]. Mild thrombocytosis and platelet hyperreactivity also accompany NS. Platelet hyperreactivity, found in ∼70% of such patients [1], is multifactorial and can be attributed to increased levels of von Willebrand factor, hyperfibrinogenemia, hypercholesterolemia and hypoalbuminemia [27]. Hypoalbuminemia leads to increased bioavailability of arachidonic acid, thus increasing thromboxane A2, thereby favoring platelet aggregation and hyperactivity [28]. Additionally, NS leads to elevated cholesterol levels that promote platelet aggregation [29]. At the level of fibrinolysis, NS is associated with a decrease in circulating plasminogen levels [30]. This decrease in plasminogen is accompanied by an increase in levels of plasminogen activator inhibitor 1 [31] and α2-plasmin inhibitor [1] that impair fibrin clearance and promote thrombus persistence. Thus underlying mechanisms of the thrombophilia of NS seems to be related to an imbalance of prothrombotic and antithrombotic factors with impaired fibrinolysis [1, 32].
Among the numerous causes of NS, only a relatively few conditions are consistently associated with an increased risk for VTE. These include membranous nephropathy (primary and secondary), membranoproliferative glomerulonephritis, minimal change disease and perhaps renal amyloidosis [1, 3, 21, 33]. In our study the association of lower serum albumin level with an increasing trend of VTE was present both in patients with glomerular disease and in patients with diabetic nephropathy. There is increasing evidence that diabetes mellitus is associated with several defects of coagulation and fibrinolysis that lead to a procoagulant, thrombogenic predisposition [34]. A study by Petrauskiene et al. [35] suggested that age-adjusted risk for VTE is more than 2-fold higher among diabetic patients than in the non-diabetic population.
In our study, the median follow-up time was around 8 years. In adults, the majority of VTE events occur within the first 6 months after NS diagnosis [18]. Prophylactic anticoagulation of asymptomatic patients who have NS and are at elevated risk for a thromboembolic event is controversial [1]. Thrombotic events carry a significant risk of morbidity and mortality; however, anticoagulation is not a benign therapy. The American College of Chest Physicians' guidelines estimate the risk of bleeding with therapeutic anticoagulation to be between 1.6 and 12.8%, depending on the presence of various risk factors including age, previous bleeding, concurrent disease states, thrombocytopenia, recent surgery, frequent falls and concurrent drug therapy such as antiplatelet agents [36, 37]. Similar bleeding risk estimates for the use of prophylactic anticoagulation have not been established. Accordingly, it is still not possible to define the risk of VTE in a certain patient and, consequently, to indicate what the ideal prophylactic therapy might consist of [22, 38]. Unfortunately, no randomized, controlled trials have been conducted to provide evidence to guide and inform nephrologists in this decision-making process. In the absence of direct clinical evidence, one would have to rely on decision analysis modeling to identify which group of patients would achieve maximal benefit from prophylactic anticoagulation. Our study, although it helps to quantify the risk of VTE in patients with NS and low albumin, does not help resolve this dilemma, as it does not address the risks of anticoagulation.
Several study limitations merit discussion. This being an observational study, we can only report associations. Baseline serum albumin measurements were not available in all patients with NS. Since the methods used for measuring serum albumin were not uniform in our cohort it is possible that there may be misclassification of patients with mild hypoalbuminemia. Additionally, models could only be adjusted for confounders for which we had available data. Therefore, we cannot rule out residual confounding. Moreover, our study population consisted of mostly male US veterans, so the results should be applied with caution to females and to patients in other countries. We also had limited information about proteinuria in our database; consequently, we cannot comment on associations between proteinuria and incident VTE. Our study used diagnostic codes to define NS and VTE events. This may have led to misclassification with regard to NS and VTE diagnosis due to the limited sensitivity and specificity of ICD-9-CM coding [39, 40]. Although some studies have used strategies to improve the specificity of these codes by using multiple diagnostic identifiers, the validity is still uncertain [41]. Additionally, asymptomatic VTE may have been missed because asymptomatic patients are not routinely screened, which may have resulted in an underestimation of the risk of VTE. Our study focused only on VTE and did not include the risk of arterial thrombotic events attributable to NS. Our study used data from a cohort that included only patients with eGFR ≥60 mL/min/1.73 m2 at baseline, which may have resulted in the exclusion of patients with more severe NS. We examined a prevalent group with NS, hence it is possible that many of the patients in our cohort developed this condition some time before entering our cohort, and hence we might not have detected VTE events occurring during the most vulnerable period of their disease. The fact that we still observed robust associations between serum albumin level and VTE risk indicates that serum albumin could be used as a potent risk marker even late during the course of NS.
Despite the above limitations, our results may have important clinical implications for the clinical care of patients with NS and for future research in this area. Our study is notable for its large sample size and for it being representative of veterans who received care in the VA system in the entire USA. To the best of our knowledge, this is the largest study to find significant associations between a diagnosis of unselected prevalent NS and VTE events. Additionally, it identifies even modest reductions in serum albumin as an independent risk factor that may aid in better risk stratification of such patients. And finally, the results of our study may help in appropriate power calculation of future prospective randomized controlled trials in NS patients with regards to thromboprophylaxis.
In summary, our study delineates high absolute risks of VTE in patients with NS, and it confirms the importance of hypoalbuminemia as a predictor of VTE events in NS. An increased risk of VTE exists even with modest reductions in serum albumin level and the risk increases proportionately with lower albumin levels. Clinical trials are needed to determine an optimal albumin level for the initiation of prophylactic anticoagulant therapy.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Praveen Potukuchi, BPharm, MSc, MS for help with preparing tables and figures. This study was supported by grant R01DK096920 to C.P.K. and K.K.-Z. and is the result of work supported with resources and the use of facilities at the Memphis VA Medical Center and the Long Beach VA Medical Center. Support for VA/CMS data are provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
AUTHORS’ CONTRIBUTIONS
G.G. was involved in data collection, data analysis, data interpretation and writing of manuscript. M.Z.M. was involved in data collection, statistical support, data interpretation and revision of manuscript. J.L.L. was involved in revision of manuscript. K.S. was involved in revision of manuscript. K.K.-Z. was involved in data interpretation and revision of manuscript. C.P.K. was involved in conception and design of study, data collection, data interpretation and revision of manuscript.
CONFLICT OF INTEREST STATEMENT
G.G., C.P.K. and K.K.-Z. are employees of the Department of Veterans Affairs. Opinions expressed in this paper are those of the authors and do not necessarily represent the opinion of the Department of Veterans Affairs. The results of this paper have not been published previously in whole or in part.
REFERENCES
- 1. Singhal R, Brimble KS. Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management. Thromb Res 2006; 118: 397–407 [DOI] [PubMed] [Google Scholar]
- 2. Remuzzi G, Mecca G, Marchesi D, et al. Platelet hyperaggregability and the nephrotic syndrome. Thromb Res 1979; 16: 345–354 [DOI] [PubMed] [Google Scholar]
- 3. Chugh KS, Malik N, Uberoi HS, et al. Renal vein thrombosis in nephrotic syndrome—a prospective study and review. Postgrad Med J 1981; 57: 566–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Llach F. Hypercoagulability, renal vein thrombosis, and other thrombotic complications of nephrotic syndrome. Kidney Int 1985; 28: 429–439 [DOI] [PubMed] [Google Scholar]
- 5. Bellomo R, Atkins RC. Membranous nephropathy and thromboembolism: is prophylactic anticoagulation warranted? Nephron 1993; 63: 249–254 [DOI] [PubMed] [Google Scholar]
- 6. Robert A, Olmer M, Sampol J, et al. Clinical correlation between hypercoagulability and thrombo-embolic phenomena. Kidney Int 1987; 31: 830–835 [DOI] [PubMed] [Google Scholar]
- 7. Lionaki S, Derebail VK, Hogan SL, et al. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol 2012; 7: 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glassock RJ. Prophylactic anticoagulation in nephrotic syndrome: a clinical conundrum. J Am Soc Nephrol 2007; 18: 2221–2225 [DOI] [PubMed] [Google Scholar]
- 9. Kumar S, Chapagain A, Nitsch D, et al. Proteinuria and hypoalbuminemia are risk factors for thromboembolic events in patients with idiopathic membranous nephropathy: an observational study. BMC Nephrol 2012; 13: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Molnar MZ, Alhourani HM, Wall BM, et al. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology 2015; 61: 1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kovesdy CP, Norris KC, Boulware LE, et al. Association of race with mortality and cardiovascular events in a large cohort of US veterans. Circulation 2015; 132: 1538–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. George LK, Molnar MZ, Lu JL, et al. Association of pre-operative albuminuria with post-operative outcomes after coronary artery bypass grafting. Sci Rep 2015; 5: 16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gosmanova EO, Molnar MZ, Alrifai A, et al. Impact of non-adherence on renal and cardiovascular outcomes in US veterans. Am J Nephrol 2015; 42: 151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251 [DOI] [PubMed] [Google Scholar]
- 16. Kendall AG, Lohmann RC, Dossetor JB. Nephrotic syndrome. A hypercoagulable state. Arch Intern Med 1971; 127: 1021–1027 [PubMed] [Google Scholar]
- 17. Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991; 151: 933–938 [PubMed] [Google Scholar]
- 18. Mahmoodi BK, ten Kate MK, Waanders F, et al. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation 2008; 117: 224–230 [DOI] [PubMed] [Google Scholar]
- 19. Andrassy K, Ritz E, Bommer J. Hypercoagulability in the nephrotic syndrome. Klin Wochenschr 1980; 58: 1029–1036 [DOI] [PubMed] [Google Scholar]
- 20. Kanfer A, Kleinknecht D, Broyer M, et al. Coagulation studies in 45 cases of nephrotic syndrome without uremia. Thromb Diath Haemorrh 1970; 24: 562–571 [PubMed] [Google Scholar]
- 21. Llach F, Papper S, Massry SG. The clinical spectrum of renal vein thrombosis: acute and chronic. Am J Med 1980; 69: 819–827 [DOI] [PubMed] [Google Scholar]
- 22. Barbour SJ, Greenwald A, Djurdjev O, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int 2012; 81: 190–195 [DOI] [PubMed] [Google Scholar]
- 23. Kauffmann RH, Veltkamp JJ, Van Tilburg NH, et al. Acquired antithrombin III deficiency and thrombosis in the nephrotic syndrome. Am J Med 1978; 65: 607–613 [DOI] [PubMed] [Google Scholar]
- 24. Vaziri ND, Paule P, Toohey J, et al. Acquired deficiency and urinary excretion of antithrombin III in nephrotic syndrome. Arch Intern Med 1984; 144: 1802–1803 [PubMed] [Google Scholar]
- 25. Cosio FG, Harker C, Batard MA, et al. Plasma concentrations of the natural anticoagulants protein C and protein S in patients with proteinuria. J Lab Clin Med 1985; 106: 218–222 [PubMed] [Google Scholar]
- 26. Rabelink TJ, Zwaginga JJ, Koomans HA, et al. Thrombosis and hemostasis in renal disease. Kidney Int 1994; 46: 287–296 [DOI] [PubMed] [Google Scholar]
- 27. Machleidt C, Mettang T, Starz E, et al. Multifactorial genesis of enhanced platelet aggregability in patients with nephrotic syndrome. Kidney Int 1989; 36: 1119–1124 [DOI] [PubMed] [Google Scholar]
- 28. Mikhailidis DP, Ganotakis ES. Plasma albumin and platelet function: relevance to atherogenesis and thrombosis. Platelets 1996; 7: 125–137 [DOI] [PubMed] [Google Scholar]
- 29. Zwaginga JJ, Koomans HA, Sixma JJ, et al. Thrombus formation and platelet-vessel wall interaction in the nephrotic syndrome under flow conditions. J Clin Invest 1994; 93: 204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomson C, Forbes CD, Prentice CR, et al. Changes in blood coagulation and fibrinolysis in the nephrotic syndrome. Q J Med 1974; 43: 399–407 [PubMed] [Google Scholar]
- 31. Hamano K, Iwano M, Akai Y, et al. Expression of glomerular plasminogen activator inhibitor type 1 in glomerulonephritis. Am J Kidney Dis 2002; 39: 695–705 [DOI] [PubMed] [Google Scholar]
- 32. Cameron JS. Coagulation and thromboembolic complications in the nephrotic syndrome. Adv Nephrol Necker Hosp 1984; 13: 75–114 [PubMed] [Google Scholar]
- 33. Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol 2012; 7: 513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jones EW, Mitchell JR. Venous thrombosis in diabetes mellitus. Diabetologia 1983; 25: 502–505 [DOI] [PubMed] [Google Scholar]
- 35. Petrauskiene V, Falk M, Waernbaum I, et al. The risk of venous thromboembolism is markedly elevated in patients with diabetes. Diabetologia 2005; 48: 1017–1021 [DOI] [PubMed] [Google Scholar]
- 36. Pincus KJ, Hynicka LM. Prophylaxis of thromboembolic events in patients with nephrotic syndrome. Ann Pharmacother 2013; 47: 725–734 [DOI] [PubMed] [Google Scholar]
- 37. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 Suppl): e419S–e494S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li SJ, Guo JZ, Zuo K, et al. Thromboembolic complications in membranous nephropathy patients with nephrotic syndrome-a prospective study. Thromb Res 2012; 130: 501–505 [DOI] [PubMed] [Google Scholar]
- 39. White RH, Garcia M, Sadeghi B, et al. Evaluation of the predictive value of ICD-9-CM coded administrative data for venous thromboembolism in the United States. Thromb Res 2010; 126: 61–67 [DOI] [PubMed] [Google Scholar]
- 40. Branchford BR, Gibson E, Manco-Johnson MJ, et al. Sensitivity of discharge diagnosis ICD-9 codes for pediatric venous thromboembolism is greater than specificity, but still suboptimal for surveillance and clinical research. Thromb Res 2012; 129: 662–663 [DOI] [PubMed] [Google Scholar]
- 41. Kerlin BA, Smoyer WE, Tsai J, et al. Healthcare burden of venous thromboembolism in childhood chronic renal diseases. Pediatr Nephrol 2015; 30: 829–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.