Abstract
Background
Lutein, a yellow xanthophyll, selectively accumulates in primate retina and brain. Lutein may play a critical role in neural and retinal development, but few studies have investigated the impact of dietary source on its bioaccumulation in infants.
Objective
We explored the bioaccumulation of lutein in infant rhesus macaques following breastfeeding or formula-feeding.
Methods
From birth to 6 mo of age, male and female rhesus macaques (Macaca mulatta) were either breastfed (BF) (n = 8), fed a formula supplemented with lutein, zeaxanthin, β-carotene, and lycopene (237, 19.0, 74.2, and 338 nmol/kg, supplemented formula-fed; SF) (n = 8), or fed a formula with low amounts of these carotenoids (38.6, 2.3, 21.5, and 0 nmol/kg, unsupplemented formula-fed; UF) (n = 7). The concentrations of carotenoids in serum and tissues were analyzed by HPLC.
Results
At 6 mo of age, the BF group exhibited significantly higher lutein concentrations in serum, all brain regions, macular and peripheral retina, adipose tissue, liver, and other tissues compared to both formula-fed groups (P < 0.001). Lutein concentrations were higher in the SF group than in the UF group in serum and all tissues, with the exception of macular retina. Lutein was differentially distributed across brain areas, with the highest concentrations in the occipital cortex, regardless of the diet. Zeaxanthin was present in all brain regions but only in the BF infants; it was present in both retinal regions in all groups but was significantly enhanced in BF infants compared to either formula group (P < 0.001). β-Carotene accumulated across brain regions in all groups, but was not detected in retina. Although lycopene was found in many tissues of the SF group, it was not detected in the brain or retina.
Conclusions
Although carotenoid supplementation of infant formula significantly increased serum and tissue lutein concentrations compared to unsupplemented formula, concentrations were still well below those in BF infants. Regardless of diet, occipital cortex showed selectively higher lutein deposition than other brain regions, suggesting lutein's role in visual processing in early life.
Keywords: carotenoids, lutein, bioaccumulation, brain, retina, rhesus macaques, infants, breast milk, infant formula
Introduction
Breast milk provides essential nutrients for growth and development, as well as other bioactive components that promote infant health. The composition of breast milk serves as a template for infant formula, but its nutrient profile is difficult to duplicate (1). Carotenoids, including lutein, zeaxanthin, canthaxanthin, β-cryptoxanthin, α-carotene, β-carotene, and lycopene, are detected in breast milk in concentrations that vary with maternal diet as well as the stage of lactation (2–7). Among the carotenoids in breast milk, lutein has attracted particular interest in recent years because of its potential role in visual and neural development (8).
Because humans cannot endogenously synthesize lutein, a yellow xanthophyll, it must be obtained from dietary sources such as dark green leafy vegetables, colorful fruits, and eggs (9). Lutein and its isomer zeaxanthin, known as macular pigments, selectively accumulate in the fovea of the primate retina, where they may provide protection from harmful short-wavelength blue light and act as antioxidants and anti-inflammatory agents (10). Epidemiologic and large-scale clinical studies have shown that increased intake of lutein and zeaxanthin is associated with a decreased risk of age-related macular degeneration, a leading cause of irreversible visual loss in older adults (11, 12). Studies of lutein and zeaxanthin supplementation have shown positive effects on visual performance in healthy subjects and those with age-related macular degeneration (13–15).
Recent evidence suggests that, compared to other carotenoids, lutein preferentially accumulates in the brains of human infants and older adults (16–18). Considering that the retina is an extension of the central nervous system, the presence of neural lutein implies a role in brain function. In intervention studies, lutein intake was associated with improved cognitive function in healthy adult subjects (19, 20). Recently, studies using fMRI or event-related potentials revealed that macular pigment optical density and serum xanthophyll concentrations were associated with enhanced neural efficiency in older adults and children (21, 22). Less is known about lutein's role in infancy. A recent metabolomic study demonstrated that lutein concentrations in infant brains were significantly correlated with amino acid neurotransmitters (23). Furthermore, higher concentrations of lutein and choline in breast milk were associated with better recognition memory in 5-mo-old infants (24).
Macaque monkeys are an appropriate animal model for investigating the potential benefits of lutein in humans because, unlike rodents, they accumulate xanthophylls in the retina and brain (25). Considering the possible roles of lutein in the developing eye and brain, and its potential life-long impact, achieving optimal lutein status in early life may be important for adult retina and brain health. Breast milk or infant formula are the sole sources of nutrition for infants before solid food is introduced. Therefore, more information is needed on the effects of dietary carotenoids from breast milk or infant formula on tissue lutein accumulation during early life.
To date, lutein bioaccumulation in response to diets during infancy has been poorly understood. Serum lutein and β-carotene concentrations, but not other carotenoids, were reported in infants fed breast milk or infant formulas with different amounts of lutein (26). In our previous study, we reported that carotenoid-supplemented infant formula increased lutein deposition in multiple tissues, including the brain and retina of young infant rhesus macaques fed for 4 mo (27). In the current study, a breastfed reference group was compared with infant formula-fed groups after 6 mo of feeding. The objective of this study was to compare how carotenoid bioaccumulation patterns in infant nonhuman primates were influenced by breast milk, an infant formula with low carotenoid levels, or a similar formula supplemented with lutein, zeaxanthin, β-carotene, and lycopene.
Methods
Animals and diets
All live animal aspects of the study were conducted at the Oregon National Primate Research Center (ONPRC) at Oregon Health and Science University. All procedures were approved by the Institutional Animal Care and Use Committee of Oregon Health and Science University and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Newborn rhesus macaques (Macaca mulatta, all of the Indian-origin subspecies) were obtained from the ONPRC breeding colony and allocated on the day after birth into 3 groups: breastfed (BF, n = 8), carotenoid-supplemented formula–fed (SF, n = 8) (Similac Advance with OptiGRO), or unsupplemented formula–fed (UF, n = 7), receiving a formula manufactured using the Similac Advance base formulation including DHA. The supplemented formula contained, by analysis, 22.1 μmol/kg of RRR-α-tocopherol and the unsupplemented formula contained 36.6 μmol/kg of all-rac-α-tocopherol. The proximate composition of infant formulas is given in Supplemental Table 1. Randomization among the groups was stratified by gender and by birth weight below or above the median (400–485 compared with 486–550 g for females and 420–506 compared with 507–610 g for males). The characteristics of the infant monkeys are described in Table 1. Ready-to-feed formulas were manufactured and labelled with a numerical code by Abbott Nutrition. Investigators and all staff working with the infants were masked to the identity of the 2 formulas until sample analysis was complete.
TABLE 1.
Summary of infant rhesus macaques characteristics1
| BF | SF | UF | Total | |
|---|---|---|---|---|
| (n = 8) | (n = 8) | (n = 7) | (n = 23) | |
| Female/male | 4/4 | 4/4 | 4/3 | 12/11 |
| Age when killed, d | 179 ± 4 | 178 ± 3 | 178 ± 3 | 178 ± 3 |
| Birth weight, kg | 0.50 ± 0.05 | 0.49 ± 0.05 | 0.52 ± 0.06 | 0.50 ± 0.05 |
| Final weight, kg | 1.41 ± 0.12 | 1.40 ± 0.10 | 1.47 ± 0.10 | 1.43 ± 0.11 |
1 Values are means ± SDs unless otherwise indicated. BF, breastfed; SF, supplemented formula-fed; UF, unsupplemented formula-fed.
BF infant monkeys were housed with their dams from birth; the dams were fed Monkey Diet Jumbo 5037 (Lab Diet) supplemented with a variety of fresh fruits and vegetables. Formula-fed infants were nursery-reared from 1 d after birth according to established protocols at ONPRC. They were housed in incubators for 2–4 wk and gradually weaned to cage-housing with another age-matched member of the same diet group and provided with blankets, stuffed toys, and a variety of enrichment devices. Infants were initially hand-fed until able to self-feed from a bottle-feeder by 3–10 d of age; feedings were provided every 2–4 h around the clock until approximately day 10 and then 3–4 times/d. Volume of intake was recorded at each feeding daily from day 1 through 12–13 wk of age and then for 5 consecutive days every 4 wk until 24 wk. Color-coded sterilized bottles were filled with age-appropriate volumes of formula 1 time/d, refrigerated until needed, and then warmed in a water bath; any formula not consumed within 4 h was removed and discarded. All infant monkeys were fed their designated diet for 6 mo (25 wk). The health and food intake of all infant monkeys was continuously monitored by veterinary staff.
Serum/plasma, tissue, and breast milk collection
Fasting 1-mL blood samples from infant monkeys were collected in EDTA and processed for plasma at baseline (1 d after birth) and after 4, 8, 12, 16, and 24 wk of breast milk or formula feeding. Breast milk samples were collected by manual expression under ketamine sedation (5–10 mg/kg intramuscular). Samples were collected directly into cryotubes, frozen on dry ice, and stored at –80°C until analysis.
After 6 mo of breastfeeding or formula feeding, infant monkeys were humanely killed by a veterinary pathologist under deep pentobarbital anesthesia, according to the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association.
At the time of death, fasting blood samples were drawn and centrifuged at 800 × g for 15 min to obtain serum. Tissues for carotenoid analysis were collected rapidly, placed in cryotubes, frozen in liquid nitrogen, and then stored at –80°C until analysis. From the brain, samples (∼0.5–1 g each) were dissected from dorsolateral prefrontal cortex, occipital cortex, superior temporal cortex, striatum, cerebellum, motor cortex, isolated frontal gray matter, frontal white matter, and hippocampus. From each retina, 4-mm diameter biopsy punches were used to obtain a macular sample centered on the fovea and samples of the peripheral retina; the vitreous was removed by blotting with filter paper, and the neural retina was gently dissected from the underlying retinal pigment epithelium and choroid. Samples were collected from other tissues including adipose tissue, liver, quadriceps, kidney, heart, lungs, spleen, and ovaries or testes. Samples of adipose tissue were collected from 4 sites: abdominal subcutaneous, mesenteric, thigh subcutaneous (TSAT), and axillary brown adipose tissues.
Carotenoid analysis
All extractions and analyses were performed under yellow light to minimize light-induced damage of carotenoids. Carotenoids were extracted from serum and plasma and multiple tissues using tissue-specific extraction methods, as described in detail in our previous publication (27).
For breast milk and formula analysis, 10 mL of 5% KOH in methanol was added to 2 mL of breast milk or formula and vortexed. Tetrahydrofuran (5 mL) was added and the mixture was vortexed. This mixture was allowed to stand at room temperature with intermittent vortexing for 30 min. Next, 10 mL of extraction solvent (dichloromethane/petroleum ether/hexane, 2:4:4) containing 0.005% BHT was added, vortexed, and centrifuged at 800 × g for 15 min. After the upper layer was removed, 10 mL of extraction solvent was added to the bottom layer and the extraction process was repeated one more time. The extract was pooled and evaporated under nitrogen, followed by adding 3 mL deionized water and 3 mL ethanol. This mixture was vortexed, sonicated, and centrifuged at 800 × g for 5 min at 4°C. The upper layer was transferred and dried under nitrogen in a 40°C water bath.
For plasma or serum analysis, ∼250 μL of sample was mixed with an equal volume of ethanol containing 0.1% BHT and then vortexed. Then, 1 mL of hexane was added, vortexed, and centrifuged at 2400 × g at 4°C (Centrifuge 5417R, Eppendorf) for 3 min. The upper hexane layer was removed. The hexane extraction process was repeated 2 more times. The extract was pooled and evaporated to dryness under argon.
For brain analysis, brain samples (0.15 g) were homogenized with 0.3 mL of 0.9% NaCl solution and 0.5 mL ethanol. Then, 100 μL of 0.8 μmol/L echinenone in ethanol (A1%1cm = 2244) was added to the homogenate as an internal standard. After incubating at 70°C for 2 min, 0.5 mL of freshly prepared 25% sodium ascorbate and 1 mL of 5% NaOH were added. The mixture was saponified in a 60°C water bath for 20 min. Then, 0.5 mL of distilled water was added and the mixture was placed on ice for 5 min. Hexane (5 mL) was added, and the mixture was vortexed for 2 min and centrifuged at 1000 × g at 4°C (Centrifuge CR3, Jouan) for 10 min. The upper hexane layer was removed and the hexane extraction process was repeated 2 more times. The extract was pooled and evaporated to dryness under argon.
For retina analysis, the tissue was minced with 1 mL 0.85% saline by using a glass rod on ice. To this, 3 mL chloroform/methanol (2:1, v/v) and echinenone were added. After vortexing, the layers were separated by centrifugation at 800 × g at 4°C (Centrifuge CR3, Jouan) for 15 min. The lower chloroform layer was transferred and evaporated to dryness under argon. The extraction was repeated using 3 mL hexane.
For adipose tissue analysis, adipose sample (125 mg) was homogenized with 400 μL of PBS. Chloroform (500 μL) and 1 mL methanol were added and vortexed for 5 min. After centrifugation at 1200 × g at 10°C (Centrifuge CR3, Jouan) for 10 min, the lower phase was collected and evaporated to dryness under argon. Next, 1 mL of 5.5% KOH in ethanol and 100 μL of freshly prepared 1.2% pyrogallol in ethanol were added to the dry residue and vortexed. After incubation at 37°C for 90 min for saponification, 1 mL distilled water, echinenone as an internal standard, and 3 mL hexane were added. Carotenoids were extracted by hexane using the above-described steps.
For other tissue (liver, quadriceps, kidney, heart, lungs, spleen, ovaries, and testes) analysis, samples were minced with ethanol containing 0.1% BHT, and 1 mL of 100% KOH saturated solution was added. After saponification in a 60°C water bath with intermittent vortexing for 30 min, 2 mL of deionized water and 6 mL of hexane were added. Carotenoids were extracted by hexane using the above-described steps.
All analyses were carried out on an Alliance HPLC system (e2695 Separation Module) equipped with Waters 2998 photodiode array detector. The extracts were separated on a reverse-phase C 30 column (4.6 × 150 mm, 3 μm; YMC) maintained at 18°C. A phase gradient method used for carotenoid separation was based on the method of Yeum et al. (28). The lower limit of detection for carotenoids is 0.2 pmol. The average recovery of the internal standard was 92.8% ± 0.5%. Carotenoids were identified via absorption spectra, retention times, and standard comparison, and quantified by an external standard curve method or internal standard curve method. Our laboratory regularly participates in the National Institutes of Standards in Technology micronutrient proficiency testing program for carotenoids and retinoids.
Statistical analysis
Data analyses were performed using SPSS version 23 (IBM SPSS Statistics) or SAS version 9.4 (SAS Institute Inc.). Unequal variances were evaluated prior to ANOVA analysis using Levene's test of homogeneity of variance. Changes in blood carotenoid concentrations, formula intake, body weight, and head circumference over time were analyzed by means of 2-way repeated-measures ANOVA. Two-way ANOVA was performed to assess effects of diet and brain region, diet and adipose region, or diet and sex on carotenoid concentrations. Carotenoid concentrations in multiple tissues were analyzed by using one-way ANOVA, with Bonferroni's multiple comparison test. In case of unequal variances, the Welch's ANOVA was used to compare group means, followed by Dunnett's T3 test for posthoc comparisons. The relationships between pairs of variables were determined by linear regression analyses. All data are presented as mean ± SD. All differences or relations were considered significant when P < 0.05.
Results
Formula intake, body weight, and organ weights
Formula intake gradually increased with age ≤6 mo, and there were no significant differences in the consumed amount of formula between the 2 formula groups (Figure 1A). No significant differences were found among dietary groups in body weight or its rate of increase with age (Figure 1B) or in head circumference (Figure 1C). In addition, no differences were observed among dietary groups for any organ weight except for modest changes for the liver (19% increase in the SF compared to the BF group, P = 0.014; 15% increase in the UF compared to the BF group, P = 0.071) and adrenal glands (28% increase in the UF compared to the BF group, P = 0.006).
FIGURE 1.
The amount of infant formula consumed per week by infant monkeys fed the 2 infant formulas for 6 mo (A). Body weight (B) and head circumference (C) of infant rhesus monkeys either BF (n = 8), SF (n = 8), or UF (n = 7) for 6 mo. Values are presented as mean ± SD. No significant group differences were found. BF, breastfed; SF, supplemented formula-fed; UF, unsupplemented formula-fed.
Carotenoid profiles of formula and breast milk
The carotenoid profiles of breast milk and the 2 infant formulas are shown in Table 2. We analyzed milk samples collected from 6 of the breastfeeding dams at 2–4 time points each from 2 to 6 mo after birth. Lutein and zeaxanthin concentrations in breast milk were 1.1-fold and 5.5-fold higher, respectively, than in the supplemented formula. The supplemented formula contained higher amounts of lutein, zeaxanthin, and β-carotene (6.1-fold, 8.5-fold, and 3.5-fold, respectively) than the unsupplemented formula. Lycopene was present in the supplemented formula but undetectable in the unsupplemented formula or in breast milk. β-Carotene and β-cryptoxanthin were undetectable in breast milk. It is possible that these carotenoids were not detected, despite their presence at low concentrations in serum and tissues of BF infants, due to the small volumes (100–1000 μL) of breast milk available for carotenoid analysis.
TABLE 2.
Carotenoid profiles of rhesus macaques breast milk and infant formulas1
| BF | SF | UF | |
|---|---|---|---|
| Lutein, nmol/kg | 251 ± 113 | 237 ± 14.5 | 38.6 ± 15.4 |
| Zeaxanthin, nmol/kg | 105 ± 46.1 | 19.0 ± 2.4 | 2.3 ± 1.1 |
| β-carotene, nmol/kg | ND2 | 74.2 ± 17.1 | 21.5 ± 9.4 |
| Total lycopene, nmol/kg | ND | 338 ± 25.1 | ND |
| β-Cryptoxanthin, nmol/kg | ND | ND | ND |
1Values are means ± SDs. BF, breastfed; ND, not detected; SF, supplemented formula-fed; UF, unsupplemented formula-fed.
2Data obtained from 2–3 batches of infant formula and breast milk samples collected from 6 dams at 2–4 different time points.
Plasma/serum carotenoids
Blood carotenoid concentrations are shown in Figure 2. At baseline, dietary groups did not differ in the concentrations of lutein and zeaxanthin. Lycopene was undetectable at baseline in any infant monkey. Blood lutein concentrations increased from baseline in the BF and SF groups, but not the UF group (Figure 2A). Breastfeeding significantly increased blood lutein concentrations compared to both formula groups. In the formula-fed groups, after 6 mo of SF feeding, blood lutein concentrations were 5-fold higher than in the UF group infants (P < 0.001), but still 5-fold lower than in BF infants. Blood zeaxanthin concentrations increased from baseline only in the BF group and were undetectable in the UF group after week 4 and in the SF group after week 12 (Figure 2B). Blood lycopene was detected throughout the feeding period only in the SF group (Figure 2C). In the BF group, a few infants had measurable blood lycopene at weeks 16 (3 infants) and 25 (1 infant), possibly because a lycopene-rich fruit or vegetable was temporarily included in the maternal diet. Blood β-carotene concentrations increased from baseline in all groups; significantly higher concentrations were found in the BF group compared to the formula-fed groups after week 4, with no significant differences between the formula groups at week 25 (Figure 2D). Blood β-cryptoxanthin concentrations increased from baseline only in the BF group and were undetectable in the formula-fed groups after week 4 (data not shown). No significant sex or diet × sex interaction effects were observed for any blood carotenoid concentrations at any individual time point (data not shown).
FIGURE 2.
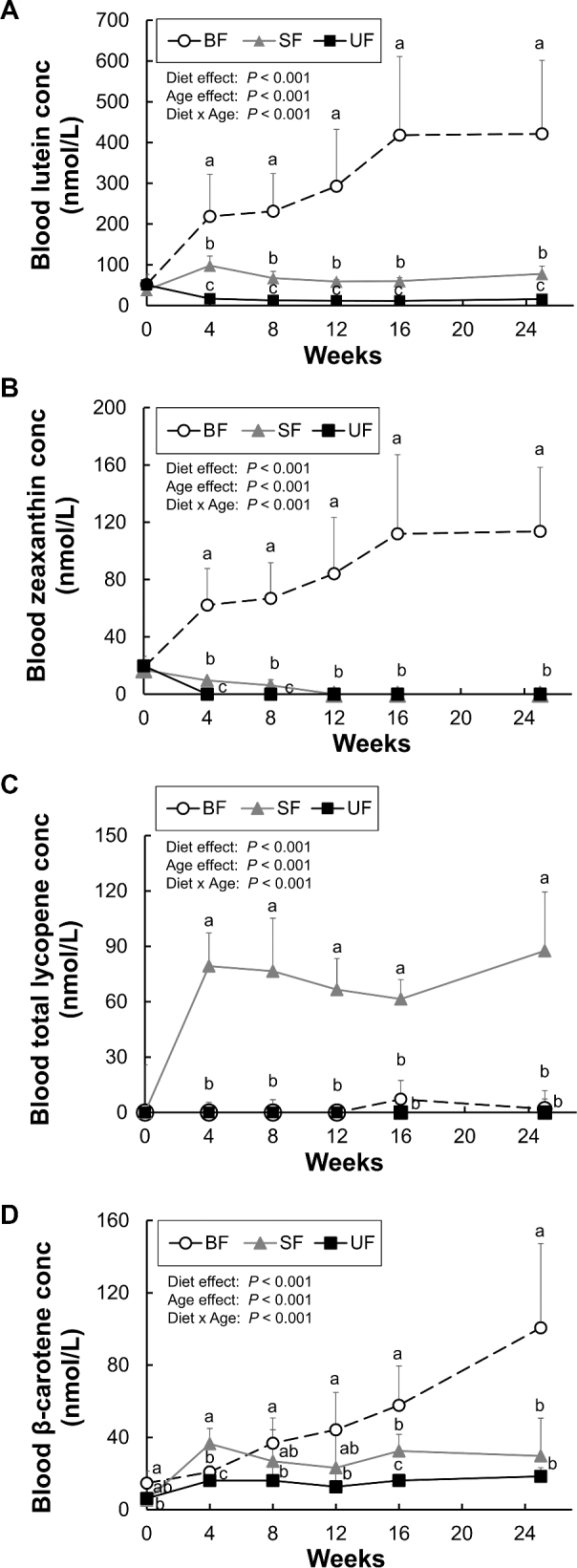
Plasma/serum lutein (A), zeaxanthin (B), total lycopene (C), and β-carotene (D) in infant rhesus monkeys either BF (n = 8), SF (n = 8), or UF (n = 7) for 6 mo. Values are presented as mean ± SD. Diet and age effects were determined by 2-way repeated measures ANOVA. Labeled means at each time point without a common letter differ, P < 0.05, by 1-way ANOVA followed by Bonferroni or Dunnett's T3 pairwise post-hoc tests. BF, breastfed; conc, concentration; SF, supplemented formula-fed; UF, unsupplemented formula-fed.
Brain carotenoids
Lutein, zeaxanthin, and β-carotene concentrations in 9 different brain regions (prefrontal cortex, occipital cortex, superior temporal cortex, striatum, cerebellum, motor cortex, gray matter, white matter, and hippocampus) are presented in Figure 3. Lutein accumulation differed among brain regions, with the highest amount in occipital cortex across all diet groups. In all brain regions analyzed, breastfeeding significantly increased lutein concentrations compared to either formula group; the SF group showed significantly higher lutein deposition compared to the UF group. A 2-way ANOVA supported these observations, with significant main effects of brain region (P < 0.001) and diet (P < 0.001), as well as a significant diet × brain region interaction (P < 0.001); post-hoc pairwise comparisons confirmed significant differences among all 3 groups in each brain region. Similarly to lutein, zeaxanthin selectively accumulated across brain regions, also with the highest amount in occipital cortex, but was detectable only in the BF group. There was a strong correlation between lutein and zeaxanthin concentrations in the brain regions of the BF group (r = 0.954, P < 0.001), suggesting that lutein and zeaxanthin may share the same uptake mechanisms into the brain.
FIGURE 3.
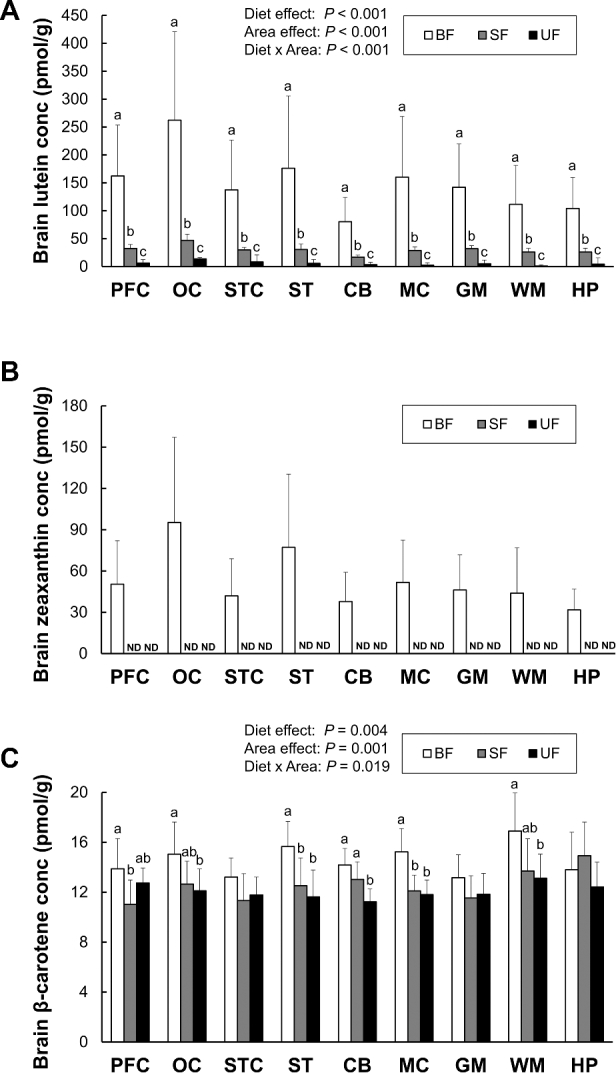
Lutein (A), zeaxanthin (B), and β-carotene (C) concentrations in each brain region of infant monkeys either BF (n = 8), SF (n = 8), or UF (n = 7) for 6 mo. Values are presented as mean ± SD. Diet and area effects were determined by 2-way ANOVA. Labeled means for each tissue without a common letter differ, P < 0.05, by 1-way ANOVA or Welch's test followed by Bonferroni or Dunnett's T3 pairwise post-hoc tests. The lower limit of detection for carotenoids is 0.2 pmol. BF, breastfed; CB, cerebellum; conc, concentration; GM, gray matter; HP, hippocampus; MC, motor cortex; ND, not detected; OC, occipital cortex; PFC, prefrontal cortex; SF, supplemented formula-fed; ST, striatum; STC, superior temporal cortex; UF, unsupplemented formula-fed; WM, white matter.
Small amounts of β-carotene (range: 8–21 pmol/g) were detected in all brain regions of all groups. The BF group had significantly higher β-carotene concentrations in all brain regions except the hippocampus, but only striatum and motor cortex reached statistical significance. A 2-way ANOVA showed significant main effects of brain region (P = 0.001) and diet (P = 0.004) as well as a significant diet × brain region interaction (P = 0.019). In pairwise comparisons, the SF group had significantly higher concentrations of β-carotene than the UF group in only one brain region, the cerebellum. Lycopene was not detected in any brain region, despite the high lycopene content in the supplemented formula.
No significant effects of sex or diet × sex interactions were seen in brain lutein and zeaxanthin concentrations in any brain region (data not shown). A significant sex difference in brain β-carotene concentrations was found only in gray matter and hippocampus, with concentrations higher in males than in females (P = 0.018 and P = 0.027, respectively).
Strong positive correlations were found between concentrations of lutein in 25-wk serum samples and in each brain region, as shown in Table 3 (R2 = 0.71–0.87, P < 0.001). Among the 9 brain regions, the slope (β) of the regression line was highest in the occipital cortex and lowest in the cerebellum, suggesting preferential uptake and lower lutein metabolism in the occipital cortex, and vice versa in the cerebellum. β-Carotene concentrations in serum and each brain region generally were more weakly correlated. The slope for serum versus brain β-carotene was lower than that of lutein in all brain regions, suggesting a selective lutein uptake mechanism from blood into the brain, or differential β-carotene metabolism in the brain.
TABLE 3.
Results of simple linear regression analyses with 25-wk serum lutein, serum β-carotene (nmol/L), or retinal lutein (pmol/g) concentrations as independent variables and brain lutein, retinal lutein, or β-carotene concentrations (pmol/g) as dependent variables (n = 23)
| Independent variable | Dependent variable | β (95% CI) | R 2 | P value |
|---|---|---|---|---|
| Lutein | ||||
| Serum | Prefrontal cortex | 0.38 (0.30, 0.45) | 0.82 | <0.001 |
| Occipital cortex | 0.63 (0.50, 0.76) | 0.84 | <0.001 | |
| Superior temporal cortex | 0.32 (0.23, 0.40) | 0.75 | <0.001 | |
| Striatum | 0.45 (0.34, 0.56) | 0.78 | <0.001 | |
| Cerebellum | 0.19 (0.16, 0.22) | 0.87 | <0.001 | |
| Motor cortex | 0.38 (0.27, 0.49) | 0.71 | <0.001 | |
| Gray matter | 0.33 (0.27, 0.39) | 0.86 | <0.001 | |
| White matter | 0.26 (0.20, 0.33) | 0.79 | <0.001 | |
| Hippocampus | 0.24 (0.19, 0.28) | 0.85 | <0.001 | |
| Macular retina | 11.7 (8.72, 14.8) | 0.76 | <0.001 | |
| Peripheral retina | 2.24 (1.90, 2.58) | 0.90 | <0.001 | |
| Macular retina | Prefrontal cortex | 0.02 (0.02, 0.03) | 0.64 | <0.001 |
| Occipital cortex | 0.04 (0.02, 0.05) | 0.59 | <0.001 | |
| Superior temporal cortex | 0.02 (0.01, 0.03) | 0.55 | <0.001 | |
| Striatum | 0.03 (0.02, 0.04) | 0.54 | <0.001 | |
| Cerebellum | 0.01 (0.01, 0.02) | 0.68 | <0.001 | |
| Motor cortex | 0.02 (0.02, 0.03) | 0.57 | <0.001 | |
| Gray matter | 0.02 (0.01, 0.03) | 0.64 | <0.001 | |
| White matter | 0.02 (0.01, 0.02) | 0.61 | <0.001 | |
| Hippocampus | 0.01 (0.01, 0.02) | 0.58 | <0.001 | |
| Peripheral retina | Prefrontal cortex | 0.14 (0.09, 0.19) | 0.65 | <0.001 |
| Occipital cortex | 0.24 (0.16, 0.32) | 0.68 | <0.001 | |
| Superior temporal cortex | 0.12 (0.07, 0.16) | 0.58 | <0.001 | |
| Striatum | 0.17 (0.11, 0.23) | 0.64 | <0.001 | |
| Cerebellum | 0.07 (0.05, 0.09) | 0.72 | <0.001 | |
| Motor cortex | 0.14 (0.08, 0.20) | 0.55 | <0.001 | |
| Gray matter | 0.13 (0.09, 0.16) | 0.69 | <0.001 | |
| White matter | 0.10 (0.07, 0.14) | 0.63 | <0.001 | |
| Hippocampus | 0.09 (0.06, 0.12) | 0.68 | <0.001 | |
| β-Carotene | ||||
| Serum | Prefrontal cortex | 0.03 (0.01, 0.04) | 0.30 | 0.01 |
| Occipital cortex | 0.03 (0.02, 0.05) | 0.43 | 0.001 | |
| Superior temporal cortex | 0.02 (0.00, 0.04) | 0.25 | 0.02 | |
| Striatum | 0.04 (0.02, 0.06) | 0.41 | 0.001 | |
| Cerebellum | 0.02 (0.00, 0.03) | 0.26 | 0.01 | |
| Motor cortex | 0.03 (0.02, 0.05) | 0.57 | <0.001 | |
| Gray matter | 0.02 (0.00, 0.03) | 0.16 | 0.06 | |
| White matter | 0.04 (0.01, 0.06) | 0.31 | 0.01 | |
| Hippocampus | 0.01 (−0.01, 0.04) | 0.05 | 0.33 | |
Retinal carotenoids
Lutein and zeaxanthin concentrations were higher in the macular retina (4-mm diameter central sample) or than in the peripheral retina (Figure 4). BF monkeys had significantly higher lutein and zeaxanthin concentrations in both retinal regions than both formula-fed groups. Carotenoid supplementation of formula significantly increased lutein concentrations in the peripheral retina but not in the macular retina. In contrast, zeaxanthin concentrations did not differ between formula groups in either retinal region.
FIGURE 4.
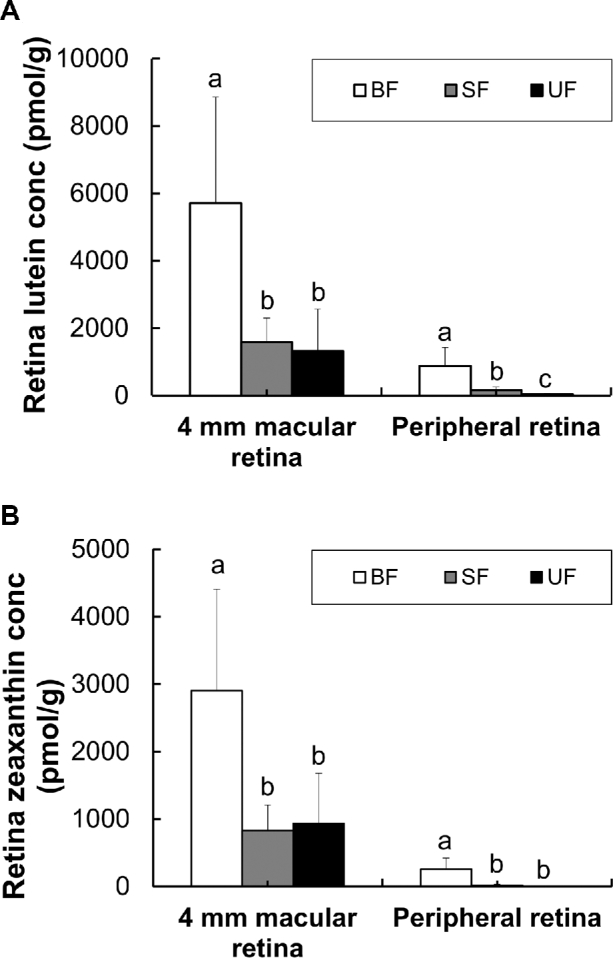
Lutein (A) and zeaxanthin (B) concentrations in each retina region of infant rhesus monkeys either BF (n = 8), SF (n = 8), or UF (n = 7) for 6 mo. Values are means ± SDs. Labeled means for each tissue without a common letter differ, P < 0.05, by Welch's ANOVA followed by Dunnett's T3 pairwise post-hoc tests. BF, breastfed; conc, concentration; SF, supplemented formula-fed; UF, unsupplemented formula-fed.
Lutein concentrations were positively correlated between 25-wk serum and each retinal region, as shown in Table 3 (R2 = 0.76 for macular retina; R2= 0.90 for peripheral retina, P < 0.001). The slope of the regression line was higher in macular retina than in peripheral retina. In addition, strong correlations were found between lutein concentrations in both retina and brain regions (Table 3; R2 = 0.54–0.72, P < 0.001).
Adipose tissue carotenoids
Carotenoid concentrations varied among the 4 adipose sites examined (Table 4). A 2-way ANOVA found a significant main effect of diet (P < 0.001), but not of adipose region (P = 0.307), nor a diet × adipose region interaction (P = 0.627). The BF group had significantly higher lutein concentrations in all adipose tissues except axillary brown adipose tissue compared with the formula-fed groups. SF significantly increased lutein accumulation in all adipose regions except TSAT, where a similar trend was observed.
TABLE 4.
Carotenoid concentrations in infant rhesus macaques in each adipose region1
| Dietary group | P values | |||||
|---|---|---|---|---|---|---|
| Abdominal area | BF | SF | UF | Diet | Adipose area | Diet × area |
| Lutein (pmol/g) | <0.001 | 0.307 | 0.627 | |||
| ASAT | 643 ± 405a | 102 ± 44.9b | 26.5 ± 22.7c | |||
| TSAT | 545 ± 370a | 88.8 ± 77.9b | 22.6 ± 26.6b | |||
| MAT | 403 ± 277a | 69.7 ± 34.7b | 8.6 ± 10.6c | |||
| BAT | 402 ± 427a | 48.6 ± 20.1a | 7.7 ± 9.7b | |||
| Zeaxanthin (pmol/g) | — | — | — | |||
| ASAT | 163 ± 102 | ND2 | ND | |||
| TSAT | 146 ± 97.5a | 2.0 ± 5.6b | ND | |||
| MAT | 100 ± 69.7 | ND | ND | |||
| BAT | 97.2 ± 101 | ND | ND | |||
| β-Carotene (pmol/g) | 0.002 | 0.001 | 0.021 | |||
| ASAT | 159 ± 26.7a | 172 ± 136a | 48.4 ± 26.1b | |||
| TSAT | 157 ± 43.3a | 147 ± 89.0a | 42.5 ± 26.2b | |||
| MAT | 118 ± 54.9a | 102 ± 41.9a | 36.0 ± 18.2b | |||
| BAT | 117 ± 54.6ab | 167 ± 78.4a | 48.4 ± 27.3b | |||
| Total lycopene (pmol/g) | — | — | — | |||
| ASAT | ND | 366 ± 107 | ND | |||
| TSAT | ND | 287 ± 57.1 | ND | |||
| MAT | ND | 209 ± 40.4 | ND | |||
| BAT | ND | 293 ± 58.1 | ND | |||
1Values are means ± SDs (n = 7–8/group). ASAT, abdominal subcutaneous adipose tissue; BAT, brown adipose tissue; BF, breastfed; MAT, mesenteric adipose tissue; ND, not detected; SF, supplemented formula-fed; TSAT, thigh subcutaneous adipose tissue; UF, unsupplemented formula-fed.
2The lower limit of detection for carotenoids is 1.6 pmol/g. Diet and area effects were determined by 2-way ANOVA. Values in a row with different superscript letters are significantly different, P < 0.05, by 1-way ANOVA or Welch's test followed by Bonferroni or Dunnett's T3 pairwise post-hoc tests.
SF significantly increased β-carotene concentrations in all adipose regions compared with the UF group. Zeaxanthin was detectable in all adipose regions of BF infants and in TSAT of one monkey fed SF. Lycopene was detectable in all adipose regions of the SF group, but not in the BF or UF groups. β-Cryptoxanthin in all adipose regions was detectable only in the BF group (data not shown). No significant sex difference or diet × sex interaction was observed in the concentrations of any of the carotenoids in any adipose region.
Carotenoids in other tissues
Lutein, zeaxanthin, β-carotene, and total lycopene concentrations in the liver, quadriceps, kidney, heart, lungs, spleen, ovaries, and testes are presented in Table 5. Lutein concentrations in these tissues were higher in the BF group than in the formula-fed groups and lower in the UF group than in the SF group. In the liver, lutein was the predominant carotenoid. The BF group showed 5-fold and 24-fold increases in liver lutein concentrations compared with the SF and the UF groups, respectively, whereas a 5-fold difference was seen between the SF and UF groups. Zeaxanthin was detected in all tissues from BF monkeys. In contrast, in the SF group, zeaxanthin was undetectable in the quadriceps, heart, and ovaries, but present in the lungs, kidney, and testes from only one monkey; and in the UF group, it was undetectable in the liver, quadriceps, kidney, lungs, spleen, ovaries, and testes, and detected in the heart of only one monkey. β-Carotene concentrations were significantly higher in the BF group in all tissues except the liver compared to the formula-fed groups. Formula supplementation enhanced β-carotene concentrations in the liver, kidney, heart, lungs, spleen, ovaries, and testes compared to UF but was not generally detected in other organs of the UF group. Lycopene was detectable in all tissues of the SF group, but in none of the UF group, with the exception of the liver of 1 subject. In BF monkeys, lycopene was present in a few individuals in some tissues. In addition, β-cryptoxanthin was detectable in all tissues only in the BF group (data not shown). No significant sex difference or diet × sex interaction was found for any carotenoid in any of the tissues.
TABLE 5.
Carotenoid concentrations in infant rhesus macaques in various tissues1
| Dietary group | ||||
|---|---|---|---|---|
| Tissue | Carotenoid (pmol/g) | BF | SF | UF |
| Liver | Lutein | 2680 ± 1008a | 530 ± 89.6b | 114 ± 88.1c |
| Zeaxanthin | 1071 ± 380a | 42.8 ± 28.7b | ND2 | |
| β-Carotene | 151 ± 137a | 65.9 ± 29.2ab | 8.9 ± 15.2b | |
| Total lycopene | 2.0 ± 5.6b | 224 ± 80.5a | 8.2 ± 21.6b | |
| Quadriceps | Lutein | 148 ± 67.0a | 24.7 ± 4.0b | 2.4 ± 4.2c |
| Zeaxanthin | 49.0 ± 30.6 | ND | ND | |
| β-Carotene | 17.4 ± 15.7 | ND | ND | |
| Total lycopene | 4.9 ± 13.9 | 16.1 ± 11.6 | ND | |
| Kidney | Lutein | 273 ± 110a | 39.8 ± 7.5b | 16.2 ± 18.9c |
| Zeaxanthin | 91.5 ± 35.0a | 1.3 ± 3.6b | ND | |
| β-Carotene | 45.2 ± 14.7 | 28.6 ± 12.2 | ND | |
| Total lycopene | ND | 53.6 ± 4.0 | ND | |
| Heart | Lutein | 189 ± 67.8a | 31.7 ± 3.6b | 19.3 ± 39.5b |
| Zeaxanthin | 69.6 ± 25.6a | ND | 1.4 ± 3.8b | |
| β-Carotene | 43.7 ± 11.3a | 22.0 ± 13.7b | ND | |
| Total lycopene | 7.0 ± 19.7b | 31.4 ± 15.1a | ND | |
| Lungs | Lutein | 536 ± 248a | 152 ± 192b | 21.6 ± 10.8b |
| Zeaxanthin | 230 ± 104a | 1.1 ± 3.1b | ND | |
| β-Carotene | 73.4 ± 23.8a | 36.5 ± 16.3b | 1.9 ± 5.0c | |
| Total lycopene | ND | 62.3 ± 17.8 | ND | |
| Spleen | Lutein | 1008 ± 386a | 199 ± 47.8b | 41.3 ± 20.7c |
| Zeaxanthin | 367 ± 148a | 15.5 ± 6.9b | ND | |
| β-Carotene | 134 ± 63.4a | 56.9 ± 23.5b | 8.8 ± 15.0c | |
| Total lycopene | 2.0 ± 5.6b | 117.1 ± 35.2a | ND | |
| Ovaries | Lutein | 600 ± 271a | 110 ± 12.9b | ND |
| Zeaxanthin | 233 ± 100 | ND | ND | |
| β-Carotene | 76.3 ± 44.9 | 68.6 ± 44.9 | ND | |
| Total lycopene | 17.0 ± 34.0b | 114 ± 81.4a | ND | |
| Testes | Lutein | 419 ± 171a | 77.3 ± 19.9b | 15.5 ± 19.9b |
| Zeaxanthin | 171 ± 72.9a | 3.4 ± 6.8b | ND | |
| β-Carotene | 59.7 ± 23.2 | 23.6 ± 18.1 | ND | |
| Total lycopene | ND | 69.9 ± 6.8 | ND | |
1Values are means ± SDs (n = 7–8/group, n = 3–4/group for ovaries and testes). BF, breastfed; ND, not detected; SF, supplemented formula-fed; UF, unsupplemented formula-fed.
2The lower limit of detection for carotenoids is 2 pmol/g. Values in a row with different superscript letters are significantly different, P < 0.05, by 1-way ANOVA or Welch's test followed by Bonferroni or Dunnett's T3 pairwise post-hoc tests.
Discussion
This study compares the effects of breastfeeding and infant formula-feeding on lutein bioaccumulation in multiple tissues of nonhuman primate infants during the first 6 mo of life. Our earlier study in infant rhesus macaques at 1–3 mo of age described the diet-dependent patterns of tissue lutein deposition resulting from 4 mo of feeding infant formulas containing high and low concentrations of carotenoids (27). However, that pilot study had a small sample size (n = 2/group), did not initiate formula feeding at birth, and lacked a BF control group. The current study demonstrates in greater detail the unique bioaccumulation patterns of carotenoids including lutein in infant monkeys in response to breast milk and infant formulas.
Breast milk is the preferred dietary vehicle for the delivery of carotenoids, especially lutein, to serum and tissues compared to either infant formula. Serum and all tissues examined in this study showed higher lutein deposition following breastfeeding compared to the formula-fed groups, despite the similar lutein concentrations in breast milk and the supplemented formula. Previously, the bioavailability of lutein from breast milk and infant formula was compared in human infants and was found to be about 4 times greater from breast milk, as assessed by serum lutein concentrations (26). This result is in line with an in vitro study using human intestinal cell lines, which showed that the higher lutein bioavailability of breast milk was primarily due to enhanced intestinal absorptive processes compared to infant formula (29). Zeaxanthin was undetectable in the brain of formula-fed infant monkeys but present in all brain regions of the BF monkeys; in this case the difference may be due to both a higher zeaxanthin concentration in breast milk and its higher bioavailability. There are many potential factors that may be responsible for enhanced carotenoid bioavailability from breast milk including food matrix, fat content, and nutrient-nutrient interactions (30, 31).
We found that breast milk enhanced lutein and zeaxanthin accumulation in both macular and peripheral retina compared to either formula, but interestingly, in the macular region accumulation was not increased by formula supplementation. Lutein and zeaxanthin have unique roles as the components of macular pigment and thus in tissue protection and enhancement of foveal vision. Therefore, enhanced macular lutein deposition from breast milk may lead to optimal retinal function with possible consequences throughout the lifespan. In contrast, lutein deposition in all other tissues was increased by formula supplementation, in agreement with our pilot study results (27). The enhanced lutein accumulation in macular retina by breast milk implies a highly targeted mechanism by which xanthophylls are efficiently delivered and concentrated in this specific location. Although both lutein and zeaxanthin are found primarily in HDL, Thomas and Harrison (32) recently reported that zeaxanthin was selectively taken up via an HDL-mediated SR-B1 pathway, while LDL more efficiently delivered lutein for entry into the retina. Furthermore, lutein is protected by binding to a specific lutein-binding protein, steroidogenic acute regulatory domain protein 3 (StARD3) (33). The effect of diet on xanthophyll uptake, as well as the protective mechanisms and lifelong impact of xanthophylls, requires further study.
We confirmed the differential lutein accumulation across brain regions of infant monkeys, with highest concentrations in the occipital cortex, the site of the primary visual cortex, in all diet groups. This distribution may imply a distinctive role of lutein in the visual system. Lutein supplementation has been reported to enhance aspects of visual processing speed that are believed to reflect visual cortical processing (14). In addition, we found the lowest amount of lutein in cerebellum of the BF and SF groups, but not the UF group. This result is in agreement with results in adult rhesus macaques, in which lower lutein accumulation was observed in cerebellum compared to concentrations in the striatum, occipital, frontal, or prefrontal cortices (25, 34). However, in human centenarians, greater lutein was deposited in the cerebellum compared to multiple cortical areas (18), a discrepancy that may be linked to age-related or pathologic cognitive decline. The unique pattern of lutein bioaccumulation may result from different lutein uptake, transport, or metabolism among brain regions.
A novel finding was that lycopene was undetectable in any brain region despite significant accumulation in serum and tissues of the SF group. This is interesting, considering that the SF had a higher content of lycopene than β-carotene, and some β-carotene did accumulate in the brain of the SF-fed infants. Thus, it may be assumed that there is a selective uptake mechanism for β-carotene and xanthophylls but not for lycopene. The absence of lycopene in the brain is consistent with a study in deceased human infants showing that lycopene was detected only in 3 of the 30 decedents, while lutein, zeaxanthin, cryptoxanthin, and β-carotene were all detectable (16). Interestingly, a small amount of lycopene accumulated in the brains of older adults, although less than β-carotene (18). In addition, it was reported that lycopene accumulated in the brains of wild-type and β-carotene 15,15′-oxygenase (BCO1) knockout mice fed lycopene-containing diets (35). The existence of lycopene in the brain may differ as a function of age and species, as a function of chronic lycopene consumption or due to altered blood-brain barrier function during aging.
Our study showed that both lutein and zeaxanthin were most highly concentrated in the macular retina, followed by liver. β-Carotene and total lycopene accumulation patterns differed from those of xanthophylls. The highest β-carotene concentrations were found in adipose tissues and the second highest in the liver and spleen, with little in the brain. In all but 2 of the SF-fed monkeys, lycopene was most concentrated in adipose tissue, followed by the liver. This differential carotenoid accumulation may be due to the different polarities of non-hydroxylated carotenes and polar hydroxylated xanthophylls (36). Moreover, the accumulation pattern might be affected by carotenoid-carotenoid interactions (31).
This study is significant in that it demonstrates lutein tissue accumulation in early life, during a critical period for retinal and neural development. One of the study's strengths is in the utilization of an appropriate animal model, rhesus macaques. One limitation of this study is that we were unable to investigate the potential molecular mechanisms underlying the unique carotenoid bioaccumulation patterns; this objective was beyond the scope of the current study.
In conclusion, we demonstrated that breastfed infant rhesus macaques had enhanced serum and tissue lutein concentrations compared to formula-fed infant rhesus macaques. Lutein concentrations in the SF group exceeded those in the UF group, but were still several-fold less than in BF infants. We confirmed that there is differential lutein accumulation across brain regions and that the occipital cortex accumulates the highest amounts of lutein, suggesting that lutein may play a critical role in visual processing in early life.
Supplementary Material
Supplemental Table 1 is available from the “Supplementary Data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Acknowledgments
The authors’ responsibilities were as follows—MN, MJK, and JWE: designed the research; SJ, KMR, EEJ, LR, and MN: conducted the research; MN, MJK, SLP, and JWE: provided essential materials; SJ, EEJ and MN: analyzed data or performed statistical analysis; SJ and JWE: wrote the paper; SJ, KMR, MN, MJK, SLP, EJJ, and JWE: contributed to revising manuscript; JWE: had primary responsibility for final content; all authors: read and approved the final manuscript.
Supported by Abbott Nutrition through the Center for Nutrition, Learning, and Memory (CNLM) at the University of Illinois at Urbana-Champaign, USDA 1950-51000-073-015 and by NIH grant P51OD011092.
Author disclosures: MJK and SLP are employed by Abbott Nutrition who funded this work; SJ, KMR, MN, EEJ, LR, EJJ, and JWE, no conflicts of interest.
Abbreviations used
- BF
breastfed
- ONPRC
Oregon National Primate Research Center
- SF
supplemented formula-fed
- TSAT
thigh subcutaneous adipose tissue
- UF
unsupplemented formula-fed
References
- 1. Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013;60:49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lietz G, Mulokozi G, Henry JC, Tomkins AM. Xanthophyll and hydrocarbon carotenoid patterns differ in plasma and breast milk of women supplemented with red palm oil during pregnancy and lactation. J Nutr 2006;136:1821–7. [DOI] [PubMed] [Google Scholar]
- 3. de Azeredo VB, Trugo NM. Retinol, carotenoids, and tocopherols in the milk of lactating adolescents and relationships with plasma concentrations. Nutrition 2008;24:133–9. [DOI] [PubMed] [Google Scholar]
- 4. Macias C, Schweigert FJ. Changes in the concentration of carotenoids, vitamin A, alpha-tocopherol and total lipids in human milk throughout early lactation. Ann Nutr Metab 2001;45:82–5. [DOI] [PubMed] [Google Scholar]
- 5. Jewell VC, Mayes CB, Tubman TR, Northrop-Clewes CA, Thurnham DI. A comparison of lutein and zeaxanthin concentrations in formula and human milk samples from Northern Ireland mothers. Eur J Clin Nutr 2004;58:90–7. [DOI] [PubMed] [Google Scholar]
- 6. Schweigert FJ, Bathe K, Chen F, Büscher U, Dudenhausen JW. Effect of the stage of lactation in humans on carotenoid levels in milk, blood plasma and plasma lipoprotein fractions. Eur J Clin Nutr 2004;43:39–44. [DOI] [PubMed] [Google Scholar]
- 7. Song BJ, Jouni ZE, Ferruzzi MG. Assessment of phytochemical content in human milk during different stages of lactation. Nutrition 2013;29:195–202. [DOI] [PubMed] [Google Scholar]
- 8. Johnson EJ. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev 2014;72:605–12. [DOI] [PubMed] [Google Scholar]
- 9. Perry A, Rasmussen H, Johnson EJ. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J Food Comp Anal 2009;22:9–15. [Google Scholar]
- 10. Kijlstra A, Tian Y, Kelly ER, Berendschot TT. Lutein: more than just a filter for blue light. Prog Retin Eye Res 2012;31:303–15. [DOI] [PubMed] [Google Scholar]
- 11. Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA 1994;272:1413–20. [PubMed] [Google Scholar]
- 12. Age-Related Eye Disease Study Research G , SanGiovanni JP, Chew EY, Clemons TE, Ferris FL, 3rd, Gensler G, Lindblad AS, Milton RC, Seddon JM, Sperduto RD. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No 22. Arch Ophthalmol 2007;125:1225–32. [DOI] [PubMed] [Google Scholar]
- 13. Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, Pei K, Tsipursky M, Nyland J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004;75:216–29. [DOI] [PubMed] [Google Scholar]
- 14. Renzi LM, Hammond BR. The effect of macular pigment on heterochromatic luminance contrast. Exp Eye Res 2010;91:896–900. [DOI] [PubMed] [Google Scholar]
- 15. Hammond BR, Jr., Wooten BR, Engles M, Wong JC. The influence of filtering by the macular carotenoids on contrast sensitivity measured under simulated blue haze conditions. Vision Res 2012;63:58–62. [DOI] [PubMed] [Google Scholar]
- 16. Vishwanathan R, Kuchan MJ, Sen S, Johnson EJ. Lutein and preterm infants with decreased concentrations of brain carotenoids. J Pediatr Gastroenterol Nutr 2014;59:659–65. [DOI] [PubMed] [Google Scholar]
- 17. Craft NE, Haitema TB, Garnett KM, Fitch KA, Dorey CK. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J Nutr Health Aging 2004;8:156–62. [PubMed] [Google Scholar]
- 18. Johnson EJ, Vishwanathan R, Johnson MA, Hausman DB, Davey A, Scott TM, Green RC, Miller LS, Gearing M, Woodard J, et al. Relationship between serum and brain carotenoids, alpha-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the georgia centenarian study. J Aging Res 2013;2013:951786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson EJ, McDonald K, Caldarella SM, Chung HY, Troen AM, Snodderly DM. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr Neurosci 2008;11:75–83. [DOI] [PubMed] [Google Scholar]
- 20. Bovier ER, Hammond BR. A randomized placebo-controlled study on the effects of lutein and zeaxanthin on visual processing speed in young healthy subjects. Arch Biochem Biophys 2015;572:54–7. [DOI] [PubMed] [Google Scholar]
- 21. Lindbergh CA, Mewborn CM, Hammond BR, Renzi-Hammond LM, Curran-Celentano JM, Miller LS. Relationship of lutein and zeaxanthin levels to neurocognitive functioning: an fMRI study of older adults. J Int Neuropsychol Soc 2017;23:11–22. [DOI] [PubMed] [Google Scholar]
- 22. Walk AM, Khan NA, Barnett SM, Raine LB, Kramer AF, Cohen NJ, Moulton CJ, Renzi-Hammond LM, Hammond BR, Hillman CH. From neuro-pigments to neural efficiency: The relationship between retinal carotenoids and behavioral and neuroelectric indices of cognitive control in childhood. Int J Psychophysiol 2017;118:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lieblein-Boff JC, Johnson EJ, Kennedy AD, Lai CS, Kuchan MJ. Exploratory metabolomic analyses reveal compounds correlated with lutein concentration in frontal cortex, hippocampus, and occipital cortex of human infant brain. PLoS One 2015;10:e0136904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheatham C, Sheppard K. Synergistic effects of human milk nutrients in the support of infant recognition memory: an observational study. Nutrients 2015;7:5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vishwanathan R, Neuringer M, Snodderly DM, Schalch W, Johnson EJ. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutr Neurosci 2013;16:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bettler J, Zimmer JP, Neuringer M, DeRusso PA. Serum lutein concentrations in healthy term infants fed human milk or infant formula with lutein. Eur J Nutr 2010;49:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jeon S, Neuringer M, Johnson E, Kuchan M, Pereira S, Johnson E, Erdman J. Effect of carotenoid supplemented formula on carotenoid bioaccumulation in tissues of infant rhesus macaques: A pilot study focused on lutein. Nutrients 2017;9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yeum KJ, Booth SL, Sadowski JA, Liu C, Tang G, Krinsky NI, Russell RM. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr 1996;64:594–602. [DOI] [PubMed] [Google Scholar]
- 29. Lipkie TE, Banavara D, Shah B, Morrow AL, McMahon RJ, Jouni ZE, Ferruzzi MG. Caco-2 accumulation of lutein is greater from human milk than from infant formula despite similar bioaccessibility. Mol Nutr Food Res 2014;58:2014–22. [DOI] [PubMed] [Google Scholar]
- 30. Reboul E, Borel P. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog Lipid Res 2011;50:388–402. [DOI] [PubMed] [Google Scholar]
- 31. van het Hof KH, West CE, Weststrate JA, Hautvast JG. Dietary factors that affect the bioavailability of carotenoids. J Nutr 2000;130:503–6. [DOI] [PubMed] [Google Scholar]
- 32. Thomas SE, Harrison EH. Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. J Lipid Res 2016;57:1865–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tanprasertsuk J, Li B, Bernstein PS, Vishwanathan R, Johnson MA, Poon L, Johnson EJ. Relationship between concentrations of lutein and StARD3 among pediatric and geriatric human brain tissue. PLoS One 2016;11:e0155488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mohn ES, Erdman JW, Jr., Neuringer M, Kuchan MJ, Johnson EJ. Brain xanthophyll content and exploratory gene expression analysis: subspecies differences in rhesus macaque. Genes Nutr 2017;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lindshield BL, King JL, Wyss A, Goralczyk R, Lu CH, Ford NA, Erdman JW, Jr.. Lycopene biodistribution is altered in 15,15′-carotenoid monooxygenase knockout mice. J Nutr 2008;138:2367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang W, Connor SL, Johnson EJ, Klein ML, Hughes S, Connor WE. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am J Clin Nutr 2007;85:762–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 is available from the “Supplementary Data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.


