Abstract
Background: To evaluate the association between diabetes mellitus (DM) and the development of incident hearing loss.
Methods: Prospective cohort study was performed in 253 301 adults with normal hearing tests who participated in a regular health-screening exam between 2002 and 2014. The main exposure was the presence of DM at baseline, defined as a fasting serum glucose ≥ 126 mg/dL, a self-reported history of DM or current use of anti-diabetic medications. Pre-diabetes was defined as a fasting glucose 100–125 mg/dL and no history of DM or anti-diabetic medication use. Incident hearing loss was defined as a pure-tone average of thresholds at 0.5, 1.0 and 2.0 kHz > 25 dB in both right and left ears.
Results: During 1 285 704 person-years of follow-up (median follow-up of four years), 2817 participants developed incident hearing loss. The rate of hearing loss in participants with normal glucose levels, pre-diabetes and DM were 1.8, 3.1 and 9.2 per 1000 person-years, respectively (P < 0.001). The multivariable-adjusted hazard ratios for incident hearing loss for participants with pre-diabetes and DM compared with those with normal glucose levels were 1.04 (95% confidence interval 0.95–1.14) and 1.36 (1.19–1.56), respectively. In spline regression analyses, the risk of incident hearing loss increased progressively with HbA1c levels above 5%.
Conclusions: In this large cohort study of young and middle-aged men and women, DM was associated with the development of bilateral hearing loss. DM patients have a moderately increased risk of future hearing loss.
Keywords: cohort study, diabetes mellitus, hearing loss, incidence, risk factors
Key Messages
The causal relationship between diabetes mellitus (DM) and hearing loss has still not been established in large longitudinal cohort studies.
In this large cohort study, participants with DM had a moderately increased risk of incident hearing loss.
Higher levels of HbA1c, representing higher long-term glucose levels and poor glycemic control, were progressively associated with hearing-loss risk.
The association of DM with hearing loss was stronger in younger (<50 years) than in older participants.
Introduction
Hearing loss is strongly associated with physical, emotional and cognitive disability, with a profound impact on social communication, quality of life, and medical and non-medical costs.1–5 The World Health Organization (WHO) estimates that over 360 million people, approximately 5% of the world’s population, has disabling hearing loss6 and the number of cases of hearing impairment is increasing because of population ageing.7 Established causes of hearing loss include genetic predisposition, vascular causes, infections, ototoxic drugs and longstanding exposure to excessive noise,8–13 but the determinants of most cases of ageing-related hearing loss are uncertain. Since sensorineural hearing loss cannot be restored, identification of preventable causes of hearing loss is a major clinical and public health goal.14
Diabetes mellitus (DM) is a common systemic metabolic disease with increasing worldwide prevalence.15 DM is associated with multiple macro- and microvascular complications, including thickening of the basal membrane of the stria vascularis capillaries on the lateral wall of the cochlea and other microvascular and neuropathic changes that could induce hearing loss.16–18 The association of hearing loss with DM, however, is still controversial. Several studies have shown no or little association between DM and hearing loss19–21 and a longitudinal study reported that DM was associated with prevalent, but not with incident, hearing loss.22
Two recent meta-analyses concluded that subjects with DM had higher risk of hearing loss compared with those without it,23,24 but this conclusion was based mostly on cross-sectional studies. The association between DM and hearing loss has still not been established in large longitudinal cohort studies. In addition, there are few prospective data on the role of pre-diabetes on hearing loss. The aim of this study was thus to conduct a prospective study of the association of DM and pre-diabetes with hearing loss in a large cohort of young and middle-aged men and women who participated in a regular health-screening programme.
Methods
Study population
The Kangbuk Samsung Health Study is a cohort study of men and women 18 years of age or older who underwent a comprehensive annual or biennial health examination at the clinics of the Kangbuk Samsung Hospital Total Healthcare Screening Center in Seoul and Suwon, South Korea, from 2002 to 2014.25,26 Over 80% of participants were employees of various companies and local governmental organizations and their spouses. In South Korea, the Industrial Safety and Health Law requires annual or biennial health-screening exams of all employees, offered free of charge. The remaining participants voluntarily purchased screening examinations at the health-screening centre.
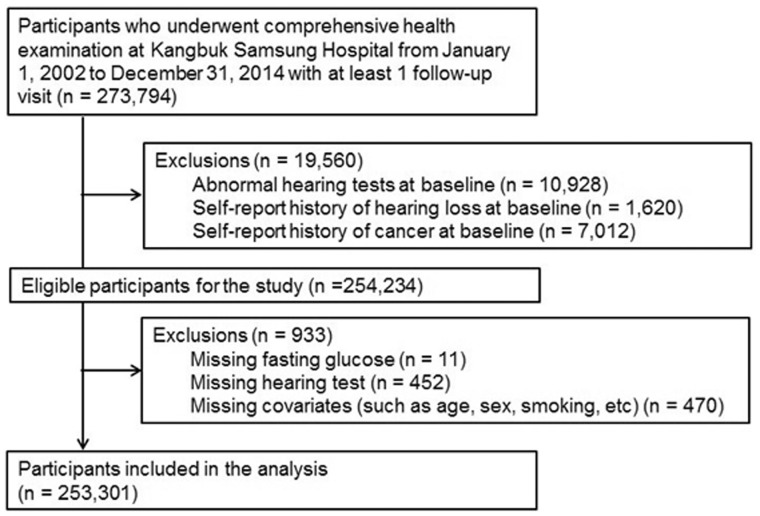
The present analysis included all study participants with at least one follow-up visit between 1 January 2002 and 31 December 2014 (n = 273 794; Figure 1). We excluded participants who had any of the following conditions at baseline: abnormal hearing tests, defined as a pure-tone air conduction average of thresholds from 0.5–2 kHz above 25 dB in both right and left ears (n = 10 928); self-reported history of hearing loss (n = 1620); or self-reported history of cancer (n = 7012). We further excluded participants with missing baseline data on fasting glucose (n = 11), hearing tests (n = 452) or any other relevant adjustment covariates (n = 470). The final sample was thus 253 301 participants (105 816 women and 147 485 men).
Figure 1.
Flow chart of study design.
The study was approved by the Institutional Review Board of the Kangbuk Samsung Hospital, which waived the requirement for informed consent, as we used only de-identified data obtained during regular health-screening exams.
Data collection
Baseline and follow-up examinations were conducted at the clinics of the Kangbuk Samsung Hospital Health Screening Center in Seoul and Suwon. At each visit, demographic characteristics, smoking status, alcohol consumption, medical history and medication use were collected through standardized self-administered questionnaires. Smoking status was categorized into never, former or current smoker, and alcohol consumption into none, moderate (≤30 g/day in men and ≤20 g/day in women) or high intake (>30 g/day in men and >20 g/day in women). Frequency of vigorous physical activity was categorized into 0, 1–3 or > 3 times/week.
Height, weight and sitting blood pressure were measured by trained nurses. Body mass index (BMI) was calculated as weight in kilograms divided by height in metres squared. Obesity was defined as BMI ≥ 25 kg/m2, a cutoff proposed for Asian populations.27 Hypertension was defined as a systolic blood pressure ≥ 140 mmHg, a diastolic blood pressure ≥ 90 mmHg, a self-reported history of hypertension or current use of anti-hypertensive medications.
Fasting blood samples were collected after at least 10 hours of fast. Serum fasting glucose levels were measured using the hexokinase method on a Advia 1,650 Autoanalyzer (Bayer Diagnostics, Leverkusen, Germany) until 2009 and Modular D systems (Roche Diagnostics; Tokyo, Japan) until 2014 in Suwon, and Advia 1650 Autoanalyzer (Bayer Diagnostics) until 2009, Cobas Integra 800 apparatus (Roche Diagnostics; Rotkreuz, Switzerland) until 2012 and Modular D systems (Roche Diagnostics) until 2013 in Seoul. DM was defined as a fasting serum glucose ≥ 126 mg/dL, a self-reported history of DM or current use of anti-diabetic medications. Pre-diabetes was defined as a fasting glucose of 100–125 mg/dL and no history of DM or anti-diabetic medication use.
Hemoglobin A1c (HbA1c) levels, available only since 1 March 2005, were determined using a turbidimetric inhibition immunoassay for hemolysed whole blood on a Cobas Integra 800 apparatus (Roche Diagnostics, Rotkreuz, Switzerland). The coefficients of variation for lower and higher level of quality-control materials were 0.95–2.68% and 0.88–2.61% during the study period, respectively. As a sensitivity analysis, we defined DM as a fasting serum glucose ≥ 126 mg/dL or HbA1c ≥ 6.5%, a self-reported history of DM or current use of anti-diabetic medications, and pre-diabetes as a fasting glucose of 100–125 mg/dL, HbA1c 5.8–6.4% and no history of DM or anti-diabetic medication use.
Laboratory methods for other analyses have been reported elsewhere.25,26 The Laboratory Medicine Department of the Kangbuk Samsung Hospital has been accredited by the Korean Society of Laboratory Medicine (KSLM) and the Korean Association of Quality Assurance for Clinical Laboratories (KAQACL) and participates in the CAP (College of American Pathologists) Survey Proficiency Testing.
Audiometric measurements
Pure-tone audiometric testing was performed at each screening visit by trained audiometric technicians using a GSI 67 audiometer (Bedford, MA, USA) equipped with TDH-39 supra-aural earphones (Telephonics Co., Farmingdale, NY, USA) in a dedicated sound-attenuating booth. Pure-tone air conduction thresholds were measured in dB hearing level (HL) for both ears at 0.5, 1.0 and 2.0 kHz and averaged for each ear. Hearing loss was defined as a pure-tone average of thresholds at 0.5, 1.0 and 2.0 kHz > 25 dB in both right and left ears.
The Korean Occupational Safety and Health Act28 requires every employee who is exposed in the workplace to a noise level of ≥ 85 dB for at least eight hours per day to have an annual hearing test at 3.0 and 4.0 kHz in addition to the regular frequencies (0.5, 1.0 and 2.0 kHz). In our study, we used having had a test at 3.0 and 4.0 kHz as a surrogate variable for occupational exposure to noise at a level ≥85 dB for at least eight hours per day.
For quality control and quality assurance, the audiometers were calibrated annually following the standards of the American National Standards Institute (ISO 389:1991) and biological calibration checks were performed daily prior to the first exam. Ambient noise level was monitored with a sound-level meter.
Statistical analysis
The study endpoint was the development of incident hearing loss over follow-up. Participants were followed from the baseline visit to the visit when hearing loss was diagnosed or to the last available visit. Follow-up was also censored when participants developed cancer. Since we started follow-up with all participants free of hearing loss at baseline, we could establish the first visit on which a participant showed hearing loss, but we could not determine the precise time of outcome development (which occurred at some point between the first visit with hearing loss and the previous visit). To take this type of interval censoring into account, we used a parametric proportional hazards model (stpm command in STATA)29 to estimate the hazard ratios (with 95% confidence intervals [CI]) for incident hearing loss comparing participants with DM and with pre-diabetes to those with normal glucose levels at baseline. In these models, the baseline log-cumulative hazards was estimated by using natural cubic splines and accounted for interval censored events. For time-varying analyses taking interval censoring into account, we used pooled logistic regression to relate time-varying DM and covariate status measured at each visit to the development of hearing loss. Pooled logistic models closely approximate time-dependent Cox proportional hazards models when the risk is low in each time interval.30
For all analyses, we used three models with increasing degrees of adjustment to account for potential confounding factors. The first model adjusted for age splines (restricted cubic spline with knots at the 5th, 35th, 65th and 95th percentiles of the age distribution to account for the potential non-linear association between age and hearing loss), sex, study centre and year of visit. The second model was further adjusted for exposure to occupational noise, BMI, smoking, alcohol and vigorous exercise. The third model was further adjusted for total and high-density lipoprotein (HDL) cholesterol, triglycerides and hypertension.
In addition, we evaluated the association between HbA1c modeled as a continuous variable using restricted cubic splines (with knots at the 5th, 35th, 65th and 95th percentiles of its sample distribution) to provide a flexible dose–response relationship between HbA1c, a marker of long-term glucose control, and hearing loss.
In sensitivity analyses, we repeated the analyses using age as the time scale and found similar results (Supplementary Table 1, available as Supplementary data at IJE online). In addition, we used alternative definitions for diabetes based on HbA1c, we used alternative definitions for hearing loss, and we examined the association between DM and hearing loss at pure-tone frequencies of 0.5, 1.0 or 2.0 kHz separately. Finally, we performed stratified analyses in pre-specified subgroups defined by age, sex, exposure to occupational noise, hypertension, BMI, smoking, alcohol use and vigorous physical activity. All analyses were performed using STATA version 12 (StataCorp LP, College Station, TX, USA).
Results
The average age of study participants at baseline was 37.6 (SD 7.7 years, inter-quartile range 31.3–41.3 years; range 18.0–87.1 years) and 58.2% of them were men (Table 1). Among all participants, the prevalence of pre-diabetes and of DM were 18.2% and 2.6%, respectively. Participants with pre-diabetes or DM were more likely to be older, male, smokers, hypertensive, to have higher BMI, total cholesterol and triglyceride levels, and to exercise more.
Table 1.
Baseline characteristics of study participants
| Characteristic | Total (n = 253 301) | Normal (n = 200 649) | Pre-diabetesa (n = 46 132) | Diabetesa (n = 6520) | P-value |
|---|---|---|---|---|---|
| Age (years) | 37.6 ± 7.8 | 36.9 ± 7.3 | 39.4 ± 8.5 | 46.1 ± 9.7 | <0.001 |
| Sex | <0.001 | ||||
| Female | 105 816 (41.8) | 92 059 (45.9) | 12 192 (26.4) | 1565 (24.0) | |
| Male | 147 485 (58.2) | 108 590 (54.1) | 33 940 (73.6) | 4955 (76.0) | |
| Study centre | <0.001 | ||||
| Seoul | 159 102 (62.8) | 127 785 (63.7) | 26 443 (57.3) | 4874 (74.8) | |
| Suwon | 94 199 (37.2) | 72 864 (36.3) | 19 689 (42.7) | 1646 (25.2) | |
| Body mass index (kg/m2) | 23.3 ± 3.2 | 22.9 ± 3.1 | 24.7 ± 3.2 | 25.5 ± 3.3 | <0.001 |
| Total cholesterol (mg/dL) | 193.7 ± 34.5 | 191.5 ± 33.7 | 201.8 ± 35.4 | 206.1 ± 40.9 | <0.001 |
| HDL cholesterol (mg/dL) | 56.2 ± 13.2 | 57.0 ± 13.3 | 53.5 ± 12.2 | 50.8 ± 11.7 | <0.001 |
| Triglycerides (mg/dL) | 120.9 ± 82.8 | 112.1 ± 73.2 | 149.0 ± 98.3 | 191.2 ± 141.5 | <0.001 |
| Smoking | <0.001 | ||||
| Never | 110 826 (43.8) | 93 505 (46.6) | 15 348 (33.3) | 1973 (30.3) | |
| Former | 65 625 (25.9) | 49 323 (24.6) | 14 250 (30.9) | 2052 (31.5) | |
| Current | 67 927 (26.8) | 50 874 (25.4) | 14 839 (32.2) | 2214 (34.0) | |
| Unknown | 8923 (3.5) | 6947 (3.5) | 1695 (3.7) | 281 (4.3) | |
| Alcohol | <0.001 | ||||
| None | 73 304 (28.9) | 61 524 (30.7) | 10 019 (21.7) | 1761 (27.0) | |
| Moderate | 141 892 (56.0) | 111 599 (55.6) | 27 031 (58.6) | 3262 (50.0) | |
| High | 23 364 (9.2) | 15 268 (7.6) | 6947 (15.1) | 1149 (17.6) | |
| Unknown | 14 741 (5.8) | 12 258 (6.1) | 2135 (4.6) | 348 (5.3) | |
| Vigorous exercise (times/week) | <0.001 | ||||
| 0 | 141 728 (56.0) | 115 176 (57.4) | 23 709 (51.4) | 2843 (43.6) | |
| 1–3 | 76 342 (30.1) | 58 735 (29.3) | 15 390 (33.4) | 2217 (34.0) | |
| >3 | 31 767 (12.5) | 24 168 (12.0) | 6290 (13.6) | 1309 (20.1) | |
| Unknown | 3464 (1.4) | 2570 (1.3) | 743 (1.6) | 151 (2.3) | |
| Hypertension | 32 165 (12.7) | 19 332 (9.6) | 10 258 (22.2) | 2575 (39.5) | <0.001 |
| Exposed to occupational noise | 26 977 (10.7) | 21 379 (10.7) | 5207 (11.3) | 391 (6.0) | <0.001 |
Values are means ± SD or numbers (%).
Diabetes status was defined by fasting glucose, history of diabetes and medication use for diabetes.
During 1 285 704 person-years of follow-up (median follow-up of 4 years, inter-quartile range 2.1–7.7 years; maximum follow-up 12.7 years), 2817 participants developed incident hearing loss. The rate of hearing loss in participants with normal glucose levels, pre-diabetes and DM were 1.8, 3.1 and 9.2 per 1000 person-years, respectively (Table 2 and Figure 2). The multivariable-adjusted hazard ratios for incident hearing loss for participants with pre-diabetes and DM at baseline compared with those with normal glucose levels were 1.04 (95% CI 0.95–1.14) and 1.36 (1.19–1.56), respectively (Table 2). When defining DM using both fasting glucose and HbA1c, the corresponding hazard ratios were 1.03 (0.95–1.13) and 1.36 (1.18–1.56), respectively. Similar associations were found when we used alternative definitions of hearing loss (Supplementary Tables 2 and 3, available as Supplementary data at IJE online).
Table 2.
Adjusted hazard ratios for incident hearing loss associated with baseline diabetes status
| Events/number of participants | Incidence rate (per 1000 person-years) | Model 1a | Model 2b | Model 3c | |
|---|---|---|---|---|---|
| Diabetes defined by glucose | |||||
| Normal | 1855/200 649 | 1.8 | Reference | Reference | Reference |
| Pre-diabetes | 699/46 132 | 3.1 | 1.10 (1.00, 1.20) | 1.04 (0.95, 1.14) | 1.04 (0.95, 1.14) |
| Diabetes | 263/6520 | 9.2 | 1.48 (1.29, 1.69) | 1.40 (1.22, 1.60) | 1.36 (1.19, 1.56) |
| Diabetes defined by glucose and HbA1c | |||||
| Normal | 1292/161 224 | 1.7 | Reference | Reference | Reference |
| Pre-diabetes | 888/63 548 | 3.3 | 1.10 (1.00, 1.20) | 1.04 (0.95, 1.14) | 1.03 (0.95, 1.13) |
| Diabetes | 287/7284 | 9.7 | 1.50 (1.31, 1.72) | 1.39 (1.21, 1.59) | 1.36 (1.18, 1.56) |
Model 1: Adjusted for age spline, sex, study centre and year of visit.
Model 2: Further adjusted for exposure to occupational noise, body mass index, smoking, alcohol and vigorous exercise.
Model 3: Further adjusted for total and HDL cholesterol, triglycerides and hypertension.
Figure 2.
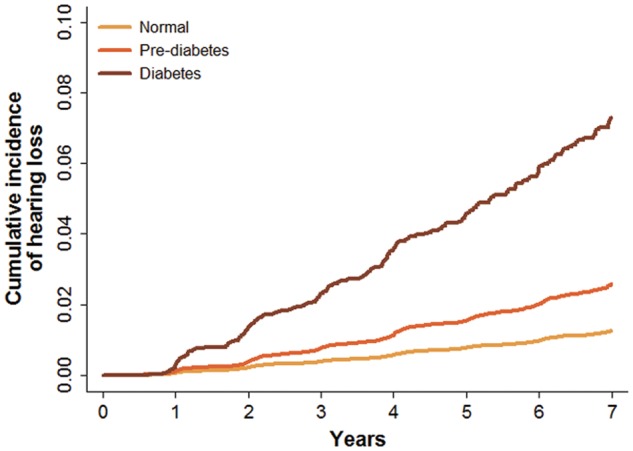
Cumulative incidence of incident hearing loss by baseline diabetes status.
When including DM status as a time-varying variable, the multivariable-adjusted hazard ratios for incident hearing loss for participants with pre-diabetes and with DM compared with those with normal glucose levels were 1.07 (0.98–1.17) and 1.34 (1.19–1.51), respectively (Table 3). The corresponding hazard ratios were 1.12 (1.02–1.23) and 1.43 (1.25–1.62), respectively, when defining DM using both fasting glucose and HbA1c.
Table 3.
Adjusted hazard ratios for incident hearing loss associated with time-varying diabetes status
| Model 1a | Model 2b | Model 3c | |
|---|---|---|---|
| Diabetes defined by glucose | |||
| Normal | Reference | Reference | Reference |
| Pre-diabetes | 1.15 (1.05, 1.26) | 1.08 (0.99, 1.18) | 1.07 (0.98, 1.17) |
| Diabetes | 1.47 (1.31, 1.65) | 1.37 (1.22, 1.54) | 1.34 (1.19, 1.51) |
| Diabetes defined by glucose and HbA1c | |||
| Normal | Reference | Reference | Reference |
| Pre-diabetes | 1.21 (1.10, 1.32) | 1.14 (1.04, 1.24) | 1.12 (1.02, 1.23) |
| Diabetes | 1.60 (1.41, 1.80) | 1.47 (1.30, 1.66) | 1.43 (1.26, 1.62) |
Model 1: Adjusted for age spline, sex, study centre and year of visit.
Model 2: Further adjusted for exposure to occupational noise, body mass index, smoking, alcohol and vigorous exercise.
Model 3: Further adjusted for total and HDL cholesterol, triglycerides and hypertension.
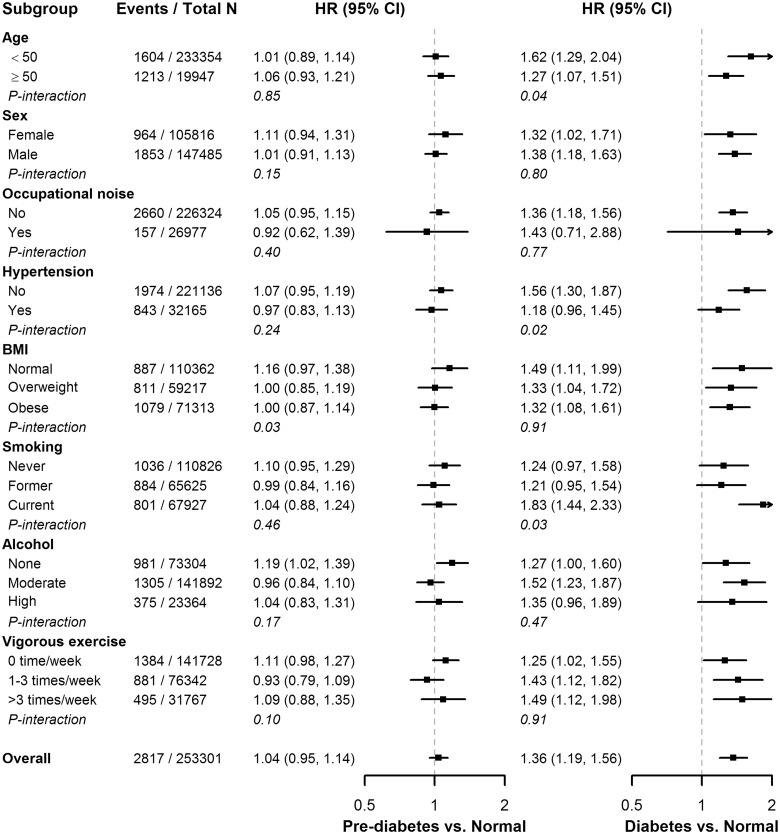
In spline regression analyses, the risk of incident hearing loss increased progressively with HbA1c levels above 5% (Figure 3). The P-value for the non-linear spline components was 0.34, indicating that the association between HbA1c levels and incident DM was approximately linear. In pre-specified subgroup analyses, the associations of pre-diabetes and DM with incident hearing loss were consistent across subgroups, except that the association with pre-diabetes was stronger among participants with normal BMI, and the association with DM was stronger among participants who were younger, normotensive and current smokers (Figure 4).
Figure 3.
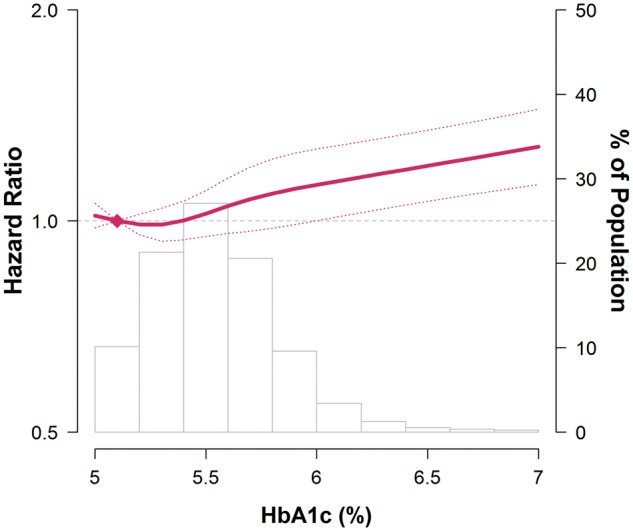
Hazard ratios for incident hearing loss by levels of baseline HbA1c.
Curves represent adjusted hazard ratio (solid line) and its 95% confidence interval (dashed lines) based on restricted cubic spline for HbA1c with knots at the 5th, 35th, 65th and 95th percentiles of its sample distribution. The reference value (diamond dot) was set at the 10th percentile of the distribution (corresponding to HbA1c = 5.1%). Model was adjusted for age spline, sex, study centre, year of visit, exposure to occupational noise, body mass index, smoking, alcohol, vigorous exercise, total and HDL cholesterol, triglycerides and hypertension. Bars represent the frequency distribution of HbA1c.
Figure 4.
Associations between baseline diabetes status and hearing loss in pre-specified subgroups.
Diabetes status was defined based on fasting glucose levels, history of diabetes and use of anti-diabetic medications. Models were adjusted for age spline, sex, study centre, year of visit, exposure to occupational noise, body mass index, smoking, alcohol, vigorous exercise, total and HDL cholesterol, triglycerides and hypertension.
Discussion
In this large cohort study, participants with DM were at an increased risk of incident hearing loss. The association of DM with hearing loss was evident even after adjusting for multiple potential confounders, including demographic characteristics, occupational noise exposure, lifestyle risk factors and other metabolic abnormalities. Furthermore, the risk of hearing loss increased progressively with increasing HbA1c levels above 5%, suggesting that glucose control in DM may play a role in the development of hearing loss. Our study indicates that physicians taking care of DM patients may have to monitor the hearing ability of their patients to identify early signs of hearing loss.
Several biologic mechanisms may explain the association between DM and hearing loss. The microvascular and neuropathic complications that affect DM patients in multiple organ systems may also affect the inner ear.31–34 Indeed, several histological changes have been observed in the inner ear in experimental DM models or in DM patients, including microangiopathic changes in the capillaries of the stria vascularis, thickening of the basilar membrane, narrowing of the internal auditory artery, loss of spiral ganglion neurons and organ of Corti cells and demyelination of the auditory nerve.16–18,35–40 Additional mechanistic research is needed to understand the relation of these changes to improved glycemic control in patients with DM.
Most studies on the association of DM with hearing loss were cross-sectional. The prevalence of hearing loss reported in patients with DM in these studies ranged from 44.0% to 60.2%41–43—a value much higher than in our study, as these prevalence estimates were derived from much older populations. Most cross-sectional studies have found an association between DM and prevalent hearing loss.23,24
Longitudinal data on DM and incident hearing loss are limited to a few studies.22,44,45 In the Blue Mountain Hearing Study (n = 1858), the five-year incidence of hearing loss was 18.7% and 18.0 % in participants with and without DM, respectively,22 and it was concluded that DM was not associated with incident hearing loss in an older population. In the Beaver Dam cohort (n = 1678), the age- and sex-adjusted hazard ratio for incident hearing loss associated with DM was 1.26 (CI 0.93–1.71)44 and a high glycosylated hemoglobin level (>12%) was associated with an increased 15-year cumulative incidence of hearing impairment. Previous longitudinal studies thus failed to find an association between DM and incident hearing loss, although they opened the possibility that poor glycemic control among diabetics could be a risk factor for hearing loss. Previous cohort studies, however, were relatively small and may have been underpowered to detect the effect of DM on hearing loss.22,44
In addition to the large sample size and the prospective design, several strengths of our study add relevance to the findings. First, we used bilateral hearing loss in the clinical audiogram range (0.5, 1.0 and 2.0 kHz) as the main study endpoint, as this endpoint is critical for speech understanding and has clear implications for quality of life and clinical management. Second, we studied an apparently healthy young and middle-aged population, with a low background rate of hearing loss. These results are thus less likely to be affected by survivor bias and biases induced by comorbidities and medication use as studies in older populations. Furthermore, hearing loss in younger populations is a major clinical and public health problem considering their life expectancy. Interestingly, in our study, the association of DM with hearing loss was stronger in younger (<50 years) than in older participants (Figure 4). A similar pattern was observed with cross-sectional data in the US National Health and Nutrition Examination Surveys.46 Since previous longitudinal studies included only participants >40 years old, the difference in age composition across studies could also explain why previous cohorts failed to identify an association between MD and incident hearing loss. Finally, our study had information on multiple lifestyle and metabolic markers, and allowed us the opportunity to adjust for several potential confounders.
Some limitations of our study also need to be considered. First, we could not evaluate high-frequency hearing impairment due to lack of data from pure-tone thresholds above 2.0 kHz. DM-related hearing loss has been described as a progressive, bilateral, sensorineural impairment with gradual onset predominantly affecting higher frequencies,47 but there are still controversies about what are the affected frequencies. Indeed, some studies showed the largest disparities in the low frequency range (<4 kHz),48,49 whereas others concluded that high frequencies were more likely to be impaired.46,50,51 Frequency-specific analysis of our data showed similar hazard ratios for the three frequencies evaluated (0.5, 1.0 and 2.0 kHz; Supplementary Tables 2 and 3, available as Supplementary data at IJE online). Second, we did not have data on leisure-time noise exposure, on the presence of otologic disease or on the use of ototoxic drugs. However, we excluded participants with hearing impairment at baseline, as well as those with a history of cancer, as oncological medications are a major group of ototoxic drugs. As a consequence, we expect that the influence of prior ear disease or ototoxic medications will be relatively small. In addition, both excessive noise and ototoxic drugs are mainly associated with high frequency (>3.0 kHz) hearing impairment.52,53 Finally, our study was performed among young and middle-aged Korean men and women participating in regular annual exams, and the findings may not be applicable to other age or race-ethnicity groups.
In conclusion, in this large cohort study of young and middle-aged Korean men and women, we found that DM was associated with the development of bilateral hearing loss. In addition, higher levels of HbA1c, representing higher long-term glucose levels and poor glycemic control, were also progressively associated with hearing-loss risk. Our data provide strong prospective evidence that hearing loss may be a consequence of DM and suggest that DM patients have a moderately increased risk of future hearing loss. Future studies should evaluate the effect of glucose-control measures in the development of hearing loss in DM patients.
Supplementary Data
Supplementary data are available at IJE online.
Conflict of interest: None declared.
Supplementary Material
References
- 1. Dalton DS, Cruickshanks KJ, Klein BE. et al. The impact of hearing loss on quality of life in older adults. Gerontologist 2003;43:661–8. [DOI] [PubMed] [Google Scholar]
- 2. Li CM, Zhang X, Hoffman HJ. et al. Hearing impairment associated with depression in US adults, National Health and Nutrition Examination Survey 2005–2010. JAMA Otolaryngol Head Neck Surg 2014;140:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin FR, Metter EJ, O’Brien RJ. et al. Hearing loss and incident dementia. Arch Neurol 2011;68:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uhlmann RF, Larson EB, Rees TS. et al. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA 1989;261:1916–19. [PubMed] [Google Scholar]
- 5. Emmett SD, Francis HW.. The socioeconomic impact of hearing loss in U.S. adults. Otol Neurotol 2015;36:545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Deafness and Hearing Loss 2015. http://www.who.int/mediacentre/factsheets/fs300/en/ (4 November 2015, date last accessed).
- 7. Nelson DI, Nelson RY, Concha-Barrientos M. et al. The global burden of occupational noise-induced hearing loss. Am J Ind Med 2005;48:446–58. [DOI] [PubMed] [Google Scholar]
- 8. Konigsmark BW. Genetic hearing loss with no associated abnormalities: a review. J Speech Hear Disord 1972;37:89–99. [DOI] [PubMed] [Google Scholar]
- 9. Kidd AR III, Bao J.. Recent advances in the study of age-related hearing loss: a mini-review. Gerontology 2012;58: 490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cole RR, Jahrsdoerfer RA.. Sudden hearing loss: an update. Am J Otol 1988;9:211–15. [PubMed] [Google Scholar]
- 11. Verhoeff M, van der Veen EL, Rovers MM. et al. Chronic suppurative otitis media: a review. Int J Pediatr Otorhinolaryngol 2006;70:1–12. [DOI] [PubMed] [Google Scholar]
- 12. Palomar Garcia V, Abdulghani Martinez F, Bodet Agusti E. et al. Drug-induced otoxicity: current status. Acta Otolaryngol 2001;121:569–72. [DOI] [PubMed] [Google Scholar]
- 13. Sliwinska-Kowalska M, Davis A.. Noise-induced hearing loss. Noise Health 2012;14:274–80. [DOI] [PubMed] [Google Scholar]
- 14. Cox RM, Johnson JA, Xu J.. Impact of advanced hearing aid technology on speech understanding for older listeners with mild to moderate, adult-onset, sensorineural hearing loss. Gerontology 2014;60:557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization. Diabetes 2015. http://www.who.int/mediacentre/factsheets/fs312/en/ (4 November 2015, date last accessed).
- 16. Nakae S, Tachibana M.. The cochlea of the spontaneously diabetic mouse. II. Electron microscopic observations of non-obese diabetic mice. Arch Otorhinolaryngol 1986;243:313–16. [DOI] [PubMed] [Google Scholar]
- 17. Raynor E, Robison WG, Garrett CG. et al. Consumption of a high-galactose diet induces diabetic-like changes in the inner ear. Otolaryngol Head Neck Surg 1995;113:748–54. [DOI] [PubMed] [Google Scholar]
- 18. Fukushima H, Cureoglu S, Schachern PA. et al. Cochlear changes in patients with type 1 diabetes mellitus. Otolaryngol Head Neck Surg 2005;133:100–6. [DOI] [PubMed] [Google Scholar]
- 19. Harner SG. Hearing in adult-onset diabetes mellitus. Otolaryngol Head Neck Surg 1981;89:322–7. [DOI] [PubMed] [Google Scholar]
- 20. Hodgson MJ, Talbott E, Helmkamp JC. et al. Diabetes, noise exposure, and hearing loss. J Occup Med 1987;29:576–9. [PubMed] [Google Scholar]
- 21. Engdahl B, Aarhus L, Lie A. et al. Cardiovascular risk factors and hearing loss: The HUNT study. Int J Audiol 2015;54: 958–66. [DOI] [PubMed] [Google Scholar]
- 22. Mitchell P, Gopinath B, McMahon CM. et al. Relationship of Type 2 diabetes to the prevalence, incidence and progression of age-related hearing loss. Diabet Med 2009;26:483–8. [DOI] [PubMed] [Google Scholar]
- 23. Akinpelu OV, Mujica-Mota M, Daniel SJ.. Is type 2 diabetes mellitus associated with alterations in hearing? A systematic review and meta-analysis. Laryngoscope 2014;124:767–76. [DOI] [PubMed] [Google Scholar]
- 24. Horikawa C, Kodama S, Tanaka S. et al. Diabetes and risk of hearing impairment in adults: a meta-analysis. J Clin Endocrinol Metab 2013;98:51–8. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Y, Chang Y, Ryu S. et al. Thyroid hormone levels and incident chronic kidney disease in euthyroid individuals: the Kangbuk Samsung Health Study. Int J Epidemiol 2014;43: 1624–32. [DOI] [PubMed] [Google Scholar]
- 26. Chang Y, Kim BK, Yun KE. et al. Metabolically-healthy obesity and coronary artery calcification. J Am Coll Cardiol 2014;63:2679–86. [DOI] [PubMed] [Google Scholar]
- 27. Wen CP, David Cheng TY, Tsai SP. et al. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr 2009;12: 497–506. [DOI] [PubMed] [Google Scholar]
- 28.National Law Information Center. Korean Occupational Safety and Health Act. http://www.law.go.kr/lsInfoP.do?urlMode =lsInfoP&lsId=001766 (20 May 2016, date last accessed).
- 29. Royston P, Parmar MK.. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med 2002;21:2175–97. [DOI] [PubMed] [Google Scholar]
- 30. D’Agostino RB, Lee ML, Belanger AJ. et al. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med 1990;9:1501–15. [DOI] [PubMed] [Google Scholar]
- 31. Friedman SA, Schulman RH, Weiss S.. Hearing and diabetic neuropathy. Arch Intern Med 1975;135:573–6. [PubMed] [Google Scholar]
- 32. Ciulla TA, Amador AG, Zinman B.. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care 2003;26:2653–64. [DOI] [PubMed] [Google Scholar]
- 33. Kato M, Natarajan R.. Diabetic nephropathy—emerging epigenetic mechanisms. Nat Rev Nephrol 2014;10:517–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vinik AI, Maser RE, Mitchell BD. et al. Diabetic autonomic neuropathy. Diabetes Care 2003;26:1553–79. [DOI] [PubMed] [Google Scholar]
- 35. Akinpelu OV, Ibrahim F, Waissbluth S. et al. Histopathologic changes in the cochlea associated with diabetes mellitus—a review. Otol Neurotol 2014;35:764–74. [DOI] [PubMed] [Google Scholar]
- 36. Lee HS, Kim KR, Chung WH. et al. Early sensorineural hearing loss in ob/ob mouse, an animal model of type 2 diabetes. Clin Exp Otorhinolaryngol 2008;1:211–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fukushima H, Cureoglu S, Schachern PA. et al. Effects of type 2 diabetes mellitus on cochlear structure in humans. Arch Otolaryngol Head Neck Surg 2006;132:934–8. [DOI] [PubMed] [Google Scholar]
- 38. Jorgensen MB. The inner ear in diabetes mellitus: histological studies. Arch Otolaryngol 1961;74:373–81. [DOI] [PubMed] [Google Scholar]
- 39. Makishima K, Tanaka K.. Pathological changes of the inner ear and central auditory pathway in diabetics. Ann Otol Rhinol Laryngol 1971;80:218–28. [DOI] [PubMed] [Google Scholar]
- 40. Makishima K. Arteriolar sclerosis as a cause of presbycusis. Otolaryngology 1978;86:322–6. [DOI] [PubMed] [Google Scholar]
- 41. Dalton DS, Cruickshanks KJ, Klein R. et al. Association of NIDDM and hearing loss. Diabetes Care 1998;21:1540–4. [DOI] [PubMed] [Google Scholar]
- 42. Aladag I, Eyibilen A, Guven M. et al. Role of oxidative stress in hearing impairment in patients with type two diabetes mellitus. J Laryngol Otol 2009;123:957–63. [DOI] [PubMed] [Google Scholar]
- 43. Sakuta H, Suzuki T, Yasuda H. et al. Type 2 diabetes and hearing loss in personnel of the Self-Defense Forces. Diabetes Res Clin Pract 2007;75:229–34. [DOI] [PubMed] [Google Scholar]
- 44. Cruickshanks KJ, Nondahl DM, Dalton DS. et al. Smoking, central adiposity, and poor glycemic control increase risk of hearing impairment. J Am Geriatr Soc 2015;63:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kiely KM, Gopinath B, Mitchell P. et al. Cognitive, health, and sociodemographic predictors of longitudinal decline in hearing acuity among older adults. J Gerontol A Biol Sci Med Sci 2012;67:997–1003. [DOI] [PubMed] [Google Scholar]
- 46. Bainbridge KE, Hoffman HJ, Cowie CC.. Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Intern Med 2008;149:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Axelsson A, Sigroth K, Vertes D.. Hearing in diabetics. Acta Otolaryngol Suppl 1978;356:1–23. [PubMed] [Google Scholar]
- 48. Ma F, Gomez-Marin O, Lee DJ. et al. Diabetes and hearing impairment in Mexican American adults: a population-based study. J Laryngol Otol 1998;112:835–9. [DOI] [PubMed] [Google Scholar]
- 49. Frisina ST, Mapes F, Kim S. et al. Characterization of hearing loss in aged type II diabetics. Hear Res 2006;211:103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vaughan N, James K, McDermott D. et al. A 5-year prospective study of diabetes and hearing loss in a veteran population. Otol Neurotol 2006;27:37–43. [DOI] [PubMed] [Google Scholar]
- 51. Agrawal Y, Platz EA, Niparko JK.. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol 2009;30: 139–45. [DOI] [PubMed] [Google Scholar]
- 52. Henderson D, Subramaniam M, Boettcher FA.. Individual susceptibility to noise-induced hearing loss: an old topic revisited. Ear Hear 1993;14:152–68. [DOI] [PubMed] [Google Scholar]
- 53. Tabuchi K, Nishimura B, Nakamagoe M. et al. Ototoxicity: mechanisms of cochlear impairment and its prevention. Curr Med Chem 2011;18:4866–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.