Abstract
Background
There is considerable debate about the benefits and risks of electronic cigarettes (ECs). To better understand the risk–benefit ratio of ECs, more information is needed about net nicotine consumption and toxicant exposure of cigarette smokers switching to ECs.
Methods
Forty cigarette smokers (≥1 year of smoking) interested in switching to ECs but not necessarily quitting smoking were enrolled in a 4-week observational study and provided an e-Go C non-variable battery and refillable atomizers and choice of eight flavors in 12 or 24 mg nicotine dosage. Measurement of urinary cotinine (metabolite of nicotine), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL; a pulmonary carcinogen), and eight volatile organic compounds (VOCs) that are toxic tobacco smoke constituents was conducted at baseline and week 4.
Results
All participants with follow-up data (92.5%) reported using the study EC. Of the 40 smokers, 16 reported no cigarettes at week 2 (40%) and six continued to report no cigarettes at week 4 (15%). Change in nicotine intake over the 4 weeks was non-significant (p = .90). Carbon monoxide (p < .001), NNAL (p < .01) and metabolites of benzene (p < .01) and acrylonitrile (p = .001) were significantly decreased in the study sample. Smokers switching exclusively to ECs for at least half of the study period demonstrated significant reductions in metabolites of ethylene oxide (p = .03) and acrylamide (p < .01).
Conclusion
Smokers using ECs over 4 weeks maintained cotinine levels and experienced significant reductions in carbon monoxide, NNAL, and two out of eight measured VOC metabolites. Those who switched exclusively to ECs for at least half of the study period significantly reduced two additional VOCs.
Implications
This study extends current literature by measuring change in smoking dependence and disease-associated biomarkers, NNAL and a panel of eight common VOCs that are toxic tobacco smoke constituents in smokers who switch to ECs. The findings support the idea of harm reduction, however some levels of toxicant exposure are still of clinical concern, particularly for dual users. Extrapolation of these results must be careful to separate the different toxic exposure results for exclusive switchers versus dual cigarette + EC users, and not to equate harm reduction with the idea that using ECs is harmless.
Introduction
There is considerable debate about the benefits and risks of electronic cigarettes (ECs). Some public health officials have recognized ECs as a potential harm reduction tool,1 with the caveat that long-term health consequences are unknown.2 On the other hand, the promise of ECs for harm reduction is challenged by concerns about the population effect of ECs through re-normalization of smoking, uptake of tobacco addiction through ECs, and overall increases in nicotine product exposure/use.3,4 Another concern is that the perception of ECs as less harmful than cigarettes is conflated with the perception that ECs are safe and carry minimal to no risk, and the latter is linked with greater EC use.5
To better understand the risk–benefit balance of ECs, more information is needed about the net tobacco consumption and tobacco smoke toxicant exposure of smokers switching to ECs. To date, reviews of the safety of ECs are inconclusive due to numerous methodological problems including variation in EC devices across studies which produce different levels of nicotine delivery, as well as most studies focusing on lab-based, rather than real-life exposure.2 In addition, it has been noted that the harm reduction benefits of dual use are contingent upon the pattern of dual use, and that identifying these patterns and associated exposure levels and harm markers are needed.6 It is therefore important to characterize the toxicant exposure of smokers who switch to ECs and determine how exposure varies overall and based on sub-groups (ie, exclusive EC use vs. dual use of cigarettes and ECs).
The present observational pilot study aims to fill a gap in the literature by evaluating changes in tobacco consumption and toxicant exposure of cigarette smokers switching to a standardized second generation EC model (e-Go C non-variable battery [3.7 volts/650 MaH] and Saturn v4i refillable atomizers [2.4 ohms]) over a 4-week time period. This will extend existing studies7–12 by measuring disease-associated biomarkers, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and a panel of common volatile organic compounds (VOCs) that are toxic environmental or tobacco smoke constituents. NNAL is a metabolite of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a tobacco-specific nitrosamine that is a potent carcinogen.13 VOCs have been identified in constituent hazard analyses as responsible for the majority of the risk of cigarette smoke.14
Method
Participants
Forty cigarette smokers who were willing to switch to EC but not necessarily interested in quitting were enrolled. The majority of the sample was male (73%), the average age was 30.08 (SD = 8.82), and half the sample was Caucasian (50%), followed by Hispanic (25%). Supplementary Table 1 provides additional sample characteristics.
Procedures
Participants were recruited from the San Diego, CA area from January to April 2015 using flyers, internet postings, and newspaper advertisements seeking smokers with a statement that “free electronic cigarettes and $100 would be provided for qualified smokers’ participation in a research study.” The study was approved by the Institutional Review Board at California State University San Marcos, and written consent was used. Please see Supplementary Table 2 for a list of eligibility and exclusion criteria, and Supplementary Figure 1 for study flow.
There were three lab visits (baseline, week 2, and week 4) and two phone visits (week 1 and week 3). Timeline follow-back (TLFB) interviewing was conducted at all visits to assess tobacco consumption, and carbon monoxide and urine samples were obtained at all in-person lab visits. The first session included brief education, training, action planning for making a complete switch to EC, and selection of e-juice flavor and dose. The second session included refilling atomizers with e-liquid. The second and final sessions included physical measurement of e-juice liquid used, and a referral to the California Smokers’ Helpline was made at the final visit. Participants were compensated a $30 Visa gift card for completing the week 2 and four lab visits respectively, and a $20 Visa gift card for bringing in at least 90% of their atomizers at these visits.
Study Material
Study supplies included a second generation EC starter kit with two e-Go C batteries (3.7 volts/650 MaH), a USB connection cord, an AC adapter, and a carrying case, and a supply of Saturn V4i atomizers (2.4 ohms) filled with liquid in their preferred flavor (28 atomizers total; 2/day).
In order to approximate natural use conditions, participants were given a choice of seven flavor categories which included tobacco, mint, fruit, candy, sweets, chocolate, and drink/soda.15 Within each flavor category, the most popular flavor in each category was obtained from an established area store (ie, mint flavor was Candy Cane; Vapure). Consistent with dosages reported by EC users,16–19 participants were provided 24 mg/mL dosage vegetable glycerin liquid in a tester sample to all participants. Those who reported the 24 mg was too strong were provided 12 mg/mL dosage liquid. See Supplementary Table 3 for flavor names and percent of participants choosing each flavor at baseline and week 2.
Measures
Biological
Breath samples were taken with a Micro + (Bedfont, Haddonfield, NJ) to measure carbon monoxide. Urine samples were frozen and shipped under refrigerated conditions to the Clinical Pharmacology Laboratory at the University of California San Francisco, and tested for (1) concentrations of NNAL measured by liquid chromatography–tandem mass spectrometry (LC–MS/MS),13 (2) metabolites of a panel of potentially toxic VOCs, including benzene (PMA), ethylene oxide (HEMA), N-nitrosodimethylamine (MMA), acrylonitrile (CNEMA), acrolein (3-HPMA), propylene oxide (2-HPMA), acrylamide (AAMA), and crotonaldehyde (HPMMA) measured by LC–MS/MS,20 and (3) cotinine, the main proximate metabolite of nicotine, measured by gas chromatography with nitrogen phosphorus detection, modified for use of a capillary column.21 Cotinine, NNAL, and VOCs were normalized for creatinine.22 Biological samples were taken at all three in-person visits (baseline, week 2, and week 4). However, due to budgetary restrictions, only the baseline and week 4 data were analyzed.
Tobacco Consumption
Consumption of cigarettes in the past 30 days was assessed by a TLFB interview at baseline and week 4. At the weeks 1, 2, and 3 visits, a 1-week recall period was used, and the use of study ECs was also included. The TLFB interview method is an assessment instrument that involves the use of anchoring events which prompt respondents to provide retrospective estimates of a behavior of interest.23,24 Criterion validity of the TLFB measure was demonstrated through a significant correlation between baseline measures of past 30-day cigarette consumption and the Wisconsin Inventory of Smoking Dependence Motives (WISDM) Primary Dependence Motives (r = 0.75), and between past 30-day EC use at week 4 and the amount of measured e-liquid used during the 4-week study (r = 0.54, p < .01).
Demographic and Smoking Characteristics
Demographic variables included race/ethnicity, sex, age, education level (dichotomized < high school graduate vs. ≥ high school graduate), monthly income (dichotomized < $1800 vs. ≥ $1800), marital status (dichotomized never married vs. all others), and sexual orientation (dichotomized lesbian, gay, or bisexual vs. heterosexual). Individuals who identified as Hispanic were classified as such regardless of race. Participants were asked whether they usually smoked menthol or non-menthol cigarettes, and how long they have smoked cigarettes. Self-efficacy to resist smoking was also assessed with a modified version of the Smoking Self-Efficacy Scale,25,26 a uni-dimensional measure (α = 0.96) consisting of 17 items reflecting certainty to resist smoking in various situations.
Nicotine dependence was assessed by the brief WISDM27; which is a 37-item measure consisting of 11 subscales.27 The subscales can be used to calculate an overall smoking dependence score, Primary Dependence Motives, and Secondary Dependence Motives scales.
In addition, two single-items were used. Time to first cigarette (dichotomized as: smoking ≤ 30 minutes after waking, and smoking > 30 minutes), as smoking within 30 minutes of waking denotes a higher level of nicotine dependence.28,29 Derived from the Cigarette Dependence Scale, participants were asked to report their level of perceived addiction to cigarettes on a scale of 0 “I am not addicted to cigarettes at all” to 100 “I am extremely addicted to cigarettes.30
An Electronic Cigarette Dependence Scale31 composed of twelve questions scored on a one (totally disagree) to five (full agree) point scale (α = 0.86) was administered at the final visit only given that using ECs was not a baseline behavior. Carrying cigarettes was assessed with the item, “When you leave your house, how often do you take cigarettes with you?” Options included: never, some of the time, most of the time, and always. A comparable question was asked about ECs at week 4 only, given that using ECs regularly was not a baseline behavior.
Statistical Analyses
Participant characteristics were summarized using descriptive statistics. TLFB measurements were used to calculate tobacco use variables. Separate analyses were conducted for the following cigarette variables: total cigarettes smoked, days using cigarettes each week, and average number of cigarettes smoked per day (CPD). To calculate CPD, the total number of cigarettes smoked over the assessed period was divided by the total number of days during this period. Lastly, the following variables were calculated for study EC use: total number of times used study EC per day (ie, “sessions” not puffs), days using EC each week, and average EC per day. Average EC per day was calculated by dividing the total number of times that the study EC was used over the period by the number of days the study EC was used during this period. Biomarker values below level of detection (LOD) were entered as LOD/sqrt(2) for analyses.32 The frequency of biomarker concentrations below LOD were: baseline NNAL (2/40, 5%), baseline PMA (6/40, 15%), week 4 PMA (9/36, 25%), baseline HEMA (6/40, 15%), week 4 HEMA (4/36, 11%), baseline MMA (8/40, 20%), week 4 MMA (5/36, 14%).
Change in counts of CPD and EC per day were assessed using generalized linear mixed effects (LME) negative binomial model.33 Paired samples t tests were used to evaluate change in biomarkers from week 0 to week 4 with log transformations to rescale non-normal distributions prior to testing. Regression models of log-transformed values at week 4 included planned covariates (age, gender, race/ethnicity) and adjustment for corresponding baseline values of examined biomarkers when exploring differences between groups with different levels of switching to e-cigarette use. Regression models included initial F tests of the set of dummy-coded indicators for between-group comparisons. In order to address Type 1 error, the overall model tests were adjusted for false discovery rate with Benjamini Hochberg.34 Post-hoc multiple between-group comparisons were performed using Tukey adjustment for multiple comparisons.35 Analyses were performed using IBM SPSS Statistics Version 22.0 and R statistical software.36
Results
Switching to ECs
Thirty-seven of 40 participants provided follow-up and all reported use of ECs during the 30-day study period. Participants reported using the study EC an average of 24.72 (7.13) days in the past 30 days. On the days they used the study EC, they reported using it an average of 11.85 (SD = 14.00) times per day. Participants were asked how many times they used the EC each day and the majority of participants reported this as a discrete time in which they took out their EC and used it for any number of puffs. This was considered a “session.” The majority of participants spontaneously reported their use in sessions as opposed to reporting individual puff counts. The amount of measured e-liquid used during the 4-week study was 15.88 mL (SD = 11.29). The nicotine content of each one mL of 24 mg/mL e-liquid corresponds to one pack of cigarettes (ie, 20 cigarettes), which (assuming all nicotine was absorbed) would equate to participants consuming the equivalent of 10.59 cigarettes per day during a 30-day period. Each atomizer held 1.6 mL. Estimating an average of 15.88 mL used during 24.72 days yields an average of 0.64 mL of e-liquid consumed per day. Dividing 0.64 mL per day by average number of sessions (11.85) yields an average amount of e-liquid consumed in one session of 0.05 mL. However, this information must be used cautiously given measurement error, including those who reported number of times used EC per day in puffs were entered as such and this likely inflated the average times used EC per day statistic, atomizer leakage, and atomizer filling slightly above or below 1.6 mL capacity due to liquid settling. Most participants chose the 24 mg nicotine dosage at baseline (97.5%) and at week 2 (91.9%) (see Supplementary Table 3).
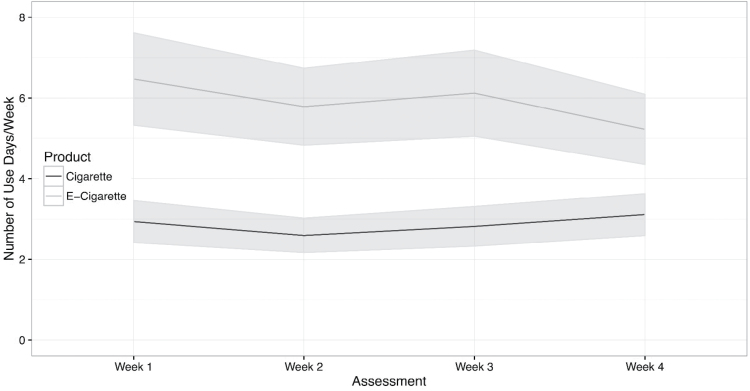
We evaluated change in frequency of EC use (arcsine transformed due to proportions given relatively beneficial effect relative to log) during the study period with LME models with planned covariates (age, gender, racial/ethnic group, and baseline cigarettes per day). Participants reported significant differences in the frequency of using EC at the 1-, 2-, 3-, 4-week assessments, F(3, 97) = 4.92, p = .003. As shown in Figure 1, when compared to the average frequency of EC use in week 1 (92.4%), use decreased in week 2 (82.6%; b = −0.17, SE = 0.08, p = .039), was similar in week 3 (87.4%; b = −0.10, SE = 0.08, p = .205), and decreased in week 4 (74.6%; b = −0.30, SE = 0.08, p = .0003). We observed similar significant decreases in the “number of times per day” (square-root transformed) participants used ECs, F(3, 97) = 5.64, p = .001). As shown in Figure 2, when compared to the average use of EC in week 1, differences were largest at the 2-week (b = −0.43, SE = 0.23, p = .06) and 4-week (b = −0.91, SE = 0.23, and p = .001) assessments. To the model above, we added terms to assess the impact of cigarette smoking on EC use. We observed that both higher levels of baseline cigarettes (b = 0.70, SE = 0.32, p = .036) and greater reduction of cigarettes per day during initial efforts to switch to EC at week 2 (b = −0.71, SE = 0.33, p = .04) were related to more frequent use of ECs at the week 2 and week 4 assessments.
Figure 1.
Average frequency of e-cigarette and cigarette use during the 4-week study period. Past 7 days was abstracted from the 30-day recall at week 4; shaded area indicates standard error.
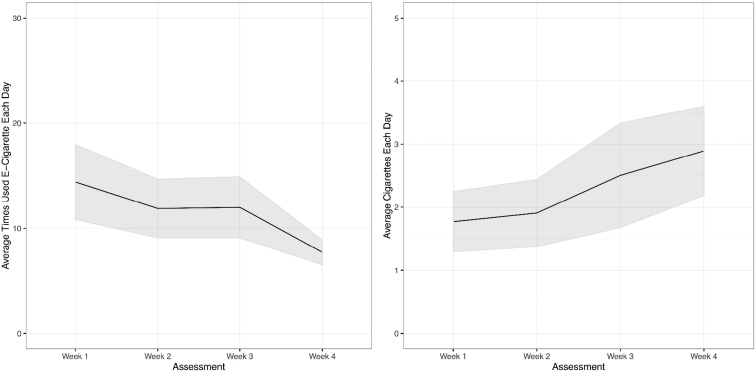
Figure 2.
Average quantity of use of cigarettes each day and average number of times using e-cigarettes each day during the 4-week study period. Past 7 days was abstracted from the 30-day recall at week 4; shaded area indicates standard error.
Change in Cigarette Consumption
When compared to the 30-days prior to the study period, smokers significantly reduced their cigarette consumption after being instructed to switch to EC during the study period (see Table 1). Smokers on average reduced their rate of smoking from baseline to week 1 by 7.1 (95% CI = 5.2, 9.1) cigarettes each day, t(32) = −7.2502, p < .001. Of the 40 smokers, 16 reported no cigarettes at week 2 (40%) and six continued to report no cigarettes at week 4 (15%).
Table 1.
Change in 30-Day Tobacco Product Consumption and Toxic Exposure from Baseline to Week 4
| Variable Mean (SD) or Median (Range)a | Baseline (N = 40) | Week 4 (N = 40)b | p |
|---|---|---|---|
| Tobacco consumption | |||
| Days smoked/past 301 | 24.83 (7.26) | 14.00 (12.31) | <.001 |
| CPD on days smoked1 | 8.76 (6.53) | 4.42 (4.06) | <.001 |
| Days used EC/past 30 | — | 24.72 (7.13) | |
| EC times on days used | — | 11.85 (14.00) | |
| Toxic exposure | |||
| Carbon monoxide (ppm) | 14.28 (12.66) | 8.93 (8.35) | <.001 |
| Cotinine (ng/mg)a,2 | 574.79 (99.53, 1417.02) | 440.84 (195.02, 1371.01) | .90c |
| NNAL/creatinine (pg/mg)a,2 | 102.75 (7.75, 291.17) | 55.85 (4.84, 234.22) | <.01c |
| VOC/creatine (ng/mg)a,2 | |||
| PMA3 | 0.71 (0.19, 2.24) | 0.59 (0.07, 1.72) | .01c |
| HEMA4 | 2.15 (0.77, 6.39) | 1.85 (0.76, 5.49) | .85c |
| MMA5 | 12.81 (3.80, 29.74) | 14.22 (9.58, 35.13) | .27c |
| CNEMA6 | 89.56 (33.69, 276.35) | 43.35 (8.39, 198.98) | <.01c |
| 3-HPMA7 | 818.90 (556.66, 818.90) | 644.46 (361.18, 2266.44) | .16c |
| 2-HPMA8 | 68.39 (32.35, 29.45) | 59.88 (29.45, 143.63) | .96c |
| AAMA9 | 192.28 (100.93, 294.92) | 168.88 (94.41, 326.97) | .67c |
| HPMMA10 | 303.35 (193.91, 480.53) | 259.83 (161.96, 765.93) | .99c |
| Nicotine dependence | |||
| WISDM11 | |||
| Total | 42.52 (12.92) | 34.23 (13.37) | <.001 |
| PDM12 | 3.83 (1.71) | 2.95 (1.51) | <.001 |
| SDM13 | 3.89 (1.04) | 3.20 (1.17) | <.001 |
| Cigarette addiction14 | 58.03 (31.35) | 38.63 (32.32) | <.001 |
| Self-efficacy to resist smoking15 | 64.92 (41.81) | 80.98 (42.69) | .025 |
CPD = cigarettes per day; EC = electronic cigarettes; SD = standard deviation; NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; VOC = volatile organic compounds.
aMedian (interquartile range) reported for cotinine, NNAL, and VOCs due to non-normal distribution.
bImputed from last observation.
cBased on analyses with log-transformed data.
1From 30-day timeline follow-back; 2normalized for creatinine; 3benzene; 4HEMA = ethylene oxide, with a possible contribution from acrylonitrile and vinyl chloride; 5N-nitrosodimethyamine; 6acrylonitrile; 7acrolein; 8propylene oxide; 9acrylamide; 10crotonaldehyde; 11Brief Wisconsin Inventory of Smoking Dependence Motives (WISDM); 12Primary Dependence Motives scale; 13Secondary Dependence Motives scale; 14Please rate your addiction to cigarettes on a scale of 0–100; 15Response options range from 0 to 10 (0 = Cannot resist smoking, 5 = Moderately certain I can resist smoking, 10 = Highly certain I can resist smoking).
Although reducing significantly from baseline levels, negative binomial GLMM of the count of cigarettes each day during the study period with adjustment for baseline level of smoking suggested participants increased the number of cigarettes per day gradually during the 4-week study period, F(3, 97) = 3.297, p < .025. When compared to week 1, participants reported a greater average number of cigarettes per day at the week-4 assessment (b = 0.45, SE = 0.17, p < 0.008). The change in numbers of cigarettes was not significantly different across 1-, 2- and 3-week assessments (p’s > .05).
Figure 1 displays trajectories of change in cigarette consumption during the evaluation period. Given compliance to the switching protocol, an estimate of the effect of change from baseline to the first week was included in the model. We evaluated change in frequency of cigarettes (arcsine transformed) during the study period with LME models with planned covariates (age, gender, racial/ethnic group, and baseline frequency of cigarette smoking days). Participants decreased the frequency of cigarette days (see Table 1) during the study period. Specifically, median frequency in cigarette days went from 97% (IQR = 47%–100%) of the past 30 days at baseline to a median of 42% (IQR = 0%–86%) at week 1. LME suggested no significant overall change in percentage of cigarette days from week 1 to week 4, F(3, 97) = 2.17, p = .097.
Change in 30-Day Nicotine Exposure
Urine cotinine levels adjusted for creatinine were used to assess change in daily nicotine consumption from baseline to week 4. Paired evaluation of log-transformed creatinine-adjusted cotinine values at baseline and week 4 were not different statistically (mean of log-differences = −0.10; 95%CI = −0.35, 0.55, p = .65). Follow-up exploratory analyses examined changes in exposure among those who initially switched exclusively at least through week-2 assessment (Initial exclusive EC users; n = 10), those who reported persistent switching exclusively to EC through week 4 (Persistent exclusive EC users; n = 6), and participants who reported dual cigarette and EC (n = 21; missing = 3) use throughout the week-4 assessment (ie, Dual Users who never exclusively used EC). Medians and inter-quartile ranges for Persistent switchers, Initial switchers, and Dual cigarette plus EC users are provided in Table 2.
Table 2.
Week 4 Toxic Exposure Levels in Exclusive EC Users Versus Dual Users
| Variable | Initial exclusive EC usersa | Persistent exclusive EC usersb | Dual usersc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (N = 10) | (N = 6) | (N = 21) | ||||||||
| Median | IQR | ES | Median | IQR | ES | Median | IQR | ES | p d | |
| Toxic exposure at week 4 | ||||||||||
| Cotinine (ng/mg)ef | 361.45 | (120.5, 710.5) | 0.32 | 266.40 | (123.6, 386.4) | 0.43 | 687.50 | (247.3, 1193) | 0.23 | .35 |
| NNAL/creatinine (pg/mg)ef | 22.15+ | (4.7, 119.3) | 0.53 | 3.50 | (2.0, 20.3) | 0.81 | 156.13 | (52.5, 320.7) | 0.16 | .01 |
| VOC/creatine (ng/mg)ef | ||||||||||
| PMA1 | 0.09+ | (0.07, 0.6) | 0.66 | 0.08 | (0.07, 0.1) | 0.65 | 1.06 | (0.6, 2.5) | 0.14 | .01 |
| HEMA2 | 0.78+ | (0.8, 1.9) | 0.68 | 1.52 | (0.9, 2.1) | 0.22 | 3.00 | (1.5, 7.4) | 0.15 | .06 |
| MMA3 | 8.48 | (3.5, 23.0) | 0.40 | 24.72 | (14.6, 36.3) | 0.54 | 16.27 | (11.4, 34.2) | 0.15 | .12 |
| CNEMA4 | 20.26++ | (8.4, 32.7) | 0.96 | 4.82++ | (2.0, 7.9) | 1.02 | 120.23 | (51.0, 422.4) | 0.03 | <.001 |
| 3-HPMA5 | 370.34 | (308.0, 518.2) | 0.32 | 390.35 | (370.4, 513.8) | 0.35 | 1014.69 | (662.2, 3346.0) | 0.07 | .11 |
| 2-HPMA6 | 38.03 | (29.2, 133.3) | 0.11 | 37.35 | (21.6, 51.3) | 0.60 | 105.08 | (62.0, 175.3) | 0.28 | .26 |
| AAMA7 | 96.52++ | (82.3, 157.3) | 0.85 | 95.31 | (69.6, 137.7) | 0.34 | 268.46 | (168.6, 394.6) | 0.23 | .01 |
| HPMMA8 | 251.63 | (157.8, 765.9) | 0.14 | 160.82 | (154.5, 169.7) | 0.40 | 305.74 | (228.6, 918.7) | 0.10 | .39 |
EC = electronic cigarettes; ES = Cohen’s d effect size for change from baseline to week 4; NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; VOC = volatile organic compounds.
aInitially switched exclusively at least through week-2 assessment.
bPersistent switching exclusively to EC through week 4.
cDual cigarette and EC use throughout the week-4 assessment (never exclusively used EC).
dBased on analyses with transformed values in models with contrasts between (1) between Initial vs. Dual Users (+p < .05, ++p < .01), (2) Persistent vs. Dual Users (++p < .01) and (3) Initial vs. Persistent Users; the p-value reflects the overall F test.
eMedian (interquartile range) reported for cotinine, NNAL, and VOCs due to non-normal distribution.
fNormalized for creatinine.
1benzene; 2ethylene oxide with a possible contribution from acrylonitrile and vinyl chloride; 3N-nitrosodimethyamine; 4acrylonitrile; 5acrolein; 66propylene oxide; 7acrylamide; 8crotonaldehyde.
Regression models of log-transformed values at week 4 included planned covariates (age, gender, race/ethnicity) and adjustment for corresponding baseline values of examined biomarkers. An overall test of adjusted means (F(2, 29) = 1.13, p = .34) revealed no significant differences between groups. A follow-up multiple comparisons exploratory analysis (see Table 2) suggested non-significant decreases in cotinine among Initial (mean difference = −0.53, SE = 0.49, p = .52) relative to dual users and a non-significant increase in Persistent exclusive EC users relative to dual users (mean difference = 0.50, SE = 0.68, p = .75) and Initial switchers (mean difference = 1.02, SE = 0.73, p = .35). When exploring the potential increase in cotinine, we observed lower levels of baseline cotinine values among the six persistent exclusive EC (Med = 80.1) compared to dual users (Med = 808.8).
Change in Tobacco Toxicant Exposure
Significant decreases were observed in carbon monoxide (p < .01) from baseline to week 4. Overall, we observed statistically significant differences in log-transformed NNAL levels (mean difference = 0.35, 95% CI = 0.09–0.69, p < .01). Of the eight VOCs measured, there were two significant decreases in log-transformed values of the benzene metabolite, PMA (mean differences = 0.57, 95%CI = 0.12–1.03, p = .01), and the acrylonitrile metabolite, CNEMA (mean differences = 0.60, 95%CI = 0.25–0.96, p = .001).
Exploratory analysis comparing initial switchers, persistent switchers, and dual users suggested that levels of NNAL (F(2, 29) = 6.3544, p = .005), the benzene metabolite PMA (F(2, 29) = 6.076, p = .006), the acrylonitrile metabolite CNEMA (F(2, 29) = 15.86, p < .001) and the acrylamide metabolite, AAMA (F(2, 29) = 7.82, p = .002) were significantly different across user groups. Group differences in HEMA were also explored (F(2, 29) = 3.79, p = .03). Post-hoc multiple comparisons suggested initial switchers (mean difference = −0.80, SE = 0.25, p = .01) had significantly greater decreases in NNAL relative to dual users. We observed a similar pattern with persistent switchers showing a decrease relative to dual users (mean difference = −0.82, SE = 0.35 p = .06), however this difference was not significant statistically. We did not observe significant differences in NNAL between initial and persistent switchers (mean difference = −0.02, SE = 0.36, p > .99). Larger decreases in PMA were detected among initial switchers (mean difference = −1.42, SE = 0.43, p = .007) relative to dual users. Observed decreases among persistent switchers were not significantly different from dual users (mean difference = −1.18, SE = 0.62, p = .15) or initial switchers (mean difference = 0.24, SE = 0.66, p = .93). Significantly greater decreases in HEMA were observed for initial (mean difference = −0.87, SE = 0.32, p = .03) but not among persistent switchers (mean difference = −0.47, SE = 0.42, p = .51). Level of HEMA after 4 weeks was not significantly different for initial and persistent switchers (mean difference = 0.40, SE = 0.45, p = .65). Decreases in CNEMA were larger for both initial (mean difference = −1.45, SE = 0.29, p < .01) and persistent switchers (mean difference = −1.63, SE = 0.42 p = .002) when compared to dual users. There were no significant differences in CNEMA at week 4 for initial and persistent switchers (mean difference = −0.18, SE = 0.43, p = .91). Larger decreases in AAMA were detected among initial switchers (mean difference = −0.82, SE = 0.21, p < .01 relative to dual users. Observed decreases among persistent switchers were not significantly different from dual users (mean difference = −0.45, SE = 0.29, p = .27) or initial switchers (mean difference = 0.37, SE = 0.31, p = .45).
We observed no significant differences in levels of MMA, HPMMA, HPEMA-2, or HPEMA-3 metabolites of ethylene oxide, N-nitrosodimethyamine, crotonaldehyde, propylene oxide, and acrolein, respectively (p’s > .05) among initial switcher, persistent switcher, or dual user groups.
Change in Nicotine Dependence and Related Attitude/Behavior
Smoking dependence as measured by a brief, multi-dimensional scale and a single item rating scale, significantly decreased from baseline to week 4 (p < .001). In addition, self-efficacy to resist smoking cigarettes significantly increased (p < .05). Scores on an EC Dependence Scale at week 4 ranged from 4 to 32 on a 60-point scale, with an average score of 16.52 (SD = 8.01). Carrying of cigarettes (always or most of the time) when leaving the house at baseline was reported by 82.5% compared to 57.5% at week 4. Carrying of EC (always or most of the time) when leaving the house at week 4 was reported by 83.3%.
Discussion
Overall, the results support the acceptability of cigarette smokers switching to second generation ECs. All participants with follow-up data (92.5%) reported using ECs in the past 30 days. Cigarette use significantly decreased from baseline to week 4, but consumption gradually rose over time, highlighting the difficulty of long-term behavior change in the absence of behavioral support. There was no significant change in cotinine over the 4 weeks, suggesting that ECs provided adequate nicotine replacement from cigarettes.
Three markers of toxic exposure were significantly decreased for the study sample at week 4: carbon monoxide, NNAL, and two VOC, benzene (PMA) and acrylonitrile (CNEMA). Smokers who switched entirely to e-cigarettes for at least half the study period demonstrated significant reduction in two additional carcinogens, metabolites of ethylene oxide/acrylonitrile/vinyl chloride (HEMA) and acrylamide (AAMA) relative to dual cigarette + EC users.
The findings provide some support for the idea of harm reduction for those who switched exclusively to ECs for at least half the study period. Those who switched exclusively to ECs significantly reduced toxic exposure levels within nonsmoker limits on two markers classified as carcinogens or possible carcinogens, CNEMA a metabolite of acrylonitrile and AAMA, a metabolite of acrylamide.37,38 Although the reduction in 3-HPMA was not statistically significant, the absolute level at week 4 was within nonsmokers limits.39 Levels of two carcinogens including PMA, a metabolite of benzene, a group 1 carcinogen40 and NNAL, a metabolite of the nicotine-derived nitrosamine, NNK, which is a highly potent pulmonary carcinogen,41 were significantly lower among exclusive EC users than dual users at week 4, but fell outside the nonsmoker range.37,41 However, it must be noted that the half-life of NNAL is such that exposure can be detected over the 6 to 12 weeks after cessation of exposure.42 Therefore, the 4-week study period was insufficient to illuminate the full extent of change in NNAL given exposure from before the study period. There was no significant difference in three VOC exposure markers (MMA, HPMMA, or 2-HPEMA) between exclusive EC users and dual cigarette + EC users. Dual users’ exposure was outside the nonsmoker range on NNAL and all VOCs for which comparative norms were available.37–39
Nicotine consumption was not significantly different from the beginning to the end of the 4-week observation period, but self-reported addiction to cigarettes decreased and self-efficacy to resist smoking cigarettes increased. This likely reflects the successful adoption of ECs in place of some or all cigarettes. With a comparable level of nicotine present following switching from cigarettes to ECs, presumably, dependence on a nicotine-delivering product remains. This dependence appears higher for cigarettes (M = 34.0, SD = 30.97) on a 0–100 scale, compared to ECs (M = 16.52, SD = 8.01) on a 60-point scale. However, the percentage of participants who reported carrying ECs always or most of the time when leaving the house was higher (83.3%) than for cigarettes (57.5%) at week 4. One could argue that although subjective dependence on cigarettes decreased, physical dependence remained and a low level of dependence on a new product developed. It will be important for future studies to monitor dependence on ECs over a longer period of time, as dependence on a product may strengthen along with the duration of product use. It will also be important for future research to use dependence scales that are compatible between cigarettes and ECs for more direct comparison.
To our knowledge, there are no other published studies of switching smokers over a period of 4 weeks to second generation ECs with measurement of disease-associated biomarkers, NNAL and a panel of common VOCs that are potentially toxic environmental or tobacco smoke constituents. The study sample experienced a significant reduction in two out of eight VOCs. Those who switched completely to ECs experienced a significant reduction in NNAL and a third VOC compared to dual users. Our results complement two studies switching cigarette smokers to first generation ECs10,43 and one to second generation ECs44 in which declines in NNAL and VOCs were observed. Extrapolation of these results must be careful to separate the different toxic exposure results for exclusive switchers versus dual EC + cigarette users, and not to equate harm reduction with the idea that using ECs is harmless.
Unique design features add to the generalizability of the study, including a choice of flavors while maintaining the control of a standard second generation EC device. In addition, the study was open to smokers at any level of intention to quit, an important feature of a study observing natural change in tobacco consumption and toxicant exposure among smokers switching to ECs. Finally, a broader panel of toxicants was evaluated than in previous studies.7–12
Limitations to the present study include the following. The 4-week study period was insufficient to see the full reduction in NNAL to nonsmoker levels due to its long half-life. It must also be noted that while including nondaily and light smokers improves the generalizability of the study, the nicotine replacement observed from ECs in the study sample might be more feasible for less frequent smokers than heavier smokers. In addition, given that cigarette use was on the rise at 4 weeks, it is imperative to conduct a longer-term follow-up study to determine whether dual users increase their toxicant exposure over time. For example, it is possible that dual users return to baseline levels of cigarette use and continue using ECs, thereby increasing exposure over baseline levels. Also, the sample size may have limited the ability to detect small effects and sub-group differences. Additionally, while it is valuable to characterize the combustible tobacco-specific toxicants associated with EC use among smokers, there are additional EC-related toxicant effects that should be studied.45 Finally, there were no instructions for eating/drinking/product use within a certain time window before urine collections. Although sessions were scheduled at the same time of day within each participant to lend consistency to ad libitum behavior patterns, the lack of standardization was a limitation.
Larger studies with a longer follow-up period are needed to confirm the EC profiles and harm exposure findings of this study and to characterize sustained change and health effects over time. The present study demonstrated feasibility of smokers switching to ECs in a short observational trial with limited behavioral support. The extension of the EC trial period and addition of a longer follow-up period would provide the next steps in determining sustained change and corresponding tobacco toxicant exposure over time.
Supplementary Material
Supplementary data are available at Nicotine & Tobacco Research online.
Funding
This study was funded by the University of Minnesota (JSA), P30 DA012393 (NLB), P50 CA180890 (NLB), and California State University San Marcos (KP).
Declaration of Interests
Benowitz is a consultant to pharmaceutical companies that market smoking cessation medications and has been an expert witness in litigation against tobacco companies. The other authors have no conflicts of interest.
Supplementary Material
Acknowledgments
This study would not have been possible without a dedicated and talented research team: lead research assistants Alyssa Ramirez, Dustin Kessler, Jon Hoerr, and Ashley Emami; lead screener Teresa Kapphahn; assistant screener and recruitment specialist Micah Savin; and assistant research assistants Marissalyn Gonzalez, Alexa Kliebenstein, Anela Amba-Pascua, Daniel Sinclair, and Brett Goudy. We are also grateful to the following individuals who assisted with data analysis Jon Hoerr, Teresa Kapphahn, Daniel Sinclair, Gabe Holguin, and Kelly McCann. In addition, we appreciate the support of Micah Savin with data interpretation and Erin Lane for biological sample storage and shipping. We are also thankful to Margaret Peng, Kristina Bello, Lawrence Chan, Christopher Havel, Trisha Mao and Lisa Yu for conducting biomarker assays and Peyton Jacob III for laboratory supervision.
References
- 1. Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes?J Public Health Policy. 2011;32(1):16–31. [DOI] [PubMed] [Google Scholar]
- 2. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med. 2014;69:248–260. [DOI] [PubMed] [Google Scholar]
- 3. Arrazola RA, Singh T, Corey CG, et al. Tobacco use among middle and high school students - United States, 2011-2014. MMWR Morb Mortal Wkly Rep. 2015;64(14):381–385. [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Notes from the field: electronic cigarette use among middle and high school students - United States, 2011–2012. Morb Mortal Wkly Rep. 2013;62(35):729–730. [PMC free article] [PubMed] [Google Scholar]
- 5. Choi K, Forster JL. Beliefs and experimentation with electronic cigarettes: a prospective analysis among young adults. Am J Prev Med. 2014;46(2):175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102(9):1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629–1637. [DOI] [PubMed] [Google Scholar]
- 9. Adriaens K, Van Gucht D, Declerck P, Baeyens F. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220–11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McRobbie H, Phillips A, Goniewicz ML, et al. Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prev Res (Phila). 2015;8(9):873–878. [DOI] [PubMed] [Google Scholar]
- 11. Polosa R, Caponnetto P, Maglia M, Morjaria JB, Russo C. Success rates with nicotine personal vaporizers: a prospective 6-month pilot study of smokers not intending to quit. BMC Public Health. 2014;14:1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tseng TY, Ostroff JS, Campo A, et al. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine Tob Res. 2016;18(10):1937–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hecht SS, Stepanov I, Carmella SG. Exposure and metabolic activation biomarkers of carcinogenic tobacco-specific nitrosamines. Acc Chem Res. 2016;49(1):106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haussmann HJ. Use of hazard indices for a theoretical evaluation of cigarette smoke composition. Chem Res Toxicol. 2012;25(4):794–810. [DOI] [PubMed] [Google Scholar]
- 15. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V. Impact of flavour variability on electronic cigarette use experience: an internet survey. Int J Environ Res Public Health. 2013;10(12):7272–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–2028. [DOI] [PubMed] [Google Scholar]
- 18. Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an internet survey. Drug Alcohol Rev. 2013;32(2):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10(6):2500–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacob P, III, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal Chem. 2008;80(21):8115–8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jacob P, III, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981;222(1):61–70. [DOI] [PubMed] [Google Scholar]
- 22. Gregg EO, Minet E, McEwan M. Urinary biomarkers of smokers’ exposure to tobacco smoke constituents in tobacco products assessment: a fit for purpose approach. Biomarkers. 2013;18(6):467–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sobell L, Maisto S, MB S. Alcohol Timeline Followback Users’ Manual. Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- 24. Steinberg K, Roffman R, Carroll K, et al. Brief Counseling for Marijuana Dependence: A Manual for Treating Adults. DHHS Publication No. (SMA) 05-4022. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2005. [Google Scholar]
- 25. Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: an integrative model. Addict Behav. 1990;15(3):271–283. [DOI] [PubMed] [Google Scholar]
- 26. Etter JF, Bergman MM, Humair JP, Perneger TV. Development and validation of a scale measuring self-efficacy of current and former smokers. Addiction. 2000;95(6):901–913. [DOI] [PubMed] [Google Scholar]
- 27. Smith SS, Piper ME, Bolt DM, et al. Development of the brief Wisconsin inventory of smoking dependence motives. Nicotine Tob Res. 2010;12(5):489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baker TB, Piper ME, McCarthy DE, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tobacco Res. 2007;9(suppl 4):S555–S570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 30. Etter JF, Le Houezec J, Perneger TV. A self-administered questionnaire to measure dependence on cigarettes: the cigarette dependence scale. Neuropsychopharmacology. 2003;28(2):359–370. [DOI] [PubMed] [Google Scholar]
- 31. Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schisterman EF, Vexler A, Whitcomb BW, Liu A. The limitations due to exposure detection limits for regression models. Am J Epidemiol. 2006;163(4):374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bates D, Maechler M, Bolker B, Walker S. Linear mixed-effects models using Eigen and S4_. R package version 1 20151–9. https://CRAN. R-project.org/package=lme4 October 23, 2016.
- 34. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc: Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 35. Tukey JW. Comparing individual means in the analysis of variance. Biometrics. 1949;5(2):99–114. [PubMed] [Google Scholar]
- 36. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. www. R-project.org/ October 23, 2016. [Google Scholar]
- 37. Ding YS, Blount BC, Valentin-Blasini L, et al. Simultaneous determination of six mercapturic acid metabolites of volatile organic compounds in human urine. Chem Res Toxicol. 2009;22(6):1018–1025. [DOI] [PubMed] [Google Scholar]
- 38. Schettgen T, Musiol A, Kraus T. Simultaneous determination of mercapturic acids derived from ethylene oxide (HEMA), propylene oxide (2-HPMA), acrolein (3-HPMA), acrylamide (AAMA) and N,N-dimethylformamide (AMCC) in human urine using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22(17):2629–2638. [DOI] [PubMed] [Google Scholar]
- 39. Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal Chim Acta. 2012;750:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. International Agency for Research on Cancer. Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. IARC Monogr. Eval. Carcinog. Risks Hum. 1987;7(suppl):1–440. [PubMed] [Google Scholar]
- 41. Bernert JT, Pirkle JL, Xia Y, Jain RB, Ashley DL, Sampson EJ. Urine concentrations of a tobacco-specific nitrosamine carcinogen in the U.S. population from secondhand smoke exposure. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2969–2977. [DOI] [PubMed] [Google Scholar]
- 42. Goniewicz ML, Eisner MD, Lazcano-Ponce E, et al. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine Tob Res. 2011;13(3):202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O’Connell G, Graff D, D’Ruiz C. Reductions in biomarkers of exposure (BoE) to harmful or potentially harmful constituents (HPHCs) following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Toxicol. Mech. Methods. 2016;26(6):443–454. doi:10.1080/15376516.2016.1196282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P, III, Benowitz NL. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine Tob Res. 2016. doi:10.1093/ntr/ntw160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mikheev VB, Brinkman MC, Granville CA, Gordon SM, Clark PI. Real-time measurement of electronic cigarette aerosol size distribution and metals content analysis. Nicotine Tob Res. 2016;18(9):1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.