The induced pluripotent stem cells (iPSC) story
In his State of the Union Address on 20 January 2015, President Barack Obama launched a new Precision Medicine Initiative to facilitate the most optimal treatment plan for every individual patient.

Although this concept is old, arguably described by Sir William Osler as early as in the 19th century when he stated, ‘If it were not for the great variability among individuals, medicine might as well be a science, not an art’, this ambitious Initiative has in fact not been possible to practice until very recently. With recent advances, precision medicine has since gathered great momentum. The principal goal is to identify novel technologies that would allow us to understand disease progression in every patient and thus lead to the development of tailor-made individualized therapies.
Although the initial targets of precision medicine were cancer and diabetes, we believe that cardiology will take the lead in setting the tone of precision medicine. Indeed, there are several factors that have allowed precision medicine to be a possibility in cardiovascular diseases. These include the resources available from the completion of the human genome project, the advancements made in next-generation sequencing and computational biology to handle ‘big data’, and the growing availability of patient’s ‘omics’ information (genomic, epigenomic, proteomics, transcriptomic, and metabolomics). The conjunction of these resources, combined with the collaborative sharing research data and enrolling participants for clinical trials among many institutes, has pushed cardiovascular precision medicine to the forefront.
Historically, the cardiovascular community has been applying precision medicine principles since the mid-1900s, with studies such as the Framingham Heart Study that utilized patient-specific data to estimate the 10-year cardiovascular risk of each individual. However, the central question of how closely the people in a study resemble the actual patients undergoing treatment remained unanswered, in almost all prior cases the subjects and patients were two different populations.
Then a decade ago, the discovery that somatic cells can be converted to stem cells by exogenous expression of a few transcription factors completely changed the landscape of medical research. These stem cells, known as induced pluripotent stem cells (iPSCs), were found to have invaluable ability to yield de novo patient-specific cells, including patient-specific cardiomyocytes (CMs). This discovery earned Dr Shinya Yamanaka and Sir John Gurdon the 2012 Noble Prize in Physiology and Medicine and has revolutionized the field of human disease modelling, with the potential to finally bring personalized diagnosis and treatment to every patient.
Several challenges need to be met for iPSCs to fully contribute to the mission of cardiovascular precision medicine. One major hurdle is how to improve differentiation protocols to achieve pure, safe, and clinically relevant iPSC-derived CMs (iPSC-CMs). With the concerted efforts of the academic community, we can now achieve more that 90% efficiency in deriving iPSC-CMs using xeno-free, chemically defined medium. This allows researchers to derive patient-specific iPSC-CMs from a just few millilitres of the patient’s blood. Armed with this revolutionary tool to create any patient’s heart muscle ‘in-a-dish’. Now, we not only can predict that patient’s heart function but also learn about key disease-associated pathways.
Another important application of iPSCs towards precision medicine has been disease modelling. Prior to the advent of iPSC technology, researchers have relied on animal models to understand the pathophysiology of cardiovascular diseases. However, because of vast differences in the genomic background between humans and animals, it is impossible to perfectly mimic the human disease phenotype in the animal model. iPSC technology-based disease modelling could make up for the imperfections of animal models and vastly enhance our understanding of disease mechanisms in humans.
Indeed, several cardiovascular diseases due to various genetic, acquired, or environmental causes have already been modelled using iPSCs, including generation of patient-specific iPSC-CMs from large family cohorts with different mutations. With the emergence of genome engineering such as clustered regularly interspaced short palindromic repeat, a genome-editing tool that can allow fast and precise changes to the genome, it is now possible to generate human cellular disease models in a precise and predictable manner. Since the availability of this tag-team technologies of iPSCs and genome editing, many laboratories are now creating cardiac disease models or correct genetic mutations in iPSC-CMs to model cardiac diseases.
Another major application of iPSC technology being used in precision medicine is in cell transplantation or regenerative medicine. The concept of personalized therapy that allows cells or tissues derived from pluripotent stem cells (PSCs) from patients to be used to help themselves has long been a dream of clinicians, because this elegant solution eliminates the need for costly, problematic, and often lifelong immunosuppression. This approach has only been possible due to the advancements in culturing and differentiation protocols that can yield clinical-grade cells with negligible risk of tumorigenesis. Indeed, several groups are planning clinical trials using PSCs, with one group (Dr Philippe Menasche, France) actively recruiting patients to test the use of human embryonic stem cell-derived cardiac progenitor cells in patients with heart failure.
Despite the excitement surrounding the use PSCs, which include both embryonic stem cells and iPSCs, the jury is still out on whether their differentiated CMs or cardiac progenitor cells could fully replace the diseased tissue in the heart. Further refinements will undoubtedly be needed and made by the growing community of researchers and clinicians using PSC technology. With the advent of new technologies such as optimal bioengineering tools that allow survival and engraftment of PSC-derived CMs in the host myocardium, the cardiovascular regenerative community has taken one step closer to achieving the goal of precision medicine in cell transplantation.
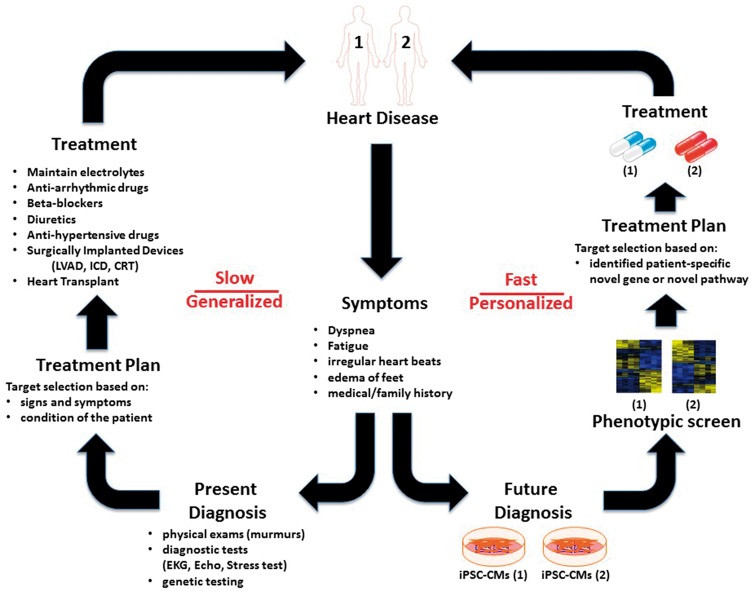
The ultimate goal of precision medicine is to deploy effective, non-toxic, patient-specific treatment plans for individual patients based on their unique genetic makeup (Figure 1). In the past, patients’ genetic differences were seen not as a blessing but a major problem, because drugs then were not and could not be developed with the end users’ genetic diversity in mind. This situation has undergone a fundamental paradigm shift with the advent of precision medicine made possible by techniques such as iPSC and gene editing. Now genetic information, rather than being a confounding hindrance, can inform clinicians on the best possible treatment for each individual patient, who will no longer be treated in the dark with one-size-fits-all therapy that may or may not work. This is possible because precision medicine considers each patient’s unique genes, environment, and life style to tailor individualized therapy.
Figure 1.
Induced pluripotent stem cell (iPSC)-based personalized medicine: a step towards future diagnosis and treatment. The conventional model for treating patients with heart disease depends on the patient’s history and clinical examination. Based on these signs and symptoms, the cardiologist usually prescribes a few different drugs as one-size-fits-all therapy that may or may not work. This generalized form of treatment is lengthy and cumbersome. In contrast, the iPSC-based model requires generation of patient-specific iPSC-CMs that may enable extensive phenotypic screening of each patient for novel genes or pathways involved in the disease. This could allow clinicians to tailor therapeutic options for each individual patient based on their genetic background. This paradigm shift towards personalized cardiovascular care could revolutionize the way we practice medicine.
The iPSC technology has given us the opportunity to execute the Precision Medicine Initiative by providing an unlimited supply of cells that faithfully mirror the patient’s own native cells. For example, iPSC-derived CMs derived from a donor can be used as a ‘mini-me surrogate’ to predict the donor’s susceptibility to certain drugs. This unique property of iPSCs will soon allow researchers to test potential cardiotoxic drugs or screen for new therapies for heart disease even before treating the patient. We expect this to revolutionize current drug screening methods, which still mostly rely on often inaccurate cardiotoxicity studies of potential new drugs using in vitro cell lines that overexpress the IKr protein hERG, which frequently lead to both false-positive and false-negative results. The adoption of iPSCs will allow pharmaceutical companies to solve the twin problems of high attrition rates and increased drug costs that plague current cardiotoxicity tests. Similarly, iPSC-based models could serve as a functional readout for the pre-clinical screening of candidates with speed and efficiency dwarfing conventional methods.
Moreover, iPSC-based medicine may fundamentally change the cumbersome way clinical trials have been run for the better part of a century, which rely on different cohorts of unrelated patients and necessary guesswork if not false hope that drugs used in the limited pool of subjects will work on patients in the population at large. Under the new approach, before a drug is given to the recruited patients, its efficacy could be tested on iPSC-derived CMs generated from the same patients. The patients whose iPSC-derived CMs show a positive response could be classified as responders and allowed to take the drug as they are moved to the next phase of the clinical trial. This iPSC-based patient stratification is expected to dramatically boost the success rate of clinical trials and reduce their cost by pre-selecting the patients recruited for the trial. These innovative ‘clinical trial in a dish’ studies are now being explored by many researchers to rapidly and economically develop personalized therapies.
So, has the time for a one-person clinical trial finally arrived? We are now just beginning to practice medicine by tailoring therapeutic options for each individual patient based on their genes and other factors. However, the days of using hit-or-miss medical treatments developed for the mythical ‘average’ patient are numbered, as optimal and optimized drugs developed for individual patients come to dominate therapy.
Cardiology will continue to play a preeminent role in precision medicine as it leverages the immense advantages of iPSC technology and other emerging techniques such as gene editing to give each patient suffering from whatever heart disease the best individualized treatment possible on a routine basis.


Conflict of interest: J.C.W. is co-founder for Stem Cell Theranostics. N.S. has no disclosures.
Cardio Pulse contact: Andros Tofield, Managing Editor. Email: docandros@bluewin.ch