Abstract
Background
A-β + ketosis-prone diabetes (KPD) is a subset of type 2 diabetes in which patients have severe but reversible β cell dysfunction of unknown etiology. Plasma metabolomic analysis indicates that abnormal arginine metabolism may be involved.
Objective
The objective of this study was to determine the relation between gut microbiome and arginine metabolism and the relation between arginine availability and β cell function in KPD patients compared with control participants.
Methods
Kinetics of arginine and related metabolites were measured with stable isotope tracers, and insulin secretory responses to arginine and glucose were determined under euglycemic and hyperglycemic conditions in 6 KPD patients and 6 age-, gender-, and body mass index–matched control participants. Glucose potentiation of arginine-induced insulin secretion was performed in a different set of 6 KPD and 3 control participants.
Results
Arginine availability was higher in KPD patients during euglycemia [53.5 ± 4.3 (mean ± SEM) compared with 40.3 ± 2.4 μmol · kg lean body mass (LBM)−1 · h−1, P = 0.03] but declined more in response to hyperglycemia (Δ 10.15 ± 2.6 compared with Δ 3.20 ± 1.3 μmol · kg LBM−1 · h−1, P = 0.041). During hyperglycemia, ornithine flux was not different between groups but after an arginine bolus, plasma ornithine AUC trended higher in KPD patients (3360 ± 294 compared with 2584 ± 259 min · μmol · L−1, P = 0.08). In both euglycemia and hyperglycemia, the first-phase insulin responses to glucose stimulation were lower in KPD patients (euglycemic insulin AUC 282 ± 108 compared with 926 ± 257 min · μU · mL−1, P = 0.02; hyperglycemic insulin AUC 358 ± 79 compared with 866 ± 292 min · μU · mL−1, P = 0.05), but exogenous arginine restored first-phase insulin secretion in KPD patients to the level of control participants.
Conclusion
Compared with control participants, KPD patients have increased arginine availability in the euglycemic state, indicating a higher requirement. This is compromised during hyperglycemia, with an inadequate supply of arginine to sustain metabolic functions such as insulin secretion. Exogenous arginine administration restores a normal insulin secretory response.
Keywords: diabetes mellitus, ketosis-prone diabetes, arginine metabolism, β cell function
Introduction
A-β + ketosis-prone diabetes (KPD, “A-β + unprovoked” subgroup) is a unique subset of diabetes in which adults present with unprovoked diabetic ketoacidosis (DKA) despite the absence of β cell autoimmunity, and subsequently experience prolonged near-normoglycemic, insulin-independent remission. The cause of the episodes of unprovoked DKA is unknown. We previously utilized a plasma metabolomics approach, coupled with stable isotope tracer kinetic validation, to determine that unique defects in the metabolism of the ketogenic amino acid leucine, and the oxidation of ketones, underlie part of the pathophysiology of ketosis in these patients (1). Additional unpublished data from that study showed that compared with control participants, KPD patients had 25% lower plasma arginine concentration (72.9 ± 5.3 compared with 91.3 ± 5.8 μmol/L, P = 0.04), and the concentration of citrulline, the precursor from which arginine is synthesized de novo, was also significantly lower (33.6 ± 2.2 compared with 26.8 ± 1.7 μmol/L, P = 0.02). In addition, Kashyap et al. (2) have previously reported that typical type 2 diabetes patients and control subjects have similar concentrations of arginine in the fasting euglycemic condition. These findings in KPD patients suggest a shortage in the supply of arginine because its rate of production is insufficient to match its rate of utilization. This constant arginine deficiency may be a contributing factor to the β cell failure and proneness to DKA during sustained hyperglycemia that is characteristic of KPD patients, as arginine is known to markedly potentiate glucose-stimulated insulin secretion in cultured β cells (3, 4). However, the in vivo effect of arginine availability on human β cell function remains unclear. Furthermore, the effect of chronic hyperglycemia on arginine metabolism and its availability in conditions such as uncontrolled diabetes is not known.
Arginine, a semiessential amino acid, becomes essential during periods of critical illness, physiologic and metabolic stress, and rapid tissue deposition (5). In the fasting state, the cellular availability of arginine depends on the balance between its inflow from the circulation plus cellular protein breakdown and de novo synthesis from citrulline, and its disposal primarily via cellular protein synthesis, hydrolysis to ornithine and urea, conversion to citrulline and nitric oxide, and synthesis of agmatine. In the present study, we tested the hypothesis that during sustained hyperglycemia, KPD patients experience a relative decrease in arginine availability due to increased arginine hydrolysis, and this deficit is associated with impaired insulin secretion. Furthermore, administration of exogenous arginine to KPD patients during hyperglycemia will normalize their insulin secretory response. In addition, the gastrointestinal tract affects dietary, and hence whole-body, arginine availability, due to its high first-pass extraction and hydrolysis by arginase activity (6). Arginase is expressed at high concentrations in the intestines, and gut bacteria could affect arginase activity and thereby alter arginine metabolism. Hence, we assessed gut microbial diversity in relation to the arginine and ornithine kinetic parameters.
Methods
Study population
The Human Studies Institutional Review Board of Baylor College of Medicine approved all protocols. Six control participants and 6 A-β + KPD patients were recruited and informed consent obtained. KPD patients were selected based on published criteria after presentation with unprovoked DKA within the previous 12 mo (7). Antidiabetic medications included metformin and insulin. Control subjects were recruited through advertisement, and were age-, gender-, and BMI-matched to the KPD patients. Study participants had no evidence of chronic or acute illness (except obesity and diabetes) as determined by medical history, physical exam, complete blood count, and comprehensive metabolic panel, including liver function tests.
All participants were studied at the Children's Nutrition Research Center Metabolic Research Unit at Baylor College of Medicine on 2 occasions with different protocols. Each protocol commenced at 0700 after a 10-h overnight fast. Protocols were separated by ≥4 d to permit isotope washout. Sterile solutions of guanidino-15N2-arginine, 5,5–2H2-citrulline, 15N2-ornithine, ring-2H5-phenylalanine, 13C-bicarbonate, and ureido-15N-citrulline (Cambridge Isotope Laboratories) were prepared using strict aseptic techniques and tested for sterility and lack of pyrogens prior to infusion. Sterile isotope solutions were prepared by fully trained and certified staff in a certified clean room at the Children's Nutrition Research Center. This facility has the highest pharmaceutical grade ratings, i.e., grade A (ISO 5) for critical areas and grade B (ISO 7) for support areas. Arginine HCl was prepared by first neutralizing the HCl with sodium hydroxide. The pH was 7.4 prior to administration. An additional protocol, glucose potentiation of arginine-induced insulin secretion (GPAIS), was performed in a different set of 6 A-β + KPD and 3 control participants. These participants were also selected using the same criteria described above without evidence of chronic or acute illness (except obesity and diabetes).
Experimental protocol 1: arginine/ornithine/citrulline kinetics in the fasting euglycemic state
Intravenous catheters were placed in one antecubital vein for infusions and in the contralateral hand for blood sampling. The sampling hand was heated to obtain arterialized blood samples. Blood glucose was checked to ensure a baseline value <120 mg/dL; if the glucose concentration was higher, a low-dose IV insulin infusion was initiated to achieve a plasma concentration of 80–120 mg/dL, followed by a 60-min washout period before proceeding. Supplemental Figure 1A outlines the protocol in schematic form.
After baseline blood samples were collected, primed-constant intravenous infusions of guanido-15N2-arginine [prime = 5 μmol/kg lean body mass (LBM); infusion = 5 μmol · kg LBM−1 · h−1), 5,5–2H2-citrulline (prime = 2 μmol/kg LBM; infusion = 1 μmol · kg LBM–1 · h–1), 15N2-ornithine (prime = 1 μmol/kg LBM; infusion = 1 μmol · kg LBM−1 · h−1), and 2H5-phenylalanine (prime = 3 μmol/kg LBM; infusion = 3 μmol · kg LBM−1 · h−1) were started and maintained for 5 h. Prime doses of 15N-citrulline (prime = 0.1 μmol/kg LBM) were also given to prime the secondary pool of citrulline with labels derived from 15N2-arginine.
Additional blood samples were taken after 3 h, and every 20 min from 4 h to 5 h. Following the isotope infusions, tests of insulin secretory response to arginine and glucose were performed as described below.
Fresh stool samples were also collected from each participant on either or both protocol days. Samples were immediately frozen at –70°C until time of analysis.
Experimental protocol 2: arginine/ornithine/citrulline kinetics in the hyperglycemic state
Protocol 1 was repeated under hyperglycemic clamp conditions. Supplemental Figure 1B depicts this in schematic form. Each participant received a 0.20 g/kg intravenous glucose bolus followed by continuous variable 20% glucose infusion to achieve a steady plasma glucose concentration of ∼325 mg/dL. Plasma glucose was clamped at this concentration for 2 h prior to administering the same 5 h isotope infusion and sampling protocol described for Protocol 1. The glucose infusion rate was then decreased rapidly until plasma glucose concentration was stable at 100–120 mg/dL. Then, tests of insulin secretory response to arginine and glucose were performed as described below.
Insulin secretion test
During Protocols 1 and 2, tests of insulin secretory response to arginine and glucose were performed as follows: 2 baseline blood samples were drawn 5 min apart. First, arginine-induced insulin secretion [acute insulin response (AIR-arg)] was assessed by injecting an intravenous pulse of 5 g arginine at time 0 min, and blood samples drawn at 2, 3, 4, 5, 6, 8, 11, 26, 31, 51, and 56 min. Four minutes after the last blood draw, glucose-stimulated insulin secretion (AIR-gluc) was assessed by intravenous administration of 20 g glucose, after which blood samples were collected at 4, 5, 6, 8, 11, 16, 21, 26, and 31 min. First-phase insulin secretion was defined as the first 10–11 min following glucose or arginine stimulation.
GPAIS
In a separate study, 6 KPD and 3 control participants underwent quantitative tests of β cell functional reserve as described by Teuscher et al. (8). Participants fasted overnight for 10 h; for the diabetic patients, the last dose of subcutaneous insulin or metformin was 12 h before the studies. All time points, including baseline samples, were tested for insulin and glucose. Following baseline sampling, insulin secretion patterns were evaluated in response to an intravenous arginine bolus, an intravenous glucose bolus, and then during GPAIS. These tests were performed in series, as described below.
First, arginine-induced insulin secretion was assessed by injecting an intravenous bolus of 5 g arginine (50 mL of 10% arginine solution in saline) at time 0. Blood samples were drawn at 2, 3, 4, 5, 7, 10, 25, and 30 min following the injection. Next, 30 min after the last blood draw, glucose-induced insulin secretion and glucose disappearance rates were assessed by administration of 20 g glucose (given as 40 mL of 50% dextrose). Blood samples were collected at 3, 4, 5, 7, 10, 15, 20, 25, 30, 115, and 120 min following the injection. Immediately thereafter, insulin secretory reserve was assessed as described by Ward et al. (9), Seaquist and Robertson (10), and Teuscher et al. (11). A 67-min infusion of a glucose solution was then administered to deliver glucose at a constant rate of 900 mg/min in order to maximally potentiate the insulin response. During the glucose infusion, at time 60 min, a 5-g intravenous arginine bolus (50 mL of 10% arginine solution) was pushed over 1 min. Blood samples were collected at 50, 55, 60, 62, 63, 64, 65, and 67 min after the start of the glucose infusion. AIR-arg was calculated as the mean of the peak 3 insulin values between 2 and 5 min following the arginine injection after subtracting the mean baseline value. AIR-gluc was calculated as the mean of the peak 3 insulin values between 2 and 5 min following the glucose injection after subtracting the mean baseline value. GPAIS was calculated as the mean of the 3 peak insulin values between 2 and 5 min following the arginine injection with the basal value (prestimulus insulin concentration after 50–60 min of glucose infusion) subtracted (8).
Laboratory analyses
Blood was drawn in prechilled tubes containing heparin or EDTA, centrifuged at 4°C, and the plasma removed and stored at –70°C for later analysis.
Plasma insulin concentrations were measured by immunoassay (Cobas e411 analyzer, Roche Diagnostics) and plasma glucose concentrations were measured by the glucose oxidase method (YSI 2300 glucose analyzer, YSI Inc.).
Mass spectrometric analysis
Isotopic measurements were made on plasma samples. For Protocols 1 and 2, the dansylated derivatives of arginine, citrulline, ornithine, and phenylalanine were prepared as previously described (12, 13) and analyzed by LC-MS/MS monitoring the following precursor and product ions: for DANS-arginine, ions m/z 410 – 392 (from guanido-15N2-arginine tracer), and 410 – 72 (for arginine derived from 5,5–2H2-citrulline); for DANS-citrulline, ions m/z 410 – 392 (from guanido-15N2-arginine tracer), and 411 – 72 (from 5,5–2H2-citrulline tracer); for DANS-ornithine ions m/z, 601 – 170 (from 15N2-ornithine tracer); for DANS-phenylalanine ions m/z 404 – 170 (from 2H5-phenylalanine tracer).
Kinetic calculations
Kinetic calculations were made using standard steady-state (for flux) and precursor-product (for conversion rates) equations as previously described by us (1, 12, 13).
Total arginine, ornithine, citrulline, and phenylalanine fluxes (or rates of appearance, QArg, QOrn QCit, Qphe) were calculated by the steady-state equation:
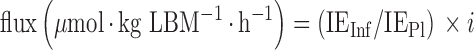 |
(1) |
where IEInf and IEPl are the isotopic enrichments of the tracer in the infusate and in plasma at isotopic steady state (M + 2 isotopomer for arginine, M + 2 isotopomer for ornithine, M + 2 isotopomer for citrulline, M + 5 isotopomer for phenylalanine) and i is the rate of infusion of 15N2-arginine, 15N2-Ornithine, 2H2-citrulline, or 2H5-phenylalanine in μmol · kg LBM−1 · h−1.
Arginine availability, i.e., arginine available for protein synthesis and synthesis of agmatine, creatine, NO, and citrulline, was calculated by subtracting endogenous ornithine flux (μmol · kg LBM−1 · h−1) from total arginine flux (μmol · kg LBM−1 · h−1) on the assumption that ornithine is only produced from arginine.
De novo arginine synthesis from citrulline was calculated from:
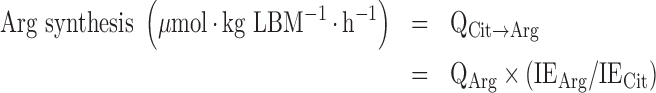 |
(2) |
where QArg is the total flux of arginine, IEArg is the plasma enrichment of the M + 2 isotopomer of arginine (i.e., derived from 2H2-citrulline), and IECit is the plasma enrichment of the M + 2 isotopomer of citrulline.
NO synthesis rate was calculated from the rate of conversion of arginine to citrulline by the NO synthase reaction as previously described (12, 13):
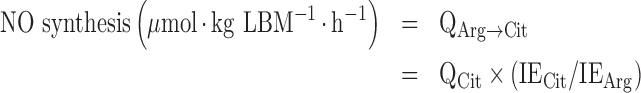 |
(3) |
where QCit is the flux of citrulline, IECit is the plasma enrichment of the M + 1 isotopomer of citrulline (i.e., ureido-15N-citrulline derived from 15N2-arginine), and IEArg is the plasma enrichment of the M + 2 isotopomer of arginine.
Gut microbiome analysis
16S rRNA gene-sequencing methods were adapted from methods developed for the NIH Human Microbiome Project (14, 15). Bacterial genomic DNA was extracted using the MO BIO PowerSoil DNA Isolation Kit (Mo Bio Laboratories).
The 16S rDNA V4 region was amplified by PCR and sequenced in the MiSeq platform (Illumina) using the 2 × 250-bp paired-end protocol, yielding pair-end reads that overlap almost completely. The primers used for amplification contain adapters for MiSeq sequencing and single-end barcodes, allowing pooling and direct sequencing of PCR products (16, 17). The read pairs were demultiplexed on the basis of unique molecular barcodes, and reads were merged using USEARCH version 7.0.1090 (18), allowing 0 mismatches and a minimum overlap of 50 bases. Merged reads were trimmed at first base with Q5. A quality filter was also applied to the resulting merged reads, and reads containing >0.05 expected errors were discarded.
Rarefaction curves of bacterial operational taxonomic units (OTUs) were constructed using sequence data for each sample to ensure coverage of the bacterial diversity present. Samples with suboptimal amounts of sequencings reads (<80% of the taxa are represented) were resequenced to ensure that most bacterial taxa were encompassed in our analyses.
16S rRNA gene sequences were clustered into OTUs at a similarity cutoff value of 97% using the UPARSE algorithm (19). OTUs were mapped to an optimized version of the SILVA Database containing only the 16S v4 region to determine taxonomies (19, 20). Abundances were recovered by mapping the demultiplexed reads to the UPARSE OTUs. A custom script constructed a rarefied OTU table from the output files generated in the previous 2 steps for downstream analyses of α diversity, β diversity, and phylogenetic trends (21, 22).
Statistical analysis
All values are reported as means ± SEMs. All between-group (control group compared with KPD) comparisons of demographic and experimental data were made using nonpaired t tests, and all within-group comparisons (euglycemia compared with hyperglycemia) were made using paired t tests for insulin and amino acid AUC after glucose and arginine bolus. Within each glycemic state, euglycemia or hyperglycemia, differences between the groups in kinetic data, and amino acid concentrations were analyzed by 2-factor repeated-measures ANOVA with group as the between factor and glycemic state as the repeated random factor. Post hoc comparisons were performed using Sidak's test. Comparisons between groups in the GPAIS protocol were made using the Mann-Whitney test. Associations between metabolic data and microbiome data were made using linear regression statistical tools. All analyses were performed with GraphPad Prism version 6 software (GraphPad Software). P values ≤0.05 were considered significant.
Results
Participant characteristics
All participants completed the stable isotope infusion protocols. Two control and 1 KPD participants did not complete the insulin secretion study following Protocol 2. Values for flux are reported per kg LBM/h.
Control and KPD participants were similar in age, sex, and BMI (Table 1). The mean duration of diabetes in the KPD group was 2.1 ± 0.4 y. There was no significant difference between groups in fasting glucose concentrations or in thyroid, renal, or liver function indices. All data in Table 1 were acquired at LabCorp (Burlington, NC).
TABLE 1.
Baseline characteristics of recent-onset KPD patients and control participants1
| Control (n = 6) | KPD (n = 6) | P | |
|---|---|---|---|
| Age, y | 45.8 ± 5.2 | 46.0 ± 1.8 | 0.98 |
| Male, % | 50.0 | 50.0 | 1.0 |
| BMI, kg/m2 | 31.3 ± 1.0 | 32.4 ± 1.2 | 0.52 |
| HbA1c, % | 5.77 ± 0.1 | 6.83 ± 0.4 | 0.04 |
| Fasting plasma glucose, mg/dL | 92.3 ± 3.5 | 101 ± 7.1 | 0.29 |
| Thyroid stimulating hormone, μIU/mL | 1.96 ± 0.3 | 2.54 ± 0.4 | 0.30 |
| Blood urea nitrogen, mg/dL | 13.5 ± 2.7 | 14.3 ± 2.1 | 0.81 |
| Creatinine, mg/dL | 0.92 ± 0.1 | 0.79 ± 0.1 | 0.27 |
| Aspartate aminotransferase, U/L | 22.3 ± 3.0 | 27.3 ± 3.4 | 0.30 |
| Alanine aminotransferase, U/L | 25.5 ± 4.8 | 34.0 ± 2.7 | 0.15 |
| Alkaline phosphatase, U/L | 75.2 ± 11.1 | 76.5 ± 7.8 | 0.92 |
1Data expressed as means ± SEMs or percentages. Between-group comparisons made by nonpaired t test. HbA1c, glycated hemoglobin; KPD, ketosis-prone diabetes.
Kinetic and plasma concentration measurements
The major metabolic pathways of arginine are via hydrolysis to urea and ornithine and via conversion to NO and citrulline. Remaining arginine is available for anabolic purposes and cellular functions such as insulin secretion.
Kinetic data prior to intravenous arginine bolus administration are presented in Table 2 and Figure 1. As shown in Table 2, there was no significant interaction between group and glycemic state for arginine flux or arginine synthesis; however, there was a significant main effect of glycemic state (euglycemic compared with hyperglycemic) on arginine flux (P < 0.0001) and arginine synthesis (P = 0.0004). Arginine flux and de novo arginine synthesis declined significantly in both groups in response to hyperglycemia (P < 0.05).
TABLE 2.
Arginine, nitric oxide, ornithine, citrulline, and phenylalanine kinetics under euglycemic and hyperglycemic conditions prior to intravenous arginine bolus administration in 6 recent-onset KPD patients and 6 age-, gender-, and BMI-matched control participants1
| Euglycemia | Hyperglycemia | |||
|---|---|---|---|---|
| Kinetics, | ||||
| μmol · kg LBM−1 · h−1 | Control (n = 6) | KPD (n = 6) | Control (n = 6) | KPD (n = 6) |
| Arginine flux2 | 55.2 ± 4.23 | 62.8 ± 3.70 | 44.5 ± 2.983 | 49.4 ± 2.203 |
| Arginine synthesis2 (from citrulline) | 7.83 ± 0.57 | 7.48 ± 0.28 | 5.30 ± 0.323 | 5.77 ± 0.673 |
| Arginine flux from synthesis, % | 14.4 ± 1.16 | 12.1 ± 0.78 | 13.1 ± 1.02 | 11.6 ± 1.09 |
| Ornithine flux2 | 24.1 ± 3.46 | 16.3 ± 1.204 | 15.0 ± 1.483 | 13.5 ± 0.81 |
| Nitric oxide synthesis | 0.18 ± 0.05 | 0.19 ± 0.02 | 0.19 ± 0.03 | 0.28 ± 0.07 |
| Citrulline flux2 | 9.17 ± 0.56 | 8.91 ± 0.45 | 7.16 ± 0.633 | 6.96 ± 0.813 |
| Phenylalanine flux5 | 35.7 ± 2.46 | 45.6 ± 2.194 | 36.6 ± 2.61 | 43.9 ± 1.56 |
1All values are means ± SEMs. Cell means were compared by repeated-measures 2-factor ANOVA. KPD, ketosis-prone diabetes; LBM, lean body mass.
2Main effect of glycemic state (euglycemic compared with hyperglycemic), P < 0.05.
3Significantly different from corresponding group in euglycemic condition, P < 0.05 (post hoc Sidak multiple comparison test).
4Significantly different from control group in corresponding clinical condition, P < 0.05 (post hoc Sidak multiple comparison test).
5Main effect of group (control compared with KPD), P < 0.05.
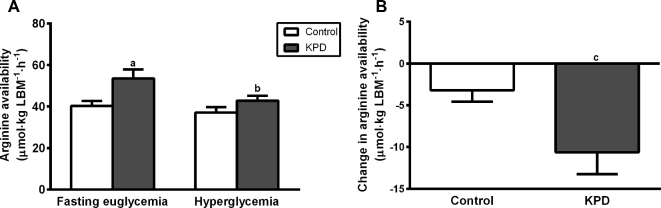
FIGURE 1.
Available arginine under euglycemic and hyperglycemic conditions (A) and the change in available arginine between the euglycemic and hyperglycemic conditions (B) in 6 recent-onset KPD patients and 6 age-, gender-, and BMI-matched control participants. Values are means ± SEMs. (A) Means were compared by repeated-measures 2-factor ANOVA. Significant interaction between group and glycemic state (P < 0.05), significant main effect of glycemic state and group (P < 0.05). Post hoc comparisons: asignificantly different from control group in corresponding clinical condition, P < 0.05 (post hoc Sidak multiple comparison test); bsignificantly different from corresponding group in euglycemic condition, P < 0.05 (post hoc Sidak multiple comparison test); csignificantly different from control group, P < 0.05 (t test). KPD, ketosis-prone diabetes; LBM, lean body mass.
When comparing plasma arginine concentration at 2 h after entering the hyperglycemic state (control group: 60.4 ± 7.15 μmol/L; KPD: 66.2 ± 7.40 μmol/L) and baseline in the euglycemic state (control group: 68.9 ± 3.68 μmol/L; KPD: 66.7 ± 6.73 μmol/L), there was no significant interaction between group and glycemic state and no main effect of group and glycemic state.
For available arginine, defined as total arginine flux minus endogenous ornithine flux, there was a significant interaction between group and glycemic state (P = 0.0413), significantly main effects of glycemic state (P = 0.0016) and group (P = 0.0497). Available arginine was significantly higher in KPD patients compared with control participants in the euglycemic condition (Figure 1A). In the hyperglycemic condition, available arginine declined significantly in KPD patients (from 53.5 ± 4.3 to 42.9 ± 2.4 μmol · kg LBM−1 · h−1, P = 0.0014). The magnitude of this change in arginine availability from the euglycemic to the hyperglycemic state was significantly greater in KPD patients compared with control participants (Figure 1B).
There was a significant main effect of glycemic state (euglycemic compared with hyperglycemic) on ornithine flux (P = 0.0029) (Table 2) and ornithine flux declined significantly only in the control group in response to hyperglycemia (P = 0.0051). In the euglycemic state, ornithine flux was significantly lower in the KPD group compared with the control group (P = 0.019). However, in the hyperglycemic state, there was no difference between the groups, because although flux decreased significantly in both control and KPD patients, the magnitude of the decline was much greater in control participants (38.2% compared with 17.2%; t test: P = 0.069). Figure 2 depicts plasma ornithine concentrations displayed as the AUC over 56 min following intravenous bolus arginine administration. Under euglycemic conditions, there was no difference in ornithine AUC between the groups following bolus arginine administration, and in response to hyperglycemia, ornithine AUC decreased significantly in both control and KPD patients. Because the magnitude of the decline in ornithine AUC in response to hyperglycemia was smaller in the KPD group compared with control participants (36% compared with 42%; t test: P = 0.905), ornithine AUC trended higher (P = 0.08) in KPD patients compared with control participants in the hyperglycemic state.
FIGURE 2.
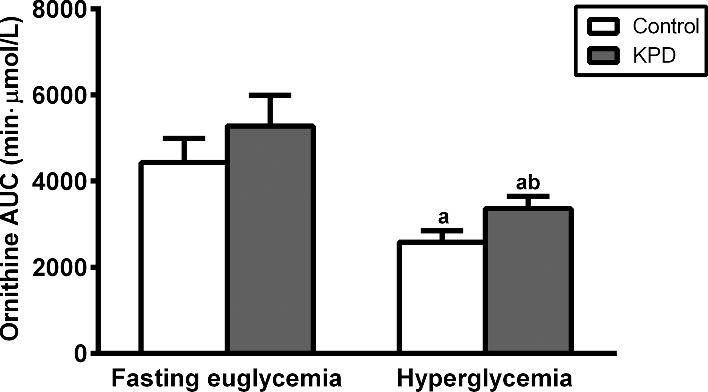
Plasma ornithine AUC from 0 to 56 min following intravenous arginine bolus in 6 recent-onset KPD patients and 6 age-, gender-, and BMI-matched control participants under euglycemic and hyperglycemic conditions. Values are means ± SEM, n = 6. aSignificantly different from corresponding group in euglycemic condition, P < 0.05 (paired t test); bsignificantly different from control group in corresponding clinical condition, P < 0.10 (nonpaired t test). KPD, ketosis-prone diabetes.
The conversion of arginine to citrulline and nitric oxide by nitric oxide synthase is an index of NO synthesis. As shown in Table 2, NO synthesis rate (measured prior to exogenous arginine administration) was similar in KPD patients and control participants in both the euglycemic and hyperglycemic conditions. KPD patients maintained a consistent production of NO in both conditions, as did control participants.
Because most citrulline circulating in the blood is derived from de novo synthesis in enterocytes (6), its flux is an estimate of its synthesis and availability for de novo arginine synthesis in other cells. As shown in Table 2, there was no significant interaction between group and glycemic state for citrulline flux; however, there was a significant main effect of glycemic state (euglycemic compared with hyperglycemic) on citrulline flux (P = 0.0021). Citrulline flux declined significantly in both control and KPD groups in response to hyperglycemia (P = 0.029 and 0.033, respectively). Citrulline concentration also declined during hyperglycemia in the control group (P = 0.019) (Table 3).
TABLE 3.
Arginine, ornithine, citrulline, phenylalanine concentrations under euglycemic and hyperglycemic conditions at 76 min after intravenous arginine bolus administration in 6 recent-onset KPD patients and 6 age-, gender-, and BMI-matched control participants1
| Euglycemia | Hyperglycemia | |||
|---|---|---|---|---|
| Concentration,2 μmol/L | Control (n = 4) | KPD (n = 5) | Control (n = 4) | KPD (n = 5) |
| Arginine | 210 ± 13.1 | 228 ± 21.5 | 157 ± 13.5 | 218 ± 12.83 |
| Ornithine | 90.6 ± 3.44 | 95.9 ± 9.29 | 59.7 ± 8.524 | 65.7 ± 4.824 |
| Citrulline | 32.6 ± 3.01 | 27.9 ± 2.61 | 23.9 ± 5.754 | 23.2 ± 2.63 |
| Phenylalanine | 45.1 ± 2.32 | 52.4 ± 2.65 | 40.1 ± 4.34 | 44.7 ± 1.724 |
1All values are means ± SEMs. Cell means were compared by repeated measures 2-factor ANOVA. KPD, ketosis-prone diabetes.
2Main effect of glycemic state (euglycemic compared with hyperglycemic), P < 0.05.
3Significantly different from control group in corresponding clinical condition, P < 0.05 (post hoc Sidak multiple comparison test).
4Significantly different from corresponding group in euglycemic condition, P < 0.05 (post hoc Sidak multiple comparison test).
For phenylalanine flux, a measure of total body protein breakdown rate, there was no significant interaction between group and glycemic state; however, there was a significant main effect of group (control compared with KPD) on phenylalanine flux (P = 0.0169). Phenylalanine flux was significantly higher in KPD patients compared with control participants during euglycemia, and flux did not change significantly in response to hyperglycemia for either group (Table 2). In response to hyperglycemia, phenylalanine concentration decreased significantly in the KPD group (P = 0.047) but not in the control group following bolus arginine administration (Table 3). As a consequence, there was no difference in plasma phenylalanine concentrations between the groups during hyperglycemia (P = 0.46).
Insulin secretion
In the euglycemic study, plasma glucose concentrations prior to the glucose bolus were 98.9 ± 4.7 mg/dL in KPD patients and 95.8 ± 4.1 mg/dL in control participants, and glucose concentrations prior to the arginine bolus were 96.2 ± 5.2 mg/dL in KPD patients and 97.8 ± 4.5 mg/dL in control participants. In the hyperglycemic study, plasma glucose concentrations prior to the glucose bolus were 92.4 ± 5.7 mg/dL in KPD patients and 96.5 ± 11.7 mg/dL in control participants, and glucose concentrations prior to the arginine bolus were 106.7 ± 6.2 mg/dL in KPD patients and 104.8 ± 4.6 mg/dL in control participants.
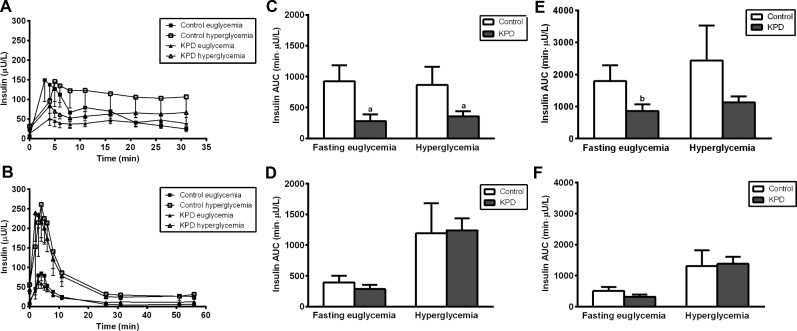
There was diminished insulin secretion in response to the glucose bolus in KPD patients compared with control participants in both the euglycemic and hyperglycemic conditions (Figure 3A), and this was manifested primarily during the first phase of insulin secretion in both conditions (Figure 3C). In contrast, the insulin secretory response to the arginine bolus in KPD patients was similar to that of control participants in both the euglycemic and hyperglycemic conditions (Figure 3B); in particular, the first-phase insulin secretory response was completely restored to normal following intravenous bolus arginine in both conditions (Figure 3D).
FIGURE 3.
Plasma insulin concentrations (secretory response) (A, B), first-phase plasma insulin AUC (C, D), and total plasma insulin AUC (E, F) after glucose bolus (A, C, E) and after arginine bolus (B, D, F) in recent-onset KPD patients and age-, gender-, and BMI-matched control participants, following euglycemic and hyperglycemic conditions. Values are means ± SEMs, n = 4 for control participants and n = 5 for KPD. aSignificantly different from control group in corresponding clinical condition: aP < 0.05; bP < 0.10 (t test). KPD, ketosis-prone diabetes.
Total insulin secretion AUC, in response to glucose, was lower in KPD patients compared with control participants in both euglycemic and hyperglycemic conditions (Figure 3E). However, in response to intravenous bolus arginine, total insulin secretion AUC was as robust in the KPD patients as in the control participants in both conditions (Figure 3F).
Collectively, these findings suggested that exogenous arginine could normalize insulin secretion under hyperglycemic conditions in KPD patients. To test this hypothesis more rigorously, GPAIS was determined in a separate cohort of 6 KPD patients with the same clinical status as the KPD patients described above, and 3 nondiabetic obese control participants.
GPAIS
In the GPAIS study, adequacy of first-phase insulin secretion was assessed as the mean of the first 3 peak insulin values. The acute insulin response to glucose was markedly blunted in the KPD patients (P = 0.02) (Figure 4); however, following bolus arginine, acute insulin secretion was not significantly different between KPD patients and control participants. Arginine stimulus superimposed upon the hyperglycemic stimulus restored the insulin secretory response in the KPD patients to the value of the control participants (P = 0.55).
FIGURE 4.
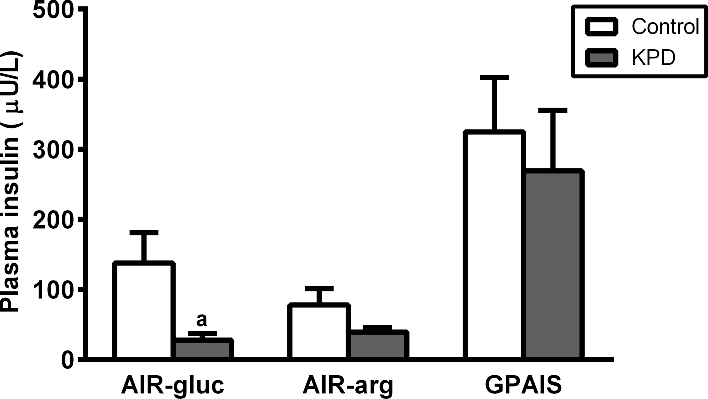
Mean of 3 peak insulin values during first-phase insulin secretion in response to glucose bolus (AIR-gluc), arginine bolus (AIR-arg), and glucose + arginine (GPAIS), in 6 recent-onset KPD patients and 3 control participants. Values are means ± SEMs. aSignificantly different from control group, P < 0.05 (t test). AIR, acute insulin response; GPAIS, glucose potentiation of arginine-induced insulin secretion; KPD, ketosis-prone diabetes.
Gut microbiome
The intestinal microbiome of KPD patients had decreased abundance of Bacteroidetes and increased abundance of Firmicutes compared with control participants (Figure 5), consistent with published literature of patterns of bacterial taxa in patients with type 2 diabetes (23). β Diversity was significantly different between control participants and KPD patients (R2 = 0.12, P = 0.03). Figure 6 shows the relation of ornithine flux to bacterial richness (defined as OTUs) (Figure 6A) and to bacterial evenness [defined by the Shannon diversity index (SDI)] (Figure 6B). Across all samples from control participants and KPD patients, ornithine flux was inversely associated with both OTUs and SDI. A significant driver of this difference was the positive correlation of relative abundance of Bacteroidetes with ornithine flux and the negative correlation of relative abundance of Firmicutes with ornithine flux (Figure 6C, D).
FIGURE 5.
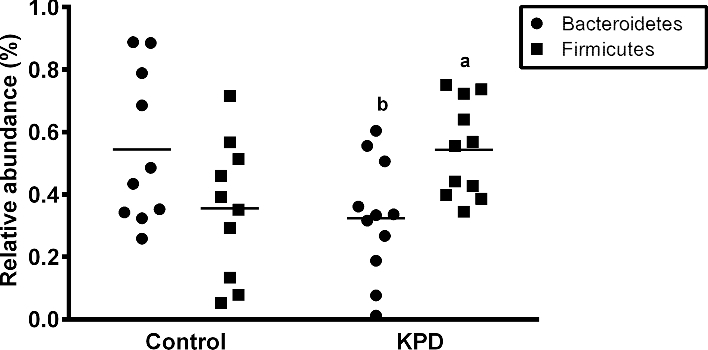
Relative abundance of Bacteroidetes and Firmicutes within the intestinal microbiome of 6 recent-onset KPD patients and 6 age-, gender-, and BMI-matched control participants. Values are individuals with mean (–). a,bSignificantly different from the control group: aP < 0.05; bP < 0.10 (paired t test). KPD, ketosis-prone diabetes.
FIGURE 6.
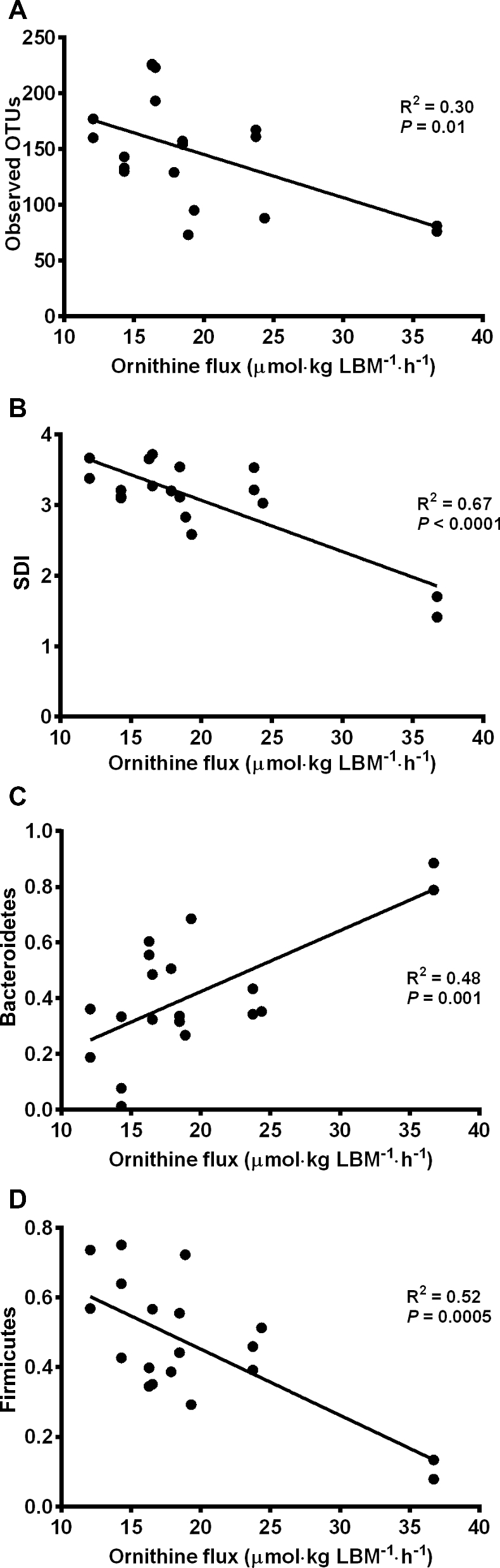
Correlation of bacterial richness using OTUs (A), bacterial evenness using SDI (B), Bacteroidetes relative abundance (C), and Firmicutes relative abundance (D) with ornithine flux in all 12 study participants [6 recent-onset KPD patients and 6 age-, gender-, and BMI-matched control participants]. KPD, ketosis-prone diabetes; LBM, lean body mass; OTU, organizational taxonomic unit; SDI, Shannon diversity index.
Discussion
KPD patients have increased arginine availability compared with the control group in the euglycemic fasting state, suggesting that they have a higher requirement for arginine to maintain physiologic and metabolic homeostasis. The significant reduction in arginine availability in response to hyperglycemia in KPD patients suggests that they may have relatively less arginine available to sustain critical physiologic and metabolic functions such as insulin secretion. Indeed, KPD patients were unable to mount a significant insulin secretory response to a bolus of glucose in the face of a hyperglycemic stress, but the administration of exogenous arginine restored their insulin secretory response to the level of control participants (Figures 3 and 4).
In the fasting state, arginine is produced primarily from protein breakdown and de novo synthesis from citrulline. Although virtually all of citrulline flux is used to synthesize arginine (24), this accounts for only 5–15% of endogenous arginine flux in humans, as whole-body protein turnover accounts for 85–95% (6). In the current study, although citrulline flux and its conversion to arginine decreased in both groups in response to hyperglycemia (Table 2), the overall effect on arginine availability was minor as its contribution to arginine flux was only ∼13% in both the euglycemic and hyperglycemic states. In agreement with the findings of Castillo et al. (24), this means that protein breakdown accounted for ∼87% of arginine produced in the fasted state in both control participants and KPD patients. Indeed, the principal contributor to the higher arginine availability in the KPD patients during euglycemia seems to be persistently increased protein breakdown, as indicated by faster phenylalanine fluxes compared with control participants. However, although KPD patients break down more protein to maintain their arginine supply and pool during euglycemia, their arginine availability is compromised because they seem to hydrolyze more arginine to ornithine in response to exogenous arginine administration.
The bulk of arginine produced is reused to synthesize body proteins, while the rest is hydrolyzed to ornithine and urea and to a lesser extent used to synthesize NO, creatine, and agmatine (6). As shown in Table 2, the amount of NO produced from arginine was minor when compared with arginine flux and its availability (Figure 1A), indicating that the nitric oxide synthase pathway is not a primary site of defective arginine metabolism in KPD patients. In the fasting euglycemic state, ornithine flux, an index of nonhepatic arginine hydrolysis, was actually lower in the KPD patients compared with control participants, suggesting that this was another arginine-sparing mechanism used to sustain a higher availability of arginine. However, in response to a relatively large exogenous dose of arginine, the greater ornithine AUCs of KPD patients during both euglycemia and hyperglycemia indicate higher rates of arginine catabolism via hydrolysis (Figure 2). Because of the relatively high activity of mucosal arginase it is known that ∼40% of dietary arginine is degraded during the first pass (25). The implication of our current finding is that after a meal, KPD patients will have even less than 60% dietary arginine available for metabolic purposes and to replenish arginine pools, because dietary-derived arginine entering the circulation will be subjected to increased hydrolysis. The potential “compensatory response” of accelerated proteolysis to provide more arginine in the fasting euglycemic state may be absent or insufficient to sustain availability of arginine in KPD patients in the fed state, when they might also experience hyperglycemia. Thus, KPD patients, especially while hyperglycemic, may have an inadequate reserve of intracellular arginine for critical metabolic functions such as insulin secretion. Indeed, we found that KPD patients were unable to mount a significant insulin secretory response to an intravenous bolus of glucose in the face of hyperglycemic stress, but a large dose of exogenous arginine restored the insulin secretory response to the value of control participants under hyperglycemic conditions (Figures 3D, 3F, and 4). These data are consistent with data from an earlier study by Gosmanov et al. (26) in patients with the phenotype of A-β + KPD which showed that an arginine bolus under hyperglycemic conditions stimulated first-phase insulin responses that were indistinguishable from those of obese, nondiabetic control subjects. Together, these findings strongly suggest that decreased arginine availability during hyperglycemic stress is a critical but readily reversible defect in KPD patients. They provide a strong rationale for a trial of supplementation of arginine or its precursor citrulline to prevent β cell decompensation and episodes of unprovoked ketoacidosis in KPD patients (6). Interestingly, this may not be the case for patients with “typical” type 2 diabetes; Ward et al. (9) showed that insulin concentrations in response to arginine supplementation were lower in such patients compared with control subjects at all matched glucose concentrations.
Bacteria in the gastrointestinal tract utilize dietary-derived amino acids for protein synthesis and production of metabolites, including NO and polyamines, products of arginine metabolism (27). In mice, dietary supplementation with arginine induces a shift in the Firmicutes-to-Bacteroidetes ratio (28). KPD patients and control participants had distinct gut microbiome compositions. In both groups, there was an inverse relation between ornithine flux and both OTUs and the SDI: as endogenous ornithine flux increased, both the number of bacterial subtypes and the evenness of their distribution decreased. Firmicutes followed this relation with a strong association between higher ornithine flux and lower relative abundance of Firmicutes. Inversely, higher Bacteroidetes relative abundance was associated with higher ornithine flux. Because arginine is hydrolyzed to ornithine, higher ornithine fluxes imply lower available arginine reserves, hence ornithine flux serves as a surrogate for arginine availability.
We have demonstrated that excess arginine hydrolysis to ornithine is likely responsible for the relative reduction in arginine supply in KPD patients; there is also an inverse relation between ornithine flux and bacterial richness and diversity as well as a corresponding trend of increasing ornithine flux associated with higher relative abundance of Bacteroidetes and lower relative abundance of Firmicutes.
Collectively, the kinetic, insulin secretion, and gut microbiome data suggest that key defects in arginine metabolism render KPD patients unable to augment insulin secretion in response to hyperglycemia. When supplied with exogenous arginine, insulin secretion in KPD patients improves to normal values. Further studies examining nutritional supplementation with arginine or its primary precursor citrulline would be important in validating these findings as well as potentially reversing a unique metabolic defect in A-β + KPD patients.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—FJ and AB: designed the research, interpreted the data, and had primary responsibility for the final content; NR: recruited participants and managed clinical care; SNM, RG, and AG: recruited the participants; SNM, RG, AG, JWH, and KMB: performed the experiments; JWH and KMB: performed the MS, biochemical analyses, and calculations; DI: performed the hormonal assays; CSH: performed autoantibody analyses to phenotype the KPD patients; NJA and JFP: performed the microbiome analyses; SNM: wrote the paper; and all authors: edited and approved the final manuscript.
Notes
Supported by a pilot award from the Alkek Center for Metagenomics and Microbiome Research at Baylor College of Medicine (to AB) and NIH RO1-DK1041411 (to FJ and AB).
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used:
- AIR
acute insulin response
- DKA
diabetic ketoacidosis
- GPAIS
glucose potentiation of arginine-induced insulin secretion
- KPD
ketosis-prone diabetes
- LBM
lean body mass
- OTU
organizational taxonomic unit
- SDI
Shannon diversity index.
References
- 1. Patel SG, Hsu JW, Jahoor F, Coraza I, Bain JR, Stevens RD, Iyer D, Nalini R, Ozer K, Hampe CS, et al. Pathogenesis of A(-)beta(+) ketosis-prone diabetes. Diabetes 2013;62:912–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kashyap SR, Lara A, Zhang R, Park YM, DeFronzo RA. Insulin reduces plasma arginase activity in type 2 diabetic patients. Diabetes Care 2008;31:134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmidt HH, Warner TD, Ishii K, Sheng H, Murad F. Insulin secretion from pancreatic B cells caused by L-arginine-derived nitrogen oxides. Science 1992;255:721–3. [DOI] [PubMed] [Google Scholar]
- 4. Floyd JC, Jr., Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest 1966;45:1487–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morris SM., Jr Arginine: beyond protein. Am J Clin Nutr 2006;83:S508–12. [DOI] [PubMed] [Google Scholar]
- 6. Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J 1998;15:336:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maldonado M, Hampe CS, Gaur LK, D'Amico S, Iyer D, Hammerle LP, Bolgiano D, Rodriguez L, Rajan A, Lernmark A, et al. Ketosis-prone diabetes: dissection of a heterogeneous syndrome using an immunogenetic and beta-cell functional classification, prospective analysis, and clinical outcomes. J Clin Endocrinol Metab 2003;88:5090–8. [DOI] [PubMed] [Google Scholar]
- 8. Teuscher AU, Kendall DM, Smets YF, Leone JP, Sutherland DE, Robertson RP. Successful islet autotransplantation in humans: functional insulin secretory reserve as an estimate of surviving islet cell mass. Diabetes 1998;47:324–30. [DOI] [PubMed] [Google Scholar]
- 9. Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1984;74:1318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seaquist ER, Robertson RP. Effects of hemipancreatectomy on pancreatic alpha and beta cell function in healthy human donors. J Clin Invest 1992;89:1761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Teuscher AU, Seaquist ER, Robertson RP. Diminished insulin secretory reserve in diabetic pancreas transplant and nondiabetic kidney transplant recipients. Diabetes 1994;43:593–8. [DOI] [PubMed] [Google Scholar]
- 12. Thame MM, Fletcher HM, Baker TM, Marini JC, Kao CC, Jahoor F. Arginine flux, but not nitric oxide synthesis, decreases in adolescent girls compared with adult women during pregnancy. J Nutr 2011;141:71–4. [DOI] [PubMed] [Google Scholar]
- 13. Kurpad AV, Kao C, Dwarkanath P, Muthayya S, Mhaskar A, Thomas A, Vaz M, Jahoor F. In vivo arginine production and nitric oxide synthesis in pregnant Indian women with normal and low body mass indices. Eur J Clin Nutr 2009;63:1091–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Human Microbiome Project C A framework for human microbiome research. Nature 2012;486:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Human Microbiome Project C Structure, function and diversity of the healthy human microbiome. Nature 2012;486:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 2012;6:1621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010;26:2460–1. [DOI] [PubMed] [Google Scholar]
- 19. Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 2013;10:996–8. [DOI] [PubMed] [Google Scholar]
- 20. Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glockner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 2013;41:D590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 2005;71:8228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J 2011;5:169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Remely M, Aumueller E, Merold C, Dworzak S, Hippe B, Zanner J, Pointner A, Brath H, Haslberger AG. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene 2014;537:85–92. [DOI] [PubMed] [Google Scholar]
- 24. Castillo L, Beaumier L, Ajami AM, Young VR. Whole body nitric oxide synthesis in healthy men determined from [15N] arginine-to-[15N]citrulline labeling. Proc Natl Acad Sci U S A 1996;93:11460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Castillo L, Chapman TE, Yu YM, Ajami A, Burke JF, Young VR. Dietary arginine uptake by the splanchnic region in adult humans. Am J Physiol 1993;265:E532–9. [DOI] [PubMed] [Google Scholar]
- 26. Gosmanov AR, Smiley D, Robalino G, Siqueira JM, Peng L, Kitabchi AE, Umpierrez GE. Effects of intravenous glucose load on insulin secretion in patients with ketosis-prone diabetes during near-normoglycemia remission. Diabetes Care 2010;33:854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dai Z, Wu Z, Hang S, Zhu W, Wu G. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol Hum Reprod 2015;21:389–409. [DOI] [PubMed] [Google Scholar]
- 28. Ren W, Chen S, Yin J, Duan J, Li T, Liu G, Feng Z, Tan B, Yin Y, Wu G. Dietary arginine supplementation of mice alters the microbial population and activates intestinal innate immunity. J Nutr 2014;144:988–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.