ABSTRACT
Backgrounds: Klotho protein interacts with the transforming growth factor β (TGF-β) receptor and Wnt, which contribute to the progression of renal disease, inhibiting their signals. Renal and circulating klotho levels are diminished in chronic kidney disease.
Methods: Experiments were performed to assess whether supplementation of klotho protein could have protective effects on the kidney. Rats were injected with adriamycin (5 mg/kg) and divided into three groups: those treated with vehicle, those treated with klotho protein and those treated with klotho plus 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD). Rats without adriamycin treatment were used as a control.
Results: Adriamycin reduced the serum klotho concentration and renal expression of klotho and E-cadherin. Adriamycin also increased the renal expression of Wnt, TGF-β, and angiotensinogen, as well as the renal abundance of β-catenin and angiotensin II. Klotho supplementation suppressed adriamycin-induced elevations of β-catenin and angiotensin II with sustained Wnt expression. Combined treatment with klotho and TDZD reversed the klotho-induced improvements in the renal abundance of β-catenin and angiotensin II as well as the expression of TGF-β and angiotensinogen without affecting E-cadherin.
Conclusions: Our data indicate that Wnt is involved in the pathogenesis of adriamycin nephropathy. Furthermore, klotho supplementation inhibited Wnt signaling, ameliorating renal angiotensin II. Finally, klotho protein appears to suppress epithelial–mesenchymal transition by inhibiting TGF-β and Wnt signaling.
Keywords: β-catenin, fibrosis, nephrin, oxidative stress, TRPC6
INTRODUCTION
Proteinuria is a strong risk factor for both end-stage renal disease and cardiovascular death [1, 2]. Podocyte injury reduces the expression of nephrin, leading to proteinuria [3]. Several types of genetic abnormalities related to podocytes cause nephrosis. Mutations in the genes encoding nephrin, podocin and transient receptor potential channel 6 (TRPC6) result in congenital nephrotic syndrome of the Finnish type, autosomal recessive steroid-resistant nephrotic syndrome and the autosomal dominant form of focal segmental glomerulosclerosis (FSGS). A gain-of-function mutation of TRPC6 in podocytes allows calcium entry that stimulates the calcineurin pathway and activates nuclear factor of activated T-cells (NFAT), playing a role in the development of FSGS [4, 5]. Acquired forms of nephropathies such as FSGS are also characterized by TRPC6 induction [6]. Nephropathies can lower vitamin D levels. Vitamin D is known to downregulate the expression of TRPC6 in podocytes [7]. Alternatively, calcium entry through TRPC is involved in efferent arteriolar constriction by angiotensin II (AngII) [8]. Indeed, various pathophysiological stimuli induce TRPC6 in podocytes, including oxidative stress, high glucose, stretch, complement, insulin and AngII [9–14].
Adriamycin nephropathy is an experimental model of FSGS [15]. FSGS frequently manifests as a steroid-resistant nephrotic syndrome, which frequently leads to end-stage renal failure. The above findings have provided an experimental basis for the clinical application of calcineurin inhibitors in the treatment of FSGS. Several lines of evidence indicate that oxidative stress, AngII and/or direct toxic action underlie the mechanism of adriamycin-induced FSGS [16, 17]. Recent findings reveal that Wnt/β-catenin signaling is involved in the pathogenesis of adriamycin nephropathy and FSGS [18–23]. Adriamycin administration induces the expression of Wnt in the kidney, along with upregulation of β-catenin in the podocytes and renal tubular cells. Thus any approaches that inhibit Wnt signaling may ultimately arrest the progression of adriamycin nephropathy, indicating a new potential treatments of choice for FSGS.
The Wnt family of proteins can be secreted, and bind to the Frizzled receptor [24]. Mutations in the Wnt pathway components lead to specific developmental defects and various human diseases, including cancer [25]. Although Wnt transduces its signal using various pathways, the classical pathway of Wnt involves the inactivation of glycogen synthase kinase-3β (GSK3β) and subsequent stabilization of β-catenin [26–28]. The latter can translocate into the nucleus to modulate the cell cycle and survival. GSK3β is a serine–threonine kinase that is constitutively active to phosphorylate glycogen synthase and β-catenin, thereby undergoing ubiquitination for degradation. Thus the inhibition of GSK3β can mimic some aspects of Wnt signaling.
Klotho, an anti-aging protein, is expressed mainly in the kidney and can be secreted into the systemic circulation [29]. The membrane-bound klotho molecule is enzymatically cleaved to release its extracellular domain into the interstitium [30, 31]. Klotho is also a well-known endogenous inhibitor of Wnt signaling [24]. Secreted klotho can tightly bind to various proteins, including Wnt [24, 30, 32], and inhibits Wnt activity upon binding. Klotho-deficient animals are used as a model of aging, and Wnt protein plays an important role in regulating self-renewal signals of stem cells [24]. Klotho expression is reduced in various kidney diseases, including adriamycin nephropathy [19]. A vicious cycle may exist between Wnt and klotho in various nephropathies. Our recent data suggest that the induction of endogenous klotho protein by either vitamin D or a calcilytic agent slows the progression of renal injury from hypertension and renal mass reduction [33–35]. Vitamin D decreased proteinuria in adriamycin nephropathy [7]. Klotho gene delivery ameliorated the Wnt-triggered activation of β-catenin, proteinuria and interstitial fibrosis as well as the reduced expression of nephrin in adriamycin nephropathy. However, the effects of exogenous klotho protein supplementation on adriamycin nephropathy have not been examined. We recently demonstrated that recombinant human klotho protein ameliorated calcium and phosphate abnormalities in klotho-deficient animals, supporting its physiological activity [30]. The present study was performed to assess whether exogenous klotho protein supplementation ameliorates adriamycin nephropathy, including the renin–angiotensin system (RAS). The influences of an inhibitor for GSK3β [27], 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD), were assessed to identify the sites of actions on RAS by exogenous klotho protein supplementation, which seems ethically more acceptable for patients than gene transfer.
MATERIALS AND METHODS
Animal model
Seven-week-old male Wistar rats were used in this study (Shizuoka Laboratory Animal Center, Shizuoka, Japan). The animals were allowed ad libitum access to tap water and standard rat chow (CE-2, Nihon CLEA, Tokyo, Japan). All experimental protocols were approved by the ethical committee for animal research of Saitama Medical University (permit number 894). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All efforts were made to minimize the suffering of animals. For acclimation, each rat was housed separately in a metabolic cage for 1 week in a temperature-controlled room with a 12-h light–dark cycle and fed the standard diet [35].
Under pentobarbital anesthesia (50 mg/kg intraperitoneally), adriamycin (5 mg/kg) was injected intravenously into 8-week-old rats [15]. The next day, animals were divided into three groups: the first group was treated with the vehicle (A; n = 8), the second group received daily subcutaneous injection of recombinant human klotho protein (516 amino acids) for 4 weeks (10 μg/kg/day, A+K; n = 8) [30] and the third group was treated daily with both klotho and TDZD (0.33 mg/kg/day, A+K+T; n = 8) [5, 27, 28]. Rats that did not receive adriamycin were treated with the vehicle alone as the control (C; n = 8). Urine was collected to measure creatinine, albumin, klotho and 8-epi-prostaglandin F2α (PGF2α) every 2 weeks [33, 36]. Blood pressure was measured by tail cuff methods. Four weeks later, animals were anesthetized with pentobarbital to harvest the kidney and blood samples. The femoral artery was cannulated with polyethylene tubing (PE50) to draw blood samples. The kidneys and thorax aorta were removed. The removed kidneys were weighed: one was quickly frozen with liquid nitrogen for chemical analysis, including measurements of AngII and klotho [37], and the other was fixed in a 4% formalin solution for pathology. The animals were then euthanized with overdoses of anesthesia. The aorta was frozen until the assay. Blood samples were centrifuged at 4°C for 10 min. Serum, plasma, urine and tissues were kept deep-frozen until the assays. Plasma AngII, renin activity (PRA), serum klotho and creatinine were measured. Creatinine clearance was used to estimate the glomerular filtration rate [33].
Data are expressed as mean ± SEM. Statistical analyses were performed using one- and two-way analysis of variance followed by the Newman–Keuls test. A P-value <0.05 was considered statistically significant.
Detailed methods are attached as Supplementary data.
RESULTS
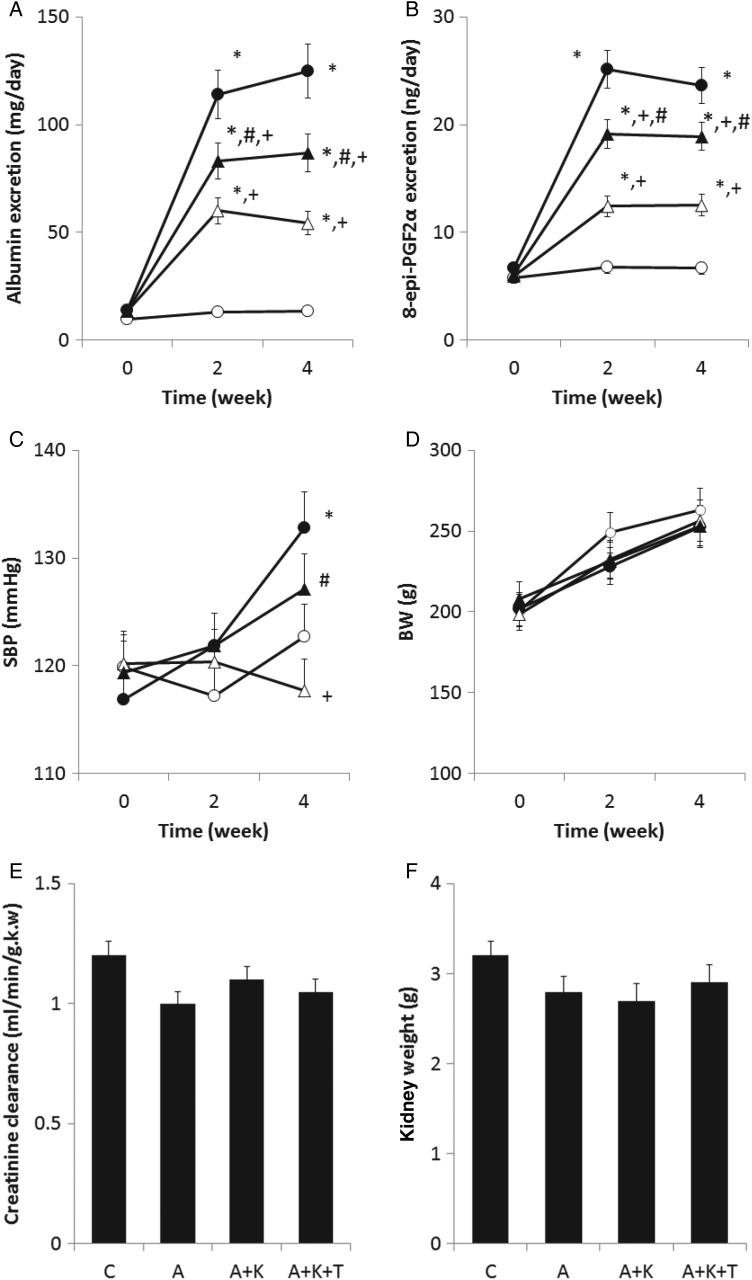
As shown in Figure 1A, albuminuria was gradually elevated over time after adriamycin injection. Group A excreted the greatest amount of albumin. Although supplemental klotho treatment significantly reduced albuminuria, albuminuria in the A+K group was higher than that in the C group. Addition of TDZD to the klotho supplement slightly reversed the klotho-induced reduction of albuminuria. Similar trends were observed with respect to PGF2α excretion (Figure 1B). Rats in group A excreted the largest amount of PGF2α, suggesting massive oxidative stress in this group. Klotho supplementation considerably reduced PGF2α excretion and the combined treatment with klotho and TDZD marginally increased PGF2α excretion. Systolic blood pressure (SBP) increased following the administration of adriamycin (Figure 1C). At experimental Week 4, SBP in group A was higher than that in group C. Furthermore, SBP in the A+K group was lower than that in group A at Week 4, suggesting that klotho supplementation decreased blood pressure. SBP in the A+K+T group was higher than that in the A+K group, indicating that additional treatment with TDZD elevated SBP. Body weights increased with time in all groups, with no significant differences among the four groups (Figure 1D). Although the A, A+K and A+K+T groups excreted a substantial amount of albuminuria, both the kidney weight and creatinine clearance were similar among the four groups (Figure 1E and F).
FIGURE 1.
Temporal profiles of (A) albuminuria and (B) 8-epi-prostaglandin (PG) F2α excretion, (C) systolic blood pressure (SBP), (D) body weight (BW), (E) creatinine clearance and (F)kidney weight. Open and closed circles and open and closed triangles indicate the C, A, A+K and A+K+T groups, respectively (n = 8 for each group). *, + and # indicate a significant difference from the C, A and A+K groups, respectively.
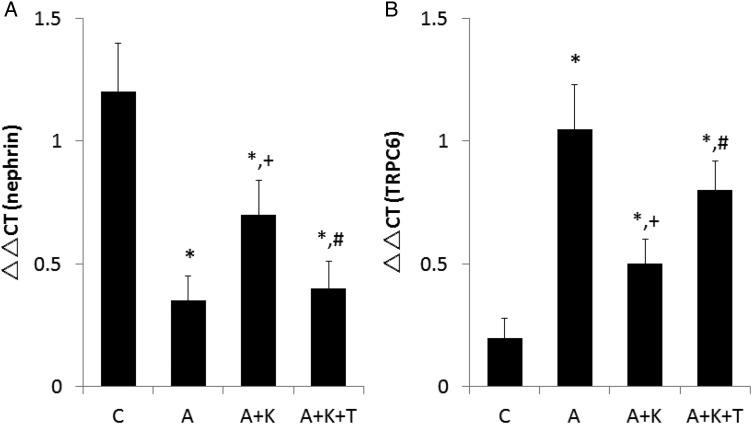
Immunohistochemistry revealed that adriamycin administration reduced the intensity of nephrin staining (Supplementary data, Figure S1). Although klotho supplementation enhanced nephrin staining to some degree, the addition of TDZD to klotho reversed this effect. Reverse transcription polymerase chain reaction (RT-PCR) analyses for nephrin gene expression showed similar trends (Figure 2A). Only weak staining of TRPC6 was observed in group C, whereas TRPC6 staining was considerably stronger in group A (Supplementary data, Figure S2). Klotho supplementation significantly attenuated the degree of TRPC6 staining, and TDZD enhanced it. TRPC6 expression assessed by RT-PCR was consistent with the immunohistochemistry results (Figure 2B). Although glomerular expression of nephrin was similar between the A and A+K+T groups, both albuminuria and PGF2α excretion in group A were higher than in the A+K+T group. Vide infra, the differences in blood pressure, plasma AngII concentration and aortic superoxide dismutase (SOD) between these two groups could contribute to the diverse results [38].
FIGURE 2.
Glomerular expression of (A) nephrin and (B) transient receptor potential channel 6 (TRPC6). *, + and # indicate a significant difference from the C, A and A+K groups, respectively (n = 8 for each group). The possibility that pathways other than TRPC6 are involved in decreasing the glomerular expression of nephrin cannot be excluded. In patients with FSGS, β-catenin was translocated to the nuclei of podocytes [21]. GSK3β phosphorylates NFAT to oppose its activation [22], and activation of NFAT alone can cause glomerulosclerosis by stimulating Wnt formation [5]. Thus, NFAT activation may form a vicious cycle with Wnt, and klotho supplementation may help break this cycle. In addition to oxidative stress itself, calcium entry through TRPC6 could activate extracellular signal-regulated kinase, contributing to the pathogenesis of FSGS [23].
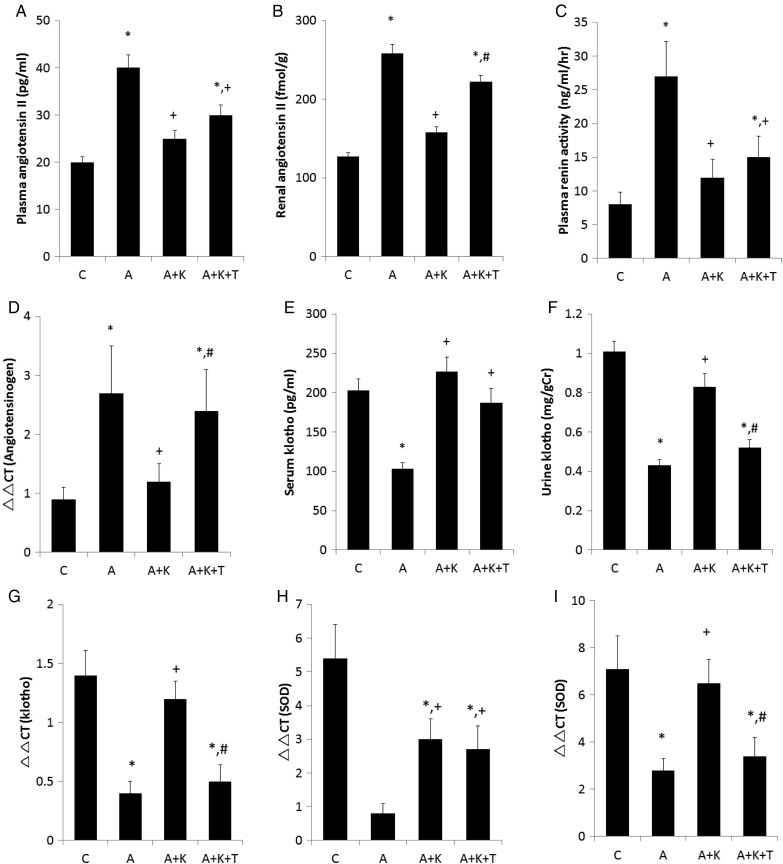
Adriamycin treatment considerably elevated PRA and AngII concentrations (Figure 3A and C). Both renal expression of angiotensinogen and AngII concentration in group A were also higher than in group C (Figure 3B and D). Exogenous klotho supplementation reduced PRA, renal expression of angiotensinogen and plasma and renal AngII levels in rats administered adriamycin. Renal expression of angiotensinogen and renal AngII concentration in the A+K+T group were substantially increased compared with those of the A+K group. There was no significant difference in PRA and plasma AngII levels between the A+K and A+K+T groups. The observation that blood pressure in the A+K+T group was higher than that of the A+K group can account for the lack of elevation of PRA in the A+K+T group and supports the notion that renal RAS is controlled independently of systemic RAS. The circulating klotho concentration was reduced by the administration of adriamycin and was normalized with exogenous klotho supplementation either in the presence or absence of TDZD (Figure 3E). Adriamycin also diminished klotho excretion, which was restored by klotho supplementation. However, klotho excretion in the A+K+T group was lower than that in the A+K group (Figure 3F). Renal klotho expression exhibited similar trends to those of klotho excretion (Figure 3G). As transcellular transport rather than glomerular filtration or direct secretion into the lumen plays an important role in klotho excretion [31], it reflects the renal interstitial klotho levels. Adriamycin reduced aortic and renal expression of SOD and klotho supplementation reversed the decrements of aortic and renal SOD (Figure 3H and I). Treatment with TDZD lowered renal but not aortic SOD. Moreover, aortic and renal expression of SOD showed similar trends with those of serum and urine klotho, respectively.
FIGURE 3.
Effects of adriamycin and klotho supplementation on (A) plasma and (B) renal angiotensin II, (C) plasma renin activity, (D) renal angiotensinogen, (E and F) serum and urine klotho, (G) renal klotho expression and superoxide dismutase (SOD) expression in the (H) aorta and (I) kidney. *, + and # indicate a significant difference from the C, A and A+K groups, respectively (n = 8 for each group).
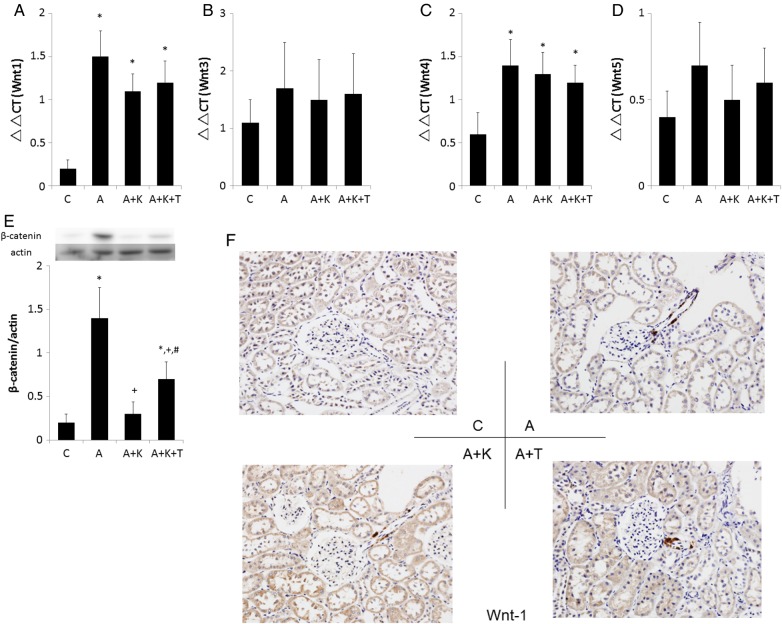
Adriamycin administration increased the renal expression of Wnt1 regardless of the presence of klotho and TDZD (Figure 4A). Adriamycin marginally enhanced renal expression of Wnt4 without significant changes in Wnt3 and Wnt5 (Figure 4B–D). Thus, the abundance of β-catenin was elevated in group A compared with that in group C (Figure 4E). However, klotho supplementation significantly reduced the abundance of β-catenin. Furthermore, β-catenin abundance in the kidneys of the A+K+T group was higher than that in the A+K group. The present findings that combined treatment with klotho and TDZD stabilized β-catenin indicated that the dose of TDZD used was sufficient to inhibit GSK3β activity [28]. The immunohistochemistry data have for the first time demonstrated that Wnt1 is expressed in the afferent arteriole (Figure 4F) as well as in the tubulointerstitium (Supplementary data, Figure S3) among all adriamycin-treated animals. However, Wnt1 was not significantly stained in the glomeruli among four groups.
FIGURE 4.
Impacts of adriamycin and klotho on (A–D) renal expression of Wnts and (E) abundance of β-catenin. (F) The trends were similar for the immunostaining of Wnt in the glomerular regions. The upregulation of Wnt signaling in podocytes disrupts the glomerular structure, resulting in a collapsing variant of FSGS [20]. Inhibition of Wnt signaling normalizes podocytes, including rapid cell-cycle exit, re-expression of differentiation markers and improved barrier function. *, + and # indicate a significant difference from the C, A and A+K groups, respectively (n = 8 for each group).
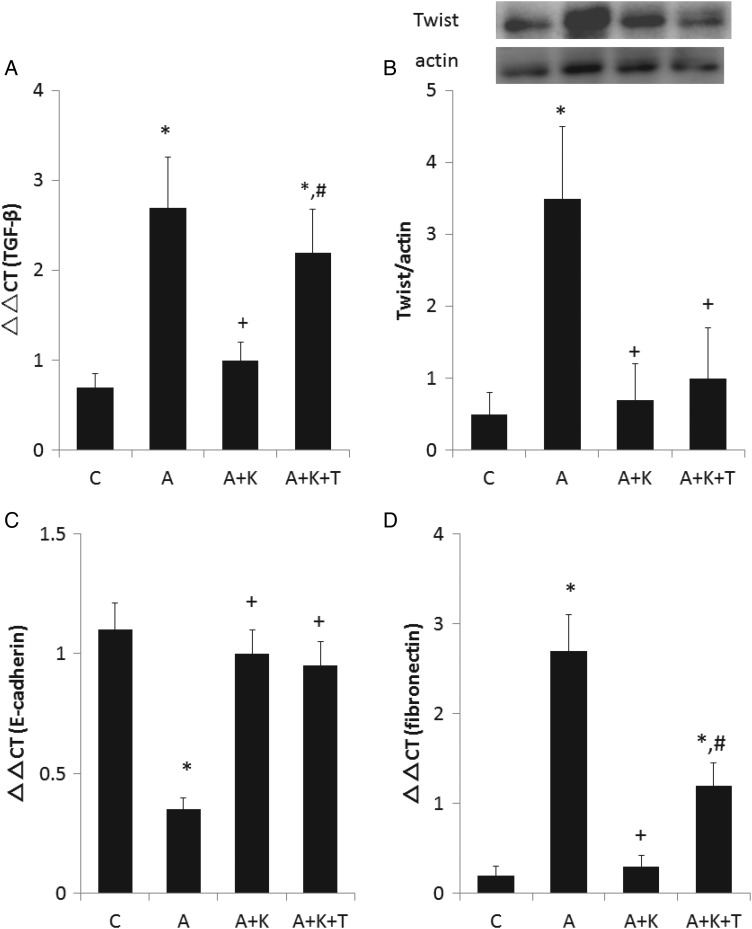
Renal expression of TGF-β was also significantly increased by adriamycin administration (Figure 5A), which was reduced by klotho supplementation. Addition of TDZD to the klotho supplementation reversed the klotho-induced decrease in TGF-β expression. Accordingly, renal abundance of Twist was markedly elevated in group A compared with group C (Figure 5B). Klotho supplementation also reduced the adriamycin-induced Twist abundance regardless of TDZD treatment. Consistent with these results, adriamycin considerably diminished the renal expression of E-cadherin (Figure 5C), and klotho supplementation restored E-cadherin expression regardless of TDZD treatment. Of note, the renal abundance of Twist remained low with preserved expression of E-cadherin in the A+K+T group, suggesting that epithelial–mesenchymal transition (EMT) was precluded also in this group. Consequently, adriamycin administration substantially elevated the renal expression of fibronectin (Figure 5D). Klotho supplementation virtually abolished the adriamycin-induced elevation of fibronectin expression. Combined treatment with TDZD and klotho reversed the klotho-induced amelioration of fibronectin expression. As shown in Supplementary data, Figure S4, adriamycin increased the fibrosis index (FI; 0.1 ± 0.1 – 2.7 ± 0.5, P < 0.01 versus group C) and klotho supplementation markedly attenuated FI (0.5 ± 0.2, P < 0.01 versus group A). The addition of TDZD to klotho marginally elevated FI (1.4 ± 0.3, P < 0.05 versus group A+K).
FIGURE 5.
Influences of adriamycin and klotho on (A) the expression of TGF-β and (B) the renal abundance of Twist, (C) the expression of E-cadherin and (D) the expression fibronectin. *, + and # indicate a significant difference from the C, A and A+K groups, respectively (n = 8 for each group).
DISCUSSION
The present observations that exposure to adriamycin induced both elevated renal expression of Wnt and increased β-catenin abundance support the recent findings that adriamycin activates the classical Wnt pathway [18]. We and others demonstrated that the kidney is a major source of circulating klotho [31, 35]. Our findings that the circulating klotho level is diminished in adriamycin nephropathy are compatible with recent data that renal klotho expression is reduced in this model [19]. Furthermore, our data extend these results and have provided the first evidence that klotho protein supplementation inhibits β-catenin stabilization in adriamycin nephropathy without inducing changes in Wnt expression. Klotho binds to Wnt protein to inhibit its signaling [24]. Collectively, these results suggest that exogenous supplementation of klotho protein suppresses the activation of classical Wnt pathway in the kidney exposed to adriamycin, where endogenous klotho is diminished.
We previously reported that angiotensinogen, renin, angiotensin-converting enzyme and AngII type 1 receptor were expressed in interstitial myofibroblasts [39]. Recent investigations revealed that Wnt signaling targets these RAS genes through LEF1 [40, 41]. The present findings constitute new demonstrations that PRA, renal and systemic AngII concentrations were elevated in adriamycin nephropathy. Surprisingly, the present data show that Wnt is expressed in the afferent arterioles as well as in the tubular cells. Furthermore, we have provided the first evidence that exogenous klotho protein supplementation reduces both plasma and renal AngII levels, accompanied by decreases in PRA and renal angiotensinogen expression. These results extend recent data that endogenous klotho enhancement by gene transfer ameliorated RAS gene expression in this model [42]. The present data also indicate that the klotho-induced reductions of renal AngII concentrations and angiotensinogen expression were reversed by GSK3β inhibition. AngII reduces renal klotho expression [43]. Taken together, these results suggest that the classical pathway of Wnt signaling through GSK3β mediates the activation of RAS and subsequent elevations of blood pressure in adriamycin nephropathy and raise the possibility that renal RAS and klotho crosstalk, especially when the Wnt/β-catenin pathway is activated in the kidney (Supplementary data, Figure S5).
Oxidative stress as well as AngII reduces renal klotho expression [44]. In contrast, klotho binds to insulin-like growth factor (IGF) receptor to induce SOD [29, 45]. Teser et al. [17] reported that proteinuria depended on free radical generation and the formation of 8-isoprostane in adriamycin nephropathy. The present data support these findings, in that the changes in albuminuria paralleled those of PGF2α excretion among the four groups, and further indicated that adriamycin administration reduced aortic and renal SOD expression, presumably contributing to the increased oxidative stress. In addition, our findings imply that the increase in albuminuria is associated with reduced glomerular expression of nephrin. Furthermore, klotho supplementation ameliorated the glomerular expression of nephrin and albuminuria. Klotho supplementation also led to the improvement of aortic and renal SOD and mitigated oxidative stress. Together, these results indicate that oxidative stress and klotho form a positive feedback loop that should hasten the progression of adriamycin nephropathy and suggest that klotho supplementation protects podocytes at least partly by diminishing oxidative stress and enhancing SOD expression.
Klotho has been shown to decrease TRPC6 expression in cardiac myocytes and podocytes by interacting with the IGF receptor and phosphoinositide 3-kinase [46, 47]. Our previous investigations indicated that subtotal nephrectomy resulted in a decrease in klotho expression and an increase in glomerular TRPC6 expression [33]. Similarly, the present results indicated that adriamycin administration lowered klotho levels and increased the glomerular expression of TRPC6, suggesting that de-repression of IGF receptors participated in the elevated expression of TRPC6 in podocytes. This notion was supported by the present observations that exogenous klotho supplementation ameliorated TRPC6 expression. Adriamycin administration elevated plasma and renal AngII concentrations, oxidative stress and blood pressure, acting as a stretch stimulus. These variables should increase calcium influx through TRPC6 to ultimately damage the podocytes [9–14]. Klotho secreted from the tubules can interstitially access the Bowman capsule and podocytes [33]. Compared with group C, renal klotho expression was diminished in the A and A+K+T groups, suggesting that secreted endogenous klotho was diminished in these groups. Glomerular expression of TRPC6 was associated with urine rather than serum klotho levels. Thus, our data suggest that renal interstitial klotho levels considerably contribute to the amelioration of TRPC6 in this experimental model.
Proteinuria induces macrophage accumulation in the renal interstitium as a source of TGF-β [2]. Consistent with this, the present study demonstrated that adriamycin induced albuminuria with resultant elevations of TGF-β, which could consequently elicit EMT using various pathways including a transcriptional factor Twist [40, 48]. In addition, AngII induces TGF-β in tubular cells [49]. Thus, the increase in AngII levels may have also contributed to the elevation of TGF-β observed in group A. Accordingly, both the increase in the renal abundance of Twist and the reduced expression of E-cadherin supported the presence of EMT in group A. Klotho supplementation reduced albuminuria and AngII concentrations as well as TGF-β expression and EMT. Klotho interacts with TGF-β receptor to inhibit its signaling [32]. Superimposition of TDZD with klotho in adriamycin nephropathy elicited moderate increases in albuminuria and considerable elevations of TGF-β expression, validating that both AngII and proteinuria are required for the full expression of TGF-β. Collectively, the present data provide the translational basis that klotho supplementation suppresses EMT in adriamycin nephropathy at least partly by inhibiting TGF-β signaling.
The origin of interstitial myofibroblasts in the fibrosing kidney is currently a topic of intense debate and investigation. The candidates are resident medullary interstitial fibroblasts, pericytes, the tubular epithelium (through EMT) and bone marrow-derived and endothelial cells [50–52]. Our recent data indicate that the contribution of EMT to renal fibrosis differs among kidney disease models and imply that the contribution of EMT to fibrosis in mouse adriamycin nephropathy is smaller than that of hydronephrosis [50]. In the present study, adriamycin induced EMT and increased fibronectin expression in the kidney, suggesting that EMT contributes to renal fibrogenesis in adriamycin nephropathy. The present data constituted new demonstrations that klotho supplementation reduced fibronectin expression as well as EMT. Although EMT was counteracted in the A+K+T group, renal expression of fibronectin was significantly increased. On the one hand, TGF-β stimulates the Wnt/β-catenin pathway [53]. However, klotho inhibits both signaling pathways, as discussed above. On the other hand, AngII directly induces profibrotic actions via hypoxia-inducible factor-1α in renal interstitial cells [54]. Taken together, these data suggest that AngII participates in renal fibrosis in both TGF-β-dependent and -independent manners in adriamycin nephropathy and support the concept that both EMT-derived and non-derived fibroblasts contribute to renal fibrosis in this model.
Caveats should be mentioned. First, no difference was observed in the glomerular expression of NFAT among the four groups (data not shown). Although we were unable to perform a western blot for NFAT in isolated glomerular samples, the results from frozen sections may provide some insights. Second, pharmacological inhibition of GSK3β was used in the present study. In a previous study, GSK3β knockdown reduced pro-inflammatory reactions in the renal tubular epithelium possibly through the NF-κB pathway [53]. Third, we failed to observe significant staining of Wnt in the glomeruli and a reduction in creatinine clearance. Although we selected an experimental period of 4 weeks, another time course might have allowed for the detection of Wnt staining in the glomeruli and changes in creatinine clearance. Further in vitro studies are warranted to elucidate these issues.
S UPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENT
The study was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology (JSPS KAKENHI 25461229) and Japan Kidney Foundation (JKFB15-57). The authors thank Maho Yamashita and Mami Fukuoka for technical assistance.
CONFLICT OF INTEREST STATEMENT
Part of the data reported here was presented at Kidney Week 2015, San Diego, CA, USA, November 2015, and published as an abstract.
REFERENCES
- 1. Damsgaard EM, Frøland A, Jørgensen OD et al. Microalbuminuria as predictor of increased mortality in elderly people. BMJ 1990; 300: 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Okada H, Moriwaki K, Kalluri R et al. Osteopontin expressed by renal tubular epithelium mediates interstitial monocyte infiltration in rats. Am J Physiol Renal Physiol 2000; 278: F110–F121 [DOI] [PubMed] [Google Scholar]
- 3. Löwik MM, Groenen PJ, Levtchenko EN et al. Molecular genetic analysis of podocyte genes in focal segmental glomerulosclerosis—a review. Eur J Pediatr 2009; 168: 1291–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 2003; 83: 253–307 [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Jarad G, Tripathi P et al. Activation of NFAT signaling in podocytes causes glomerulosclerosis. J Am Soc Nephrol 2010; 21: 1657–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Möller CC, Wei C, Altintas MM et al. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol 2007; 18: 29–36 [DOI] [PubMed] [Google Scholar]
- 7. Sonneveld R, Ferrè S, Hoenderop JG et al. Vitamin D down-regulates TRPC6 expression in podocyte injury and proteinuric glomerular disease. Am J Pathol 2013; 182: 1196–1204 [DOI] [PubMed] [Google Scholar]
- 8. Takenaka T, Suzuki H, Okada H et al. Transient receptor potential channels in rat renal microcirculation: actions of angiotensin II. Kidney Int 2002; 62: 558–565 [DOI] [PubMed] [Google Scholar]
- 9. Anderson M, Kim EY, Hagmann H et al. Opposing effects of podocin on the gating of podocyte TRPC6 channels evoked by membrane stretch or diacylglycerol. Am J Physiol Cell Physiol 2013; 305: C276–C289 [DOI] [PubMed] [Google Scholar]
- 10. Kim EY, Anderson M, Dryer SE. Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species. Am J Physiol Renal Physiol 2012; 302: F298–F307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim EY, Anderson M, Wilson C et al. NOX2 interacts with podocyte TRPC6 channels and contributes to their activation by diacylglycerol: essential role of podocin in formation of this complex. Am J Physiol Cell Physiol 2013; 305: C960–C971 [DOI] [PubMed] [Google Scholar]
- 12. Kistler AD, Singh G, Altintas MM et al. Transient receptor potential channel 6 (TRPC6) protects podocytes during complement-mediated glomerular disease. J Biol Chem 2013; 288: 36598–36609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu BC, Song X, Lu XY et al. High glucose induces podocyte apoptosis by stimulating TRPC6 via elevation of reactive oxygen species. Biochim Biophys Acta 2013; 1833: 1434–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nilius B, Owsianik G, Voets T et al. Transient receptor potential cation channels in disease. Physiol Rev 2007; 87: 165–217 [DOI] [PubMed] [Google Scholar]
- 15. Lee VW, Harris DC. Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology (Carlton) 2011; 16: 30–38 [DOI] [PubMed] [Google Scholar]
- 16. de Mik SM, Hoogduijn MJ, de Bruin RW et al. Pathophysiology and treatment of focal segmental glomerulosclerosis: the role of animal models. BMC Nephrol 2013; 14: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tesar V, Zima T, Jirsa M Jr et al. Influence of losartan and enalapril on urinary excretion of 8-isoprostane in experimental nephrotic syndrome. Med Sci Monit 2002; 8: BR69–BR74 [PubMed] [Google Scholar]
- 18. He W, Kang YS, Dai C et al. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 2001; 22: 90–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou L, Li Y, Zhou D et al. Loss of klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol 2013; 24: 771–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shkreli M, Sarin KY, Pech MF et al. Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat Med 2011; 18: 111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Naves MA, Requião-Moura LR, Soares MF et al. Podocyte Wnt/ß-catenin pathway is activated by integrin-linked kinase in clinical and experimental focal segmental glomerulosclerosis. J Nephrol 2012; 25: 401–409 [DOI] [PubMed] [Google Scholar]
- 22. Puri S, Magenheimer BS, Maser RL et al. Polycystin-1 activates the calcineurin/NFAT (nuclear factor of activated T-cells) signaling pathway. J Biol Chem 2004; 279: 55455–55464 [DOI] [PubMed] [Google Scholar]
- 23. Chiluiza D, Krishna S, Schumacher VA et al. Gain-of-function mutations in transient receptor potential C6 (TRPC6) activate extracellular signal-regulated kinases 1/2 (ERK1/2). J Biol Chem 2013; 288: 18407–18420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu H, Fergusson MM, Castilho RM et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 2007; 317: 803–806 [DOI] [PubMed] [Google Scholar]
- 25. Chen S, Yao X, Li Y et al. Histone deacetylase 1 and 2 regulate Wnt and p53 pathways in the ureteric bud epithelium. Development 2015; 142: 1180–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Metcalfe C, Bienz M. Inhibition of GSK3 by Wnt signalling—two contrasting models. J Cell Sci 2011; 124 (Pt 21): 3537–3544 [DOI] [PubMed] [Google Scholar]
- 27. Yoshino J, Monkawa T, Tsuji M et al. Snail1 is involved in the renal epithelial-mesenchymal transition. Biochem Biophys Res Commun 2007; 362: 63–68 [DOI] [PubMed] [Google Scholar]
- 28. Whittle BJ, Varga C, Pósa A et al. Reduction of experimental colitis in the rat by inhibitors of glycogen synthase kinase-3beta. Br J Pharmacol 2006; 147: 575–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kurosu H, Yamamoto M, Clark JD et al. Suppression of aging in mice by the hormone Klotho. Science 2005; 309: 1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takenaka T, Inoue T, Miyazaki T et al. Xeno-Klotho inhibits parathyroid hormone signaling. J Bone Miner Res 2016; 31: 455–462 [DOI] [PubMed] [Google Scholar]
- 31. Hu MC, Shi M, Zhang J et al. Renal production, uptake, and handling of circulating αKlotho. J Am Soc Nephrol 2016; 27: 79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Doi S, Zou Y, Togao O et al. Klotho inhibits transforming growth factor-β1 signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 2011; 286: 8655–8665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takenaka T, Inoue T, Miyazaki T et al. Antialbuminuric actions of calcilytics in the remnant kidney. Am J Physiol Renal Physiol 2015; 309: F216–F226 [DOI] [PubMed] [Google Scholar]
- 34. Takenaka T, Inoue T, Ohno Y et al. Calcitriol supplementation improves endothelium-dependent vasodilation in rat hypertensive renal injury. Kidney Blood Press Res 2014; 39: 17–27 [DOI] [PubMed] [Google Scholar]
- 35. Takenaka T, Watanabe Y, Inoue T et al. Fibroblast growth factor 23 enhances renal klotho abundance. Pflugers Arch 2013; 465: 935–943 [DOI] [PubMed] [Google Scholar]
- 36. Morizane R, Fujii S, Monkawa T et al. miR-363 induces transdifferentiation of human kidney tubular cells to mesenchymal phenotype. Clin Exp Nephrol 2016; 20: 394–401 [DOI] [PubMed] [Google Scholar]
- 37. Kato Y, Arakawa E, Kinoshita S et al. Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun 2000; 267: 597–602 [DOI] [PubMed] [Google Scholar]
- 38. Kobori H, Ozawa Y, Suzaki Y et al. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol 2005; 16: 2073–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Okada H, Inoue T, Kanno Y et al. Interstitial fibroblast-like cells express renin-angiotensin system components in a fibrosing murine kidney. Am J Pathol 2002; 160: 765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim K, Lu Z, Hay ED. Direct evidence for a role of β-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int 2002; 26: 463–476 [DOI] [PubMed] [Google Scholar]
- 41. Zhou L, Li Y, Hao S et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J Am Soc Nephrol 2015; 26: 107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou L, Mo H, Miao J et al. Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin-angiotensin system. Am J Pathol 2015; 185: 3211–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mitani H, Ishizaka N, Aizawa T et al. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 2002; 39: 838–843 [DOI] [PubMed] [Google Scholar]
- 44. Mitobe M, Yoshida T, Sugiura H et al. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp Nephrol 2005; 101:e67–e74 [DOI] [PubMed] [Google Scholar]
- 45. Wang Y, Sun Z. Antiaging gene Klotho regulates endothelin-1 levels and endothelin receptor subtype B expression in kidneys of spontaneously hypertensive rats. J Hypertens 2014; 32: 1629–1636 [DOI] [PubMed] [Google Scholar]
- 46. Xie J, Cha SK, An SW et al. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun 2012; 3: 1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim JH, Xie J, Hwang KH et al. Klotho may ameliorate proteinuria by targeting TRPC6 channels in podocytes. J Am Soc Nephrol 2016. pii: ASN.2015080888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Runyan CE, Hayashida T, Hubchak S et al. Role of SARA (SMAD anchor for receptor activation) in maintenance of epithelial cell phenotype. J Biol Chem 2009; 284: 25181–25189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wolf G, Mueller E, Stahl RA et al. Angiotensin II-induced hypertrophy of cultured murine proximal tubular cells is mediated by endogenous transforming growth factor-beta. J Clin Invest 1993; 92: 1366–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Inoue T, Umezawa A, Takenaka T et al. The contribution of epithelial-mesenchymal transition to renal fibrosis differs among kidney disease models. Kidney Int 2015; 87: 233–238 [DOI] [PubMed] [Google Scholar]
- 51. Xavier S, Vasko R, Matsumoto K et al. Curtailing endothelial TGF-β signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD. J Am Soc Nephrol 2015; 26: 817–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lovisa S, LeBleu VS, Tampe B et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med 2015; 21: 998–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dai C, Wen X, He W et al. Inhibition of proinflammatory RANTES expression by TGF-β1 is mediated by glycogen synthase kinase-3β-dependent β-catenin signaling. J Biol Chem 2011; 286: 7052–7059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang Z, Tang L, Zhu Q et al. Hypoxia-inducible factor-1α contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney Int 2011; 79: 300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.