Abstract
Aims
We have shown that extracellular vesicles (EVs) secreted by embryonic stem cell-derived cardiovascular progenitor cells (Pg) recapitulate the therapeutic effects of their parent cells in a mouse model of chronic heart failure (CHF). Our objectives are to investigate whether EV released by more readily available cell sources are therapeutic, whether their effectiveness is influenced by the differentiation state of the secreting cell, and through which mechanisms they act.
Methods and results
The total EV secreted by human induced pluripotent stem cell-derived cardiovascular progenitors (iPSC-Pg) and human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CM) were isolated by ultracentrifugation and characterized by Nanoparticle Tracking Analysis, western blot, and cryo-electron microscopy. In vitro bioactivity assays were used to evaluate their cellular effects. Cell and EV microRNA (miRNA) content were assessed by miRNA array. Myocardial infarction was induced in 199 nude mice. Three weeks later, mice with left ventricular ejection fraction (LVEF) ≤ 45% received transcutaneous echo-guided injections of iPSC-CM (1.4 × 106, n = 19), iPSC-Pg (1.4 × 106, n = 17), total EV secreted by 1.4 × 106 iPSC-Pg (n = 19), or phosphate-buffered saline (control, n = 17) into the peri-infarct myocardium. Seven weeks later, hearts were evaluated by echocardiography, histology, and gene expression profiling, blinded to treatment group. In vitro, EV were internalized by target cells, increased cell survival, cell proliferation, and endothelial cell migration in a dose-dependent manner and stimulated tube formation. Extracellular vesicles were rich in miRNAs and most of the 16 highly abundant, evolutionarily conserved miRNAs are associated with tissue-repair pathways. In vivo, EV outperformed cell injections, significantly improving cardiac function through decreased left ventricular volumes (left ventricular end systolic volume: −11%, P < 0.001; left ventricular end diastolic volume: −4%, P = 0.002), and increased LVEF (+14%, P < 0.0001) relative to baseline values. Gene profiling revealed that EV-treated hearts were enriched for tissue reparative pathways.
Conclusion
Extracellular vesicles secreted by iPSC-Pg are effective in the treatment of CHF, possibly, in part, through their specific miRNA signature and the associated stimulation of distinct cardioprotective pathways. The processing and regulatory advantages of EV could make them effective substitutes for cell transplantation.
Keywords: Heart failure , Extracellular vesicles , miRNA , Regenerative medicine , Acellular therapy
Translational perspectives
Induced pluripotent stem cells (iPSC) are currently used for drug screening and disease modelling and, to a lesser extent, for experimental cell therapy. Here, we show that iPSC-derived cardiovascular progenitors release extracellular vesicles (EVs) that are effective therapeutics for mice in chronic heart failure. In vitro analyses suggest that EV may promote cell survival, proliferation of resident cardiac cells, and angiogenesis thereby improving left ventricular function. These effects, combined with the logistic, safety, and regulatory advantages of a cell-free approach, make EV a potentially viable option for the treatment of heart failure and reveal a new role for iPSC as in vitro ‘biofactories’.
Introduction
Cardiovascular disease remains the leading cause of death in the developed world, with chronic heart failure (CHF) among the top causes of cardiovascular morbidity. While the evidence-based guidelines mandated by the European Society of Cardiology1 have contributed to the improved management of patients with heart failure, some of them remain refractory to treatment. When exploring alternatives to conventional therapies, cell transplantation remains an attractive option. However, while there is some indication of the potential for efficacy, this approach still fails to demonstrate clinically meaningful improvements in outcomes.2 Data generated by this research have led to a major shift in our understanding of the mechanism of action of transplanted cells; while the original hypothesis was that implanted cells could directly replace damaged tissue, it has become increasingly apparent that the therapeutic effects of transplanted cells are largely mediated by the release of factors which stimulate endogenous regeneration or repair pathways.3,4 There is mounting evidence that most of these paracrine factors are found in extracellular vesicles (EVs), which are membrane-bound vesicles containing proteins, bioactive lipids, messenger RNA (mRNA), microRNA (miRNA) and other macromolecules.5
Given their ability to target specific cell types, possibly through the presence of distinctive surface markers, and deliver bio-active macromolecules, EV have now been successfully tested in preclinical models of stroke,6 acute kidney injury,7 myocardial infarction,8–11 myocardial infarction/reperfusion injury,12,13 and hind limb ischaemia,14 among others. Clinical trials in this field are in their infancy, but antitumour therapies based on EV derived from dendritic cells have already entered phase II human clinical trials.15
We have previously demonstrated that the EV secreted by human embryonic stem cell (hESC)-derived cardiovascular progenitor cells could replicate the therapeutic effects of their parent cells in a mouse model of CHF.8 While these results open new clinical perspectives for cell-free therapies for CHF, there are ethical, logistic, and practical concerns that may limit the implementation of therapies dependent on hESC sources. In this study, we have thus considered an alternative source of pluripotent stem cells, namely human induced pluripotent stem cells and used both in vitro and in vivo approaches to investigate whether the EV released by human induced pluripotent stem cell-derived cardiovascular progenitors (iPSC-Pg) and human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CM) are therapeutic, whether their effectiveness is influenced by the differentiation state of the secreting cell, and through which mechanisms they may act.
Methods
Detailed materials and methods are given in the Supplementary material online.
Extracellular vesicle preparation
Human iPSC-Pg and iPSC-CM were purchased from Cellular Dynamics International (Madison, WI, USA). Conditioned media were collected and ultracentrifuged for 16 h at 100 000 g to pellet all EV. At the end of the culture period, cells were harvested and characterized by quantitative PCR (qPCR) and immunofluorescence to confirm their differentiation state. Extracellular vesicles were characterized by Nanoparticle Tracking Analysis (NTA3.2, Malvern, Malvern, UK) and cryo-transmission electron microscopy (cryo-TEM), and the presence of exosomes was confirmed by western blot. The miRNA contents of iPSC-Pg and their EV were examined by Affymetrix miRNA Array (ArrayExpress accession number E-MTAB-5165).
In vitro bioactivity assays
To determine the pro-survival effects of iPSC-Pg-EV, serum deprived H9c2 cardiac myoblasts were incubated with iPSC-Pg-EV and the number of viable cells at the end of 27 h was determined. The potential for these EV to be internalized by target cells was examined by observing the fluorescence of H9c2 cells after 20 h of incubation with calcein-AM-labelled iPSC-Pg-EV by imaging flow cytometry, confocal microscopy and by fluorescence video microscopy (spinning disk). The pro-angiogenic potential of iPSC-Pg-EV was determined using human umbilical vein endothelial cell (HUVEC) wound healing and Matrigel tube formation assays. Human embryonic stem cell derived-cardiomyocytes (hESC-CM) were used to study the pro-proliferative effect of iPSC-Pg-EV.
Animal experiments
Myocardial infarction was induced in 199 nude mice by permanent left anterior coronary artery occlusion. Three weeks later, mice were assessed echocardiographically and assigned to receive transcutaneous echo-guided peri-infarct injections of phosphate-buffered saline (PBS), iPSC-CM cells, iPSC-Pg cells, or iPSC-Pg-EV. Functional improvement was assessed again by echocardiography (blinded to treatment groups) 7 weeks after treatment. After sacrifice, approximately half of the animals were evaluated for infarct size, percent fibrosis, and vascular density by histology on cryo-sections. The remaining hearts were evaluated for differentially expressed genes when compared with PBS controls by Affymetrix mouse mRNA array (ArrayExpress accession E-MTAB-5164).
Statistical analyses
Data are summarized using mean (standard deviation) or median (q1–q3) for continuous variables when appropriate and n (%) for categorical variables. Comparisons of LV function variables across groups were performed using analysis of variance; multiple comparisons were done using pairwise comparison with Tukey correction for post hoc tests. For multiple comparisons with a control group, Dunnett correction was used. Across groups, the change of LV function variables between baseline and 7 weeks post-treatment were compared using a Student test. The reliability of measurements of heart function by the same echocardiographer was assessed using the intra class correlation coefficient (ICC). Comparisons of histological parameters between groups were performed using a mixed model analysis of variance-covariance taking into account the intraheart correlation. For all analyses, a two-tailed P-value <0.05 was considered statistically significant. Analyses were conducted using SAS 9.4 (Statistical Analysis System, Cary, NC, USA).
Results
Characterization of induced pluripotent stem cell-derived cardiovascular progenitors and cardiomyocytes
Thawed human iPSC-Pg were cultured for 4 days in serum-free media supplemented with bFGF. At this time point, their cardiovascular progenitor identity was confirmed by both qPCR and immunostaining which showed the reduced expression of the pluripotency markers NANOG, SOX2, OCT3/4, the expression of the early cardiovascular lineage markers ISL-1, MEF2C, NKX2.5 and GATA4, and the absence of mature cardiomyocyte markers such as TBX20, TNNT2, PLN, MYH6, MYH7, MYL2 (see Supplementary material online, Figures S1 and S2, Tables S1 and S2). Thawed human iPSC-CM were plated and cultured 7 days, at the end of which time they expressed markers of mature cardiomyocytes (see Supplementary material online, Figures S1 and S3).
Extracellular vesicle isolation and characterization
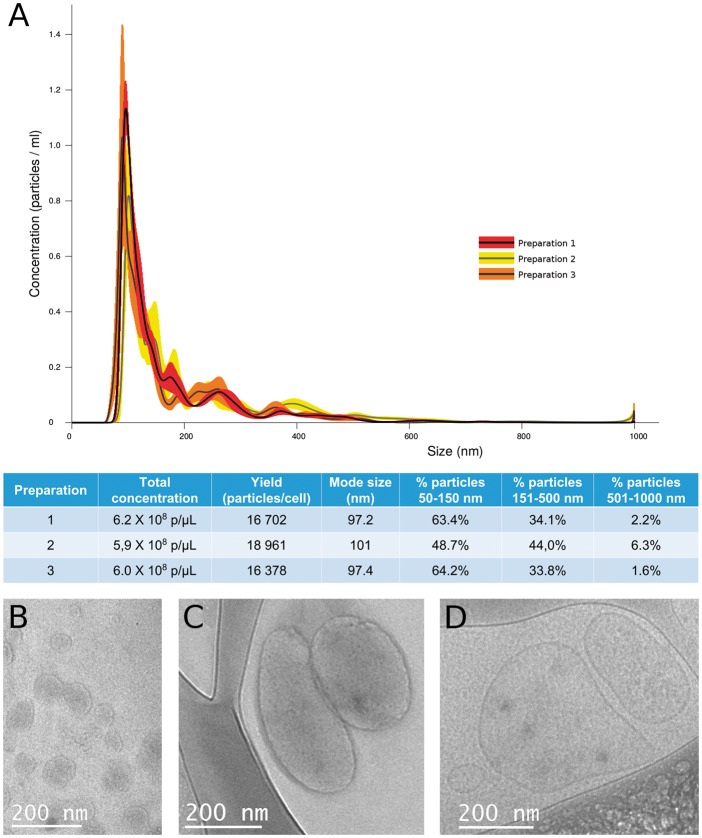
Extracellular vesicle were isolated from iPSC-Pg conditioned media on Day 2 (for in vitro experiments only) and on Day 4 (for in vitro and in vivo experiments) of culture. Both time points yielded consistently large quantities of iPSC-Pg-EV (Figure 1A). This was in contrast to iPSC-CM, which produced no detectable EV in any of the six culture conditions tested, including three different media types under normoxic and hypoxic (3% O2) conditions (see Supplementary material online, Figure S4). Nanoparticle tracking analysis of iPSC-Pg-EV preparations detected a poly-disperse population of particles ranging primarily from approximately 50–550 nm, with a small population at around 1000 nm. The majority of particles in our preparations (approximately 68% of detected particles) were in the size range of exosomes (approximately 50–150 nm). These results were consistent with cryo-TEM, which identified primarily particles of the size of exosomes, many microparticles and a few apoptotic bodies (Figure 1B–D). This mixed population of particles was expected since our isolation protocol (ultracentrifugation at 100 000 g for 16 hours) pellets total EV, and does not distinguish between EV sub-types. The presence of exosomes was confirmed by western blot, which identified both CD81 and CD63 exosomal markers in our iPSC-Pg-EV Day 4 preparations (two independent EV preparations, Supplementary material online, Figure S5).
Figure 1.
Characterization of induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicle preparations. (A) Similar overall concentrations and yields of particles per cell were found in three separate preparations of induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicles by Nanoparticle Tracking Analysis. Cryo-transmission electron microscopy of these extracellular vesicles identified membrane-bound vesicles of the size of (B) exosomes, (C) microparticles, and (D) large vesicular bodies that may be apoptotic bodies.
In vitro bio-activity of extracellular vesicles
Induced pluripotent stem cell-derived cardiovascular progenitor-extracellular vesicles promote survival of stressed cardiac cells
Serum deprivation of H9c2 rat cardiac myoblasts for 27 h led to a dramatic decrease in the number of viable cells. Conversely, iPSC-Pg-EV increased viability in a seemingly dose-dependent fashion (Figure 2). This effect was highly reproducible for numerous independent iPSC-Pg-EV preparations. While the virgin media contains no detectable particles of the size of EV, other components, such as growth factors or proteins, in the media could have accounted for some of the effect of the EV preparations. To control for this possibility, virgin complete media, was incubated 48 h in coated culture flasks in the CO2 incubator (without cells) and ultracentrifuged; the bottom of the tube was then thoroughly rinsed with PBS in a volume equivalent to the one used in our iPSC-Pg-EV preparations and tested in an H9c2 survival assay. This preparation had no positive effect on cell survival (see Supplementary material online, Figure S6).
Figure 2.
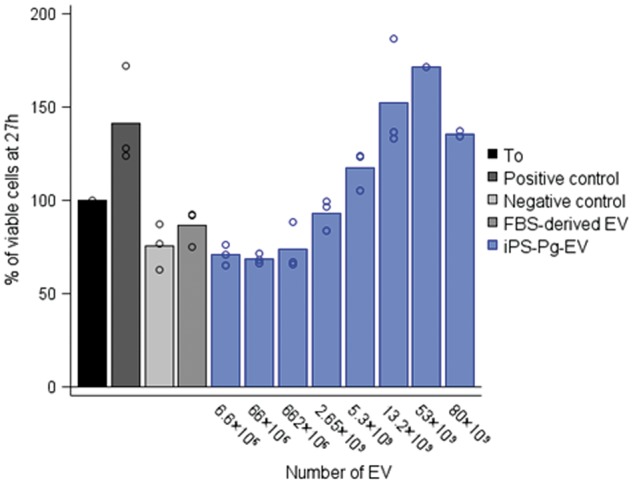
Induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicles improve cardiac cell survival of immortalized H9c2 rat cardiac myoblasts. At T0, media was refreshed with complete media (positive control, dark grey bar), stressful serum free media (Dulbecco's Modified Eagle Medium glutamax, +1% penicillin/streptomycin/Amphotericin B) (negative control, bright grey bar), or serum free media plus 5.3 × 109 foetal bovine serum-derived extracellular vesicles (medium grey bar) or induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicles at Day 4 of culture at increasing concentrations (blue bars). At T = 27 h, the number of viable cells from each condition was determined and normalized to the number of cells at the start of the stress (T0 = 100%). Results are the average of 1–3 independent experiments, each in duplicate. Extracellular vesicle concentrations were determined by Nanoparticle Tracking Analysis at high camera sensitivity (camera level 15).
Induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicles induce angiogenic responses
Scratch wound assay: The percent wound invasion was determined at numerous time points, with the 18-h time point showing clear and consistent differences between positive and negative controls (97% and 10% healing, respectively). Human umbilical vein endothelial cell invasion was increased by iPSC-Pg-EV incubation, even at the lowest concentration tested, with a seemingly dose-dependent effect (Figure 3). Tube formation assay: Tube formation, as measured by total segment length and number of nodes after 24 h, was also increased by iPSC-Pg-EV, though in contrast to the scratch wound assay, this effect was without a clear dose-dependent response (see Supplementary material online, Figure S7, Results).
Figure 3.
Induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicles exhibit pro-angiogenic effects in a scratch wound assay. The potential for induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicles to affect human umbilical vein endothelial cell migration was evaluated by the scratch wound assay. A scratch was induced in a human umbilical vein endothelial cell monolayer. (A) The percent wound invasion was determined at numerous time points, with the 18-h time point showing clear and consistent differences between (B) positive control (complete media = endothelial cell growth medium plus endothelial cell growth medium supplement pack; dark grey bar), and (C) negative control (poor media = endothelial cell growth medium; bright grey bar) with 97% and 10% healing, respectively. (D) Human umbilical vein endothelial cell invasion was increased by extracellular vesicle incubation (poor media + extracellular vesicles), even at the lowest concentration tested, with a seemingly dose-dependent increase in cell migration, reaching 80% invasion with 1 × 1010 extracellular vesicles per 15 000 human umbilical vein endothelial cells.
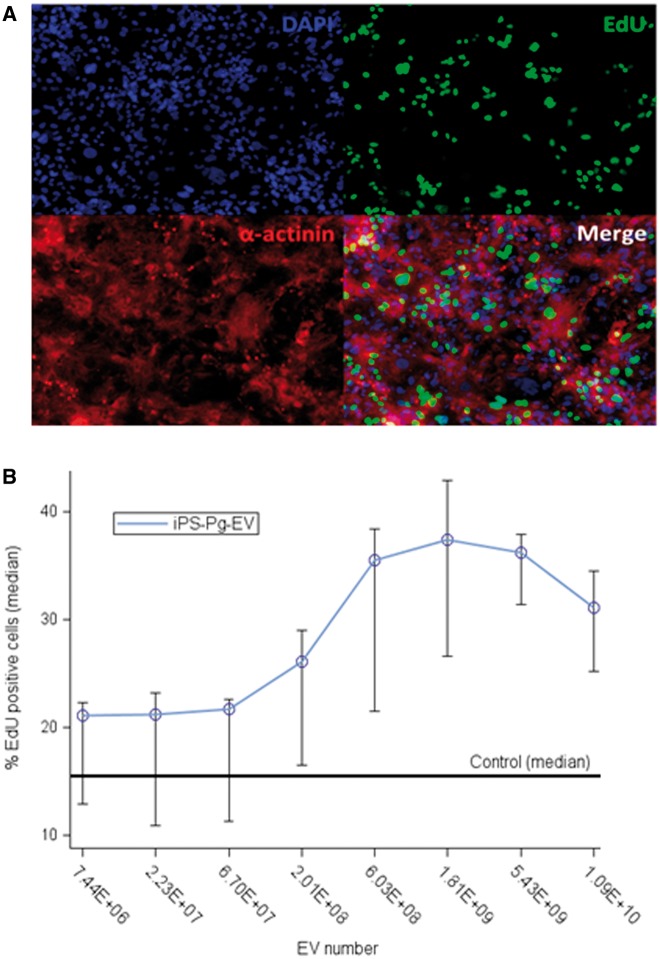
Induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicles increase cardiomyocyte proliferation
The number of proliferating cells was determined by EdU incorporation. After 3 days of iPSC-Pg-EV incubation, the percentage of proliferating hESC-CM cells increased in a dose-dependent manner from 15% (basal rate) up to 35% (Figure 4, Supplementary material online, Results).
Figure 4.
Induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicles stimulate human embryonic stem cell derived-cardiomyocyte proliferation. EdU incorporation was used to assess the potential for extracellular vesicles to stimulate cardiomyocyte proliferation. After 48 h of extracellular vesicle incubation, human embryonic stem cell derived-cardiomyocytes were incubated with EdU for an additional 24 h. (A) An α-actinin staining confirmed the cell phenotype. (B) The basal rate of proliferative cells was 15% which increased in a seemingly dose-dependent manner up to 35% of cells at 1.81 × 109 extracellular vesicles per 10 000 human embryonic stem cell derived-cardiomyocyte.
Induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicles are internalized by cardiac cells
In order to determine whether EV were taken up by H9c2 target cells, EV were first labelled with calcein-AM green fluorescent dye. Extracellular vesicle labelling was confirmed by ImageStream (see Supplementary material online, Figure S8A-B). Labelled EV were incubated with serum-deprived H9c2 cells which, after 20 h of incubation, were themselves analysed by ImageStream. H9c2 cells incubated with labelled EV were more fluorescent than any of the negative controls [see Supplementary material online, Figure S8E and F 1-complete medium; 2-poor medium; 3-poor medium + unlabelled foetal bovine serum (FBS)-EV; 5-poor medium + unlabelled-iPSC-Pg-EV; 6-poor medium + wash], with fluorescence not reaching the level of intensity as high as cells directly labelled with calcein dye (see Supplementary material online, Figure S8E and F: 7-cells incubated in poor medium with unlabelled-FBS-EV and directly labelled with 1 µM calcein AM from T19h-T20h; 8-cells incubated in poor medium with unlabelled-FBS-EV and directly labelled with 1 µM calcein AM from T0-T20h). These data suggest that labelled iPSC-Pg-EV were internalized by or closely associated with H9c2 cells. In order to distinguish between these two possibilities, cells were imaged by confocal microscopy. Punctate green signals were detected in cells incubated with labelled iPSC-Pg-EV (see Supplementary material online, Figure S8G). Z-stack analysis confirmed that the green fluorescence was indeed within the cell body and not on the surface of the cells. Green fluorescence was not detected in the negative controls (see Supplementary material online, Figure S8H: H9c2 and non-labelled iPSC-Pg-EV; Supplementary material online, Figure S8I: H9c2 and wash to control for possible contaminating free calcein-AM). The internalisation of calcein-labeled iPS-Pg-EV by H9c2 cells was also captured by fluorescence video microscopy (see Supplementary material online, Video S1).
Induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicles contain microRNAs associated with a cardioprotective potential
Bioanalyser analyses of small RNAs showed that iPSC-Pg-EV were enriched in miRNAs when compared with their parent cells, with an average of 53% of small RNAs of the size of miRNAs in EV and 17% in iPSC-Pg cells (average of three independent iPSC-Pg cultures and their corresponding EV preparations). Furthermore, Affymetrix array showed that at the highest detection thresholds, the number of miRNAs in EV exceeded that in their parent cells, thereby suggesting that certain miRNAs are specifically targeted to EV, which are not simply a random sampling of cytosolic content (see Supplementary material online, Figure S9, Results).
In order to identify miRNAs potentially relevant to the therapeutic effects of the iPSC-Pg-EV, we first considered only the most highly abundant miRNAs (log intensity threshold 9). Next, since the human derived iPSC-Pg-EV had an effect on rat and human cells in vitro and on mouse hearts in vivo, we selected only miRNAs broadly conserved across species as annotated in Target Scan (v 7.1, released June 2016). Sixteen miRNAs were then identified that met these criteria (Table 1, Supplementary material online, Results), of which 11 have previously been reported to be stably present in the heart or cardiovascular cells and have predicted biological functions expected to benefit the failing heart (cell differentiation, proliferation, survival, development, and angiogenesis).
Table 1.
Top 16 conserved miRNA and their respective families
| miRNA | Intensities (log) |
Family | |||
|---|---|---|---|---|---|
| S1 | S2 | S3 | Avg | ||
| hsa-miR-6088 | 12.4 | 12.6 | 13.0 | 12.7 | miR-143-3p |
| hsa-miR-92a-3p | 11.8 | 12.1 | 12.0 | 12.0 | miR-25-3p/32-5p/92-3p/363-3p/367-3p |
| hsa-miR-17-5p | 10.6 | 11.7 | 11.4 | 11.3 | miR-17-5p/20-5p/93-5p/106-5p/519-3p |
| hsa-miR-106a-5p | 10.6 | 11.6 | 11.3 | 11.2 | |
| hsa-miR-20a-5p | 9.7 | 11.3 | 10.8 | 10.7 | |
| hsa-miR-93-5p | 9.8 | 10.8 | 10.0 | 10.3 | |
| hsa-miR-20b-5p | 8.7 | 10.0 | 9.7 | 9.6 | |
| hsa-miR-302d-3p | 9.6 | 10.4 | 10.7 | 10.3 | miR-302-3p/372-3p/373-3p/520-3p |
| hsa-miR-103a-3p | 10.1 | 10.1 | 10.5 | 10.3 | miR-103-3p/107 |
| hsa-miR-107 | 8.7 | 9.5 | 10.0 | 9.5 | |
| hsa-miR-24-3p | 9.8 | 10.8 | 9.6 | 10.2 | miR-24-3p |
| hsa-miR-191-5p | 9.0 | 10.0 | 10.2 | 9.8 | miR-191-5p |
| hsa-miR-26a-5p | 6.4 | 9.7 | 10.6 | 9.7 | miR-26-5p |
| hsa-miR-23a-3p | 8.2 | 10.4 | 9.8 | 9.7 | miR-23-3p |
| hsa-miR-16-5p | 7.8 | 9.9 | 9.4 | 9.2 | miR-15-5p/16-5p/195-5p/424-5p/497-5p |
| hsa-miR-130b-3p | 8.8 | 9.8 | 8.4 | 9.1 | miR-130-3p/301-3p/454-3p |
Avg, log of the average of the intensities; S1, EV sample 1; S2, EV sample 2; S3, EV sample 3.
Functional assessment in the chronic heart failure model
At the final 7-week time point, the study group included 72 mice: PBS (vehicle control, n = 17); iPSC-CM cells (1.4 × 106 cells per mouse, n = 19); iPSC-Pg cells (1.4 × 106 cells per mouse, n = 17); and the iPSC-Pg-EV secreted by the same batch of iPSC-Pg cells over 48 h (Day 2–4 of culture; n = 19) (see Supplementary material online, Results). ANOVA showed no baseline differences between groups for left ventricular end systolic volume (LVESV) (PBS: 48.94 ± 6.61 µL; iPSC-CM cells: 47.92 ± 7.30 µL; iPSC-Pg cells: 50.87 ± 8.16; iPSC-Pg-EV: 50.71 ± 7.07 µL), left ventricular end diastolic volume (LVEDV) (PBS: 78.84 ± 6.62 µL; iPSC-CM cells: 76.31 ± 8.20 µL; iPSC-Pg cells: 79.28 ± 8.52 µL; iPSC-Pg-EV: 79.55 ± 7.57 µL), or ejection fraction (EF) (PBS: 38.08 ± 4.09%; iPSC-CM cells: 37.36 ± 5.23%; iPSC-Pg cells: 36.06 ± 4.70%; iPSC-Pg-EV: 36.43 ± 4.13%).
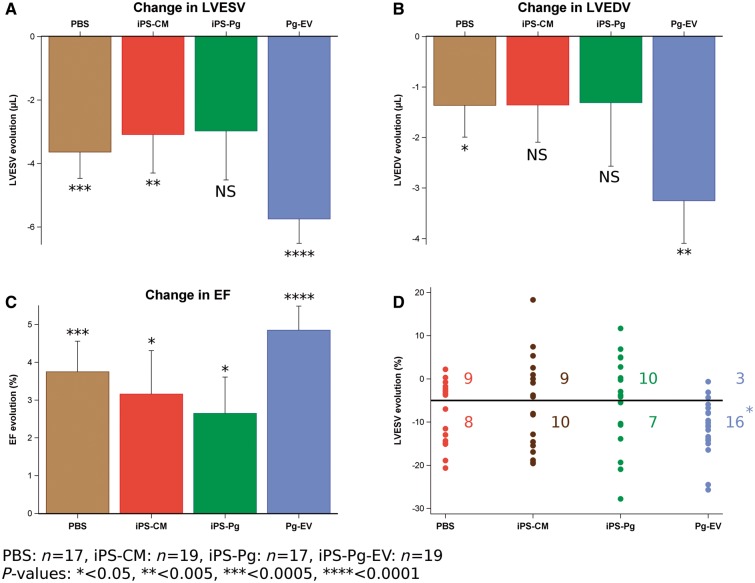
After 7 weeks, the best preservation of LV function was found in the EV-injected hearts with LVESV significantly decreased (by 11%) compared with baseline values (change: −5.75 µL, P < 0.0001) (Figure 5A–C) along with a decrease of 4% in LVEDV (−3.25 µL vs. baseline, P = 0.001). Because of these parallel reductions in LV volumes, EV treatment resulted in the highest post-treatment EF with an increase of 14% over baseline (+4.85%, P < 0.0001), with no difference between animals treated with fresh or frozen EV (P = 0.34). A randomly selected subset of echocardiographic data were re-assessed, blinded to previous results, to confirm these results and a high ICC was found for all functional parameters (ICC range: 0.97–0.99).
Figure 5.
Induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicle treatment significantly improves cardiac function. Left ventricular end systolic volume, left ventricular end diastolic volume, and ejection fraction were determined 3 weeks after myocardial infarction (baseline) and 7 weeks after treatment. All groups showed some decrease in ventricular volumes and increase in ejection fraction. For the extracellular vesicle group, there is a clear and highly significant improvement in all three functional parameters, representing a decrease in (A) left ventricular end systolic volume and (B) left ventricular end diastolic volume of 11% and 4% relative to baseline, respectively, and an increase in (C) ejection fraction of 14% relative to baseline. (D) Considering left ventricular end systolic volume changes of individual mice in each group, significantly more mice responded to treatment (>5% decrease, ‘responders’, below the line) in the extracellular vesicle group than in any other group (P = 0.04, χ2 test). Analysis of variance indicates that the evolution of individual mice in the extracellular vesicle group is significantly more homogeneous than the evolution of mice in the induced pluripotent stem cell-derived cardiomyocytes and progenitor cell groups.
Of note, significantly more mice in the EV group responded positively to treatment (LVESV improvements greater than 5%) compared with the other groups (P = 0.04, χ2 test) (Figure 5D). Furthermore, while individual functional improvements were well clustered in the EV group, they were more variable in the iPSC-Pg group. Likewise, analysis of variance found that the iPSC-CM group also had significantly greater variances in the distributions of LVESV changes than the EV group (P = 0.02).
Gross macroscopic and histological analyses of heart tissue
No significant differences were detected between groups for any of the histological or gross macroscopic analyses of heart tissue. This is illustrated in Supplementary material online, Figure S10, where the box plots indicate that there are no differences in the distribution of the parameters between groups.
Transcriptomic analysis of heart tissue
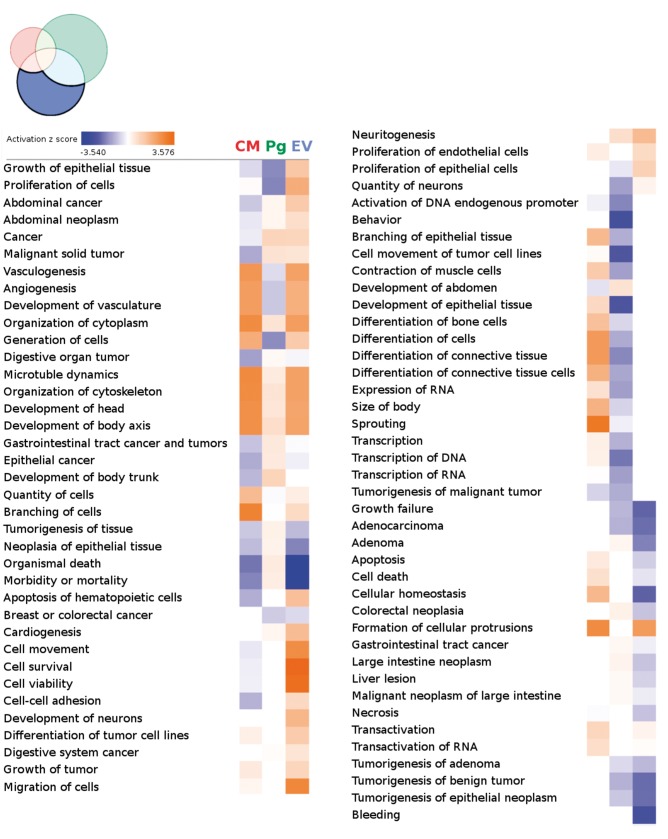
Differential gene expression analysis comparing each treatment group to the PBS vehicle controls revealed distinct gene expression profiles following each treatment (Figure 6).
Figure 6.
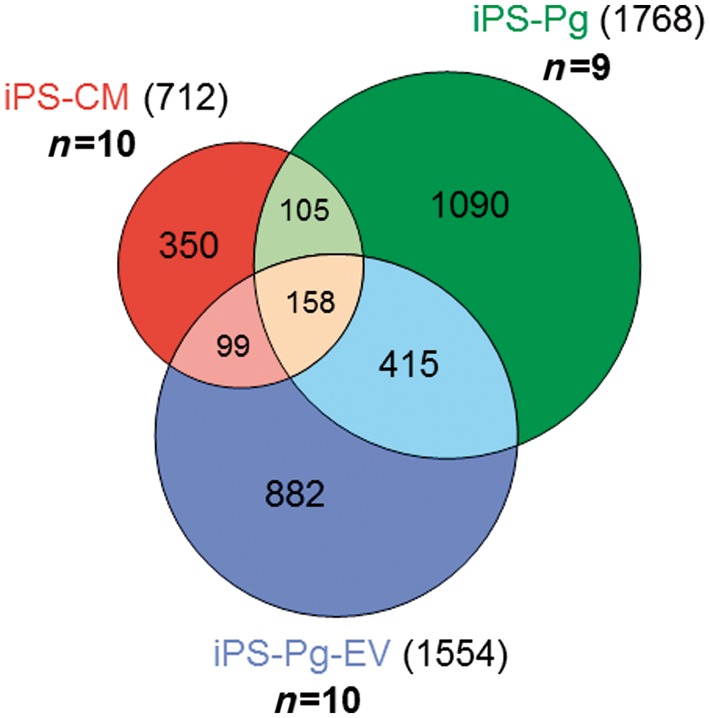
Induced pluripotent stem cell-derived cardiomyocytes, induced pluripotent stem cell-derived cardiovascular progenitors, and induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicle treatments lead to distinct gene expression patterns 7 weeks after treatment. Gene expression patterns in mouse hearts 7 weeks after treatment were analysed via mRNA array. Genes differentially expressed in each group when compared with the control phosphate-buffered saline group were identified and gene lists were compared with one another to identify common and specific differentially expressed genes. One-hundred and fifty-eight genes were common to all three treatments, whereas induced pluripotent stem cell-derived cardiomyocytes, induced pluripotent stem cell-derived cardiovascular progenitors, and induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicles differentially expressed 350, 1090, and 882 genes, respectively.
The differentially expressed genes were then analysed by Ingenuity Pathway Analysis (IPA, Qiagen, https://www.qiagenbioinformatics.com/). The three treatments were found to be associated with distinct gene expression patterns with the iPSC-Pg-EV group being more enriched for pathways thought to be beneficial to hearts in chronic failure, such as increased growth, proliferation, survival, metabolism, angiogenesis, vasculogenesis, and decreased organismal morbidity and mortality (Figure 7, Supplementary material online, Figure S11, Results).
Figure 7.
Heat map of differential functions by group—specific gene lists. Differentially expressed genes from each treatment group were assessed by Ingenuity to predict upregulated (warm colours) and downregulated (cool colours) diseases and biological functions when compared with phosphate-buffered saline controls. Each column examines differentially expressed genes uniquely present in the indicated treatment group (left—induced pluripotent stem cell-derived cardiomyocytes; middle—induced pluripotent stem cell-derived cardiovascular progenitors; right—induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicles). Induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicle treated hearts were the most enriched for functions consistent with increased cell proliferation/growth, cardiogenesis, and vessel formation. The list is sorted to have the greatest difference in enrichment between the extracellular vesicle group and the other groups at the top of the list. This list was truncated after the 78th line.
Discussion
Rationale
In parallel to conducting our current clinical trial testing the epicardial delivery of SSEA-1+ ISL-1+ cardiovascular progenitors embedded into a fibrin gel,16 we investigated the mode of action of these cells and have data supporting a paracrine signalling mechanism; in a mouse model of CHF, the functional benefits of these cardiovascular progenitors could be duplicated by the sole administration of the EV that they had released under stress conditions.8 This study builds on these previous data by further assessing the effects of EV released by cardiac progenitors, but with an important switch in the cell source from ESC to iPSC for clinically relevant practical reasons: unlimited availability of iPSC, better suitability for industrial, robust, reproducible and scalable cell culture, limited ethical issues and, importantly, reduced safety issues as long as the cells are now intended to be exclusively used for producing the EV and not for being transplanted to patients. The switch to these cells, with the necessary evaluation of the therapeutic potential of their EV was, in our view, a crucial step, necessary for the development of a therapeutic with the potential for widespread clinical use.
Functional data
The main result of this study is that EV released by iPSC-Pg yielded the best functional outcomes 7 weeks after treatment. These data support the protective effects of EV previously documented in reperfused12,13 and non-reperfused8–11 myocardial infarctions. In our experiments, the EV-related preservation of LV function was best illustrated by the significant decrease in LVESV from baseline values, which is clinically relevant in view of the correlation between changes in LVESV and patient outcomes.17 Extracellular vesicle treatment was also the only one to cause a highly significant decline in LVEDV, thereby reflecting an attenuation of adverse LV remodelling. Of note, the benefits of EV were similar in the fresh and cryo-frozen subsets, consistent with the idea that exosomes are stable across a wide range of temperatures,5,9,18 which reinforces their attractiveness as an off-the-shelf product readily available for clinical use.
An unexpected finding of our study was that the EV globally outperformed their iPSC-Pg parent cells, largely because the latter was associated with a greater scattering of cardiac functional changes. Possible explanations for this heterogeneous pattern include different rates of cell death during injections, different rates of post-injection retention depending on the locally present inflammatory cues, or large variation in the number of cells delivered to each mouse due to clumping or settling out of cells within the syringe during the injection process (contrasting with a highly homogeneous EV suspension which does not settle out at all during the injection process). Another possible explanation for the apparent superiority of EV therapy over cell injection could be related to a dose-effect. Since these cells are only transiently present in the host tissue,19 the number of EV released by the dwindling numbers of iPSC-Pg cells in situ could be dramatically reduced from the number they had released after 48 h of healthy culture in vitro. Indeed, our in vitro results are consistent with the possibility of a dose effect. One potential corollary to this could be that multiple injections over time could increase therapeutic effectiveness of EV and provide better long-term effects.
We also found that hearts injected with iPSC-CM failed to recover cardiac function to a greater extent than PBS-injected controls. Although it would be over simplistic to relate therapeutic effectiveness to EV release, it is noteworthy that we only found a minimal release of EV from our mature CM, a finding also reported by Malik et al.20
Mechanistic insights
The protective effects of EV are generally attributed to the transfer of their content to target cells and the subsequent intracellular activation of repair pathways.21,22 This idea is supported in this study by three main lines of evidence. First, EV were shown to be internalized into cultured cardiac cells. Second, most of the miRNAs identified in the iPSC-Pg-EV were associated with tissue repair pathways. These miRNAs were preferentially packaged in EV when compared with their parent cells, indicating a selective enrichment of the transcripts as has been described previously in the literature.5,23,24 The predicted targets of these miRNA are consistent with the improved viability of stressed cardiomyocytes, the vascular repair of the scratch-induced wound and the increased proliferation of hESC-CM seen in our in vitro bioassays. These effects are also consistent with the strong pro-survival, pro-angiogenic and pro-proliferative roles of EV derived from diverse cellular sources, including undifferentiated ESC.11 Third, gene transcription profiles in hearts treated by cells or EV suggest that both interventions affect myocardial cell gene expression but that EV treatment induced the greatest transcriptional changes associated with cardiac functional improvement. These changes primarily consisted of an up-regulation of signalling pathways known to be possible targets of the EV miRNA content, such as cell proliferation/growth, cardiogenesis, and vessel formation.
We have not explored here the relative therapeutic importance of each of the 16 promising miRNA identified in our study. While others have indeed found links between EV therapeutic effectiveness and a single miRNA,10,25 at this juncture, we feel that it could be counterproductive to deconstruct the EV cargo into single components as it is likely that it is a combination of molecules acting synergistically which account for the biological effects of EV. Furthermore, even the identification of particularly effective miRNAs would only give a truncated picture of the overall mechanism of action of EV because of the likely additional involvement of proteins and lipid rafts.23,25 Finally, it should be emphasized that one of the great advantages of EV as a therapeutic is their inherent ability to deliver cargo much more efficiently than if the individual components of that cargo were to simply encounter the target cells in a ‘naked’ form.26 Indeed this capacity of EV for shuttling a full cargo directly to the recipient cell in which it is internalized is increasingly exploited for targeted delivery of drugs or other therapeutics.27
Limitations
We acknowledge that this study has several limitations. Firstly, we have only investigated cardiovascular progenitor-derived EV on the premise that cardiac repair is best served by cardiac-committed cells.28 However, we cannot exclude that another source of parent cells, particularly mesenchymal stem cells,29 could yield equally effective EV. Secondly, we used total EV preparations, partly because of a lack of consensus regarding the selective isolation of EV sub-populations and lack of consistent markers to determine sub-fraction purity, and also because it is unknown which sub-population is endowed with the greatest cardioprotective potential. Future studies are warranted to identify the most effective EV fraction, characterize specific biomarkers allowing its isolation,30 and develop more discriminatory purification methods. Third, the uptake of EV by target cells was demonstrated in vitro and remains to be demonstrated in vivo. However, as we have previously documented the efficacy of our transcutaneous echo-guided injections for delivering EV in infarcted hearts using fluorescent dye injection,8 it is reasonable to speculate that the improved functional recovery yielded by EV injections is a consequence of their actual presence in the target sites. Future work is nevertheless warranted to identify the specific cell-type(s) which internalizes the EV. Lastly, we used here an animal model prone to a high rate of spontaneous recovery and a late study Endpoint of 7 weeks after treatment, which may have resulted in missing some early histological or transcriptomic changes in the treated hearts. Indeed, the lack of histological evidence for an increase in vessel density at the 7-week post-treatment time point contrasts with the in vitro findings that EV stimulate endothelial cell migration and tube formation. The explanation might be the rapid rate of spontaneous collateral revascularisation of the mouse species31 which could then make it difficult to show a benefit in the EV treatment group at a late time point because of the ‘catch-up’ of angiogenesis in controls.
Translation of extracellular vesicle-therapies to clinical use
Currently, iPSC-derived differentiated cells have been used for drug screening and disease modelling while ‘regenerative’ therapy remains a more lofty goal. This is largely due to the fact that potential genetic and epigenetic changes incurred by these cells during the reprogramming process raise safety issues that may hamper approval from regulatory agencies. Our results open the way for possibly reconciling the advantages of iPSC-based therapies and patient safety by assigning a new role to these cells, i.e. that of an in vitro factory of therapeutically effective EV acting as cell surrogates. In addition to circumventing the safety issues that might be associated with direct transplantation of iPSC-derived cells in patients, this strategy features multiple clinically relevant advantages: greater standardization and reproducibility of manufacturing, scalability, more accurate product characterization, greater suitability for regulation-compliant quality controls and therefore better alignment with a pharmaceutical production model. Consequently, pharmaceutical companies may be stimulated to invest in the development of this field facilitating the translation of EV-therapies to the clinic. The obvious pre-requisite for such a development is to validate the safety of the final EV formulation to ensure a lack of arrhythmias, immune reaction (particularly if time-release formulations or multiple EV injections are considered) and tumourigenesis. This last point is particularly important considering that some of the miRNA identified in the iPSC-Pg-EV are associated both with regeneration and cancer pathways.
Overall, we have found that iPSC-Pg-derived EV are effective in the treatment of CHF, possibly, in part, through their specific miRNA signature and the associated stimulation of cardioprotective pathways (Take home figure). The production and regulatory advantages of EV could make them appealing alternatives to cell transplantation.
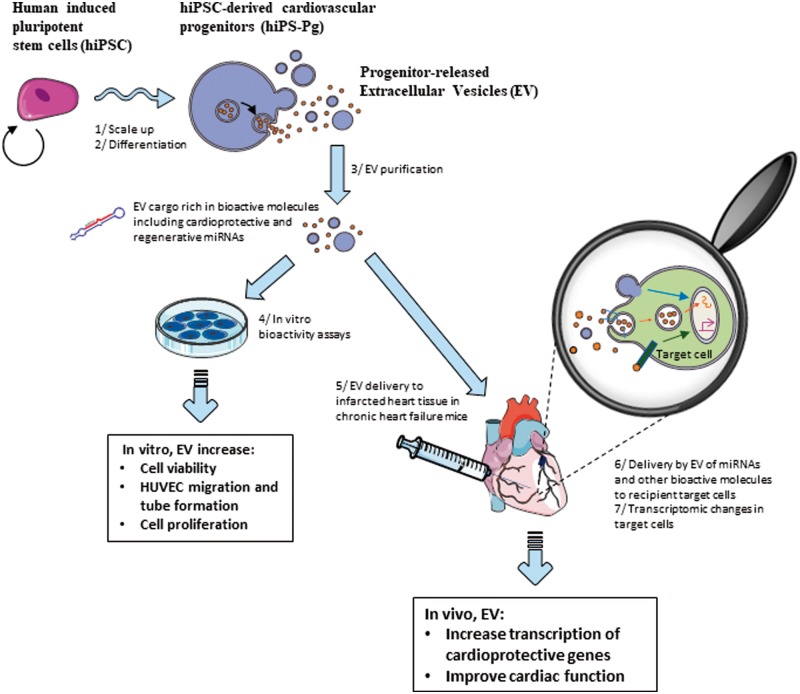
Take home figure.
Human induced pluripotent stem cell-derived cardiovascular progenitor cell ‘biofactories’ produce therapeutic extracellular vesicles that benefit cells and tissues, improving outcomes. Human induced pluripotent stem cells are 1-expanded and 2-differentiated to cardiovascular progenitors. Under the appropriate culture conditions, the latter release extracellular vesicles which can be 3-concentrated and purified, eliminating the secretory cells. The human induced pluripotent stem cell-derived cardiovascular progenitor cell–extracellular vesicles contain a unique miRNA signature, including miRNAs associated with cardio protective and reparative pathways. In vitro, 4-these extracellular vesicles are internalized by target cells leading to cellular effects consistent with cardio-protection or repair. When 5-extracellular vesicles are delivered to the infarcted heart tissue of mice in chronic heart failure, human induced pluripotent stem cell-derived cardiovascular progenitor–extracellular vesicles appear to stimulate transcriptomic changes leading to improved cardiac function.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors thank the Plateforme Génomique of the Institut Cochin (INSERM U1016-CNRS UMR8104-Université Paris Descartes, Paris, France) for performing all the microarray experiments and Ghislaine Frébourg and the Electron Microscopy Facility (FR 3631-CNRS-UPMC) for performing cryo-electron microscopy. We would like to thank Daniel Balvay (Plateforme d'Imagerie du Vivant de Paris Descartes) for his help with the macroscopic image preparation and the assessment of infarct size using his physio3D3 software, Sean Spiering for the preparation of human embryonic stem cell-derived cardiomyocytes and Thomas Mathivet (INSERM U970 PARCC, Paris, France) for his help with the confocal microscopy.
Funding
Institut National de la Santé et de la Recherche Médicale (INSERM); Université Paris Descartes; the LabEx REVIVE (ANR-10-LABX-73); the Fondation pour la Recherche Médicale (DEQ20160334910); the Fondation de France (FDF/2014 00047970); and the LeDucq Foundation (SHAPEHEART network). PhD students were supported by fellowships from the Ecole Doctorale, Université Paris Descartes (NE), the LabEX REVIVE (AK), the Université Pierre et Marie Curie (SP), and the AFM-Téléthon (YH).
Conflict of interest: none declared.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Fisher SA, Doree C, Mathur A, Taggart DP, Martin-Rendon E.. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev 2016;12:CD007888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, de Kleijn DPV.. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res 2008;1:129–137. [DOI] [PubMed] [Google Scholar]
- 4. Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, van Oorschot AAM, Goumans MJ, Strijder C, Sze SK, Choo A, Piek JJ, Doevendans PA, Pasterkamp G, de Kleijn DPV.. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res 2011;6:206–214. [DOI] [PubMed] [Google Scholar]
- 5. Vlassov AV, Magdaleno S, Setterquist R, Conrad R.. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 2012;1820:940–948. [DOI] [PubMed] [Google Scholar]
- 6. Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M.. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 2013;33:1711–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bruno S, Porta S, Bussolati B.. Extracellular vesicles in renal tissue damage and regeneration. Eur J Pharmacol 2016;790:83–91. [DOI] [PubMed] [Google Scholar]
- 8. Kervadec A, Bellamy V, El Harane N, Arakélian L, Vanneaux V, Cacciapuoti I, Nemetalla H, Périer M-C, Toeg HD, Richart A, Lemitre M, Yin M, Loyer X, Larghero J, Hagège A, Ruel M, Boulanger CM, Silvestre J-S, Menasché P, Renault NKE.. Cardiovascular progenitor-derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J Heart Lung Transplant 2016;35:795–807. [DOI] [PubMed] [Google Scholar]
- 9. Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G.. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res 2014;103:530–541. [DOI] [PubMed] [Google Scholar]
- 10. Ibrahim AG-E, Cheng K, Marbán E.. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep 2014;2:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VNS, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, Kishore R.. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 2015;117:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marbán L, Ghaleh B, Marbán E.. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 2016;38:201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DPV, Lim SK.. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 2010;4:214–222. [DOI] [PubMed] [Google Scholar]
- 14. Leroyer AS, Ebrahimian TG, Cochain C, Récalde A, Blanc-Brude O, Mees B, Vilar J, Tedgui A, Levy BI, Chimini G, Boulanger CM, Silvestre J-S.. Microparticles from ischemic muscle promotes postnatal vasculogenesis. Circulation 2009;119:2808–2817. [DOI] [PubMed] [Google Scholar]
- 15. Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, O’Driscoll L, Fais S, Falcon-Perez JM, Felderhoff-Mueser U, Fraile L, Gho YS, Görgens A, Gupta RC, Hendrix A, Hermann DM, Hill AF, Hochberg F, Horn PA, Kleijn D. D, Kordelas L, Kramer BW, Krämer-Albers E-M, Laner-Plamberger S, Laitinen S, Leonardi T, Lorenowicz MJ, Lim SK, Lötvall J, Maguire CA, Marcilla A, Nazarenko I, Ochiya T, Patel T, Pedersen S, Pocsfalvi G, Pluchino S, Quesenberry P, Reischl IG, Rivera FJ, Sanzenbacher R, Schallmoser K, Slaper-Cortenbach I, Strunk D, Tonn T, Vader P, Balkom B. W M V, Wauben M, Andaloussi SE, Théry C, Rohde E, Giebel B.. Applying extracellular vesicles based therapeutics in clinical trials—an ISEV position paper. J Extracell Vesicles 2015;4:30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Menasché P, Vanneaux V, Hagège A, Bel A, Cholley B, Parouchev A, Cacciapuoti I, Al-Daccak R, Benhamouda N, Blons H, Agbulut O, Tosca L, Trouvin J-H, Fabreguettes J-R, Bellamy V, Charron D, Tartour E, Tachdjian G, Desnos M, Larghero J.. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. JACC 2018;71:429–438. [DOI] [PubMed] [Google Scholar]
- 17. McManus DD, Shah SJ, Fabi MR, Rosen A, Whooley MA, Schiller NB.. Prognostic value of left ventricular end-systolic volume index as a predictor of heart failure hospitalization in stable coronary artery disease: data from the Heart and Soul Study. J Am Soc Echocardiogr 2009;22:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ge Q, Zhou Y, Lu J, Bai Y, Xie X, Lu Z.. miRNA in plasma exosome is stable under different storage conditions. Mol Basel Switz 2014;19:1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bellamy V, Vanneaux V, Bel A, Nemetalla H, Emmanuelle Boitard S, Farouz Y, Joanne P, Perier M-C, Robidel E, Mandet C, Hagège A, Bruneval P, Larghero J, Agbulut O, Menasché P.. Long-term functional benefits of human embryonic stem cell-derived cardiac progenitors embedded into a fibrin scaffold. J Heart Lung Transplant 2015;34:1198–1207. [DOI] [PubMed] [Google Scholar]
- 20. Malik ZA, Kott KS, Poe AJ, Kuo T, Chen L, Ferrara KW, Knowlton AA.. Cardiac myocyte exosomes: stability, HSP60, and proteomics. Am J Physiol Heart Circ Physiol 2013;304:H954–H965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, Farber DB.. Transfer of microRNAs by embryonic stem cell microvesicles. PloS One 2009;4:e4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Waldenström A, Gennebäck N, Hellman U, Ronquist G, Qin G.. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PloS One 2012;7:e34653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bobis-Wozowicz S, Kmiotek K, Sekula M, Kedracka-Krok S, Kamycka E, Adamiak M, Jankowska U, Madetko-Talowska A, Sarna M, Bik-Multanowski M, Kolcz J, Boruczkowski D, Madeja Z, Dawn B, Zuba-Surma EK.. Human induced pluripotent stem cell-derived microvesicles transmit RNAs and proteins to recipient mature heart cells modulating cell fate and behavior. Stem Cells 2015;33:2748–2761. [DOI] [PubMed] [Google Scholar]
- 24. Ogawa Y, Taketomi Y, Murakami M, Tsujimoto M, Yanoshita R.. Small RNA transcriptomes of two types of exosomes in human whole saliva determined by next generation sequencing. Biol Pharm Bull 2013;36:66–75. [DOI] [PubMed] [Google Scholar]
- 25. Cervio E, Barile L, Moccetti T, Vassalli G.. Exosomes for intramyocardial intercellular communication. Stem Cells Int 2015;2015:482171.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G.. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 2007;110:2440–2448. [DOI] [PubMed] [Google Scholar]
- 27. Ohno S-I, Drummen GPC, Kuroda M.. Focus on extracellular vesicles: development of extracellular vesicle-based therapeutic systems. Int J Mol Sci 2016;17:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fernandes S, Chong JJH, Paige SL, Iwata M, Torok-Storb B, Keller G, Reinecke H, Murry CE.. Comparison of human embryonic stem cell-derived cardiomyocytes, cardiovascular progenitors, and bone marrow mononuclear cells for cardiac repair. Stem Cell Rep 2015;5:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lai RC, Yeo RWY, Lim SK.. Mesenchymal stem cell exosomes. Semin Cell Dev Biol 2015;40:82–88. [DOI] [PubMed] [Google Scholar]
- 30. Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C.. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA 2016;113:E968–E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang H, Faber JE.. De-novo collateral formation following acute myocardial infarction: dependence on CCR2+ bone marrow cells. J Mol Cell Cardiol 2015;87:4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.