Abstract
Diverticulitis is a chronic disease of the colon in which diverticuli, or outpouching through the colonic wall, become inflamed. Although recent observations suggest that genetic factors may play a significant role in diverticulitis, few genes have yet been implicated in disease pathogenesis and familial cases are uncommon. Here, we report results of whole exome sequencing performed on members from a single multi-generational family with early onset diverticulitis in order to identify a genetic component of the disease. We identified a rare single nucleotide variant in the laminin β 4 gene (LAMB4) that segregated with disease in a dominant pattern and causes a damaging missense substitution (D435N). Targeted sequencing of LAMB4 in 148 non-familial and unrelated sporadic diverticulitis patients identified two additional rare variants in the gene. Immunohistochemistry indicated that LAMB4 localizes to the myenteric plexus of colonic tissue and patients harboring LAMB4 variants exhibited reduced LAMB4 protein levels relative to controls. Laminins are constituents of the extracellular matrix and play a major role in regulating the development and function of the enteric nervous system. Reduced LAMB4 levels may therefore alter innervation and morphology of the enteric nervous system, which may contribute to colonic dysmotility associated with diverticulitis.
Introduction
Diverticulosis is characterized by diverticuli, which are herniations of the mucosal and submucosal layers of the colon through the muscular component of the bowel wall. Inflamed diverticuli result in diverticulitis (1,2). Only a subset of those with diverticulosis (1–4%) develop diverticulitis as assessed over an 11 year follow up study (3). These patients may need surgery if medical management fails (4). Diverticulosis is a common condition that increases in prevalence with age, affecting <5% of those under 40 but approximately 50% of people over 70 years of age (5,6). Diverticulitis tends to have a more severe clinical course in younger patients (7).
Both genetic and environmental factors appear to play a role in the susceptibility to diverticulitis. Family and twin studies suggest that genetic predisposition may account for 40–53% of disease incidence (8–11). However, TNFSF15 is the only gene that has been associated with the pathogenesis of diverticulitis to date (12). Cases of familial diverticulitis with early age of onset also suggest the existence of rare, highly penetrant variants predisposing individuals to the disease (13). Thus, the identification of families with multiple affected members with early age of onset provides an opportunity to investigate the genetic basis of diverticulitis.
The pathophysiology of diverticulitis offers several hypotheses for the underlying etiology of the disease (12). Diverticulosis is a prerequisite for diverticulitis. Alterations to the basement membrane and extracellular matrix can contribute to the development of diverticuli (14,15). Individuals with diverticulosis may be asymptomatic, but the diverticuli may develop a chronic low-grade inflammatory state (16). Subsequent inflammation of the diverticuli results in abdominal tenderness, alterations in bowel elimination, abdominal swelling and often fever (17). Such inflamed diverticuli can progress to the point of abscess formation or perforation, which may require surgical intervention. Genetic factors could contribute to the disease pathogenesis at several steps, from the development of the diverticuli themselves, to the subsequent complication of abscess formation, and/or increased susceptibility to the inflammatory process (12).
The laminin family of heterotrimeric glycoproteins comprises major constituents of the extracellular matrix. Each laminin molecule contains one α-, one β-, and one γ-chain subunit and each subunit is encoded by multiple distinct genes (18). Laminins perform a variety of functions, including mechanical scaffolding for tissues, cellular adhesion, differentiation, neuronal development and regulation of gene expression (19,20). In particular, laminins play a major role in regulating the enteric nervous system (21,22). Abnormal functioning of the enteric nervous system has been previously associated with diverticulitis (23).
In this study, we used exome sequencing to identify a rare single nucleotide variant (SNV) missense mutation in LAMB4 that caused a D435N substitution that encodes one of the β chains of laminin. This variant segregated with disease presentation in a pedigree with several members diagnosed with early onset diverticulitis. Subsequent targeted sequencing of LAMB4 in 148 non-familial, sporadic diverticulitis patients identified additional variants. We found that individuals harboring some of these variants displayed decreased LAMB4 protein levels within the myenteric plexus of their colonic tissue. These findings suggest that certain SNVs in LAMB4 contribute to altered intestinal innervation of the enteric nervous system affecting colonic motility and therefore possibly predisposing patients to diverticulosis and consequently diverticulitis.
Results
Exome sequencing identified a rare variant in the LAMB4 gene that segregated with diverticulitis
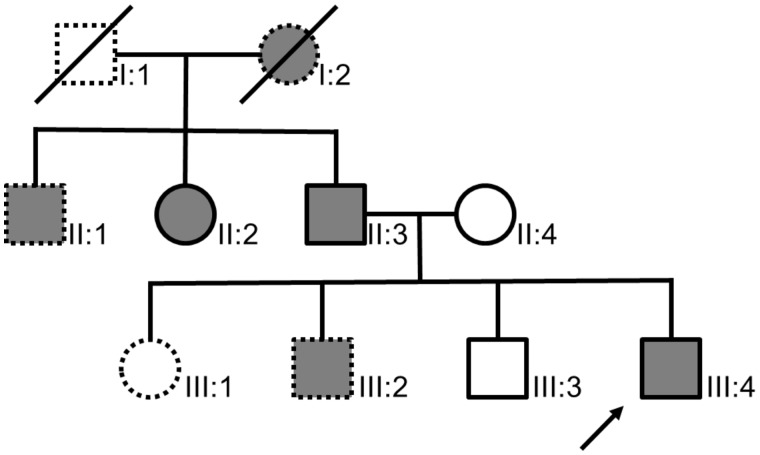
We identified and recruited members of a family who showed an autosomal dominant pattern of inheritance of early onset diverticulitis (Fig. 1). Five DNA samples from the blood of three affected and two unaffected members of the family were collected and exome sequenced in order to identify SNVs that segregated with disease. The average read depth was 25X across the exons of all five patients. Variant calling identified approximately 366,000 SNVs per patient, which was reduced to approximately 60,000 variants per patient after filtering for a read depth of at least 10, a genotype quality score of at least 15, and read ratios for variant calls of ≤0.20 for reference/reference, ≥0.35 to ≤0.65 for variant/reference, and ≥0.80 for variant/variant, respectively. Retaining only SNVs that segregated with diverticulitis reduced the number to 1765 variants. Excluding non-coding and synonymous coding SNVs reduced the number to 213. Since cases of early onset diverticulitis are relatively rare (5,6), only SNVs present in less than 5% of a racially comparable population were considered, yielding 6 variants at a population frequency of 1 - 5%, 20 at a frequency of <1%, and 6 that have no reported population frequency (Supplementary Material, Table S1).
Figure 1.
Pedigree of the recruited family. A total of five family members were analyzed, including three affected by disease (filled circles and squares, II:2, II:3, and III:4) and two unaffected (open circles and squares, II:4, III:3). Dotted outlines designate those members of the pedigree who were not recruited for the study. The index case (indicated by the arrow) was diagnosed with diverticulitis at age 36. Subjects II:2 and II:3 were diagnosed at the ages of 52 and 50 years, respectively. All three had surgical interventions.
To focus on those variants likely to have phenotypic consequences, we examined the scaled CADD scores for each of the 32 variants with population frequencies under 5%. We focused on the 10 variants that were predicted to be the most damaging (Table 1). In particular, we focused on LAMB4, since it is selectively expressed in the colon and is known to be altered in other diseases affecting the gastrointestinal tract (24). LAMB4 encodes one of the β-subunits of laminin, which are trimeric proteins containing one each of the α-, β-, and γ-chain subunits. Laminins play a major role in the proper functioning of the enteric nervous system, further suggesting its potential involvement in diverticulitis (25,26). The LAMB4 variant at position 107738905 on chromosome 7 is predicted to cause an aspartate (D) to asparagine (N) transition at amino acid 435 with a scaled CADD score of 23.9 (Table 1). DNA isolated from the proband (patient III:4) and the four other family members in the study were subjected to Sanger sequencing to confirm the results of exome sequencing (Supplementary Material, Fig. S1).
Table 1.
Genetic variants co-segregating with early onset diverticulitis in the recruited family. Listed are those genetic variants with population wide allele frequencies less than 5%, based on Exome Aggregation Consortium data, which segregated with disease in the proband’s family. Variants are ordered by their predictive damaging CADD score
| Chr. | Location | Gene | Gene Function | Phred Score | ExAC Frequency |
|---|---|---|---|---|---|
| 12 | 50727870 | FAM186A | Unknown function | 35 | 0.008088 |
| 4 | 113359702 | ALPK1 | Regulation of motor coordination | 34 | 0.01036 |
| 14 | 45403699 | KLHL28 | Transcriptional regulation via control of chromatin structure and function | 32 | 0.01219 |
| 21 | 15561623 | LIPI | Lysophosphatidic acid production | 28.1 | 0.004918 |
| 7 | 72985148 | TBL2 | Stress Signaling and Cell Survival | 25.8 | 0.04004 |
| 9 | 91994026 | SEMA4D | Receptor binding and transmembrane signaling receptor activity | 25.5 | 0.00208 |
| 12 | 55725974 | OR6C3 | Olfactory GPCR | 25.3 | 0.001689 |
| 7 | 107738905 | LAMB4 | Extracellular matrix protein | 23.9 | N/A |
| 17 | 76694990 | CYTH1 | Regulates protein sorting and membrane trafficking | 22.5 | 0.00001651 |
| 15 | 42565572 | TMEM87A | Transmembrane protein of unknown function | 22.5 | 0.000008266 |
LAMB4 variants are associated with decreased LAMB4 protein expression in colonic myenteric plexus
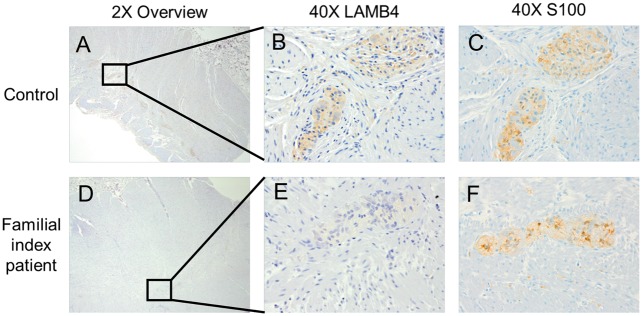
In order to address the role of LAMB4 in the colon and to assess the consequence of the D435N variant on LAMB4 function, we examined LAMB4 expression in the colon by immunohistochemistry. Prior to conducting immunohistochemical analysis, we confirmed the specificity of our antibodies and found that staining using only primary or secondary antibodies produced no signal (Supplementary Material, Fig. S2). Moreover, the anti-LAMB4 antibody detected LAMB4 protein in an immunoblot analysis of HEK293 cells that were transiently transfected with a plasmid expressing LAMB4 cDNA but not in cells transfected with the vector alone (Supplementary Material, Fig. S3). We determined the distribution and abundance of LAMB4 by conducting immunohistochemical analysis of four controls, non-diseased colonic tissues. We noted a strong staining pattern of LAMB4 protein that was consistent with location to the myenteric plexus (Fig. 2A–C). This localization was confirmed in a higher magnification image and by staining of a subsequent tissue section with the glial cell marker S100 (Fig. 2C). We found that LAMB4 staining within the myenteric plexus localized to the endoneurium rather than the epineurium, which was defined by staining for Collagen IV (Supplementary Material, Fig. S4) (27). This localization pattern for LAMB4 is consistent with a previous report that localized laminin in the myenteric plexus (28). No staining of LAMB4 was observed in the submucosal plexus or epithelial layers of the colon. Immunohistochemical analysis of colon tissue from the index patient in the recruited family also found that LAMB4 localized to the myenteric plexus (Fig. 2D–F). However, the intensity of the staining was noticeably decreased in the index patient tissue compared to the control tissues.
Figure 2.
LAMB4 localization and expression in colonic tissue from control and the index diverticulitis patient. Full thickness cross sections of human colon were immunohistologically stained for LAMB4 protein and imaged at 2X magnification for both control (A) and the index patient (D). 40X images of regions from A and D showing LAMB4 staining in individual myenteric plexus in control (B) and index patient (E). Staining of glial marker S100 as a marker of myenteric plexus on the subsequent cut of tissue for both control (C) and index patient (F).
Additional rare LAMB4 variants are present in sporadic diverticulitis patients
To determine whether patients with sporadic diverticulitis also harbored variants in LAMB4, we performed targeted sequencing of the LAMB4 gene on DNA isolated from blood or saliva of 148 patients who had confirmed diverticulitis with no reported family history of the disease as well as the 5 members of the family examined in this study. We found that 52 of the 153 individuals (34%) carried one of nine different nonsynonymous coding variants in LAMB4 (Table 2 and Fig. 3). Six of the variants: rs2074749, rs2240445, rs147992634, rs149874137, rs9690688, and rs1627354 have been previously identified and were present at a frequency consistent with the minor allele frequency of those variants in the general population (Exome Aggregation Consortium (ExAC), Cambridge, MA (http://exac.broadinstitute.org; date last accessed May 30, 2017)) (Table 2) (29). However, the ExAC database consists of 60,706 unrelated individuals sequenced as part of various disease-specific and population genetic studies who were not phenotyped for diverticulitis, thus it is not known whether the individuals with these variants in ExAC have or will develop diverticulitis. Three variants had not been previously reported in dbSNP and were therefore over-represented in the 153 diverticulitis patients that underwent targeted sequencing.
Table 2.
Nonsynonymous variants identified through targeted sequencing of the LAMB4 gene. LAMB4 was sequenced from genomic DNA isolated from 148 patients with sporadic diverticulitis and the five members of the proband family. Not including the variant identified in the proband family, seven variants with scaled CADD scores above 10 were identified in 52 of the samples. All the variants were confirmed by Sanger sequencing. Variant in the proband family is number 4 on the list.
| Chr. | Position | Reference Nucleotide | Alternate Nucleotide | Reference Amino Acid | Alternate Amino Acid | Residue Number | Scaled CADD | RS Number | ExAC Freq. | Our Data Set Freq. | Our data set | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 107746432 | G | A | H | Y | 234 | 25.8 | rs2074749 | 0.029 | 0.026 | 4 |
| 2 | 7 | 107706895 | T | C | N | S | 866 | 25.1 | rs2240445 | 0.028 | 0.0128 | 2 |
| 3 | 7 | 107696101 | A | C | V | G | 1244 | 24.8 | rs147992634 | 0.005 | 0.00641 | 1 |
| 4 | 7 | 107738905 | C | T | D | N | 435 | 23.9 | 0.0192 | 3 | ||
| 5 | 7 | 107746309 | G | A | R | C | 275 | 23.4 | 0.0004 | 0.00641 | 1 | |
| 6 | 7 | 107749668 | C | G | R | T | 117 | 17.75 | rs149874137 | 0.015 | 0.0128 | 2 |
| 7 | 7 | 107720162 | C | A | V | F | 591 | 15.13 | rs9690688 | 0.095 | 0.128 | 20 |
| 8 | 7 | 107720090 | G | T | P | T | 615 | 13.76 | 0.0003 | 0.00641 | 1 | |
| 9 | 7 | 107677984 | G | A | H | Y | 1510 | 8.412 | rs1627354 | 0.097 | 0.115 | 18 |
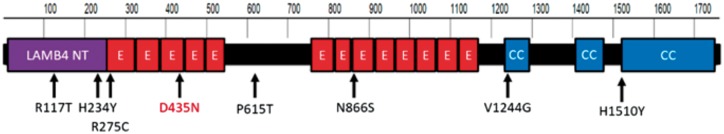
Figure 3.
Human LAMB4 protein structure and positions of variants identified. LAMB4 consists of 1761 amino acids in three separate domain types: LAMB4 N-Terminal (LAMB4 NT), EGF-Like (E), and coiled coil (CC). Shown are missense variants identified from diverticulitis patients through targeted sequencing of LAMB4. Red: variant in index patient.
Patients with diverticulitis and variants in LAMB4 showed decreased expression of LAMB4 in the myenteric plexus
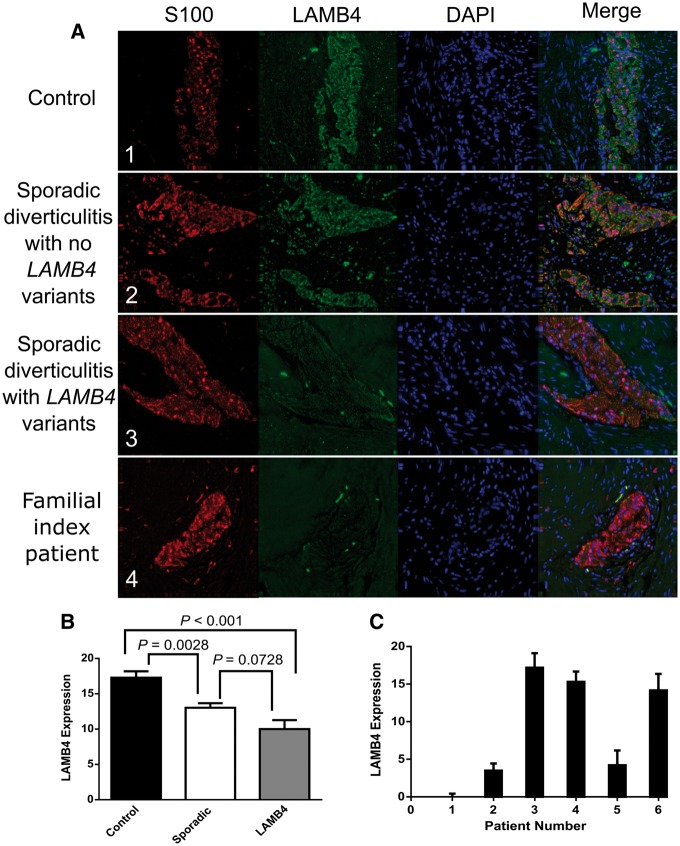
We next determined whether the potentially damaging variants we identified in LAMB4 affected either the localization or levels of LAMB4 using indirect immunofluorescence of resected sigmoid colon tissue sections. We assessed tissues from eleven sporadic cases of diverticulitis lacking LAMB4 variants and tissues from five sporadic cases with a missense variant in LAMB4. Tissue from index patient with the damaging variant in LAMB4 was also examined. Colonic tissue samples from the other four family members were not available. Finally, we examined LAMB4 levels in tissue from four control patients without diverticulitis or variants in LAMB4. These patients did not have colorectal cancer (CRC), as LAMB4 levels are decreased in CRC (24), a finding we confirmed in CRC tissues in our bank (data not shown).
In comparison to controls, the intensity and staining pattern of LAMB4 staining were unaffected in the sporadic patients lacking LAMB4 variants (compare Fig. 4A1 and A2). However, the intensity of LAMB4 staining was significantly decreased in tissue from a sporadic cases that contained the rs2074749 variant in LAMB4 and was essentially absent in tissue from the proband (Fig. 4A3 and A4). When the staining levels were combined and averaged, individuals carrying LAMB4 variants were significantly lower than controls (P < 0.0001, as determined by post-hoc Tukey-Kramer HSD pairwise comparisons, Fig. 4B). Interestingly, when we performed the same analysis on tissue from patients with sporadic diverticulitis but no variants in LAMB4, we noted a similar decrease in the intensity of LAMB4 staining (Sporadic vs Control P = 0.0028, Fig. 4B). We observed that patients with diverticulitis and no variants in LAMB4 displayed higher levels of LAMB4 than patients with diverticulitis and variants in LAMB4; however, this trend did not reach statistical significance (P = 0.0728) (Fig. 4B). Tissue from the index patient from our study family had the lowest level of LAMB4 expression observed (Fig. 4C, patient 1).
Figure 4.
LAMB4 protein levels in myenteric plexus of patients with LAMB4 variants. (A) 40X images of representative myenteric plexus in colonic tissue stained for S100, LAMB4 or DAPI from a non-diverticulitis control patient (1), a patient with sporadic diverticulitis but no damaging variants in LAMB4 (2), a patient with sporadic diverticulitis and heterozygous for the rs2074749 variant in LAMB4 (3) and the index patient (4). (B) Average LAMB4 expression in myenteric plexus in colonic tissue from Control, Sporadic and LAMB4 patients. LAMB4 expression was calculated for each patient sample as the average value of the total level of LAMB4 immunofluorescence in 13 separate myenteric plexus on average for each sample after subtracting the level of fluorescence in the surrounding tissue. Bars show the average of those values for four patients without diverticulitis (Control), eleven patients with diverticulitis but without a variant within the LAMB4 coding region (Sporadic) and six patients with diverticulitis carrying a variant within LAMB4 (LAMB4). Each bar represents the mean (± the standard error of the mean) of the average values from multiple plexus from the sampled tissues. (C) LAMB4 expression levels in colonic sections from individual patients who carried variants in LAMB4. LAMB4 expression was calculated for each patient sample as the average value of the total level of LAMB4 immunofluorescence in at least 13 separate myenteric plexus for each sample after subtracting the level of fluorescence in the surrounding tissue. Any value below zero was set to zero. Patient 1, index patient; patient 2, rs149874137; patient 3, chr7:107746309; patient 4, rs147992634; patient 5, rs2074749; patient 6, chr7:107720090.
We also noted a trend in the correlation of CADD score of the LAMB4 variant with age of diverticulitis diagnosis in patients carrying a LAMB4 variant, with higher CADD scores trending toward an earlier age of diverticulitis diagnosis within our cohort (P <0.065) (Supplementary Material, Fig. S5A). However, the abundance of LAMB4 protein expression as characterized by the intensity of LAMB4 staining did not significantly correlate with the CADD score of the variant (P = 0.7127) (Supplementary Material, Fig. S5B) or the ages of diagnosis for patients with variants in LAMB4 (P = 0.7557) (Supplementary Material, Fig. S5C). Thus, not all variants in LAMB4 have a pathological effect, which is not surprising given the frequency of their occurrence in the population relative to the prevalence of diverticulitis.
Discussion
In order to identify variants associated with diverticulitis, we performed exome-sequencing analysis of five members of a family in which the disease occurred with early ages of onset in multiple members over at least three generations. Given the apparent low frequency of diverticulitis pedigrees with a Mendelian pattern of inheritance, our hypothesis was that a dominant acting highly penetrant rare variant would underlie the susceptibility to disease in this family. We identified rare and low minor allele frequency variants in several genes that segregated with disease, so we cannot unequivocally assign a causative mutation from these results alone. However, we focused on the LAMB4 SNV because prior studies have shown that LAMB4 protein is expressed predominantly in the colon and specifically in the myenteric plexus (30). This expression pattern supported LAMB4 as a diverticulitis candidate gene since the myenteric plexus innervates the gut between the circular and longitudinal muscle layers and serves as a primary regulator of gut motility. Previous studies have suggested the myenteric plexus is significantly smaller and less organized in patients with diverticulitis (31–33). A second reason to suspect the LAMB4 as a potential causative allele was that somatic mutations of LAMB4 are present in a number of colon carcinomas, suggesting that LAMB4 plays a significant role in normal colonic function (24). This is the only prior reported association of LAMB4 with any disease. Finally, the LAMB4 SNV had the strongest prediction for a damaging effect on protein function based on the CADD score of the rarest (<0.1 MAF) SNVs. Our immunofluorescent analysis of LAMB4 confirmed that the protein was localized to the myenteric plexus and the level of the protein was reduced in patients with diverticulitis and further reduced in patients carrying variants of LAMB4. The control and sporadic diverticulitis patients without variants in LAMB4 showed considerably less variation in LAMB4 abundance compared to patients with variants in LAMB4.
The pattern of early onset diverticulitis in the index family was consistent with an autosomal dominant pattern of inheritance, suggesting that the affecting allele should be present in as heterozygote in the affected members of the pedigree, as was the case for LAMB4 D435N variant. This raises the question as to the mechanism that would yield a dominant phenotype for a heterozygous missense variant, for which our expression data suggests an explanation. Three of the LAMB4 SNVs identified were associated with normal levels of the protein in the myenteric plexus in colonic sections, while two yielded approximately half as much protein as seen in normal tissue, as might be expected from haplo-insufficiency due to a loss of function allele (Supplementary Material, Fig. S5B) . In contrast, the D435N variant was associated with substantially reduced LAMB4 levels, suggesting that the variant protein interfered with the expression of the wild type protein in a dominant negative manner, leading to complete loss of gene function even though the variant was only present in one allele. Further mechanistic studies will be required to test that hypothesis.
LAMB4 is a subunit of laminin, a major component of the extracellular matrix (ECM), which provides structural support for tissues, facilitates extracellular signaling between cells, and promotes cellular differentiation (34,35). In particular, the ECM potentiates signaling during development of the enteric nervous system (ENS) (21) such that alterations within the ECM disrupt the development of the ENS (36,37). The ENS initially develops from neural crest cells (NCCs) (38,39), which differentiate and follow particular migration paths to innervate the developing gut (40,41). The ECM helps to regulate the migration pattern of NCCs (42,43). Dysfunctional differentiation of NCCs is associated with congenital disorders of the ENS such as Hirschsprung’s disease (37,44). The role of laminins in the development of the ENS is also supported by the temporal expression of the laminin receptor, which occurs in NCCs only when they reach the bowel (45–47). Thus, diminished or altered function of LAMB4 might be expected to result in reduced elaboration of the ENS and a corresponding alteration in normal gut motility.
Altered motility and variations in luminal colonic pressure are known to contribute to the pathogenesis of diverticulosis (48,49). Altered activity of the sigmoid colon in diverticulosis was originally demonstrated by Arfwidsson in 1964 (50) and more recently confirmed by Bassotti et al. (51). Sigmoid diverticulosis has been shown to be associated with increased intraluminal pressure (52). Moreover, the neuromuscular interactions between the colonic muscles and the ENS are often morphologically different in patients with diverticulitis (53,54). In particular, the number of glial cells and interstitial cells of Cajal of the myenteric plexus, external submucosal plexus and internal submucosal plexus are reduced in patients with diverticulosis and diverticulitis (33,55,56). These observations support the role of altered colonic pressure and motility in diverticulosis onset and progression and provide a mechanism by which LAMB4 dysfunction could increase the likelihood of diverticulitis by causing diverticulosis at an earlier age.
LAMB4 variants may therefore contribute to the development and predisposition of diverticulosis through effects on the organization and morphology of the myenteric plexus during development of the ENS with subsequent effects on the motility of the gut. Laminins are important for proper glial cell function within the myenteric plexus, and therefore alterations in LAMB4 function or expression could contribute to the observed loss of glial cells in diverticulitis and chronic dysmotility of the gut (57). However, LAMB4 is only one of a complex network of proteins that comprise the ECM and that contribute to the development and function of the ENS, thus variants in other genes almost certainly play a role and similarly may contribute to the pathogenesis of diverticulosis.
We cannot exclude that variants in exonic or non-exonic regions not interrogated in our exome analysis were missed. Discovery of these potential variants would require whole genome sequencing. However, LAMB4 has significant biological support as a diverticulitis candidate gene, the predicted damaging D435N variant segregated with disease and was not found in 148 sporadic diverticulitis patients, suggesting that the D435N variant is a rare private mutation, perhaps specific to the family analyzed. In addition, we did find two other rare LAMB4 SNVs predicted to be damaging in sporadic diverticulitis patients. We believe our data support future studies on determining the precise role of LAMB4 and the D435N missense variant in diverticulitis.
Materials and Methods
Patients and samples
Samples were obtained from The Penn State Health Inflammatory Bowel Disease Center biorepository, which contains a large collection of blood and surgical tissue samples acquired for research from patients with diverticulitis. The biorepository also contains samples from control patients confirmed clinically not to have diverticular disease. The index case for this study was a 36 yr old male who required surgery for early onset diverticulitis. Two family members from different generations also had early onset diverticulitis. Blood samples were collected from the proband and four family members, two diagnosed with diverticulitis at 50 yr and 52 yr of age, respectively, and two with no clinical evidence of diverticulosis/diverticulitis. Genomic DNA was extracted from blood using the NucleoSpin Blood L Kit (Macherey-Nagel, Cat No. 740954.20) and quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Life Technologies, Cat No. P7589). Blood samples were also available on 148 sporadic clinical diverticulitis cases with no known affected family members. The research was carried out according to The Code of Ethics of the World Medical Association (Declaration of Helsinki), with all participants providing written informed consent. The Institutional Review Board of the Penn State College of Medicine approved the research (HY98-057).
Whole exome sequencing
Genomic DNA samples were sheared to an average length of 260 bp using a E220 focused-ultrasonicator (Covaris). End repair, ligation of adapters and index sequences and fragment amplification were performed on 0.5 – 1 µg of sheared DNA in an Apollo 324 NGS Library Prep System (Wafergen). Exonic sequences were enriched by hybridization capture of the amplified and indexed samples using a NimbleGen 64 Mb exon capture kit (Roche) with 2.1 million probes and then sequenced using a HiSeq 2500 (Illumina) in rapid mode with paired end 100 bp read lengths.
Bioinformatics pipeline
Single nucleotide variants (SNVs) were identified using the Genome Analysis Tool Kit (GATK) (58) bioinformatics pipeline, which first assessed FASTQ files for quality by FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/; date last accessed May 31, 2017) software and then aligned reads to the hg19 reference human genome with the Burrows-Wheeler Aligner (59). The aligned files were converted to SAM (Sequence Alignment/Map) and BAM (binary Alignment/Map) formats and PCR duplicates removed with Picard (http://picard.sourceforge.net; date last accessed May 31, 2017). The pipeline then performed local realignment around indels using local realignment tools from the GATK and then recalibrated the base quality score with CountCovariates and TableRecalibration from GATK. SNVs were called with GATK UnifiedGenotyper and saved as an output VCF file.
Variant filtering
SNVs were filtered using SNP & Variation Suite v8.4 (Golden Helix, Inc., Bozeman, MT, www.goldenhelix.com). SNVs were filtered by excluding those whose minor allele frequency was greater than 5% in the admixed AMR super population from the 1000 Genomes Project Consortium (60). SNVs that did not segregate with diverticulitis were also excluded. A protein damaging likelihood score was assigned to each remaining SNVs based on CADD analysis and those with a scaled CADD score over 10 (most highly damaging) were retained (61).
Targeted and confirmation sequencing
Sequencing of the LAMB4 gene in 148 sporadic cases of diverticulitis and the 5 recruited familial patients was performed using the Seq-Ready TE SmartChip Multisample nano dispenser (Wafergen) and SmartChip TE PCR cycler (Wafergen). The sporadic cases ranged in age of surgery from 30 to 83 years with an average age of 57 years and were 52% female and 48% male. The custom amplicon set was designed for the exons of ENSG00000091128 (LAMB4). The resultant amplicons were sequenced on a MiSeq (Illumina) platform using 2 X 250 bp read lengths. The SNVs identified in LAMB4 from this group were confirmed using Sanger sequencing (Supplementary Material, Fig. S1). The primers used are listed in Supplementary Material, Table S2.
Immunohistochemistry of LAMB4
Banked colonic tissue samples were collected after surgical resection with portions of the bowel sectioned by a pathologist and fixed in formalin solution within 60 min of resection. Fixed samples were then embedded in paraffin and sectioned on to glass slides by the Penn State Hershey Molecular and Histopathology Core. Sigmoid colon tissue samples from six diverticulitis patients with variants in LAMB4 and eleven diverticulitis patients who did not have variants in LAMB4 were assessed. Four sigmoid colon tissue samples were collected from patients with a traumatic injury or cancer not affecting the colon and without a history of diverticulitis or colon cancer and who did not carry any of the identified LAMB4 variants. Slides with colon tissue sections from patients and controls were incubated for 1 h at 50 °C and deparaffinized and hydrated by two washes of 100% xylene, two washes of 100% EtOH, two washes of 95% EtOH, two washes of 75% EtOH, and three washes of deionized H2O for 2 min per wash. Antigen retrieval was performed in a rice cooker using a 10 mM sodium citrate buffer (Sigma-Aldrich, Cat #C9999), pH 6.0, at 95 °C for 20 min. The slides were removed and allowed to cool for 20 min.
To minimize background staining, slides were washed in deionized water, followed by a wash buffer (0.3% Tween 20 in 1X PBS), and incubated in a humidity chamber with Ultravision Protein Block (Thermofisher scientific Cat #PBQ 150602) for 5 min. Slides were then washed twice in wash buffer and incubated with the primary antibody diluted in 2% bovine serum albumin (BSA) (Supplementary Material, Table S3) for 1 h at room temperature. After another three washes with wash buffer, samples were incubated for 2 h either with fluorescent labeled or HRP conjugated secondary antibodies diluted in 2% BSA (Supplementary Material, Table S3). Following the incubation, fluorescent-labeled slides were washed twice with wash buffer followed by three washes with deionized water. Slides used for immunohistochemistry were developed using DAB (Vector Cat #Sk-4100) and counterstained using hematoxylin (Thermofisher, scientific Cat #TA-125-MH). Slides were then washed three times with water, twice in 75% EtOH, twice in 95% EtOH, twice in 100% EtOH, and twice in 100% Xylene. Coverslips were mounted using hardset mounting medium with DAPI (Vector, Burlingame, CA). S100 immunohistological staining was done using a DAKO autostainer.
Quantification of images
Images were captured using Deltavision (Applied Biosystems; Olympus IX71) as 20 z sections with 0.4 µm spacing using a 20X objective lens. Cy-5, FITC, and DAPI excitation and emission filters were used with exposure times and percent transmission of: 1 s and 32%, 0.5 s and 10%, and 0.08 and 10%, respectively. Images were deconvolved and projected using softWoRx 5.5 imaging analysis system and exported as tiff files. The tiff files were then analyzed using Image J software (62). Myenteric plexus were identified using the S100 marker in the red channel, and the average intensity was measured within and outside the plexus of each image. The values for the plexus were obtained by subtracting the background value from the intensity value over each plexus. For each patient, we calculated the protein level in all available (average of 13) plexus within a tissue section and determined an average expression level for that tissue section. We determined the level of LAMB4 protein within the myenteric plexus of sigmoid colon tissue from control patients and patients carrying LAMB4 variants by quantification of immunofluorescent staining, normalized to the level of S100 staining within the same region. The levels of expression within myenteric plexus were consistent within a single tissue sample.
Accession Number
Exome sequence data used in this study are available in the Short Read Archive, Accession Number SRP072190.
Supplementary Material
Supplementary Material is available at HMG online.
Supplementary Material
Acknowledgements
The authors would like to thank the patients who have generously agreed to participate in the research studies and the Exome Aggregation Consortium and the groups that provided exome variant data for comparison. A full list of contributing groups can be found at http://exac.broadinstitute.org/about.
Conflict of Interest statement. None declared
Funding
Carlino fund for Inflammatory Bowel Disease research at Pennsylvania State University, Pennsylvania Department of Health using Tobacco CURE funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
References
- 1. Stollman N., Raskin J.B. (2004) Diverticular disease of the colon. Lancet, 363, 631–639. [DOI] [PubMed] [Google Scholar]
- 2. Hughes L.E. (1969) Postmortem survey of diverticular disease of the colon. I. Diverticulosis and diverticulitis. Gut, 10, 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shahedi K., Fuller G., Bolus R., Cohen E., Vu M., Shah R., Agarwal N., Kaneshiro M., Atia M., Sheen V.. et al. (2013) Long-term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin. Gastroenterol. Hepatol., 11, 1609–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matrana M.R., Margolin D.A. (2009) Epidemiology and pathophysiology of diverticular disease. Clin. Colon. Rectal. Surg., 22, 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Everhart J.E., Ruhl C.E. (2009) Burden of digestive diseases in the United States part II: lower gastrointestinal diseases. Gastroenterology, 136, 741–754. [DOI] [PubMed] [Google Scholar]
- 6. Murphy T., Hunt R.H., Fried M., Krabshuis J.H. (2007), World Gastroenteology Organization (WGO).
- 7. Strate L.L., Modi R., Cohen E., Spiegel B.M. (2012) Diverticular disease as a chronic illness: evolving epidemiologic and clinical insights. Am. J. Gastroenterol., 107, 1486–1493. [DOI] [PubMed] [Google Scholar]
- 8. Claassen A.T., Mourad-Baars P.E., Mearin M.L., Hilhorst-Hofstee Y., Gerritsen van der Hoop A. (2006) Two siblings below the age of 20 years with diverticular disease. Int. J. Colorectal. Dis., 21, 190–191. [DOI] [PubMed] [Google Scholar]
- 9. Granlund J., Svensson T., Granath F., Hjern F., Ekbom A., Blomqvist P., Schmidt P.T. (2011) Diverticular disease and the risk of colon cancer - a population-based case-control study. Aliment. Pharmacol. Ther., 34, 675–681. [DOI] [PubMed] [Google Scholar]
- 10. Strate L.L., Erichsen R., Baron J.A., Mortensen J., Pedersen J.K., Riis A.H., Christensen K., Sorensen H.T. (2013) Heritability and familial aggregation of diverticular disease: a population-based study of twins and siblings. Gastroenterology, 144, 736–742. e731; quiz e714. [DOI] [PubMed] [Google Scholar]
- 11. Borsch G., Pusch H., Borger G. (1986) Perforated sigmoid diverticulitis in identical twins. Dig. Dis. Sci., 31, 558.. [DOI] [PubMed] [Google Scholar]
- 12. Connelly T.M., Berg A.S., Hegarty J.P., Deiling S., Brinton D., Poritz L.S., Koltun W.A. (2014) The TNFSF15 gene single nucleotide polymorphism rs7848647 is associated with surgical diverticulitis. Ann. Surg., 259, 1132–1137. [DOI] [PubMed] [Google Scholar]
- 13. Ambrosetti P., Gervaz P., Fossung-Wiblishauser A. (2012) Sigmoid diverticulitis in 2011: many questions; few answers. Colorectal. Dis., 14, e439–e446. [DOI] [PubMed] [Google Scholar]
- 14. Klinge U., Rosch R., Junge K., Krones C.J., Stumpf M., Lynen-Jansen P., Mertens P.R., Schumpelick V. (2007) Different matrix micro-environments in colon cancer and diverticular disease. Int. J. Colorectal. Dis., 22, 515–520. [DOI] [PubMed] [Google Scholar]
- 15. Mimura T., Bateman A.C., Lee R.L., Johnson P.A., McDonald P.J., Talbot I.C., Kamm M.A., MacDonald T.T., Pender S.L. (2004) Up-regulation of collagen and tissue inhibitors of matrix metalloproteinase in colonic diverticular disease. Dis. Colon Rectum., 47, 371–378. discussion 378-379. [DOI] [PubMed] [Google Scholar]
- 16. Tursi A., Brandimarte G., Elisei W., Giorgetti G.M., Inchingolo C.D., Danese S., Aiello F. (2008) Assessment and grading of mucosal inflammation in colonic diverticular disease. J. Clin. Gastroenterol., 42, 699–703. [DOI] [PubMed] [Google Scholar]
- 17. Vennix S., Morton D.G., Hahnloser D., Lange J.F., Bemelman W.A. (2014) Systematic review of evidence and consensus on diverticulitis: an analysis of national and international guidelines. Colorectal Dis., 16, 866–878. [DOI] [PubMed] [Google Scholar]
- 18. Domogatskaya A., Rodin S., Tryggvason K. (2012) Functional diversity of laminins. Annu. Rev. Cell. Dev. Biol., 28, 523–553. [DOI] [PubMed] [Google Scholar]
- 19. Li S., Edgar D., Fassler R., Wadsworth W., Yurchenco P.D. (2003) The role of laminin in embryonic cell polarization and tissue organization. Dev. Cell, 4, 613–624. [DOI] [PubMed] [Google Scholar]
- 20. Tzu J., Marinkovich M.P. (2008) Bridging structure with function: structural, regulatory, and developmental role of laminins. Int. J. Biochem. Cell Biol., 40, 199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rauch U., Schafer K.H. (2003) The extracellular matrix and its role in cell migration and development of the enteric nervous system. Eur. J. Pediatr. Surg., 13, 158–162. [DOI] [PubMed] [Google Scholar]
- 22. Paulus J.D., Halloran M.C. (2006) Zebrafish bashful/laminin-alpha 1 mutants exhibit multiple axon guidance defects. Dev. Dyn., 235, 213–224. [DOI] [PubMed] [Google Scholar]
- 23. Bassotti G., Villanacci V., Sidoni A., Nascimbeni R., Dore M.P., Binda G.A., Bandelloni R., Salemme M., Del Sordo R., Cadei M.. et al. (2015) Myenteric plexitis: A frequent feature in patients undergoing surgery for colonic diverticular disease. United European Gastroenterol. J., 3, 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi M.R., An C.H., Yoo N.J., Lee S.H. (2015) Laminin gene LAMB4 is somatically mutated and expressionally altered in gastric and colorectal cancers. Apmis, 123, 65–71. [DOI] [PubMed] [Google Scholar]
- 25. Bronner-Fraser M. (1986) An antibody to a receptor for fibronectin and laminin perturbs cranial neural crest development in vivo. Dev. Biol., 117, 528–536. [DOI] [PubMed] [Google Scholar]
- 26. Nakazawa N., Miyahara K., Okawada M., Yamataka A., Suzuki R., Akazawa C., Tomikawa-Ichikawa N., Arikawa-Hirasawa E. (2013) Laminin-1 promotes enteric nervous system development in mouse embryo. Pediatr. Surg. Int., 29, 1205–1208. [DOI] [PubMed] [Google Scholar]
- 27. Zilic L., Garner P.E., Yu T., Roman S., Haycock J.W., Wilshaw S.P. (2015) An anatomical study of porcine peripheral nerve and its potential use in nerve tissue engineering. J. Anat., 227, 302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bannerman P.G., Mirsky R., Jessen K.R., Timpl R., Duance V.C. (1986) Light microscopic immunolocalization of laminin, type IV collagen, nidogen, heparan sulphate proteoglycan and fibronectin in the enteric nervous system of rat and guinea pig. J. Neurocytol., 15, 733–743. [DOI] [PubMed] [Google Scholar]
- 29. Sherry S.T., Ward M.H., Kholodov M., Baker J., Phan L., Smigielski E.M., Sirotkin K. (2001) dbSNP: the NCBI database of genetic variation. Nucleic. Acids Res., 29, 308–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berglund L., Bjorling E., Oksvold P., Fagerberg L., Asplund A., Szigyarto C.A., Persson A., Ottosson J., Wernerus H., Nilsson P.. et al. (2008) A genecentric Human Protein Atlas for expression profiles based on antibodies. Mol. Cell. Proteomics, 7, 2019–2027. [DOI] [PubMed] [Google Scholar]
- 31. Wood J.D. (1975) Neurophysiology of Auerbach's plexus and control of intestinal motility. Physiol. Rev., 55, 307–324. [DOI] [PubMed] [Google Scholar]
- 32. Sharkey K.A. (2015) Emerging roles for enteric glia in gastrointestinal disorders. J. Clin. Invest., 125, 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bassotti G., Battaglia E., Bellone G., Dughera L., Fisogni S., Zambelli C., Morelli A., Mioli P., Emanuelli G., Villanacci V. (2005) Interstitial cells of Cajal, enteric nerves, and glial cells in colonic diverticular disease. J. Clin. Pathol., 58, 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bonnans C., Chou J., Werb Z. (2014) Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol., 15, 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rozario T., DeSimone D.W. (2010) The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol., 341, 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Breau M.A., Pietri T., Eder O., Blanche M., Brakebusch C., Fassler R., Thiery J.P., Dufour S. (2006) Lack of beta1 integrins in enteric neural crest cells leads to a Hirschsprung-like phenotype. Development, 133, 1725–1734. [DOI] [PubMed] [Google Scholar]
- 37. Fujimoto T., Hata J., Yokoyama S., Mitomi T. (1989) A study of the extracellular matrix protein as the migration pathway of neural crest cells in the gut: analysis in human embryos with special reference to the pathogenesis of Hirschsprung's disease. J. Pediatr. Surg., 24, 550–556. [DOI] [PubMed] [Google Scholar]
- 38. Gershon M.D., Chalazonitis A., Rothman T.P. (1993) From neural crest to bowel: development of the enteric nervous system. J. Neurobiol., 24, 199–214. [DOI] [PubMed] [Google Scholar]
- 39. Nishiyama C., Uesaka T., Manabe T., Yonekura Y., Nagasawa T., Newgreen D.F., Young H.M., Enomoto H. (2012) Trans-mesenteric neural crest cells are the principal source of the colonic enteric nervous system. Nat. Neurosci., 15, 1211–1218. [DOI] [PubMed] [Google Scholar]
- 40. Theveneau E., Mayor R. (2012) Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev. Biol., 366, 34–54. [DOI] [PubMed] [Google Scholar]
- 41. Sasselli V., Pachnis V., Burns A.J. (2012) The enteric nervous system. Dev Biol., 366, 64–73. [DOI] [PubMed] [Google Scholar]
- 42. Perris R., Perissinotto D. (2000) Role of the extracellular matrix during neural crest cell migration. Mech. Dev., 95, 3–21. [DOI] [PubMed] [Google Scholar]
- 43. Endo Y., Ishiwata-Endo H., Yamada K.M. (2012) Extracellular matrix protein anosmin promotes neural crest formation and regulates FGF, BMP, and WNT activities. Dev. Cell, 23, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heanue T.A., Pachnis V. (2007) Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat. Rev. Neurosci., 8, 466–479. [DOI] [PubMed] [Google Scholar]
- 45. Pomeranz H.D., Sherman D.L., Smalheiser N.R., Tennyson V.M., Gershon M.D. (1991) Expression of a neurally related laminin binding protein by neural crest-derived cells that colonize the gut: relationship to the formation of enteric ganglia. J. Comp. Neurol., 313, 625–642. [DOI] [PubMed] [Google Scholar]
- 46. Parikh D.H., Tam P.K., Van Velzen D., Edgar D. (1992) Abnormalities in the distribution of laminin and collagen type IV in Hirschsprung's disease. Gastroenterology, 102, 1236–1241. [PubMed] [Google Scholar]
- 47. Parsons M.J., Pollard S.M., Saude L., Feldman B., Coutinho P., Hirst E.M., Stemple D.L. (2002) Zebrafish mutants identify an essential role for laminins in notochord formation. Development, 129, 3137–3146. [DOI] [PubMed] [Google Scholar]
- 48. Parks T.G., Connell A.M. (1969) Gut, Vol. 10, p 534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Commane D.M., Arasaradnam R.P., Mills S., Mathers J.C., Bradburn M. (2009) Diet, ageing and genetic factors in the pathogenesis of diverticular disease. World J. Gastroenterol., 15, 2479–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arfwidsson S., Knock N.G., Lehmann L., Winberg T. (1964) Pathogenesis of multiple diverticula of the sogmoid colon in diverticular disease. Acta. Chir. Scand. Suppl., 63, Suppl 342, 341–368. [PubMed] [Google Scholar]
- 51. Bassotti G., Battaglia E., Spinozzi F., Pelli M.A., Tonini M. (2001) Twenty-four hour recordings of colonic motility in patients with diverticular disease: evidence for abnormal motility and propulsive activity. Dis. Colon Rectum., 44, 1814–1820. [DOI] [PubMed] [Google Scholar]
- 52. Trotman I.F., Misiewicz J.J. (1988) Sigmoid motility in diverticular disease and the irritable bowel syndrome. Gut, 29, 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Macbeth W.A., Hawthorne J.H. (1965) Intramural ganglia in diverticular disease of the colon. J. Clin. Pathol., 18, 40–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Iwase H., Sadahiro S., Mukoyama S., Makuuchi H., Yasuda M. (2005) Morphology of myenteric plexuses in the human large intestine: comparison between large intestines with and without colonic diverticula. J. Clin. Gastroenterol., 39, 674–678. [DOI] [PubMed] [Google Scholar]
- 55. Mattii L., Ippolito C., Segnani C., Battolla B., Colucci R., Dolfi A., Bassotti G., Blandizzi C., Bernardini N. (2013) Altered expression pattern of molecular factors involved in colonic smooth muscle functions: an immunohistochemical study in patients with diverticular disease. PLoS One, 8, e57023.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wedel T., Busing V., Heinrichs G., Nohroudi K., Bruch H.P., Roblick U.J., Bottner M. (2010) Diverticular disease is associated with an enteric neuropathy as revealed by morphometric analysis. Neurogastroenterol Motil., 22, 407–414. e493-404. [DOI] [PubMed] [Google Scholar]
- 57. Raghavan S., Gilmont R.R., Bitar K.N. (2013) Neuroglial differentiation of adult enteric neuronal progenitor cells as a function of extracellular matrix composition. Biomaterials, 34, 6649–6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M.. et al. (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res., 20, 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li H., Durbin R. (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics, 25, 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Abecasis G.R., Auton A., Brooks L.D., DePristo M.A., Durbin R.M., Handsaker R.E., Kang H.M., Marth G.T., McVean G.A. (2012) An integrated map of genetic variation from 1,092 human genomes. Nature, 491, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kircher M., Witten D.M., Jain P., O'Roak B.J., Cooper G.M., Shendure J. (2014) A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet., 46, 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schneider C.A., Rasband W.S., Eliceiri K.W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods, 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.