Abstract
Background.
Regular CD4 count testing is often used to monitor antiretroviral therapy efficacy. However, this practice may be redundant in children with a suppressed human immunodeficiency virus (HIV) viral load.
Methods
Study end points were as follows: (1) a CD4 count <200 cells/mm3 followed by a CD4 count ≥200 cells/mm3 (transient CD4 <200); (2) CD4 count <200 cells/mm3 confirmed within 6 months (confirmed CD4 <200); and (3) a new or recurrent World Health Organization (WHO) stage 3 or 4 illness (clinical failure). Kaplan–Meier curves and Cox regression were used to evaluate rates and predictors of transient CD4 <200, confirmed CD4 <200, and clinical failure among virally suppressed children aged 5–15 years who were enrolled in the TREAT Asia Pediatric HIV Observational Database.
Results
Data from 967 children were included in the analysis. At the time of confirmed viral suppression, median age was 10.2 years, 50.4% of children were female, and 95.4% were perinatally infected with HIV. Median CD4 cell count was 837 cells/mm3, and 54.8% of children were classified as having WHO stage 3 or 4 disease. In total, 18 transient CD4 <200 events, 2 confirmed CD4 <200 events, and10 clinical failures occurred at rates of 0.73 (95% confidence interval [95% CI], 0.46–1.16), 0.08 (95% CI, 0.02–0.32), and 0.40 (95% CI, 0.22–0.75) events per 100 patient-years, respectively. CD4 <500 cells/mm3 at the time of viral suppression confirmation was associated with higher rates of both CD4 outcomes.
Conclusions
Regular CD4 testing may be unnecessary for virally suppressed children aged 5–15 years with CD4 ≥500 cells/mm3.
Keywords: CD4 cell count monitoring, pediatrics, viral suppression.
Human immunodeficiency virus (HIV) viral load monitoring accurately detects treatment failure before CD4 cell count decline or clinical deterioration, thereby providing an early indication of the need to enhance adherence support or switch antiretroviral therapy (ART) [1]. The World Health Organization (WHO) recommends regular viral load testing as the preferred method of monitoring ART [2], and substantial efforts are being made to support the scale-up of viral load capacity in resource-limited areas. An important aspect of this scale-up involves redirecting resources that could be used more efficiently.
CD4 count is a strong predictor of disease progression and mortality in people with HIV [3–10]. It is also an important tool for assessing ART eligibility and the need to initiate opportunistic infection prophylaxis [2]. Regular CD4 count testing can be used to monitor ART efficacy; however, this role is increasingly considered unnecessary in patients who are known to be virally suppressed [11–17].
In 2014, the WHO released a policy brief which stated that CD4 monitoring could be stopped in virally suppressed patients for whom routine viral monitoring is available [18]. However, there are limited data supporting this recommendation in children, and it remains unclear whether this guidance applies to those with a low CD4 cell count on ART. A recent report from South Africa found that there was a 2.8% probability of severe immunosuppression over 3 years of follow-up in HIV-positive children aged ≥2 years who, at the time of viral suppression, had minimal immunosuppression and had been on ART for <18 months [17]. Pediatric data from Asia are currently lacking. We addressed this issue by analyzing the TREAT Asia Pediatric HIV Observational Database (TApHOD).
METHODS
Study Population
The TApHOD data collection is based on a standardized set of demographic, monitoring, and treatment variables that have been described previously [19]. Recruitment started in 2008 and involves 16 pediatric centers in Cambodia (n = 1), India (n = 1), Indonesia (n = 2), Malaysia (n = 4), Thailand (n = 5), and Vietnam (n = 3). These sites are predominantly public or university-based pediatric HIV referral clinics. Ethics approval has been obtained at the sites, the coordinating center (TREAT Asia/amfAR), and the data management and statistical analysis center (The Kirby Institute, University of New South Wales Australia). Patient consent is deferred to the individual participating sites and their institutional review boards.
Children were eligible for inclusion in this analysis if they (1) were receiving ≥3 antiretroviral agents for ≥6 months, (2) had confirmed viral suppression, defined as 2 consecutive viral load measurements <400 copies/mL and a CD4 count ≥200 cells/mm3, all within a 390-day period, (3) were aged 5–15 years at the time of confirmed viral suppression, (4) had a minimum of 1 subsequent viral load measurement <400 copies/mL at least 12 months after confirmed viral suppression, and (5) had a minimum of 1 CD4 measurement 6 months after confirmed viral suppression. Follow-up time was only counted while virally suppressed patients had CD4 count monitoring every 6 months. A break in regular CD4 count testing resulted in censoring. A 3-month window on either side of the 6-monthly CD4 cell count date was used. Patients may have had more than 1 CD4 count measurement within this period, but the measurement closest to the 6-month date was considered the primary measurement. All subsequent CD4 measurements were evaluated when determining the CD4 outcomes described below.
Outcomes and Collected Data
Study end points were as follows: (1) a CD4 count <200 cells/mm3 followed by a CD4 count ≥200 cells/mm3 (transient CD4 <200); (2) CD4 count <200 cells/mm3 confirmed within 6 months (confirmed CD4 <200); and (3) a new or recurrent WHO stage 3 or 4 illness (clinical failure). A CD4 count threshold of 200 cells/mm3 was used because measurements that drop below this level indicate the need for opportunistic infection prophylaxis in all settings [2]. Subanalysis was also undertaken to evaluate the sensitivity of our findings when 350 cells/mm3 was used as the CD4 count threshold in children with a CD4 count ≥350 cells/mm3 at baseline. The subanalysis end points were as follows: (1) a CD4 count <350 cells/mm3 followed by a CD4 count ≥350 cells/mm3 (transient CD4 <350); and (2) CD4 count <350 cells/mm3 confirmed within 6 months (confirmed CD4 <350).
Data included age, sex, mode of HIV exposure, orphan status, primary care giver status, history of previous acquired immune deficiency syndrome diagnoses, height, weight, hemoglobin, hepatitis B and C serologies, and ART regimen. Baseline was defined as the date of confirmed viral suppression. Anemia was defined according to the age- and sex-specific limits defined by the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events [20].
Statistical Analysis
Rates and predictors of transient CD4 <200, confirmed CD4 <200, transient CD4 <350, confirmed CD4<350, and clinical failure were analyzed by Kaplan–Meier curves and Cox regression. Patients could only contribute a single outcome in each analysis. If the last biannual CD4 count was <6 months before an ART class change or a final viral load measurement <400 copies/mL, then follow-up time was censored at the date of ART class change or final suppressed viral load (whichever occurred first). If the last biannual CD4 count occurred >6 months before an ART class change or a final viral load of <400 copies/mL, then follow-up time was censored 6 months after the final biannual CD4 count. Antiretroviral therapy class change was defined as a substitution or cessation of ≥1 antiretroviral drug class in the regimen being used at the beginning of viral suppression. Univariate and multivariate predictors were considered significant if they exhibited a P value ≤.05. Stata statistical software (version 14.1; StataCorp, College Station, Texas) was used for all statistical analysis.
RESULTS
As of September 2014, a total of 5609 children were enrolled in TApHOD. Of these, 1792 had been using ART for ≥6 months and had documentation of confirmed viral suppression between age 5 and 15 years; 967 had a follow-up viral load and at least 6-monthly CD4 monitoring and were therefore eligible for this analysis (see Supplementary Figure 1). Baseline characteristics of the study population are shown in Table 1. The median rate of viral load monitoring while enrolled in TApHOD and using ART was 1.4 (interquartile range, 1.1–2.0) tests/year among eligible children.
Table 1.
Baseline Characteristicsa
| Characteristic | Total Patients = 967 |
|---|---|
| Age in years, median (IQR) | 10.2 (7.8–12.6) |
| Female | 487 (50.4) |
| Mode of HIV infection | |
| Perinatal | 922 (95.4) |
| Other | 10 (1.0) |
| Unknown | 35 (3.6) |
| Orphan status | |
| Both parents alive | 237 (24.5) |
| Single parent alive | 276 (28.5) |
| Neither parent alive | 358 (37.0) |
| Unknown | 96 (9.9) |
| Primary care giver | |
| Parents | 372 (38.5) |
| Grandparent | 183 (18.9) |
| Relative/nonrelative/foster care | 234 (24.2) |
| Unknown | 178 (18.4) |
| CD4 cell count in cells/mm3, median (IQR) | 837 (602–1092) |
| WHO category | |
| 1/2 | 437 (45.2) |
| 3 | 333 (34.4) |
| 4 | 197 (20.4) |
| Height-for-age z-score, median (IQR), N = 941 | −1.7 (−2.5 to −1.0) |
| Weight-for-age z-score, median (IQR), N = 942 | −1.7 (−2.5 to −0.9) |
| Anemic, n (%tested), N = 619 | 47 (7.6) |
| HBsAg positive, n (%tested), N = 761 | 36 (4.7) |
| Hepatitis C antibody positive, n (%tested), N = 182 | 5 (2.7) |
| Antiretroviral therapy | |
| NRTI-NNRTI | 760 (78.6) |
| NRTI-PI | 189 (19.5) |
| Other | 18 (1.9) |
Abbreviations: HBsAg, hepatitis B surface antigen; HIV, human immunodeficiency virus; IQR, interquartile range; N, number of patients with data available; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; WHO, World Health Organization.
aValues are n (%total) unless otherwise specified.
Overall, and in children with a baseline CD4 count >750 cells/mm3, median CD4 count declined over time but never fell below 650 cells/mm3. In those with a baseline CD4 count between 500 and 750 cells/mm3, median CD4 count remained stable between 598 and 659 cells/mm3. Among children with a baseline CD4 count between 200 and 499 cells/mm3, median CD4 count rose from 397 to 556 cells/mm3 in the first 18 months of viral suppression and remained approximately 550 cells/mm3 thereafter.
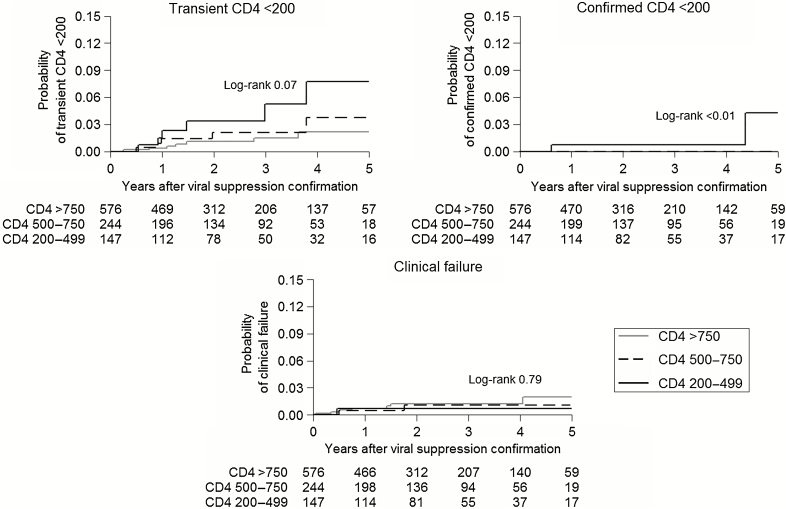
In total, 18 transient CD4 <200 events, 2 confirmed CD4 <200 events, and 10 clinical failures occurred at rates of 0.73 (95% confidence interval [95% CI], 0.46–1.16), 0.08 (95% CI, 0.02–0.32), and 0.40 (95% CI, 0.22–0.75) events per 100 patient-years, respectively. Figure 1 shows the cumulative probability of transient CD4 <200, confirmed CD4 <200, and clinical failure by baseline CD4 category. After 5 years, the probabilities of transient CD4 <200 in patients with baseline CD4 count 200–499, 500–750, and >750 cells/mm3 were 7.7%, 3.7%, and 2.1%, respectively (log-rank test 0.07). Confirmed CD4 <200 only occurred in children with a baseline CD4 count between 200 and 499 cells/mm3; the 5-year cumulative probability of confirmed CD4 <200 in this group was 4.3% (log-rank test <0.01). The 5-year probabilities of clinical failure with baseline CD4 count 200–499, 500–750, and >750 cells/mm3 were 0.7%, 1.1%, and 2.0%, respectively (log-rank test 0.79). Given the low number of events, our regression analyses were limited to investigating transient CD4 <200. The only baseline factor significantly associated with transient CD4 <200 in univariate analysis was CD4 count (hazard ratio [HR] = 1.09 for every 50 cells/mm3 lower; 95% CI, 1.01–1.18; P = .03).
Figure 1.
Kaplan–Meier curves showing the probability of transient CD4 <200, confirmed CD4 <200, and clinical failure by baseline CD4 category. Numbers underneath each time point represent the number of children at risk by baseline CD4 category.
In a subanalysis of patients with a baseline CD4 ≥350 cells/mm3 (n = 917), 58 transient CD4 <350 events occurred at a rate of 2.57 (95% CI, 1.99–3.33) per 100 patient-years, and 11 confirmed CD4 <350 events occurred at a rate of 0.47 (95% CI, 0.26–0.84) per 100 patient-years. After 5 years, the probabilities of transient CD4 <350 in patients with baseline CD4 count 350–499, 500–750, and >750 cells/mm3 were 25.9%, 16.2%, and 7.5%, respectively (log-rank test <0.01). The 5-year probabilities of confirmed CD4 <350 in patients with baseline CD4 count 350–499, 500–750, and >750 cells/mm3 were 15.8%, 4.4%, and 0.7%, respectively (log-rank test <0.01) (see Supplementary Figure 2). The only baseline factors significantly associated with transient CD4 <350 in univariate analysis were age (HR = 1.16 for every year older; 95% CI, 1.06–1.28; P < .01) and CD4 count (HR = 1.17 for every 50 cells/mm3 lower; 95% CI, 1.10–1.24; P < .01). In multivariate analysis, baseline age became nonsignificant after adjustment for baseline CD4 cell count.
DISCUSSION
Virally suppressed children in this study generally had stable CD4 count measurements over time, and overall rates of transient CD4 <200, confirmed CD4 <200, confirmed CD4 <350, and clinical failure were less than 1% per year. Median CD4 count remained lowest over 5 years of follow-up in those with a low baseline CD4 count. Accordingly, children with a low baseline CD4 count were at the greatest risk of transient and confirmed CD4 <200 and transient and confirmed CD4 <350. Rates of clinical failure were ≤2% in all baseline CD4 count categories evaluated.
CD4 count tends to increase gradually in virally suppressed adults regardless of baseline CD4 count [11]. In contrast, we found overall median CD4 count declined after baseline, which was driven by children with a baseline CD4 >750 cells/mm3. This is most likely explained by the biologic decline in CD4 count observed during childhood [21–23]. Our results provide reassurance that, even though CD4 count may decline in virally suppressed children with a high baseline CD4 level, the risk of serious immune suppression and subsequent need to consider opportunistic infection prophylaxis remains low. Furthermore, although children with a baseline CD4 count ≥500 cells/mm3 exhibit a moderate risk of a transient CD4 count <200 cells/mm3 and a reasonably high risk of a transient CD4 count <350 cells/mm3, there seems to be a low probability that such levels of immune suppression will be sustained while viral load is undetectable. More importantly, the clinical relevance of a single low CD4 count is questionable in HIV-infected patients because it is usually reversed without intervention and often associated with physiological variation, acute illness, or the use of bone marrow-suppressing medication [12, 17, 24].
Our results add to those of the study by Davies et al [17] on virally suppressed, HIV-infected children living in South Africa. In their study, median age was 5.8 years and the probability of a single CD4 count indicating severe immune suppression was 6.6% over 3 years of follow-up (2.2%/year). The authors also reported that severe immune suppression resolved in 86% of children with a subsequent CD4 count. It is notable that the rate of transient CD4 <200 in our study (0.73%/year) was lower than that of severe immune suppression in the African cohort. This could be because children with a CD4 count <200 cells/mm3 and no subsequent CD4 count were censored in our analysis. The older age of our study population may also have been an influence, because Davies et al [17] reported that the rate severe immune suppression was lower among children aged ≥2 years at baseline compared with those aged <2 years. Regardless of these results, the overarching message from both studies is that clinically relevant CD4 decline is rare among virally suppressed children.
Our study had several limitations. First, our definition of viral suppression was based on 2 consecutive measurements <400 copies/mL. This was to account for the different viral load test thresholds over the history of the TApHOD cohort. However, current testing methods are able to detect virus present at >20 copies/mL, meaning that some patients with a detectable viral load at this threshold may have been included in our analysis. Our rates of CD4 decline and clinical failure might have been even lower if we could have reliably defined a lower cutoff point for viral suppression. In addition, the observational nature of TApHOD data collection limited the assessment of specific events, medications, or interventions that may have been related to the CD4 declines documented.
CONCLUSIONS
Virally suppressed children aged 5–15 years in our Asian cohort with a baseline CD4 cell count ≥500 cells/mm3 had a low risk of clinically relevant CD4 count decline or clinical failure. Reducing or discontinuing regular CD4 count monitoring in this population may be a safe intervention that would help pediatric HIV programs with viral load capacity to utilize their resources more effectively. This could also enhance the feasibility of expanding routine viral load monitoring across the region. Future work should seek to establish the safety of reduced CD4 count monitoring in children <5 years of age and in children with a baseline CD4 measurement <500 cells/mm3.
The TREAT Asia Pediatric HIV Network
P. S. Ly* and V. Khol, National Centre for HIV/AIDS, Dermatology and STDs, Phnom Penh, Cambodia; J. Tucker, New Hope for Cambodian Children, Phnom Penh, Cambodia; N. Kumarasamy,* S. Saghayam, and E. Chandrasekaran, YRGCARE Medical Centre, CART CRS, Chennai, India; D. K. Wati*, L. P. P. Atmikasari, and I.Y. Malino, Sanglah Hospital, Udayana University, Bali, Indonesia; N. Kurniati* and D. Muktiarti, Cipto Mangunkusumo General Hospital, Jakarta, Indonesia; S. M. Fong,*† M. Lim, and F. Daut, Hospital Likas, Kota Kinabalu, Malaysia; N. K. Nik Yusoff* and P. Mohamad, Hospital Raja Perempuan Zainab II, Kelantan, Malaysia; K. A. Razali*, T. J. Mohamed, and NADR Mohammed, Pediatric Institute, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; R. Nallusamy* and K. C. Chan, Penang Hospital, Penang, Malaysia; T. Sudjaritruk*, V. Sirisanthana, L. Aurpibul, and P. Oberdorfer, Department of Pediatrics, Faculty of Medicine, Chiang Mai University and Research Institute for Health Sciences, Chiang Mai, Thailand; R. Hansudewechakul,* S. Denjanta, W. Srisuk, and A. Kongphonoi, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; P. Lumbiganon*‡, P. Kosalaraksa, P. Tharnprisan, and T. Udomphanit, Division of Infectious Diseases, Department of Pediatrics, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; G. Jourdain, PHPT-IRD UMI 174 (Institut de Recherche pour le Développement and Chiang Mai University), Chiang Mai, Thailand; T. Bunupuradah*, T. Puthanakit, W. Prasitsuebsai, and W. Chanthaweethip, HIV-NAT, The Thai Red Cross AIDS Research Centre, Bangkok, Thailand; K. Chokephaibulkit*, K. Lapphra, W. Phongsamart, and S. Sricharoenchai, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; K. H. Truong*, Q. T. Du, and C. H. Nguyen, Children's Hospital 1, Ho Chi Minh City, Vietnam; V. C. Do*, T. M. Ha, and V. T. An, Children's Hospital 2, Ho Chi Minh City, Vietnam; L. V. Nguyen*, D. T. K. Khu, A. N. Pham, and L. T. Nguyen, National Hospital of Pediatrics, Hanoi, Vietnam; O. N. Le, Worldwide Orphans Foundation, Ho Chi Minh City, Vietnam; A. H. Sohn*, N. Durier, and C. Sethaputra, TREAT Asia/amfAR — The Foundation for AIDS Research, Bangkok, Thailand; D. A. Cooper, M. G. Law*, and A. Kariminia, The Kirby Institute, UNSW Australia, Sydney, Australia; Q. T. Du, C. H. Nguyen, Children's Hospital 1, Ho Chi Minh City, Vietnam; V. C. Do*, T. M. Ha, V. T. An, Children's Hospital 2, Ho Chi Minh City, Vietnam; L. V. Nguyen*, D. T. K. Khu, A. N. Pham, L. T. Nguyen, National Hospital of Pediatrics, Hanoi, Vietnam; O. N. Le, Worldwide Orphans Foundation, Ho Chi Minh City, Vietnam; A. H. Sohn* and C. Sethaputra, TREAT Asia/amfAR — The Foundation for AIDS Research, Bangkok, Thailand; D. A. Cooper, M. G. Law*, and A. Kariminia, The Kirby Institute, UNSW Australia, Sydney, Australia. *Steering Committee members; †Current Steering Committee Chair; ‡Co-Chair.
Supplementary Data
Supplementary materials are available at Journal of the Pediatric Infectious Diseases Society online.
Supplementary Material
Notes
Disclaimer. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Financial support. This work was funded by TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with backing from the US National Institutes of Health's National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute as part of the International Epidemiologic Databases to Evaluate AIDS (grant U01AI069907) and the AIDS Life Association. The Kirby Institute is funded by the Australian Government Department of Health and Ageing and is affiliated with the Faculty of Medicine, University of New South Wales (UNSW) Australia.
Potential conflicts of interest. D. C. B. has received research funding from Gilead Sciences and has previously been employed by Merck Serono and Bayer Schering Pharma. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rutherford GW, Anglemyer A, Easterbrook PJ et al. . Predicting treatment failure in adults and children on antiretroviral therapy: a systematic review of the performance characteristics of the 2010 WHO immunologic and clinical criteria for virologic failure. AIDS 2014; 28(Suppl 2):S161–9. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach – June 2013. Available At: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1. Accessed January 3, 2014. [PubMed] [Google Scholar]
- 3. Fahey JL, Taylor JM, Detels R et al. . The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med 1990; 322:166–72. [DOI] [PubMed] [Google Scholar]
- 4. Fauci AS, Macher AM, Longo DL et al. . NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med 1984; 100:92–106. [DOI] [PubMed] [Google Scholar]
- 5. Goedert JJ, Biggar RJ, Melbye M et al. . Effect of T4 count and cofactors on the incidence of AIDS in homosexual men infected with human immunodeficiency virus. JAMA 1987; 257:331–4. [PubMed] [Google Scholar]
- 6. Hogg RS, Yip B, Chan KJ et al. . Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA 2001; 286:2568–77. [DOI] [PubMed] [Google Scholar]
- 7. Pedersen C, Gerstoft J, Tauris P et al. . Trends in survival of Danish AIDS patients from 1981 to 1989. AIDS 1990; 4:1111–6. [DOI] [PubMed] [Google Scholar]
- 8. Phillips AN, Lee CA, Elford J et al. . Serial CD4 lymphocyte counts and development of AIDS. Lancet 1991; 337:389–92. [DOI] [PubMed] [Google Scholar]
- 9. Polk BF, Fox R, Brookmeyer R et al. . Predictors of the acquired immunodeficiency syndrome developing in a cohort of seropositive homosexual men. N Engl J Med 1987; 316:61–6. [DOI] [PubMed] [Google Scholar]
- 10. Saah AJ, Hoover DR, He Y et al. . Factors influencing survival after AIDS: report from the Multicenter AIDS Cohort Study (MACS). J Acquir Immune Defic Syndr 1994; 7:287–95. [PubMed] [Google Scholar]
- 11. Ahn JY, Boettiger D, Law M et al. . Implementation and operational research: effects of CD4 monitoring frequency on clinical end points in clinically stable HIV-infected patients with viral suppression. J Acquir Immune Defic Syndr 2015; 69:e85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gale HB, Gitterman SR, Hoffman HJ et al. . Is frequent CD4+ T-lymphocyte count monitoring necessary for persons with counts >=300 cells/muL and HIV-1 suppression? Clin Infect Dis 2013; 56:1340–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Girard PM, Nelson M, Mohammed P et al. . Can we stop CD4+ testing in patients with HIV-1 RNA suppression on antiretroviral treatment? AIDS 2013; 27:2759–63. [DOI] [PubMed] [Google Scholar]
- 14. Reekie J, Mocroft A, Sambatakou H et al. . Does less frequent routine monitoring of patients on a stable, fully suppressed cART regimen lead to an increased risk of treatment failure? AIDS 2008; 22:2381–90. [DOI] [PubMed] [Google Scholar]
- 15. Sax PE. Editorial commentary: can we break the habit of routine CD4 monitoring in HIV care? Clin Infect Dis 2013; 56:1344–6. [DOI] [PubMed] [Google Scholar]
- 16. Ford N, Meintjes G, Pozniak A et al. . The future role of CD4 cell count for monitoring antiretroviral therapy. Lancet Infect Dis 2015; 15:241–7. [DOI] [PubMed] [Google Scholar]
- 17. Davies MA, Ford N, Rabie H et al. . Reducing CD4 monitoring in children on antiretroviral therapy with virologic suppression. Pediatr Infect Dis J 2015:1361–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: what's new. Available at: http://www.who.int/hiv/pub/arv/policy-brief-arv-2015/en/. Accessed December 7, 2015. [Google Scholar]
- 19. Kariminia A, Chokephaibulkit K, Pang J et al. . Cohort profile: the TREAT Asia pediatric HIV observational database. Int J Epidemiol 2011; 40:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, US Department of Health and Human Services. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.0., November 2014. Available at: http://rsc.tech-res.com/Document/safetyandpharmacovigilance/DAIDS_AE_GRADING_TABLE_v2_NOV2014.pdf. Accessed January 19, 2015. [Google Scholar]
- 21. Comans-Bitter WM, de Groot R, van den Beemd R et al. . Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr 1997; 130:388–93. [DOI] [PubMed] [Google Scholar]
- 22. Erkeller-Yuksel FM, Deneys V, Yuksel B et al. . Age-related changes in human blood lymphocyte subpopulations. J Pediatr 1992; 120:216–22. [DOI] [PubMed] [Google Scholar]
- 23. Hannet I, Erkeller-Yuksel F, Lydyard P et al. . Developmental and maturational changes in human blood lymphocyte subpopulations. Immunol Today 1992; 13:215–8. [DOI] [PubMed] [Google Scholar]
- 24. Ford N, Stinson K, Davies MA et al. . Is it safe to drop CD4+ monitoring among virologically suppressed patients: a cohort evaluation from Khayelitsha, South Africa. AIDS 2014; 28:2003–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.