Abstract
Study Objectives
To determine whether defining two subtypes of sleep-disordered breathing (SDB) events—with or without hypoxia—results in measures that are more strongly associated with hypertension and sleepiness.
Methods
A total of 1022 participants with 2112 nocturnal polysomnograms from the Wisconsin Sleep Cohort were analyzed with our automated algorithm, developed to detect breathing disturbances and desaturations. Breathing events were time-locked to desaturations, resulting in two indices—desaturating (hypoxia-breathing disturbance index [H-BDI]) and nondesaturating (nonhypoxia-breathing disturbance index [NH-BDI]) events—regardless of arousals. Measures of subjective (Epworth Sleepiness Scale) and objective (2981 multiple sleep latency tests from a subset of 865 participants) sleepiness were analyzed, in addition to clinically relevant clinicodemographic variables. Hypertension was defined as BP ≥ 140/90 or antihypertensive use.
Results
H-BDI, but not NH-BDI, correlated strongly with SDB severity indices that included hypoxia (r ≥ 0.89, p ≤ .001 with 3% oxygen-desaturation index [ODI] and apnea hypopnea index with 4% desaturations). A doubling of desaturation-associated events was associated with hypertension prevalence, which was significant for ODI but not H-BDI (3% ODI OR = 1.06, 95% CI = 1.00–1.12, p < .05; H-BDI OR 1.04, 95% CI = 0.98–1.10) and daytime sleepiness (β = 0.20 Epworth Sleepiness Scale [ESS] score, p < .0001; β = −0.20 minutes in MSL on multiple sleep latency test [MSLT], p < .01). Independently, nondesaturating event doubling was associated with more objective sleepiness (β = −0.52 minutes in MSL on MSLT, p < .001), but had less association with subjective sleepiness (β = 0.12 ESS score, p = .10). In longitudinal analyses, baseline nondesaturating events were associated with worsening of H-BDI over a 4-year follow-up, suggesting evolution in severity.
Conclusions
In SDB, nondesaturating events are independently associated with objective daytime sleepiness, beyond the effect of desaturating events.
Keywords: Pattern recognition, automated, Outcome Assessment (Health Care), sleep apnea syndromes
Statement of Significance
This study objectively verified the clinical importance of nondesaturating sleep-disordered breathing events through a population-based sample (the Wisconsin Sleep Cohort [WSC]). Using AASM guidelines, an automated sleep-disordered breathing (SDB) scoring algorithm identified flow-reducing breathing events, with and without oxygen desaturations. Different breathing disturbance indices (desaturating and nondesaturating) are associated with different clinical outcomes. Consistent with current evidence, oxygen-desaturating breathing disturbances were associated with prevalent hypertension and sleepiness. Nondesaturating events were associated only with objective sleepiness, for the first time objectively confirming clinical practice. Longitudinal models pointed to conversion from nondesaturating to desaturating breathing events over 4-year follow-up. Together the data suggest that nondesaturating breathing disturbances are a milder SDB phenotype, warranting further investigation into their pathophysiology and optimal treatments.
INTRODUCTION
The most common manifestation of sleep-disordered breathing (SDB), obstructive sleep apnea (OSA), affects approximately one-fourth of men and approximately one-tenth of women.1 With recent increases in obesity, OSA prevalence has increased 14%–55% over the last 2 decades, resulting in millions of untreated individuals.1,2 Health consequences of OSA range from increased all-cause mortality, cardiovascular morbidity, psychiatric illness, traffic accidents, and lost productivity.3,4
The apnea hypopnea index (AHI)-based definition of OSA has changed over time and is inconsistently applied, making the clinical impact of sleep-disordered breathing difficult to interpret. Because cardiovascular consequences have been associated with oxygen desaturation, Medicare criteria mandate that hypopneas include ≥4% oxyhemoglobin desaturation. However, many participants—especially the young, nonobese, and women—have SDB without desaturations.5 Reflecting this, the most recent American Academy of Sleep Medicine (AASM) recommendations define hypopneas by association with either arousal or ≥3% desaturation.6 The Medicare and AASM 2007 standard criteria for scoring OSA events result in substantially different prevalence rates at equivalent frequency cutoffs.7,8 Even individuals with the same AHI have vast differences in SDB characteristics.9,10 Recent studies have highlighted that the oxygen-desaturation index (ODI)11 or event duration9,12 may be more predictive of cardiovascular outcomes.
Similarly, daytime sleepiness is an important symptom of OSA, imparting a significant psychosocial burden.3,4 Yet, large epidemiologic studies have found only 35% of participants with moderate OSA and 37% with severe OSA reported excessive daytime sleepiness, the cardinal daytime symptom of OSA.13In a comparison among OSA, upper airway resistance syndrome (UARS), and controls, the subjective and objective measures of sleepiness were worse in the participants, compared with controls.14 However, there were no appreciable differences in sleepiness measures between the participant groups (OSA and UARS).14Furthermore, both groups had similar rates of breathing disturbances (RDI for UARS and AHI for OSA), suggesting that the chronicity of the breathing problem in participants with OSA may result in a blunting of the arousal response (as measured by visually scored and electroencephalogram [EEG] spectral power changes), fundamentally changing the nature and pathophysiology of the events comprising the indices as disease severity progresses. This hypothesis has found support in the blunted canine arousal responses to airway occlusion resulting from sleep fragmentation or sleep apnea,15,16 as well as in AHI increases caused by sleep fragmentation in humans.17 Moreover, despite normal nocturnal oxygenation, participants with snoring18,19 and UARS20 have shown improvements in daytime function with continuous positive airway pressure (CPAP). And when hypoxemia was experimentally induced despite sleep breathing improvement with CPAP, the improvement in MSL was no different from CPAP usage with normoxia, pointing to a nondesaturating mechanism causing objective sleepiness.21 Taken together, these findings highlight that data-driven polysomnographic analyses could lead to better understanding of the different pathophysiologies like OSA and their consequences.
Additionally, a major obstacle to OSA treatment is poor adherence to gold-standard therapy: CPAP. Despite dose-response symptomatic improvements with usage, compliance is unpredictable22 and correlates weakly with AHI, cardiovascular morbidity, and sleepiness.23,24 Even clinical outcomes are hard to confirm as trial results show weak effects of CPAP on blood pressure, metabolic derangements, inflammation, and obesity.25 This also may relate to the difficulty of assessing disease severity with a single metric: the AHI.26,27
There are complex anatomical and physiological interactions that contribute to OSA, such as upper airway fat deposition and anatomy, muscle control, loop gain, and arousal threshold variations.28–33 Because different pathophysiologic mechanisms presumably result in different consequences of breathing disturbances, and these different types of breathing disturbances may result in different clinical outcomes, the aim of the current study was to use an automated breathing-disturbance detection algorithm to differentiate breathing events (eg, with desaturation or not) in order to ascertain differences in clinical outcomes (eg, sleepiness and hypertension) associated with these distinct breathing event types.
METHODS
Population and Sample
The University of Wisconsin-Madison Health Sciences and Stanford University Institutional Review Boards approved the study (Stanford #19027; University of Wisconsin 2012-0084). Protocols for the WSC Study have been described previously.34 Briefly, in 1988, all employees of five state agencies in Wisconsin, aged 30–60 years, were mailed a survey about sleep habits, health, and demographic characteristics. Initial response rate was 73% (5091/6947). Invitation to participate in overnight polysomnography (PSG) (repeated every 4 years) was extended to a stratified random sample of 2884 individuals (nonpregnant and without recent airway cancer, airway surgery, or decompensated cardiopulmonary disease). Of these, 1545 agreed to participate in at least one overnight protocol. For this analysis, there were 1022 eligible participants from the cohort that provided computer-recorded sleep studies. The majority of participants underwent at least two (maximum six) sleep studies, all of which were used for this analysis.
Two multiple sleep latency test (MSLT) protocols were conducted between June 1989 and August 2003: a research protocol (1839 studies) was conducted for an average of 3 weeks after PSG and a clinical protocol (1142 studies) was conducted in the morning following PSG. In both protocols, participants completed a week-long sleep diary and went to bed at their sleep-diary-based habitual bedtime on the night prior to testing. Both MSLT protocols were conducted in accordance with established practice parameters35,36 modified as follows: (1) all participants proceeded with the MSLT, even if total sleep time on the preceding night was <6 hours; (2) all participants continued medications, without change, before or during the MSLT; (3) clinical protocol participants began the MSLT 1.5 hours after awakening from overnight PSG and were allowed to sleep for 15 minutes of clock time after sleep onset in a nap period; (4) research protocol participants began the MSLT at 10:00 and were awakened immediately and the nap trial ended immediately after sleep onset was established.
Measurements
Sleep studies were performed at the Clinical Research Unit of the University of Wisconsin. Sleep studies included PSG and other assessments, including blood pressure and body mass index (BMI). At each polysomnography study, hypertension was defined by one of the two methods: clinically assessed auscultatory blood pressure equal to or above 140/90 mm Hg (measured as described in Ref.37) or use of antihypertensive medications (“clinical hypertension”). Data on medical history, medication use, alcohol use, smoking habits, depression, and self-reported sleep habits, problems, and daytime sleepiness were obtained using questionnaires. Subjective daytime sleepiness was assessed using the Epworth Sleepiness Scale (ESS), which asks participants to rate their likelihood of falling asleep in a variety of common situations and assigns a score from 0 (least sleepy) to 24 (sleepiest), with the commonly defined threshold for daytime sleepiness set at a cutoff of >10.36 Objective daytime sleepiness was assessed using the MSLT, which allows for 4–5, 20-minute nap periods to determine whether there is rapid sleep onset (MSL <8 minutes) and/or the presence of sleep-onset rapid eye movement (REM) periods (REM sleep onset <15 minutes in a nap period).36 Depression was assessed by either a Zung score of ≥5038 or antidepressant use. Insomnia was defined as a habitual sleep mean <6 hours per night based on the weeklong sleep diaries completed by participants prior to the MSLT.
Sleep state and respiratory/cardiac parameters were assessed with polysomnography (Grass Instruments, Quincy, MA, USA). Each 30-second epoch of the polysomnographic records was scored for sleep stage by trained sleep technicians, using conventional criteria incorporating electroencephalography, electrooculography, and electromyography.39 Oxyhemoglobin saturation was recorded using a photoplethysmograph (Ohmeda 3740, Englewood, CO, USA). Oral airflow was recorded using a thermocouple (ProTec, Hendersonville, TN, USA). Air pressure at the nares was recorded using a pressure transducer (Validyne Engineering Corp., Northridge, CA, USA). Chest and abdomen excursions were recorded using respiratory inductance plethysmography (Respitrace, Ambulatory Monitoring, Ardsley, NY, USA).
Detection of Desaturation Events
The photoplethysmograph hemoglobin saturation (SaO2) signal was used for desaturation detection. Initially, the saturation signal was down-sampled to 100 Hz, and fluctuations due to the signal’s discrete values were minimized by smoothing the signal with a moving average window of 2 seconds (25% overlap) (Supplementary Figure S1). Signal was limited to the 5th–95th percentiles, to eliminate extreme peaks. The algorithm was optimized on PSGs using the manually scored, ≥3%-desaturation criterion. Local maxima were detected and subsequent drops of >2% lasting >4 seconds were identified as desaturation events, based on this algorithm optimization (Supplementary Figure S2). Also, based on optimization, transient increases of 0.4% during an event were allowed. Resaturation was detected using a similar strategy. Fivefold cross-validation on 40 randomly selected participants (10 PSGs from each subgroup: AHI < 5, AHI 5–15, AHI 15–30, AHI > 30) demonstrated good desaturation algorithm performance in all standard measures of model robustness40 using the desaturation/resaturation parameters outlined above:
Artifact Removal
Nasal pressure (NP), airflow thermocouple (oral flow), and chest/abdomen respiratory inductance plethysmography (RIP) belts were used in the detection of stable and reduced breathing. NP signals were removed with hardcoded thresholds to eliminate out-of-bound signals and periods with lack of pressure signal (suggesting cannula dislodgement), based on trial-and-error optimization with 80 randomly selected PSGs. Fast Fourier transform, spectral power comparisons between high and low frequencies allowed for removal of artifacts caused by loud snoring (a high-frequency artifact) on low-flow breathing.
Detection of Nasal Pressure Drops
RIP belts were bandpass filtered from 0.1–5 Hz. Five-minute windows (50% overlap) were evaluated for NP maxima/minima as well as start of inspiration and expiration, based on signal zero-crossing. NP and RIP signals were aligned (with the chest belt taking priority in cases of paradoxical breathing) to check the NP signal and calculate breathing frequency. After correction for inverted signals, NP maximum (NPmax) and minimum (NPmin) values were detected from the nontransformed signal, based on inspiratory peaks and expiratory troughs, respectively. Outliers beyond the 5th–95th percentile range were eliminated and a moving 1-minute averaging window, which assigns low weight to outliers, was applied to establish a baseline not impacted by artifacts. Using the AASM 2012 standard,6 when compared with the baseline, if there was a >30% decrease in NP amplitude (NPmax–NPmin) of breaths over >10 second period, without a commensurate twofold increase in oral breathing, then an event was scored (Supplementary Figure S3). Exclusion of rare central events was based on RIP belt activity that dropped below 75% baseline amplitude for >50% of event. These calculations resulted in a breathing disturbance index (BDI) for each participant, independent of associated arousal or hypoxia events.
Event Linking
All signals were cleaned by either an artifact detection algorithm or digital filtration. If more than 75% of the recording was contaminated with artifacts, the signal was excluded from further analysis. Desaturation events were time-locked to NP drops if there was an overlap of the NP event with the 10 seconds preceding or following the start of the desaturation. Events that could be linked to a 2% desaturation comprised the hypoxia-BDI (H-BDI), others the nonhypoxia-BDI (NH-BDI) (Figure 1). The focus on nonassociated breathing disturbances was intentional: allowing exploration of the potential clinical associations of the breathing disturbances themselves (particularly those that may not manifest with an EEG arousal or desaturation) and avoiding variability in scoring of associated polysomnographic phenomena (ie, EEG arousals).
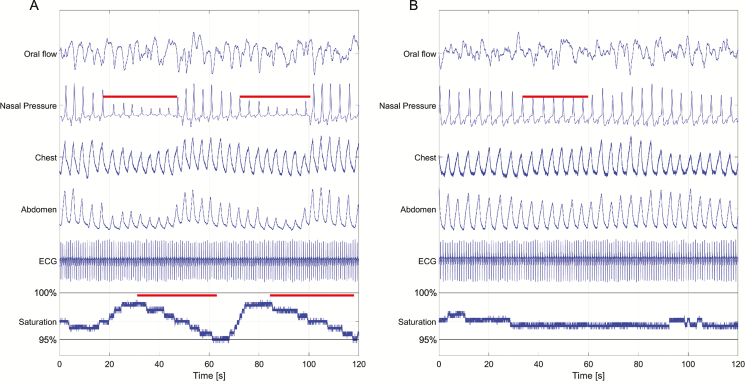
Figure 1.
Demonstration of algorithm-tagged events. Two 120-second epochs of sleep from the same individual, demonstrating (A) two desaturation-associated breathing disturbances (contributing to H-BDI) and (B) one nondesaturating breathing disturbance (contributing to NH-BDI).
Statistical Analysis
Analyses were performed with SAS software v9.4 (SAS Institute Inc., Cary, NC, USA). Indices were log2-transformed to enhance model fit. Group means were compared using two-tailed t-tests with the assumption of unequal variances between groups. A threshold of 0.05 was used to minimize type I error; however, the p-value was used in combination with effect sizes and alternate methods of analysis to ensure the validity of findings. Pearson product moments were used to assess linear correlations between indices. Generalized estimating equations (for binary outcomes) and mixed modeling (for continuous outcomes) were used to estimate robust standard errors for significance testing, allowing for incorporation of repeated measures into analyses. Odds ratios were calculated from logistic regression to assess association with prevalent hypertension (dichotomous). Linear regression was used to determine the associated degree of subjective (ESS score) and objective (MSL on MSLT) sleepiness. Finally, a generalized linear model was used to assess the associated change in 3% ODI over a 4-year follow-up period.
RESULTS
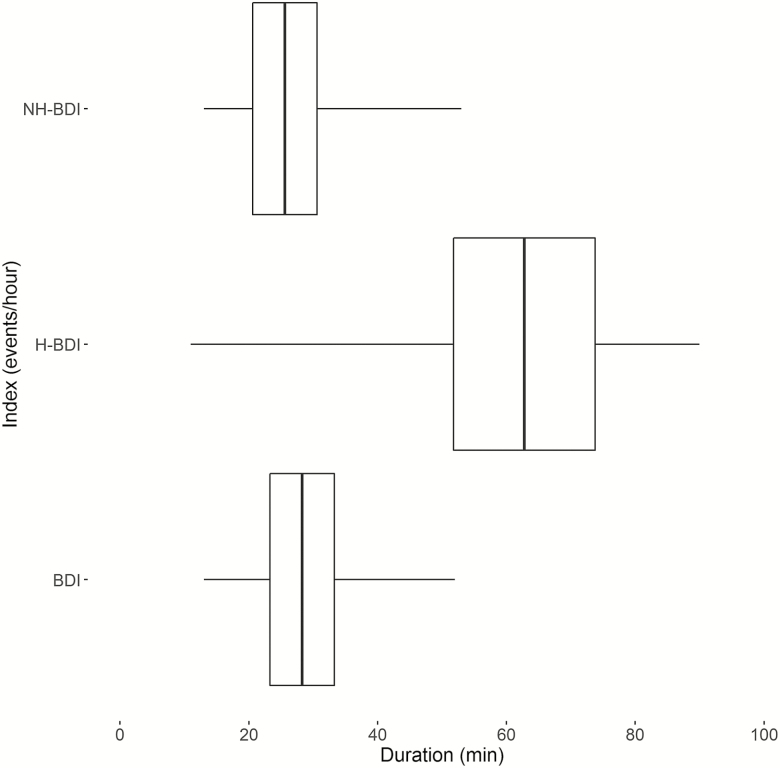
The total sample was comprised of 1022 unique individuals, with 2112 sleep studies. Age (59.7 ± 8 vs 58.9 ± 8) and proportion of men (53% vs 52%) were not different between the entire sample and the subset who underwent MSLT (n = 865); other variables of interest were similar between the groups (Supplementary Table S1). Table 1 indicates that H-BDI has strong correlation with all traditional measures of OSA severity: ODI with 3% desaturation threshold and AHI with 4% hypopnea criteria (Supplementary Figure S4). Suggesting that it is an independent measure of SDB, NH-BDI showed no correlation with any of the traditional OSA metrics. This explains the weakening of correlations noted in the BDI (a combined measure of H-BDI and NH-BDI) compared with H-BDI alone. Pairwise comparisons between each of the breathing disturbance indices further highlight the distinction between H-BDI and NH-BDI based on mean event duration (62.8 ± 11 vs 25.6 ± 5, p < .05) (Figure 1).
Table 1.
Correlations of Variables of Interest After log2-transformation.
| Mean (SD) | Correlation coefficient | |||||
|---|---|---|---|---|---|---|
| AHI 4 | ODI3 | BDI | H-BDI | NH-BID | ||
| AHI 4 | 2.7 (2.1) | × | 0.94* | 0.70* | 0.89a | 0.002b |
| ODI 3 | 2.7 (2.0) | × | × | 0.69* | 0.90* | −0.04b |
| BDI | 4.5 (0.9) | × | × | × | 0.81* | 0.52* |
| H-BDI | 2.7 (1.9) | × | × | × | × | 0.10a |
| NH- BDI | 3.7 (0.8) | × | × | × | × | × |
Using the last visit for each participant; n = 1022. *p < .0001, ap = .001, bnot significant. Abbreviations: AHI 4 = 4%-hypopnea-criterion apnea-hypopnea index; ODI3 –=3% desaturation ODI criterion; BDI = total breathing disturbance index; H-BDI = breathing disturbance index with associated oxygen desaturations; NH-BDI = breathing disturbance index without associated oxygen desaturations.
The relationship of SDB severity to hypertension has been assessed in this longitudinal cohort before, indicating that a threshold of five events per hour for desaturating events (H-BDI) is most appropriate, given the high correlation with the measure initially used to establish this association: AHI with 4% desaturations (AHI 4).41,42 Moreover, because desaturating sleep-disordered breathing events are generally considered more severe, qualifying individuals for a diagnosis of OSA according to all accepted guidelines, a cutoff of five events per hour was felt to be appropriate in this study for comparing groups by the H-BDI, whereas a more conservative cutoff of 15 events per hour was used for NH-BDI stratification, due to independence of the index from the desaturating events and a lack of identified consequences (ie, arousal), through our algorithm. Stratification revealed age, BMI, the presence of snoring, and neck girth were significantly associated with more frequent desaturations, regardless of NH-BDI level (Table 2, and Supplementary Tables S2 and S3). Additionally, the high H-BDI group was subjectively sleepier, despite no objective evidence of increased sleep propensity on MSLT. The prevalence of hypertension was higher in the H-BDI > 5 group. Individuals with either an H-BDI > 5 or an NH-BDI > 15 were more likely to be male. Moreover, those with high NH-BDI (>15) in addition to a high H-BDI (>5) were more likely to be males than those with just an elevated H-BDI.
Table 2.
Characteristics of H-BDI and NH-BDI Stratifications.
| Low H-BDI (< 5) | High H-BDI (>5) | |||
|---|---|---|---|---|
| Low NH-BDI (<15) | High NH-BDI (≥15) | Low NH-BDI (<15) | High NH-BDI (≥15) | |
| N | 526 | 252 | 598 | 736 |
| Age, years, mean (SD) | 57.6 (9) | 59.0 (9)* | 59.7 (8)*,a | 61.3 (8)*,a |
| Body mass index, kg/m2, mean (SD) | 28.3 (6) | 27.4 (5) | 34.0 (7)*,a | 31.8 (6)*,a |
| MSLT, minutes, mean (SD) | 12.3 (5) | 11.7 (5) | 11.7 (5) | 11.2 (5)* |
| Epworth, mean (SD) | 7.8 (4) | 8.1 (4) | 9.1 (4)*,a | 9.0 (4)*,a |
| Number of alcoholic drinks per week, mean (SD) | 3.3 (5) | 3.6 (5) | 3.6 (5) | 4.2 (6) |
| Neckgirth, centimeters, mean (SD) | 36.3 (4) | 36.6 (3) | 39.6 (4)*,a | 39.4 (4)*,a |
| Male, n (%) | 193 (37) | 133 (53)* | 325 (54)* | 475 (65)*,a,b |
| Hypertension, n (%) | 210 (40) | 96 (38) | 369 (62)*,a | 426 (58)*,a |
| Current smoker, n (%) | 63 (12) | 30 (12) | 61 (10) | 45 (6)*,a,b |
| Depression, n (%) | 148 (28) | 64 (25) | 176 (30) | 171 (23) |
| Frequent snoring, n (%) | 134 (28) | 71 (34) | 307 (58)*,a | 391 (60)*,a |
| Insomnia, n (%) | 264 (50) | 131 (52) | 299 (50) | 366 (50) |
*p < .05, significantly different from low H-BDI/low NH-BDI group; ap < .05, significantly different from low H-BDI/high NH-BDI group; bp < .05, significantly different from high H-BDI/low NH-BDI group.
Odds ratios were calculated to determine the increased odds of hypertension based on severity of sleep-disordered-breathing events, as measured by various indices (Table 3). After correction for age, sex, BMI, alcohol consumption, and smoking status, only the log2-transformed 3% ODI was associated with a mildly increased risk of hypertension (OR = 1.06, 95% CI 1.00–1.12, p < .05). While in the correct direction (OR = 1.04, 95% CI 0.98–1.12, p = .19), the H-BDI did not reach statistical significance in association with prevalent hypertension, probably due to the more liberal 2% desaturation used to define the measure. Conversely, a stronger association with objective sleepiness was noted in the nondesaturating events (NH-BDI), with a half minute shorter MSL for every doubling of the nondesaturating event rate (β = −0.52 minutes MSL per doubling of event rate, 95% CI −0.81 to −0.23, p = .004). The NH-BDI contributed most significantly to the strong objective and subjective sleepiness associations with BDI, with beta coefficients in the linear models suggesting a 0.39- (0.20–0.57, p < .0001) point increase in the ESS and 0.73- (0.44–1.03, p < .0001) minute decrease in MSL on MSLT for every doubling of the BDI (Table 4). A similar, but weaker, association was found for the desaturation-associated breathing events (ie, 3% ODI and H-BDI).
Table 3.
Relationship of log2-transformed Breathing Variables With Hypertension.
| Model parameters for hypertension* (for a twofold change in breathing disturbance indices) | |||
|---|---|---|---|
| Odds ratio | 95% confidence interval | p-Value | |
| ODI 3% | 1.06 | (1.00, 1.12) | .0496 |
| BDI | 0.98 | (0.85, 1.12) | .7287 |
| H-BDI | 1.04 | (0.98, 1.10) | .1931 |
| NH-BDI | 0.93 | (0.83, 1.05) | .2439 |
*Adjusted for age, sex, body mass index, smoking, alcohol.
Table 4.
Relationship of log2-transformed Breathing Variables With Subjective and Objective Sleepiness.
| Model parameters (for a twofold change in breathing disturbance indices) | ||||||
|---|---|---|---|---|---|---|
| Epworth Sleepiness Scalea | MSLb | |||||
| Beta Coefficient | 95% confidence interval | p-Value | Beta coefficient | 95% confidence interval | p-Value | |
| ODI 3% | 0.20 | (0.11, 0.29) | <.0001 | −0.17 | (−0.31, −0.04) | .0146 |
| NH-BDI | 0.12 | (−0.03, 0.27) | .1043 | −0.52 | (−0.81, −0.23) | .0004 |
| H-BDI | 0.20 | (0.11, 0.30) | <.0001 | −0.20 | (−0.35, −0.05) | .0086 |
| BDI | 0.39 | (0.20, 0.57) | <.0001 | −0.73 | (−1.03, −0.44) | <.0001 |
Adjusted for aage, sex, body mass index, habitual sleep, depression; bage, body mass index, sex, habitual sleep duration, sleep efficiency, minutes of sleep the two nights preceding clinical MSLT.
In order to determine whether NH-BDI represented a less severe form of sleep-disordered breathing, a general linear model was used to assess the associated change in H-BDI over a 4-year time interval (ΔH-BDI) in the 654 individuals with follow-up sleep studies. The model incorporated commonly associated clinical variables, including baseline BMI, age, sex, alcohol consumption (number of drinks), smoking status (current, never, previous), neck girth, log2-transformed NH-BDI, log2-transformed H-BDI baseline, and the change in BMI over the same time period (added in order to account for the known effect of BMI on desaturating event rate8,43). Age, alcohol consumption, and NH-BDI were the only significant baseline factors, with NH-BDI β-coefficient = 1.96, indicating an estimated, co-variate adjusted 4-year increase in the H-BDI of nearly two events per hour for every doubling of baseline NH-BDI, p = .0002. Expectedly, the change in BMI (β = 1.14 events/hour for every unit increase in BMI, p < .0001) had a strong influence on ΔH-BDI (Table 5).
Table 5.
General Linear Model of Change in H-BDI Over a 4-Year Follow-up.
| Parameter | Estimate | Standard error | t value | Pr > |t| |
|---|---|---|---|---|
| Age | −0.10 | 0.05 | −2.00 | 0.0455 |
| log2(NH-BDI) | 1.96 | 0.52 | 3.74 | 0.0002 |
| log2(H-BDI) | −1.02 | 0.23 | −4.44 | <0.0001 |
| ΔBMI | 0.97 | 0.17 | 5.68 | <0.0001 |
| R 2 = 0.104817 | ||||
| df = 10 | ||||
| F = 7.53 | ||||
| Root MSE = 9.133602 | ||||
Change in H-BDI, 2.50 ± 9.58 events/hour. Model corrected for baseline BMI, age, sex (binary), number of alcoholic drinks per week, smoking status (categorical: current, never, past), neck girth, and log2-transformed H-BDI, as well as change in BMI over the same 4-year follow-up. Abbreviations: ΔBMI = change in BMI from baseline.
DISCUSSION
Automated scoring provides a means by which SDB events can be consistently scored, without the inter-/intra-rater variability of human scorers. The scoring algorithm of the present study identified breathing disturbances with (H-BDI or ODI) and without (NH-BDI) associated oxyhemoglobin desaturations. Indeed, H-BDI correlated strongly with ODI measures and the Medicare AHI 4% (Table 1), indicating this index adequately reflects hypopneas and apneas with desaturations. In contrast, the NH-BDI did not correlate with these measures, indicating that it reflects a distinct entity from desaturation-based sleep-disordered breathing (Table 1, Figure 2, and Supplementary Figure S4). As both indices independently correlated with objective daytime sleepiness, as measured with the MSLT (Table 4), it is probably both types of events that disturb sleep and, although this was not verified in this study, induce significant EEG or subcortical arousals that disrupt sleep. Alternatively, the associated sleepiness may reflect a characteristic of an underlying sleep disturbance that predisposes to sleep-disordered breathing.
Figure 2.
Participants’ mean event duration, stratified by index. Boxplot of event durations with central line demonstrating mean of individual means, the box ends demonstrating 2 SD around the mean, and the whiskers demonstrating range of individual means. All pairwise comparisons between indices were significantly different, p < .05. Abbreviations: BDI = breathing disturbance index, H-BDI = desaturation-associated breathing disturbance index, NH-BDI = nondesaturation-associated breathing disturbance index.
This is consistent with the suggested associations in the literature. Although Martin and colleagues found that arousals correlated with MSL, no relationship was found between arousals and ESS in participants with OSA.44 However, contrary to our findings, the AHI was most strongly associated with changes in MSL.44 Similar to our findings, work by Roehrs and colleagues showed that arousal indices were more significantly correlated with MSL than hypoxic measures; again this was in a clinical population of participants with OSA.45 Nonetheless, their results suggest that sleep fragmentation accounts for about 16% of the variance in daytime sleepiness.45 Additionally, many participants with OSA have a similar number of cortical arousals as normal participants when studied in a sleep laboratory;46 thus, it does not appear to be the cortical arousals that are the cause of the increased sleepiness, per se. It is also important to note that many of these studies have explored arousal events that have a significant overlap with desaturating breathing disturbances, suggesting a high degree of collinearity among the measures studied in the existing literature.47 Furthermore, not all sleep-related apneas and hypopneas are terminated by cortical arousals, and thus, our efforts to identify the clinical impact of non-desaturating sleep breathing disturbances—regardless of the polysomnographic associations—highlight the value of incorporating these events into indices, in addition to suggesting a need for further exploration of their pathophysiologic basis.
Of interest was the comparison of participants with high versus low NH-BDI, and high versus low desaturating indices (eg, H-BDI) (Table 2). Older participants and men are known to have more severe SDB,5 a phenomenon reflected here by the fact that older individuals and a higher percentage of men had both higher H-BDI and NH-BDI. Although participants with high NH-BDI were more likely to be older and male (regardless of H-BDI), it was those individuals with high H-BDI (vs low H-BDI) that had slightly higher BMIs, concordant with the findings that increased weight is associated with a desaturating sleep-disordered breathing.8,43 This relationship was also supported by our longitudinal model (Table 5), as increases in desaturations after 4-year follow-up (ΔH-BDI) were not only associated with baseline H-BDI, age, and BMI, but also by baseline frequency of nondesaturating events (NH-BDI) and changes in BMI that have occurred over 4 years (ΔBMI). The fact that participants with higher H-BDI are generally more obese and have greater neck girth8,43 is also in agreement with our observation that increased body weight contributes to the emergence of desaturating breathing disturbances. Breathing disturbances associated with oxygen drops (H-BDI) were also significantly longer in duration (Figure 1) suggesting that with time and increased BMI, participants may be less arousable, leading to longer, more severe pauses that have time to produce significant desaturations. Other contributors could involve a higher prevalence of pulmonary or hematologic (eg, anemia) pathology with age and increased BMI.
Hypertension was substantially more prevalent (60% vs 39%) in individuals with high H-BDI compared with those with low ODI, a trend that was not observed in relation to nondesaturating breathing disturbances (high NH-BDI vs low NH-BDI: 53% vs 52%). Logistic regression models (Table 3) confirmed the association of oxygen-desaturation events with the odds of hypertension: a doubling of the ODI 3% was significantly associated with 6% increased odds of prevalent hypertension (OR 1.06, 95% CI 1.00–1.12, p < .05). A weaker relationship was found with H-BDI (OR 1.04, 95% CI 0.98–1.10, p = .19), presumably due to the use of a 2% desaturation threshold to define the index. These findings are in accord with prior animal and human work that points to higher cardiovascular risk mostly with oxygen desaturations (typically 4%) and not sleep distruptions.11,48,49 Moreover, studies of multiple polysomnographic parameters have similarly found strong primary associations between duration of sleep with low blood oxygen levels and cardiovascular risk.50,51 The mechanistic links to cardiovascular risk appear to be mediated by oxidative stress, systemic inflammation, and endothelial dysfunction in the setting of chronic, intermittent hypoxia.52
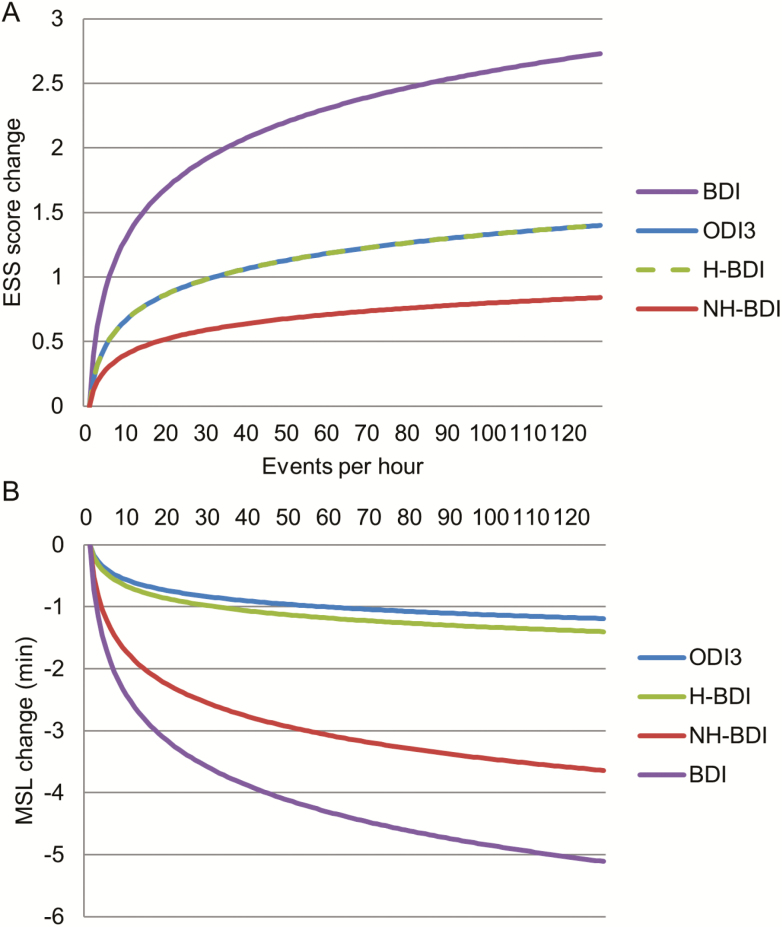
A different picture emerged with regard to the relationship of these indices with daytime sleepiness. As expected, participants with H-BDI > 5 were subjectively (ESS: 9.0 ± 4 vs 7.9 ± 4) sleepier (Supplementary Table S2) regardless of the severity of NH-BDI, though they were not found to be objectively sleepier on MSLT. In fact, it was only individuals with both high NH-BDI and ODI who had significantly lower MSL on the MSLT compared with the “normal” group with low ODI and low NH-BDI (11.2 ± 5 vs 12.3 ± 5 minutes). This was also confirmed by our multivariate model (Table 4, Figure 3), which showed strong associations of nondesaturating events with objectively measured daytime sleepiness (MSLT). Essentially, for every doubling of the NH-BDI, there was a >30-second decrease in MSL (Figure 3). Interestingly, associations of nondesaturating events with subjective sleepiness were more variable and nonsignificant (although trending toward significance), suggesting that sleep disruptions caused by the breathing disturbances above and beyond the desaturation-associated events are associated with objective daytime sleepiness, and that desaturations have a greater effect on subjective daytime sleepiness. These findings suggest that sleep fragmentation or another feature of sleep-disordered breathing not captured by the AHI (or ODI) and not hypoxia explains, in part, objective and subjective sleepiness, particularly given that the current algorithm did not differentiate desaturating events with arousals from those without.45,53
Figure 3.
Relationship of breathing variables with subjective and objective sleepiness. Visual demonstration of the impact of changes in (A) Epworth sleepiness scale (ESS) score and (B) mean sleep latency (MSL) on multiple sleep latency testing, based on back-transformation of the log2-transformed predictor variables (the indices) used in the original linear modeling (Table 4). The log2-transformation results in changes in the outcomes of interest (ESS score and MSL) for every doubling of event rate. A broken line was introduced in (A) because of overlap of the models for ODI3 and H-BDI. Abbreviations: ODI3 = 3% desaturation ODI criterion; BDI = total breathing disturbance index; H-BDI = breathing disturbance index with associated oxygen desaturations; NH-BDI = breathing disturbance index without associated oxygen desaturations.
Prior studies have explored these questions through the impact of sleep fragmentation. Significant decreases in MSL have been noted after nonvisible sleep fragmentation, with no difference in subjective daytime sleepiness as measured by the Stanford Sleepiness Scale.53 This is in line with the reported discordance between subjective and objective sleepiness measures,54,55 and our findings. In fact, more recent studies of OSA populations have suggested that desaturating events appear to correlate with subjective,56 but not objective, sleepiness.55 The correlation between sleep fragmentation measures and daytime sleepiness frequently accounts for only a small part of the total variance,47 which is why we initially sought to explore nondesaturating breathing disturbances, regardless of association, as a means of determining whether the sleep-breathing events, in and of themselves, justify their inclusion into the AHI. The lack of correlation of the NH-BDI with any other traditional measure of SDB in this population-based sample suggests that the events in question are truly distinct from the standard measures of SDB studied in the literature to date. Our results prompt further investigations into the mechanisms by which the breathing disturbances are associated with objective sleepiness, whether it is through EEG/autonomic arousals or other, potentially bidirectional, pathophysiologic processes.
Additionally, snoring appeared to be more common among individuals with an H-BDI > 5 compared with those <5 (59% vs 30%, p < .05). However, upon comparing NH-BDI <15 with ≥15 (53% vs 44%, p < .05), it was revealed that snoring is more prevalent regardless of the metric used to define SDB severity, proving it to be a poor differentiator. Similarly, insomnia and depression showed no major differences between any subgroup.
There are a number of limitations to the current method, which will be improved upon in future projects. The most evident is the usage of hard-coded algorithms that rely upon existing scoring standards, which will likely continue to evolve as our understanding of sleep-disordered breathing grows. Additionally, the NH-BDI was defined as all nondesaturating breathing disturbances; however, detection of other specific consequences such as cortical or autonomic arousals and increased effort of breathing and flow limitations may help delineate the breathing events more effectively. Similarly, arousal-associated desaturating events were not differentiated from nonarousal associated events, which will be addressed with future scoring algorithms. Furthermore, in order to ensure high quality of the NP signal, large amounts of data were excluded due to artifacts (eg, periods with severe snoring in combination with low amplitude NP), but such phenomena are hallmarks of OSA in certain participants. Another limitation is that the findings may not be generalizable beyond the WSC, a middle-to-older-aged, predominantly white sample with relatively few leanparticipants. Fortunately, software-based algorithms can easily be applied to new cohorts for future verification of their validity and reliability.
To the best of our knowledge, this is the first study that has differentiated desaturating and nondesaturating SDB events in a population-based sample, in order to identify differential associations with cross-sectional and longitudinal clinical outcomes. This was possible through a hard-coded automated scoring algorithm that could reproducibly quantify all breathing disturbances, regardless of association, allowing for time-locking of breathing disturbances to automatically scored desaturation events. Future studies can further differentiate the subtypes of breathing disturbances, based on their polysomnographic associations, in order to more precisely phenotype sleep-disordered breathing. Subphenotypes may offer insights into the pathobiology of sleep-disordered breathing, thereby resulting in optimization of therapeutic choice(s) and clearer associations to clinical outcomes.
SUPPLEMENTARY MATERIAL
Supplementary material is available at SLEEP online.
FUNDING
This work was supported by NS2372 and by grants from the Lundbeck Foundation, Technical University of Denmark, and Danish Center for Sleep Medicine. PEP, EH, and LAF were supported by R01 HL062252 and UL1 RR025011. Instrumentation provided by grants S10 RR021086 and S10 OD010569. The funding institutes played no role in the design and conduct of the study; no role in the collection, management, analysis, or interpretation of data; and no role in the preparation, review, or approval of the manuscript.
AUTHOR CONTRIBUTIONS
Concept and design: HK, LDS, PEP, EWH, HBDS, PJ, and EJMM. Data acquisition: HK, EWH, and PEP. Analysis: all authors. Interpretation, draft and review, and final approval: all authors. HK, LDS, and EJMM had full access to the study data and take responsibility for the integrity of the data and accuracy of the analyses.
WORK PERFORMED
Department of Psychiatry and Behavioral Medicine, Stanford University Center for Sleep Sciences and Medicine, Stanford University, CA.
DISCLOSURE STATEMENT
HK has nothing to disclose. LDS reports grants from NIH, during the conduct of the study. LAF has nothing to disclose. EBL has nothing to disclose. PEL reports grants from NIH, during the conduct of the study and personal fees from ResMed, outside the submitted work. EH reports grants from NIH, during the conduct of the study. HBDS has nothing to disclose. PJ has nothing to disclose. EM reports grants from NIH, during the conduct of the study, and grants and personal fees from Jazz Pharmaceutical, personal fees from Actelion, grants from The Lundbeck Foundation, other from ALPCO, other from Federal Trade Commission, grants from GSK, other from Novo Nordisk, grants from Sunovion, grants from Merck, other from Resmed, other from INC, other from Google/Verily, other from Balance, other from Airweave, and other from Flamel, outside the submitted work.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Terry Young for her work in establishing the Wisconsin Sleep Cohort Study as well as Lotte Trap, Amanda Rasmuson, Kathryn Pluff, Robin Stubbs, Hyatt Moore, and Oscar Carillo for assistance in data collection, organization, and statistics. HK and LDS contributed equally to this work.
REFERENCES
- 1. Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009; 108(5): 246–249. [PMC free article] [PubMed] [Google Scholar]
- 2. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013; 177(9): 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The Price of Fatigue: The Surprising Costs of Unmanaged Obstructive Sleep Apnea. Boston, MA: McKinsey & Co; 2010. [Google Scholar]
- 4. AlGhanim N, Comondore VR, Fleetham J, Marra CA, Ayas NT. The economic impact of obstructive sleep apnea. Lung. 2008; 186(1): 7–12. [DOI] [PubMed] [Google Scholar]
- 5. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008; 5(2): 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berry RB, Budhiraja R, Gottlieb DJ et al. . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5): 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ho V, Crainiceanu CM, Punjabi NM, Redline S, Gottlieb DJ. Calibration model for apnea-hypopnea indices: impact of alternative criteria for hypopneas. Sleep. 2015; 38(12): 1887–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ernst G, Bosio M, Salvado A, Dibur E, Nigro C, Borsini E. Difference between apnea-hypopnea index (AHI) and oxygen desaturation index (ODI): proportional increase associated with degree of obesity. Sleep Breath. 2016; 20(4): 1175–1183. [DOI] [PubMed] [Google Scholar]
- 9. Muraja-Murro A, Nurkkala J, Tiihonen P et al. . Total duration of apnea and hypopnea events and average desaturation show significant variation in patients with a similar apnea-hypopnea index. J Med Eng Technol. 2012; 36(8): 393–398. [DOI] [PubMed] [Google Scholar]
- 10. Kulkas A, Tiihonen P, Julkunen P, Mervaala E, Töyräs J. Novel parameters indicate significant differences in severity of obstructive sleep apnea with patients having similar apnea-hypopnea index. Med Biol Eng Comput. 2013; 51(6): 697–708. [DOI] [PubMed] [Google Scholar]
- 11. Punjabi NM, Caffo BS, Goodwin JL et al. . Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009; 6(8): e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Butler MP, Smales C, Wu H et al. . The circadian system contributes to apnea lengthening across the night in obstructive sleep apnea. Sleep. 2015; 38(11): 1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carter GS. Screening for improvement of health outcomes in asymptomatic obstructive sleep apnea. JAMA Neurol. 2017; 74(4): 394. [DOI] [PubMed] [Google Scholar]
- 14. Guilleminault C, Do Kim Y, Chowdhuri S, Horita M, Ohayon M, Kushida C. Sleep and daytime sleepiness in upper airway resistance syndrome compared to obstructive sleep apnoea syndrome. Eur Respir J. 2001; 17(5): 838–847. [DOI] [PubMed] [Google Scholar]
- 15. Espinoza H, Thornton AT, Sharp D, Antic R, McEvoy RD. Sleep fragmentation and ventilatory responsiveness to hypercapnia. Am Rev Respir Dis. 1991; 144(5): 1121–1124. [DOI] [PubMed] [Google Scholar]
- 16. Brooks D, Horner RL, Kimoff RJ, Kozar LF, Render-Teixeira CL, Phillipson EA. Effect of obstructive sleep apnea versus sleep fragmentation on responses to airway occlusion. Am J Respir Crit Care Med. 1997; 155(5): 1609–1617. [DOI] [PubMed] [Google Scholar]
- 17. Sériès F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994; 150(2): 481–485. [DOI] [PubMed] [Google Scholar]
- 18. Guilleminault C, Stoohs R, Duncan S. Snoring (I). Daytime sleepiness in regular heavy snorers. Chest. 1991; 99(1): 40–48. [DOI] [PubMed] [Google Scholar]
- 19. Bennett LS, Langford BA, Stradling JR, Davies RJ. Sleep fragmentation indices as predictors of daytime sleepiness and nCPAP response in obstructive sleep apnea. Am J Respir Crit Care Med. 1998; 158(3): 778–786. [DOI] [PubMed] [Google Scholar]
- 20. Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest. 1993; 104(3): 781–787. [DOI] [PubMed] [Google Scholar]
- 21. Colt HG, Haas H, Rich GB. Hypoxemia vs sleep fragmentation as cause of excessive daytime sleepiness in obstructive sleep apnea. Chest. 1991; 100(6): 1542–1548. [DOI] [PubMed] [Google Scholar]
- 22. Weaver TE, Maislin G, Dinges DF et al. . Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007; 30(6): 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Delanote I, Borzée P, Belge C, Buyse B, Testelmans D. Adherence to cpap therapy: comparing the effect of three educational approaches in patients with obstructive sleep apnoea. Clin Respir J. 2016. doi:10.1111/crj.12491. [DOI] [PubMed] [Google Scholar]
- 24. Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS). Sleep Med Rev. 2003; 7(1): 81–99. [DOI] [PubMed] [Google Scholar]
- 25. Zhao YY, Redline S. Impact of continuous positive airway pressure on cardiovascular risk factors in high-risk patients. Curr Atheroscler Rep. 2015; 17(11): 62. [DOI] [PubMed] [Google Scholar]
- 26. Punjabi NM. COUNTERPOINT: is the apnea-hypopnea index the best way to quantify the severity of sleep-disordered breathing? No. Chest. 2016; 149(1): 16–19. [DOI] [PubMed] [Google Scholar]
- 27. Rapoport DM. POINT: is the apnea-hypopnea index the best way to quantify the severity of sleep-disordered breathing? Yes. Chest. 2016; 149(1): 14–16. [DOI] [PubMed] [Google Scholar]
- 28. Eckert DJ, Owens RL, Kehlmann GB et al. . Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond). 2011; 120(12): 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013; 188(8): 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Owens RL, Edwards BA, Eckert DJ et al. . An integrative model of physiological traits can be used to predict obstructive sleep apnea and response to nonpositive airway pressure therapy. Sleep. 2015; 38(6): 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol (1985). 1988; 64(2): 789–795. [DOI] [PubMed] [Google Scholar]
- 32. White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005; 172(11): 1363–1370. [DOI] [PubMed] [Google Scholar]
- 33. Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001; 163(5): 1181–1190. [DOI] [PubMed] [Google Scholar]
- 34. Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009; 108(5): 246–249. [PMC free article] [PubMed] [Google Scholar]
- 35. Littner MR, Kushida C, Wise M et al. ; Standards of Practice Committee of the American Academy of Sleep Medicine Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005; 28(1): 113–121. [DOI] [PubMed] [Google Scholar]
- 36. Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000; 9(1): 5–11. [DOI] [PubMed] [Google Scholar]
- 37. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000; 342(19): 1378–1384. [DOI] [PubMed] [Google Scholar]
- 38. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965; 12(1): 63–70. [DOI] [PubMed] [Google Scholar]
- 39. Hori T, Sugita Y, Koga E et al. . Proposed supplements and amendments to “A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects”, the Rechtschaffen & Kales (1968) standard. Psychiatry Clin Neurosci. 2001; 55(3): 305–310. [DOI] [PubMed] [Google Scholar]
- 40. Powers DM. Evaluation: from precision, recall and F-measure to ROC, informedness, markedness and correlation. J Mach Learn Technol. 2011; 2(1):37–63. [Google Scholar]
- 41. Hla KM, Young TB, Bidwell T, Palta M, Skatrud JB, Dempsey J. Sleep apnea and hypertension. A population-based study. Ann Intern Med. 1994; 120(5): 382–388. [DOI] [PubMed] [Google Scholar]
- 42. Hla KM, Young T, Finn L, Peppard PE, Szklo-Coxe M, Stubbs M. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep. 2008; 31(6): 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ling IT, James AL, Hillman DR. Interrelationships between body mass, oxygen desaturation, and apnea-hypopnea indices in a sleep clinic population. Sleep. 2012; 35(1): 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martin SE, Engleman HM, Kingshott RN, Douglas NJ. Microarousals in patients with sleep apnoea/hypopnoea syndrome. J Sleep Res. 1997; 6(4): 276–280. [DOI] [PubMed] [Google Scholar]
- 45. Roehrs T, Zorick F, Wittig R, Conway W, Roth T. Predictors of objective level of daytime sleepiness in patients with sleep-related breathing disorders. Chest. 1989; 95(6): 1202–1206. [DOI] [PubMed] [Google Scholar]
- 46. Mathur R, Douglas NJ. Frequency of EEG arousals from nocturnal sleep in normal subjects. Sleep. 1995; 18(5): 330–333. [DOI] [PubMed] [Google Scholar]
- 47. Bonnet MH, Doghramji K, Roehrs T et al. . The scoring of arousal in sleep: reliability, validity, and alternatives. J Clin Sleep Med. 2007; 3(2): 133–145. [PubMed] [Google Scholar]
- 48. Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997; 99(1): 106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parker JD, Brooks D, Kozar LF et al. . Acute and chronic effects of airway obstruction on canine left ventricular performance. Am J Respir Crit Care Med. 1999; 160(6): 1888–1896. [DOI] [PubMed] [Google Scholar]
- 50. Turhan M, Bostanci A, Bozkurt S. Estimation of cardiovascular disease from polysomnographic parameters in sleep-disordered breathing. Eur Arch Otorhinolaryngol. 2016; 273(12): 4585–4593. [DOI] [PubMed] [Google Scholar]
- 51. Kendzerska T, Gershon AS, Hawker G, Leung RS, Tomlinson G.. Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: a decade-long historical cohort study. PLoS Med. 2014; 11(2):e1001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014; 383(9918): 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martin SE, Wraith PK, Deary IJ, Douglas NJ. The effect of nonvisible sleep fragmentation on daytime function. Am J Respir Crit Care Med. 1997; 155(5): 1596–1601. [DOI] [PubMed] [Google Scholar]
- 54. Roth T, Hartse KM, Zorick F, Conway W. Multiple naps and the evaluation of daytime sleepiness in patients with upper airway sleep apnea. Sleep. 1980; 3(3-4): 425–439. [PubMed] [Google Scholar]
- 55. Li Y, Zhang J, Lei F, Liu H, Li Z, Tang X. Self-evaluated and close relative-evaluated Epworth Sleepiness Scale vs. multiple sleep latency test in patients with obstructive sleep apnea. J Clin Sleep Med. 2014; 10(2):171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen R, Xiong KP, Lian YX et al. . Daytime sleepiness and its determining factors in Chinese obstructive sleep apnea patients. Sleep Breath. 2011; 15(1): 129–135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.