Abstract
Objective:
Older adults frequently report sleep problems and are at increased risk of cardiometabolic disruption. Experimental sleep restriction of younger adults has suggested that cortisol may be on the pathway between sleep restriction and cardiometabolic disease. We investigated whether the natural variation in sleep among older adults is associated with daytime cortisol level.
Methods:
Salivary cortisol samples and actigraphy sleep data were collected from a random subsample of participants in the National Social Life, Health and Aging Project, a nationally representative probability sample of adults aged 62–90 (N = 672). Salivary cortisol was measured with 3 timed samples at the beginning, middle, and end of a 2-hr in-home interview. Sleep characteristics were derived from wrist actigraphy (fragmentation, wake after sleep onset [WASO], and duration) and from survey responses about usual sleep duration and sleep problems. For each individual, a single summary daytime cortisol level was estimated by fitting a marginal longitudinal model for the 3 time-stamped cortisol samples. The resulting estimates were then regressed on each sleep measure, adjusting for sociodemographics, health behaviors, and comorbidities.
Results:
From actigraphy, both higher fragmentation score (β = 0.02; 95% confidence interval [CI] = 0.00 to 0.03) and longer WASO (β = 0.27; 95% CI = 0.04 to 0.51) were significantly associated with higher daytime cortisol; sleep duration was not. Self-reported sleep duration and sleep problems were also not associated with cortisol.
Conclusion:
Actigraph measures of sleep disturbance are associated with higher daytime cortisol among older adults. However, cross-sectional data cannot distinguish causal direction or whether cortisol and sleep disruption have a common cause.
Keywords: sleep, cortisol, fragmentation, WASO, random effects model.
INTRODUCTION
Cortisol, a steroid, is part of the larger family of glucocorticoids produced by the hypothalamic–pituitary–adrenal axis. In blood serum, cortisol is 85%–95% bound to carriers such as corticosteroid-binding globulin and albumin, while the remaining 5%–15% is free, unbound cortisol1 which can be easily measured via saliva; salivary cortisol and blood serum cortisol are highly correlated, with equilibrium between the 2 achieved in less than 5 minutes.1,2 Increased blood cortisol levels counteract insulin, suppress the immune system, and contribute to the metabolism of carbohydrates, fats, and proteins.3 Prolonged exposure to elevated cortisol levels has been associated with many adverse physiological effects, including weight gain,4 abdominal obesity,5 decreased bone mineral density,6 impaired short-term memory,7 increased risk of cardiovascular disease,8,9 and increased diabetes-related complications.10 Many of these same outcomes have been associated with poor or short sleep.11–14
Cortisol is a complicated biomarker to measure because it follows a diurnal pattern. Peak nightly levels normally occur during the second half of sleep, and the daytime peak level occurs approximately 30 minutes after waking.15,16 After this initial peak, cortisol steadily decreases over the remainder of the day.17,18 In a meta-analysis examining the cortisol diurnal pattern among individuals aged 18–83 (n = 90), aging has been shown to blunt the amplitude and increase the timing of circadian elevation in cortisol, both of which may be involved in the etiology of sleep disorders.19 Besides measuring cortisol in blood or saliva, it is also possible to assess urinary free cortisol, which is measured over a 24-hour period, one complete circadian cycle.
Experimental studies have examined how sleep characteristics relate to nighttime or daytime cortisol; these studies were conducted in sleep labs, generally among young adults and with sleep opportunity manipulated. For nighttime cortisol levels, there are relatively consistent findings that poor sleep quality and sleep disruption are associated with elevated nighttime cortisol.20–22 Several studies have found that after severe sleep restriction, such as 4 hours per night, afternoon and evening cortisol levels are also elevated.23–25
Two very large observational studies have examined how sleep variation in the population relates to cortisol levels, with somewhat inconsistent findings that may be related to how sleep and cortisol were assessed. The MrOS study included 3100 older men and found that longer self-reported usual sleep duration was associated with lower 24-hour urinary free cortisol, but there was no significant association between actigraph-measured sleep duration averaged over several nights.26 The Whitehall II study, a large occupational cohort, collected 6-timed cortisol measures throughout the day.27 Sleep measures were duration from a sleep log for the night before the cortisol data collection and insomnia symptoms. They found that evening cortisol was higher in those reporting short sleep duration the night before and reporting more sleep disturbances. Chronic insomnia symptoms and shorter sleep duration on at least 3 occasions were also associated with a steeper rise in the cortisol awakening response and higher levels of cortisol later in the day, respectively. A much smaller study of 14 middle-aged adults tracked daily variation in self-reported sleep duration and ratings as well as daily cortisol over 4 weeks and found no associations for individuals between each night’s sleep measure and daytime cortisol.28 Our study includes actigraph and self-reported sleep measures for both sleep duration and sleep disruption for a large nationally representative sample of older adults in the United States. We took 3 daytime salivary cortisol measures for each respondent. We hypothesized that actigraph sleep characteristics will be associated with daytime cortisol levels and that shorter sleep durations and more disturbed sleep will both be associated with higher daytime cortisol levels.
METHODS
The National Social Life, Health and Aging Project (NSHAP) is a longitudinal, nationally representative survey of individuals born in 1920 through 1947. The first wave of data collection occurred in 2005 through 2006 and the second wave in 2010 through 2011. Both included in-home interviews and biomarker collection. Wave 2 added the coresident spouses and partners of Wave 1 respondents. The study has been described in detail elsewhere.29,30 Deidentified NSHAP data are publicly available; information on data access can be found at http://www.icpsr.umich.edu/icpsrweb/NACDA/studies/20541.
Sample Selection
The current study used data collected in Wave 2 and in an ancillary study to Wave 2 that included additional sleep data collection. Several biomeasures were collected from participants during the in-home Wave 2 interviewer visit. Two-thirds of participants were randomly selected to provide salivary cortisol samples, resulting in usable samples from 2240 individuals. A 50% random sample of the cortisol subsample was invited to participate in the ancillary sleep study, and about 80% of them agreed to do so (n = 823). Because arranging for a time to send the actigraph required recontacting participants a few days after the interview, 780 (94.8%) of the 823 ultimately participated.
Our analysis focused on adults aged 60–90, and we excluded those spouses/partners of Wave 1 participants who were not in that age-group (n = 29). Also, those with salivary cortisol measurements >35 nmol/L (n = 73) were eliminated, as these likely reflected sample contamination (e.g., with blood). Six indiviudals whose main sleep periods were during the day were also omitted, as described subsequently. The final analytic sample included 672 participants.
Saliva Collection and Cortisol Measurement
Three salivary cortisol samples were collected by a trained interviewer from each participant: at the beginning of the interview, partway through the interview, and at the completion of the interview. Interviews were scheduled at the convenience of the participant, and therefore the salivary cortisol samples were collected across the day: 499 (24.6%) samples in the morning, 1199 (58.9%) in the afternoon, and 336 (16.5%) in the evening, with 1820 (89.5%) between the hours of 9 am and 6 pm. On average, 42.1 minutes elapsed between the first and second samples and 67.2 minutes elapsed between the second and third samples. Approximately 90% of interviews lasted between 1 and 3 hours.
The samples were obtained by asking participants to chew on a single Salivette® swab (Salivette®-Cortisol; Catalog No. 51.1534.500; SARSTEDT Group, Nümbrecht, Germany) for 1 minute, after which the participant inserted the Salivette® into a tube. Samples were then immediately placed in a cooled container with a refrigerant pack and mailed overnight to the McClintock Survey Biomeasures Laboratory at the University of Chicago where they were stored at −80°C. The samples were then shipped for analysis to Dresden Lab Service GmbH, Germany, where they were centrifuged and saliva extracted to measure free salivary cortisol concentration (Cortisol Luminescence Immunoassay, RE62011; IBL International, Hamburg, Germany). Cortisol data collection and analysis are described in more detail in O’Doherty et al.29
Sleep Measurement
The sleep ancillary study included wearing a wrist actigraph for 72 consecutive hours (Actiwatch Spectrum model, Philips Respironics). The actigraph contains an omnidirectional accelerometer that was set to record activity counts over 15-second epochs. The actigraphy protocol was limited to 72 hours because of a concern that a more burdensome ancillary study might reduce Wave 3 participation. A previous study found 3 nights of sleep characteristics were sufficient in order to estimate average sleep for older adults although not to estimate variability.31 The magnitude and pattern of activity counts were used to infer sleep characteristics. In addition, the actigraph recorded the level of ambient light. Participants were asked to press an event marker button on the actigraph to mark the times when they went to bed and woke up, and they were also asked to record this information in a sleep log. On the third day (after a 72-hour period of recording), the actigraphs were returned in a prepaid mailer. The protocol for collecting the data, setting the rest and sleep intervals, and analyzing the data from the actigraphs has been previously described32 and is summarized subsequently.
For each 24-hour period, a single major rest interval was first determined using the manufacturer’s software (Actiware software V.5.59), based on the activity pattern. Each rest interval was reexamined and edited by the investigators who prioritized the event marker and ambient light data, which the software did not consider. The event marker, when present, was considered the best source to indicate rest interval. Because some individuals forgot to press the event marker, we do not use the rest interval (sometimes considered “time in bed”) as a sleep characteristic in this study, since it was not uniformly measured across the study population. We focus on the sleep interval, which is the duration from first sleep to last awakening, to assess sleep characteristics because that was more uniformly measured. The software was then used to calculate measures characterizing the quantity and quality of sleep during the sleep interval. The main predictors of interest were sleep duration (total sleep time), fragmentation, and minutes of wake after sleep onset (WASO). The default threshold of 40 activity counts was used to score each epoch, with sleep status determined not only on the activity count in the focal epoch but also the adjoining epochs. Therefore, some epochs scored as sleep were epochs with activity counts. Philips Respironics used this threshold for their validation and therefore recommends it for analyses, including setting the sleep start and end times within the rest interval, which are based on contiguous epochs scored as sleep. The default setting of 10 minutes of immobile epochs was used to define sleep onset and offset (i.e., the sleep interval). Duration refers to the total duration of all epochs scored as sleep during the sleep interval. Sleep fragmentation is a commonly used measure of sleep consolidation from actigraphy.33–35 It is calculated by the Actiware software as the sum of 2 percentages: the percentage of the sleep interval during which the participant is mobile (versus immobile) and the percentage of immobile periods that are only 1-minute long. WASO is the total minutes spent awake during the sleep interval. Each of these 3 summary sleep measures was averaged over the 3 nights of the sleep assessment for each participant. A few individuals wore the actigraph for fewer than three nights, and for them the averages of available nights were used. In this cohort of older individuals, most of whom were no longer employed, there were no systematic day-of-the-week differences in sleep characteristics.36
The sleep ancillary study included a booklet with 4 widely used sleep problem questions. The 4 questions were “How often do you feel really rested when you wake up in the morning?”; “How often do you have trouble falling asleep?”; “How often do you have trouble with waking up during the night?”; and “How often do you have trouble with waking up too early and not being able to fall asleep again?” Response categories were rarely/never, sometimes, or most of the time. Following previous work with these questions, they were combined to form a single “troubled sleep” scale with “feeling rested” reverse-coded37; higher scores indicated more sleep problems. Self-reported sleep duration was reported as number of hours slept on average per night.
Covariates
Adjustd models included sociodemographic, health, and lifestyle factors as well as season of data collection and diurnal type. Sociodemographic variables included age, sex, race, ethnicity, and education and were chosen because previous studies have found them to be correlated with actigraph-measured sleep characteristics.38,39 Race was measured using 5 self-reported categories: white/caucasian, black/African American, American Indian/Alaskan Native, Asian or Pacific Islander, and other. Ethnicity was defined as whether the participant identified as Hispanic. Education was classified into 4 categories: less than high school degree, high school degree or equivalent, vocational certificate/some college/associate degree, and bachelor’s degree or higher. Lifestyle risk factors included current cigarette and alcohol use as well as self-reported level of physical activity. Alcohol consumption was based on the average number of drinks consumed per day and was categorized into 3 groups: 0, 1–2, and 3+. Physical activity was categorized into 3 groups based on reported frequency of vigorous physical activity lasting 30 minutes or more: never, less than once per week, and one or more times per week. Participants were asked whether they had been diagnosed by a doctor for each of the following conditions grouped here by categories: cardiovascular diseases (hypertension, any heart problem, previous heart attack, congestive heart failure, previous procedure to treat coronary heart disease, or stroke), cancer (not including skin cancer), endocrine (diabetes), bone health (osteoporosis, hip fracture, rheumatoid arthritis, or osteoarthritis), respiratory (emphysema, asthma, chronic bronchitis, or chronic obstructive pulmonary disease), incontinence (urinary or stool incontinence, or other urinary problems), and Parkinson’s disease. The number of possible conditions was 19, and the numbers reported ranged from 0 to 12, with higher scores indicating more comorbidities. Comorbidities were classified into 3 groups: 0, 1–2, and 3+. Depressive symptoms were assessed utilizing a modified Center for Epidemiological Studies-Depression scale40 and was a continuous variable. Hormone replacement therapy (HRT) use among women was categorized as current use of HRT or not. Season of sleep data collection was categorized as winter (January, February, and March), spring (April, May, and June), summer (July, August, and September), or autumn (October, November, and December). Individuals were also categorized by diurnal type based on their actigraph sleep times. While we did not have a survey-based categorization of diurnal type, some previous studies have used the observed sleep interval to estimate diurnal type.41,42 We defined diurnal type using the average midpoint of the sleep interval over the 3-day actigraph study period. Individuals whose average sleep midpoint was between 9:00 am and 7:59 pm, who may have been shift workers, were removed from all analyses (n = 6). The distribution of sleep midpoints was divided into tertiles, keeping divisions at whole hours. The 3 groups had the following ranges of sleep midpoints: 8:00 pm to 1:59 am, 2:00 am to 2:59 am, and 3:00 am to 8:59 am.
Statistical Analyses
Cortisol Modeling
There were 3 salivary cortisol samples from each participant, and each had a time stamp. Let Yij represent the log-transformed cortisol value for the jth sample from the ith respondent and tij the time at which the sample was taken. We fit the following model to the data:
where f(∙) is a restricted cubic spline with knots at 10:18, 12:09, 14:07, 15:46, and 19:02 (corresponding to the 5th, 27.5th, 50th, 72.5th, and 95th percentiles as recommended by Harrell et al.43), and αi is a respondent-level deviation from the mean with distribution N(0, σα2). The error term ϵij is assumed to be independent with distribution N(0,σ2). The model was fit using maximum likelihood, and the Best Linear Unbiased Predictions (BLUPs) of the αi were obtained.44 Model (1) is clearly simplistic in that it does not provide for respondent-specific differences in the rate of change during the interview. However, augmenting the model by including respondent-specific slopes yields highly similar estimates of respondent-level deviations about the mean.
To understand whether NSHAP data with just 3 cortisol samples could be used to estimate an overall mean cortisol level, we drew on a study that collected cortisol measures every 20–30 minutes over a 24-hour period and used that to simulate what we would have observed had the cortisol data collection been the same as actually occurred in NSHAP with respect to number of samples and time of day. We estimate the correlation between the mean derived from the 3 cortisol measures and the overall 24-hour level to be a highly significant 0.5, which is likely to be an underestimate (see Technical Appendix).
Regression
Unadjustesd and adjusted multiple linear regression45 were used to estimate the relationship between the standardized cortisol level value as dependent variable and each of 5 sleep measures, the 3 actigraph-measures and the 2 survey-based sleep measures, as independent variables. Each model included one of the sleep measures. The adjusted models include all of the sociodemographic, behavioral, and health covariates described earlier. In addition, interaction terms between the sleep variable and each covariate were examined to determine whether that covariate moderated the individual-level effect. These models were fit using the survey weights distributed with the data set that accounts for differential probabilities of selection and differential nonresponse. Design-based standard errors were obtained using the linearization method46 as implemented in the Stata statistical software package version 13.1.47
RESULTS
The distributions of the sociodemographic, behavioral, health, and sleep variables among the analytic sample are presented in Table 1. Mean age was 71.5 years, and 54% of the sample was female. The racial and ethnic distribution was similar to that of the U.S. population of older adults, with 84% reporting themselves as non-Hispanic white. The average WASO was 0.7 (SD: 0.4) hours, while the average duration of actigraph total sleep time was 7.2 (SD: 1.3) hours. The mean fragmentation index was 14.3% (SD: 6.2). WASO was significantly correlated with both sleep fragmentation (r = 0.78; p < 0.001) and sleep duration (r = 0.32; p < .001). Sleep fragmentation and sleep duration were not significantly correlated (r = −0.01; p = .849). Individuals reported usual sleep durations averaging 7.4 hours, and the average trouble sleep score was 2.9 (out of 8 possible).
Table 1.
Weighted distributions of sociodemographic, behavioral, health, and sleep characteristics among the analytic sample measured at Wave 2 in the National Social Life, Health and Aging Project (NSHAP) from August 2010 to May 2011a.
| Characteristic | Value |
|---|---|
| Age, mean (IQR) | 71.5 (66–78) |
| Female, n (%) | 364 (53.7) |
| Race, n (%) | |
| White, non-Hispanic | 564 (83.2) |
| White, Hispanic | 26 (3.9) |
| African American, non-Hispanic | 46 (6.8) |
| African American, Hispanic | 0 (0.0) |
| Other | 42 (6.2) |
| Education, n (%) | |
| Less than high school | 95 (14.0) |
| High school or equivalent | 170 (25.1) |
| Voc cert/some college/Assoc | 260 (38.3) |
| Bachelors or higher | 154 (22.7) |
| Drink alcohol, n (%) | |
| Does not drink | 287 (42.3) |
| 1–2 drinks/day | 329 (48.5) |
| 3+ drinks/day | 63 (9.2) |
| Smoke cigarettes, n (%) | |
| Current smoker | 96 (14.1) |
| Physical activity, n (%) | |
| No physical activity | 136 (20.1) |
| <3 time/month | 139 (20.5) |
| 1+ times/week | 403 (59.4) |
| Modified Charlson Comorbidity, n (%) | |
| 0 | 69 (10.1) |
| 1–2 | 314 (46.2) |
| 3+ | 296 (43.6) |
| Depressive symptoms,b mean (IQR) | 4.32 (1–7) |
| Hormone replacement therapy,cn (%) | |
| No | 611 (91.9) |
| Yes | 54 (8.1) |
| Diurnal type,dn (%) | |
| 8 pm– 1:59 am | 105 (19.3) |
| 2 am–2:59 am | 194 (35.6) |
| 3 am–8:59 am | 246 (45.1) |
| Season, n (%) | |
| Winter | 117 (17.4) |
| Spring | 56 (8.3) |
| Summer | 175 (26.0) |
| Autumn | 324 (48.2) |
| Currently Employed, n (%) | |
| No | 515 (76.6) |
| Yes | 157 (23.4) |
| Actigraph-Measured Sleep, mean (IQR) | |
| Wake after sleep onset (WASO), hours | 0.6 (0.4–0.8) |
| Duration, hours | 7.8 (7.1–8.7) |
| Fragmentation (%) | 14.3 (10.3–18.4) |
| Self-Reported Sleep, mean (IQR) | |
| Duration, hours | 7.4 (6–8) |
| Troubled Sleep Scalee | 2.9 (2–4) |
Abbreviations: IQR, interquartile range; CES-D, Center for Epidemiological Studies-Depression.
a N = 672.
bAssesed utilizing a modified CES-D scale.
cAmong women only.
dDiurnal type was defined using the average midpoint of the sleep interval over the 3-day ancillary study period.
eTroubled sleep Scale is a combined metric (0 = Never/rarely, 1 = Sometimes, 2 = Most of the time) from 4 questions: feeling rested in the morning, trouble falling asleep, trouble waking during the night, and trouble waking too early.
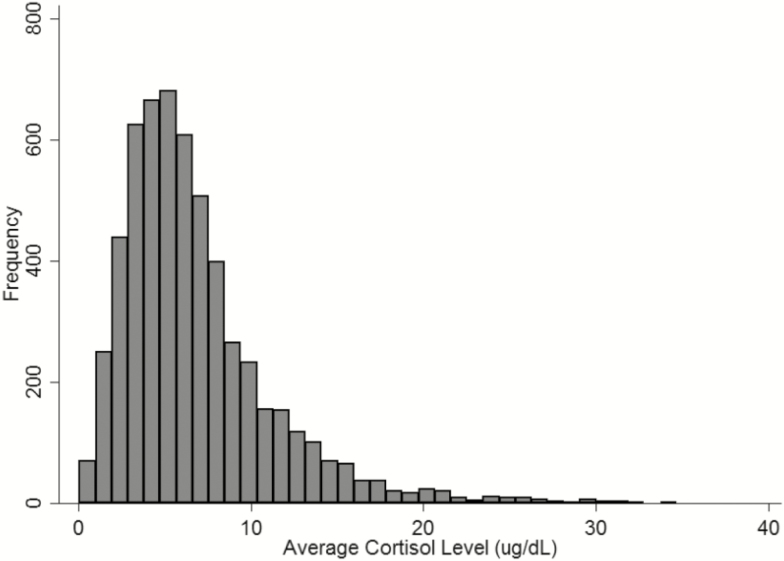
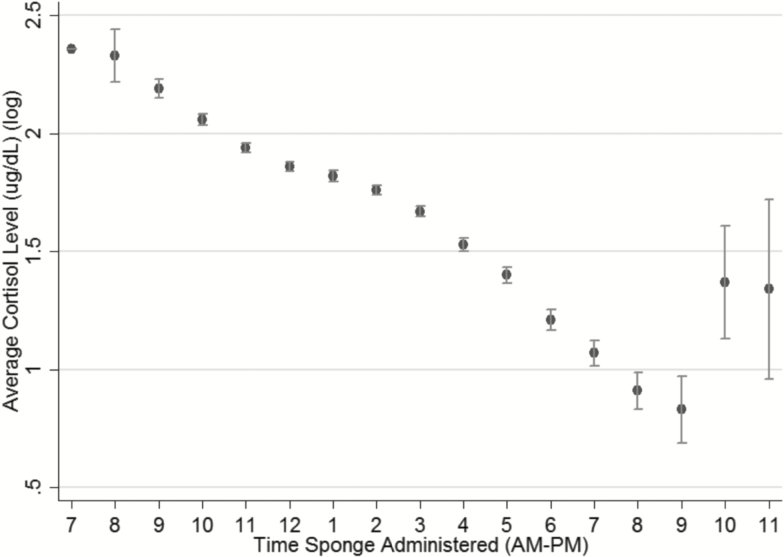
Figure 1 shows the distribution of cortisol averaged over the 3 measurements. Figure 2 shows the diurnal pattern of cortisol, with a steady decrease in log average cortisol levels throughout the day.
Figure 1.
Distribution of (A) average cortisol level (nmol/L) and (B) log average cortisol level (nmol/L) from the National Social Life Health and Aging Project (NSHAP) from August 2010 to May 2011, N = 672.
Figure 2.
Distribution of mean log average cortisol (nmol/L) level by hour of collection (whiskers indicate standard deviation), rounded to the nearest hour, from the National Social Life Health and Aging Project (NSHAP) from August 2010 to May 2011, N = 672.
Unadjusted and adjusted analyses are shown in Tables 2–4. Both actigraph-measured sleep fragmentation (β = 0.02; 95% confidence interval [CI] = 0.01 to 0.04) and WASO (β = 0.26; 95% CI = 0.09 to 0.43) were significantly and positively associated with individual-level cortisol effects in the unadjusted models such that those with more sleep disruption showed higher levels of daytime cortisol (Table 2). Actigraph-measured sleep duration, however, was not significantly associated with cortisol level (β = 0.01; 95% CI = −0.05 to 0.07). For the self-reported sleep measures, there was a trend toward significance for the troubled sleep scale in the unadjusted model, with report of more sleep problems associated with higher cortisol, but no evidence for an association between reported duration and cortisol (Table 2).
Table 2.
Unadjusted linear regression models of individual-level cortisol effect regressed on sleep measures for the National Sleep Life, Health and Aging (NSHAP) project Wave 2 from August 2010 to May 2011a.
| Variableb | β | p | 95% CI |
|---|---|---|---|
| Actigraph-measured sleep | |||
| Average sleep fragmentation, % | 0.02 | .002 | 0.01 to 0.03 |
| Average WASO, hours | 0.26 | .004 | 0.09 to 0.43 |
| Average sleep duration, hours | 0.01 | .8 | −0.05 to 0.07 |
| Self-reported sleep | |||
| Average sleep duration, hours | −0.02 | .9 | −0.03 to 0.03 |
| Troubled sleep scalec | 0.04 | .07 | −0.00 to 0.09 |
Abbreviations: CI, confidence interval; WASO, wake after sleep onset.
a N = 672.
bEach variable is a separate model.
cTroubled sleep scale is a combined metric (0 = never/rarely, 1 = sometimes, 2 = most of the time) including trouble falling asleep, trouble waking during the night, and trouble waking too early. Scores may range from 0 to 8, and the β estimates the effect of a 1-unit change in the score.
Table 4.
Linear regression models of individual-level cortisol effect on self-reported sleep measures in Wave 2 of the National Social Life, Health and Aging Project (NSHAP), N = 672, adjusted for all listed covariatesa.
| Variable | Model 4 | Model 5 | ||||
|---|---|---|---|---|---|---|
| β | p | 95% CI | β | p | 95% CI | |
| Self-reported sleep duration, hours | 0.00 | .7 | −0.03 to 0.03 | – | – | – |
| Troubled sleep scaleb | – | – | – | 0.02 | .5 | −0.05 to 0.09 |
| Age | 0.02 | .004 | 0.01 to 0.04 | 0.02 | .002 | 0.01 to 0.04 |
| Gender | ||||||
| Malec | ref | – | – | ref | – | – |
| Female | −0.49 | <.001 | −0.72 to −0.25 | −0.46 | .001 | −0.73 to −0.20 |
| Race | ||||||
| White, non-Hispanicc | ref | – | – | ref | – | – |
| White, Hispanic | 0.17 | .5 | −0.30 to 0.64 | 0.20 | .5 | −0.37 to 0.76 |
| African American, non-Hispanic | −0.12 | .5 | −0.46 to 0.22 | −0.16 | .4 | −0.51 to 0.20 |
| Other | 0.09 | .6 | −0.22 to 0.41 | 0.17 | .4 | −0.27 to 0.61 |
| Education | ||||||
| High school or equivalentc | ref | – | – | ref | – | – |
| Less than high school | −0.14 | .4 | −0.47 to 0.20 | −0.23 | .2 | −0.56 to 0.11 |
| Voc cert/some college/assoc | −0.15 | .3 | −0.45 to 0.14 | −0.17 | .2 | −0.46 to 0.12 |
| Bachelors or more | −0.19 | .3 | −0.52 to 0.14 | −0.20 | .2 | −0.53 to 0.13 |
| Alcohol use | ||||||
| Does not drinkc | ref | – | – | ref | – | – |
| 1–2 drinks/day | 0.14 | .3 | −0.14 to 0.41 | 0.12 | .4 | −0.17 to 0.41 |
| 3+ drinks/day | 0.15 | .3 | −0.15 to 0.46 | 0.21 | .2 | −0.10 to 0.52 |
| Tobacco use | ||||||
| Noc | ref | – | – | ref | – | – |
| Yes | −0.07 | .7 | −0.40 to 0.27 | −0.05 | .8 | −0.42 to 0.32 |
| Physical activity | ||||||
| No physical activityc | ref | – | – | ref | – | – |
| <3 time/month | 0.26 | .1 | −0.05 to 0.57 | 0.27 | .1 | −0.09 to 0.63 |
| 1+ times/week | 0.09 | .4 | −0.13 to 0.31 | 0.05 | .6 | −0.17 to 0.28 |
| Modified Charlson Comorbidity | ||||||
| 0c | ref | – | – | ref | – | – |
| 1–2 | −0.02 | .9 | −0.30 to 0.26 | −0.05 | .7 | −0.36 to 0.26 |
| 3+ | 0.11 | .4 | −0.18 to 0.41 | 0.11 | .5 | −0.21 to 0.43 |
| Diurnal typed | ||||||
| 8 pm–1:59 am | ref | – | – | ref | – | – |
| 2 am–2:59 am | 0.21 | .2 | −0.11 to 0.52 | 0.06 | .7 | −0.29 to 0.41 |
| 3 am–8:59 am | 0.24 | .1 | −0.06 to 0.55 | 0.15 | .3 | −0.17 to 0.47 |
| Season | ||||||
| Winter | ref | – | – | ref | – | – |
| Spring | 0.13 | .4 | −0.21 to 0.48 | 0.24 | .2 | −0.17 to 0.67 |
| Summer | −0.23 | .2 | −0.55 to 0.09 | −0.12 | .5 | −0.52 to 0.27 |
| Autumn | −0.30 | .07 | −0.62 to 0.02 | −0.26 | .2 | −0.65 to 0.12 |
| Depressive symptomse | 0.01 | .4 | −0.02 to 0.04 | 0.00 | .9 | −0.03 to 0.03 |
| Hormone replacement therapyf | ||||||
| No | ref | – | – | ref | – | – |
| Yes | −0.18 | .3 | −0.49 to 0.13 | −0.30 | .07 | −0.63 to 0.03 |
| Currently employed | ||||||
| No | ref | – | – | ref | – | – |
| Yes | 0.05 | .6 | −0.16 to 0.27 | −0.01 | .9 | −0.24 to 0.21 |
| Constant | 0.52 | .1 | −0.14 to 1.18 | |||
Abbreviations: WASO, wake after sleep onset; CES-D, Center for Epidemiological Studies-Depression; CI, confidence interval.
aThe 2 models each include one of the sleep measures and all covariates.
bTroubled sleep scale is a combined metric (0 = never/rarely, 1 = sometimes, 2 = most of the time) including trouble falling asleep, trouble waking during the night, and trouble waking too early. Scores may range from 0 to 8, and the β estimates the effect of a 1-unit change in the score.
cReference group.
dDiurnal type was defined using the average midpoint of the sleep interval over the 3-day ancillary study period.
eAssesed utilizing a modified CES-D scale.
fAmong women only.
Adding all of the covariates to the regression models had little effect on the coefficients for fragmentation, WASO, and duration (Table 3). Both fragmentation and WASO remained statistically significant (Table 3). Cortisol levels increased with age and were lower for women than for men. However, no cortisol differences were observed between racial or education groups nor were there any associations with the behavioral or health covariates. In the adjusted models, neither of the self-reported sleep measures were significantly associated with daytime cortisol (Table 4).
Table 3.
Linear regression models of individual-level cortisol effect on actigraph-based sleep measures in Wave 2 of the National Social Life, Health and Aging Project (NSHAP), n = 672, adjusted for all listed covariatesa.
| Variable | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | |
| Average sleep fragmentation, % | 0.02 | .03 | 0.00 to 0.03 | – | – | – | – | – | – |
| Average WASO, hours | – | – | – | 0.27 | .02 | 0.04 to 0.51 | – | – | – |
| Average sleep duration, hours | – | – | – | – | – | – | −0.01 | .9 | −0.08 to 0.07 |
| Age | 0.03 | .002 | 0.01 to 0.04 | 0.03 | .001 | 0.01 to 0.04 | 0.03 | .001 | 0.01 to 0.04 |
| Gender | |||||||||
| Maleb | ref | – | – | ref | – | – | ref | – | – |
| Female | −0.41 | <.001 | −0.063 to −0.019 | −0.43 | <.001 | −0.66 to −0.21 | −0.44 | <.001 | −0.66 to −0.22 |
| Race | |||||||||
| White, non-Hispanicb | ref | – | – | ref | – | – | ref | – | – |
| White, Hispanic | 0.21 | .4 | −0.027 to 0.68 | 0.21 | .4 | −0.27 to 0.69 | 0.19 | .4 | −0.30 to 0.67 |
| African American, non-Hispanic | −0.09 | .6 | −0.42 to 0.25 | −0.08 | .6 | −0.42 to 0.26 | −0.05 | .8 | −0.37 to 0.28 |
| Other | 0.09 | .6 | −0.26 to 0.43 | .11 | 0.5 | −0.21 to 0.44 | 0.11 | .5 | −0.20 to 0.43 |
| Education | |||||||||
| High school or equivalentb | ref | – | – | ref | – | – | ref | – | – |
| Less than high school | −0.28 | .1 | −0.66 to 0.10 | −0.33 | .1 | −0.71 to 0.05 | −0.29 | .1 | −0.67 to 0.09 |
| Voc cert/some college/assoc | −0.17 | .3 | −0.47 to 0.13 | −0.17 | .3 | −0.47 to 0.13 | −0.17 | .2 | −0.48 to 0.13 |
| Bachelors or more | −0.14 | .4 | −0.47 to 0.19 | −0.15 | .4 | −0.48 to 0.19 | −0.18 | .3 | −0.51 to 0.16 |
| Alcohol use | |||||||||
| Does not drinkb | ref | – | – | ref | – | – | ref | – | – |
| 1–2 drinks/day | 0.09 | .5 | −0.18 to 0.37 | 0.10 | .5 | −0.18 to 0.37 | 0.09 | .5 | −0.19 to 0.37 |
| 3+ drinks/day | 0.10 | .5 | −0.20 to 0.40 | 0.09 | .5 | −0.21 to 0.38 | 0.09 | .5 | −0.2 to 0.39 |
| Tobacco use | |||||||||
| Nob | ref | – | – | ref | – | – | ref | – | – |
| Yes | −0.03 | .9 | −0.37 to 0.31 | −0.02 | .9 | −0.36 to 0.32 | −0.02 | .9 | −0.37 to 0.32 |
| Physical activity | |||||||||
| No physical activityb | ref | – | – | ref | – | – | ref | – | – |
| <3 time/month | 0.25 | .1 | −0.06 to 0.55 | 0.23 | .1 | −0.07 to 0.53 | 0.22 | .2 | −0.09 to 0.52 |
| 1+ times/week | 0.14 | .2 | −0.06 to 0.33 | 0.13 | .2 | −0.08 to 0.33 | 0.10 | .3 | −0.10 to 0.31 |
| Modified Charlson Comorbidity | |||||||||
| 0b | ref | – | – | ref | – | – | ref | – | – |
| 1–2 | −0.02 | .9 | −0.33 to 0.29 | −0.04 | .8 | −0.34 to 0.27 | −0.06 | .7 | −0.36 to 0.23 |
| 3+ | 0.14 | .4 | −0.19 to 0.47 | 0.13 | .5 | −0.21 to 0.46 | 0.11 | .5 | −0.21 to 0.43 |
| Depressive Symptomsc | 0.00 | .9 | −0.03 to 0.03 | 0.00 | .9 | −0.03 to 0.03 | 0.01 | .7 | −0.02 to 0.03 |
| Hormone replacement therapyd | |||||||||
| No | ref | – | – | ref | – | – | ref | – | – |
| Yes | −0.19 | .3 | −0.54 to 0.15 | −0.17 | .3 | −0.53 to 0.18 | −0.19 | .3 | −0.55 to 0.17 |
| Diurnal typee | |||||||||
| 8 pm–1:59 am | ref | – | – | ref | – | – | ref | – | – |
| 2 am–2:59 am | 0.12 | .5 | −0.20 to 0.44 | 0.13 | .4 | −0.19 to 0.45 | 0.12 | .5 | −0.21 to 0.45 |
| 3 am–8:59 am | 0.19 | .2 | −0.13 to 0.50 | 0.21 | .2 | −0.12 to 0.52 | 0.20 | .2 | −0.13 to 0.52 |
| Season | |||||||||
| Winter | ref | – | – | ref | – | – | ref | – | – |
| Spring | 0.00 | .9 | −0.35 to 0.36 | 0.00 | 1.0 | −0.35 to 0.36 | 0.01 | 1.0 | −0.35 to 0.37 |
| Summer | −0.25 | .1 | −0.60 to 0.10 | −0.24 | .2 | −0.60 to 0.12 | −0.26 | .2 | −0.61 to 0.10 |
| Autumn | −0.38 | .03 | −0.72 to −0.04 | −0.37 | .04 | −0.72 to −0.03 | −0.39 | .03 | −0.72 to 0.04 |
| Currently employed | |||||||||
| No | ref | – | – | ref | – | – | ref | – | – |
| Yes | 0.10 | .4 | −0.13 to 0.33 | 0.10 | .4 | −0.12 to 0.33 | 0.09 | .4 | −0.14 to 0.32 |
| Constant | 0.29 | .4 | −0.38 to 0.95 | 0.40 | .2 | −0.27 to 1.08 | 0.67 | .2 | −0.33 to 1.68 |
Abbreviations: WASO, wake after sleep onset; CES-D, Center for Epidemiological Studies-Depression; CI, confidence interval.
aThe three models each include one of the sleep measures and all covariates.
bReference group.
cAssesed utilizing a modified CES-D scale.
dAmong women only.
eDiurnal type was defined using the average midpoint of the sleep interval over the 3-day ancillary study period.
DISCUSSION
In a nationally representative sample of older adults in the United States, we found significant associations between actigraph-estimated measures of sleep quality, specifically WASO and sleep fragmentation, and daytime cortisol level, adjusting for the time of day when saliva was sampled. These findings indicate that more sleep disruption is associated with elevated daytime cortisol levels among older adults. However, we found no significant associations between actigraph-measured sleep duration and daytime cortisol levels. Nor did we find significant associations between self-reported sleep duration and sleep problems with daytime cortisol level.
These findings add to previous results from large observational cohorts that so far do not coalesce into a consistent set of associations, complicated by the different approaches to measuring both cortisol and sleep. Our lack of a significant relationship between actigraph-measured sleep duration and cortisol is similar to the MrOS Sleep Study, although they had a different cortisol measure: 24-hour urinary cortisol levels.26 The MrOS study did however find a significant association between self-reported sleep duration and cortisol level which we did not. The Whitehall II study (2009) measured cortisol throughout the day with six timed measures, so that they could examine more features of the cortisol response over the day.48 They found an association between sleep duration from the previous night’s sleep log and both the wakening and evening cortisol levels. Unfortunately, our sleep logs and actigraphy were collected several days after the cortisol measure so we could not examine the one-day effects of sleep. Further, the Whitehall II study reported that the survey responses about sleep disturbance were also related to cortisol, specifically a higher evening cortisol level and shallower slope during the day. In more recent analysis of the Whitehall II data that used survey responses about usual sleep duration and disturbance across different years of data collection, they found that those repeatedly reporting short sleep durations had higher evening cortisol.27 Our daytime cortisol associations with actigraph sleep disturbance measures are broadly similar to the Whitehall II finds, although Whitehall II did not include actigraphy. Both MrOS and Whitehall II were very large studies and included over 2000 participants; they therefore had greater power to detect associations than our study. Our results suggest that sleep disturbance measures from actigraphy are more strongly related to daytime cortisol than reports of sleep disturbances or sleep duration, whether measured by actigraphy or self-report.
Vgontzas et al. found insomnia effects on 24-hour cortisol secretion in a study that included 11 young insomniacs (mean age 31.4 ± 6.7 years) and 13 healthy controls (mean age 27.7 ± 6.87 years).49 Insomniacs, who also had high amount of objectively observed sleep disruption, had significantly higher 24-hour cortisol secretions, compared to the healthy controls. Our finding of no significant association between cortisol level and an insomnia symptom scale may be related to the differences in the age range or in the cortisol measures, or it may be commonly reported insomnia symptoms among older adults which do not represent the same types or severity of problems as among younger adults.
We did not find evidence of seasonal effects in cortisol. A previous study compiled data from 15 independent field studies providing a database of 104623 salivary cortisol samples collected from 18698 participants (age range: 0.5–98.5, mean = 48.3 years) and found that cortisol levels were strongly influenced by the season in which they were collected. Our sample size may not have been adequately large to detect these effects.
Our findings of significant associations between 2 actigraph measures of sleep disruption and daytime cortisol level could result from several different underlying scenarios. Our data are cross-sectional, and we cannot conclude that worse sleep caused higher cortisol levels. It is also possible that higher cortisol levels disrupt sleep. Another explanation could be that there is a common cause of both higher daytime cortisol and more disrupted sleep, such as stress.50 While longitundal observational data might provide better evidence about the causal direction, more definitive evidence would come from interventions that improve disrupted sleep.
Our study has several important limitations. The salivary and actigraphy data were not collected on the same days. Salivary cortisol samples were collected during the in-home interview, and sleep was measured a few days to a few weeks later. Our results therefore assume that the mean sleep characteristics over 3 nights represent habitual sleep characteristics similar to the preceding weeks. If sleep duration is more variable than fragmentation or WASO, our null findings for duration could be related to the separation in time between the sleep and the cortisol data collection. Having only 3 nights of actigraphy is also a limitation. However, previous work has shown 3 nights is adequate to estimate means for older adults.31 The fragmentation index from actigraphy does not have clear correspondence to measures of sleep disruption from polysomnography, although there is evidence that both are similarly elevated in patients with pathologies affecting sleep.33 Three salivary samples, while sufficient to estimate an individual mean level adjusted for time of sample collection, did not allow us to assess the cortisol awakening and evening responses. Further, salivary samples were collected over a 2-hour window, and thus we were unable to characterize the diurnal cortisol pattern for each respondent. Because the interviews were scheduled across the day and evening, we did not separate evening and morning effects of sleep.
Despite these limitations, our results provide new evidence that sleep quality, as measured by actigraphy, is associated with higher daytime cortisol levels in the general population of older adults. These cross-sectional data cannot distinguish causal direction or whether both cortisol and sleep disruption have a common cause. Longitudinal data and interventions would be needed to identify a causal relationship.
FINANCIAL SUPPORT
This work was supported by R01AG042164 from the National Institute of Aging and the Basic Behavioral and Social Sciences Research Opportunity Network (OppNet) at the National Institutes of Health. The core data collection in the National Social Life and Aging Project (NSHAP) was supported by R01 AG021487 and R01 AG033903 from the National Institute of Aging at the National Institutes of Health. The ancillary sleep data collection for NSHAP received additional support from Phillips Respironics and the Health and Retirement Survey.
DISCLOSURE STATEMENT
None disclosed.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Eve Van Cauter for providing the cortisol data from healthy subjects.
TECHNICAL APPENDIX
Determination of Cortisol Levels
In this note, we evaluate the performance of the procedure used in this analysis by conducting a small simulation study. Our model as described in the methods section:
| (1) |
We began with the following 1-harmonic cosinor model often used to analyze repeated cortisol measurements:
| (2) |
where is the overall (i.e., 24-hour) mean, is the amplitude, and is the acrophase, all specific to individual .1 As in Model (1), is assumed to be independent and normally distributed. Although additional harmonics are typically required to describe precisely the change in cortisol over a 24-hour period, Model (2) represents the periodic nature of cortisol fluctuation as well as the main sources of variability between individuals.
Model (2) was fit to a subset of the data reported in Van Cauter et al.2 to which we had access. This consisted of plasma cortisol measurements taken every 20–30 minutes over a 24-hour period from 62 healthy adults (42% female, median age 55). The model was fit using the approach described in Mikulich et al.3 which involves applying a linearizing transformation to Equation (2) and then fitting a linear mixed model. Back-transforming the BLUPs of the random effects yielded individual estimates of the overall means (), amplitudes (), and acrophases () with means of 4.20, 0.63, and −0.56, and standard deviations of 0.28, 0.23 and 0.36, respectively. The standard deviation of the residuals was 0.46.
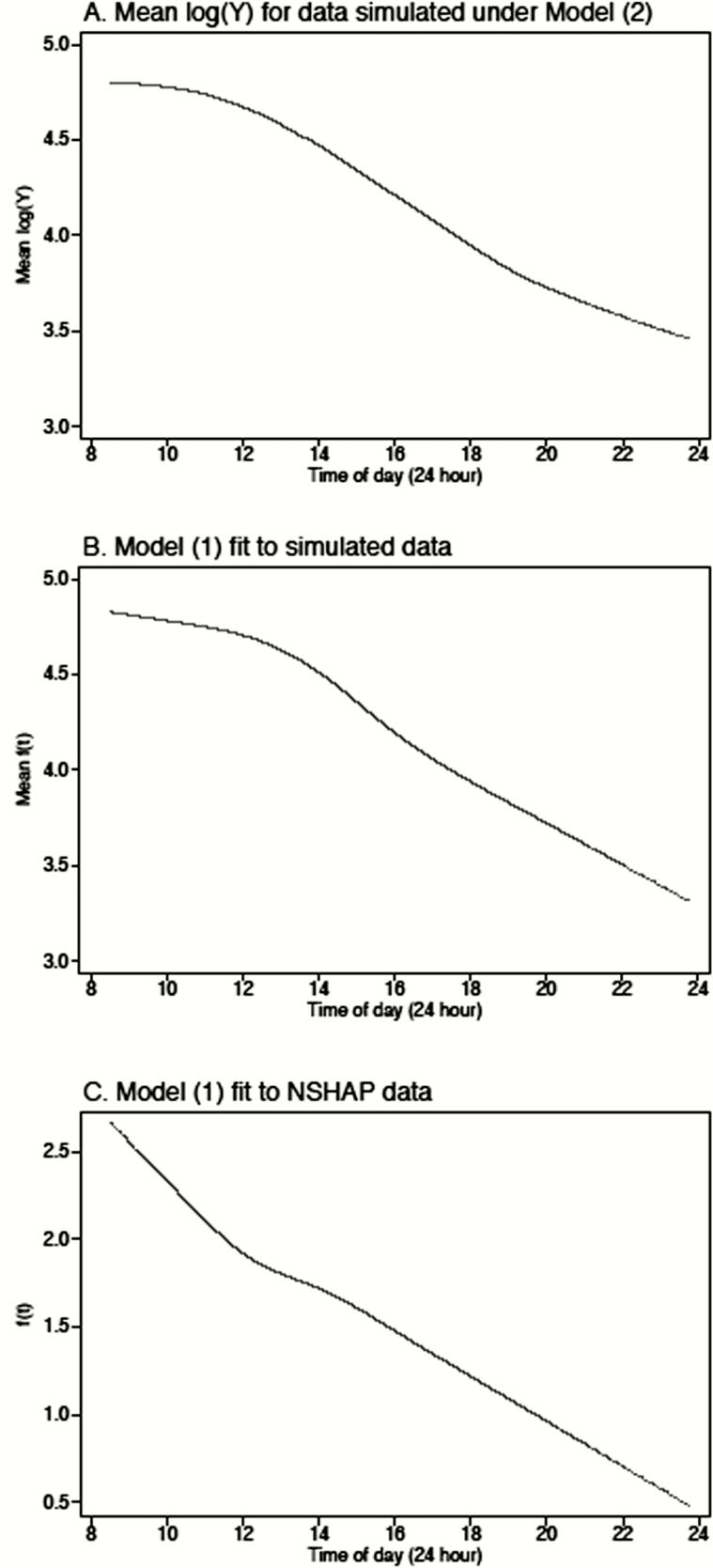
Next, we used the fitted model to simulate data for 625 hypothetical individuals, using the same sample collection times as observed in the National Social Life, Health and Aging Project (NSHAP) data set. Specifically, for each individual, we drew values for and from their estimated distributions and then used Model (2) together with the observed collection times for an NSHAP respondent to generate log cortisol values (). The overall mean (averaged across all individuals) is plotted in Appendix Figure 1A over the observed range of times. Model (1) was then fit to the simulated data set, and the correlations between the and the true, known values of , and were computed. This process was repeated 250 times. The mean correlation between and was 0.475 (95% CI = 0.471 to 0.479), between and was −0.039 (95% CI = −0.044 to −0.034)) and between and was 0.248 (95% CI = 0.243 to 0.254). Appendix Figure 1B plots the estimated curve for the simulated data, which in general resemble the mean curve. Interestingly, the estimated curve for the NSHAP data shows a similar slope before 12:00 as after, perhaps reflecting a systematic increase in the initial cortisol measurements triggered by anxiety in anticipation of the interview.
Figure 1.
Plots of the estimated curve f(t) for the simulated data (A) mean log(Y) for data simulated under model (2), (B) model (1) fit to simulated data, and (C) model (1) fit to NSHAP data.
In sum, this exercise demonstrates that fitting Model (1) to data from healthy adults collected using the NSHAP sampling scheme yields estimates that have a correlation of 0.48 with subjects’ overall means. There is also a smaller correlation with the acrophase such that those with a cortisol rhythm shifted forward relative to the population have on average higher cortisol levels at a given time within the observed time span.
One limitation of this simulation is the fact that the control data were obtained from serum samples (instead of saliva). More important, perhaps, is the possibility that the healthy subjects differed systematically from the NSHAP respondents. For example, the NSHAP respondents are both older and represent the entire home-dwelling population (including individuals with illness), both of which would be expected to increase their mean cortisol and likely also the variability in mean cortisol between individuals. For example, the ratio for the simulated data is only 0.095/0.215 = 0.44, when compared to 0.247/0.086 = 2.87 for the NSHAP data. This substantially higher variability between individuals should increase the correlation between the estimated and the respondents’ true, underlying mean cortisol levels, suggesting that the correlation of 0.48 found in this simulation may underestimate the actual correlation for the NSHAP sample.
REFERENCES
1. Halberg F, Tong YL, Johnson EA. Circadian system phase—an aspect of temporal morphology. In: von Mayersbach H, editor. Cell. Asp. Biorhythms. Berlin: Springer; 1967. p. 20–48.
2. Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab 1996;81:2468–2473. http://dx.doi.org/10.1210/jc.81.7.2468.
3. Mikulich SK, Zerbe GO, Jones RH, Crowley TJ. Comparing linear and nonlinear mixed model approaches to cosinor analysis. Stat Med 2003;22:3195–211. http://dx.doi.org/10.1002/sim.1560.
REFERENCES
- 1. Kirschbaum C, Hellhammer D. Salivary cortisol. Encycl Stress. 2000; 3:379–383. [Google Scholar]
- 2. Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. 1983; 20 (Pt 6): 329–335. [DOI] [PubMed] [Google Scholar]
- 3. Marieb E, Hoehn K. Human Anatomy and Physiology. 7th ed Upper Saddle River, NJ: Pearson Prentice Hall; 2006. [Google Scholar]
- 4. Epel ES, McEwen B, Seeman T, et al. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 62(5):623–632. http://www.ncbi.nlm.nih.gov/pubmed/11020091 Accessed April 22, 2016. [DOI] [PubMed] [Google Scholar]
- 5. Rosmond R, Dallman MF, Björntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998; 83(6): 1853–1859. [DOI] [PubMed] [Google Scholar]
- 6. Michelson D, Stratakis C, Hill L, et al. Bone mineral density in women with depression. N Engl J Med. 1996; 335(16): 1176–1181. [DOI] [PubMed] [Google Scholar]
- 7. Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Brain Res Rev. 1997;24(1):1–27. http://www.ncbi.nlm.nih.gov/pubmed/9233540 Accessed April 22, 2016. [DOI] [PubMed] [Google Scholar]
- 8. Terzolo M, Bovio S, Pia A, et al. Midnight serum cortisol as a marker of increased cardiovascular risk in patients with a clinically inapparent adrenal adenoma. Eur J Endocrinol. 2005; 153(2): 307–315. [DOI] [PubMed] [Google Scholar]
- 9. März W, Tiran B, Seelhorst U, et al. ; LURIC Study Team. N-terminal pro-B-type natriuretic peptide predicts total and cardiovascular mortality in individuals with or without stable coronary artery disease: the Ludwigshafen Risk and Cardiovascular Health Study. Clin Chem. 2007; 53(6): 1075–1083. [DOI] [PubMed] [Google Scholar]
- 10. Chiodini I, Adda G, Scillitani A, et al. Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care. 2007; 30(1): 83–88. [DOI] [PubMed] [Google Scholar]
- 11. Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006; 164(10): 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. López-García E, Faubel R, León-Muñoz L, Zuluaga MC, Banegas JR, Rodríguez-Artalejo F. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. Am J Clin Nutr. 2008; 87(2): 310–316. [DOI] [PubMed] [Google Scholar]
- 13. Chee MWL, Chuah YML. Functional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivation. Proc Natl Acad Sci. 2007; 104(22): 9487–9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002; 166(2): 159–165. [DOI] [PubMed] [Google Scholar]
- 15. Krieger D. Rhythms in CRF, ACTH and Corticosteroids. New York, NY: Raven Press; 1979. [Google Scholar]
- 16. Born J, Muth S, Fehm HL. The significance of sleep onset and slow wave sleep for nocturnal release of growth hormone (GH) and cortisol. Psychoneuroendocrinology. 1988; 13(3): 233–243. http://www.ncbi.nlm.nih.gov/pubmed/3406323 Accessed April 22, 2016. [DOI] [PubMed] [Google Scholar]
- 17. Alford FP, Baker HW, Burger HG, et al. Temporal patterns of integrated plasma hormone levels during sleep and wakefulness. I. Thyroid-stimulating hormone, growth hormone and cortisol. J Clin Endocrinol Metab. 1973; 37(6): 848–854. [DOI] [PubMed] [Google Scholar]
- 18. Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009; 72(1): 67–73. [DOI] [PubMed] [Google Scholar]
- 19. Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996; 81(7): 2468–2473. [DOI] [PubMed] [Google Scholar]
- 20. Vgontzas AN, Zoumakis M, Bixler EO, et al. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab. 2003; 88(5): 2087–2095. [DOI] [PubMed] [Google Scholar]
- 21. Späth-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry. 1991; 29(6): 575–584. [DOI] [PubMed] [Google Scholar]
- 22. Schüssler P, Uhr M, Ising M, et al. Nocturnal ghrelin, ACTH, GH and cortisol secretion after sleep deprivation in humans. Psychoneuroendocrinology. 2006; 31(8): 915–923. [DOI] [PubMed] [Google Scholar]
- 23. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999; 354(9188): 1435–1439. [DOI] [PubMed] [Google Scholar]
- 24. Reynolds AC, Dorrian J, Liu PY, et al. Impact of five nights of sleep restriction on glucose metabolism, leptin and testosterone in young adult men. PLoS One. 2012; 7(7): e41218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014; 99(8): 2861–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rao MN, Blackwell T, Redline S, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group. Association between sleep duration and 24-hour urine free cortisol in the MrOS Sleep Study. PLoS One. 2013; 8(9): e75205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abell JG, Shipley MJ, Ferrie JE, Kivimäki M, Kumari M. Recurrent short sleep, chronic insomnia symptoms and salivary cortisol: A 10-year follow-up in the Whitehall II study. Psychoneuroendocrinology. 2016; 68: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dahlgren A, Kecklund G, Theorell T, Akerstedt T. Day-to-day variation in saliva cortisol–relation with sleep, stress and self-rated health. Biol Psychol. 2009; 82(2): 149–155. [DOI] [PubMed] [Google Scholar]
- 29. O’Doherty K, Jaszczak A, Hoffmann JN, et al. Survey field methods for expanded biospecimen and biomeasure collection in NSHAP Wave 2. J Gerontol B Psychol Sci Soc Sci. 2014;69Suppl 2: S27–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Muircheartaigh C, English N, Pedlow S, Kwok PK. Sample design, sample augmentation, and estimation for Wave 2 of the NSHAP. J Gerontol B Psychol Sci Soc Sci. 2014;69Suppl 2: S15–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rowe M, McCrae C, Campbell J, et al. Actigraphy in older adults: comparison of means and variability of three different aggregates of measurement. Behav Sleep Med. 2008; 6(2): 127–145. [DOI] [PubMed] [Google Scholar]
- 32. Lauderdale DS, Philip Schumm L, Kurina LM, et al. Assessment of sleep in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2014;69Suppl 2: S125–S133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rotem AY, Sperber AD, Krugliak P, Freidman B, Tal A, Tarasiuk A. Polysomnographic and actigraphic evidence of sleep fragmentation in patients with irritable bowel syndrome. Sleep. 2003; 26(6): 747–752. [DOI] [PubMed] [Google Scholar]
- 34. Rimmer J, Downie S, Bartlett DJ, Gralton J, Salome C. Sleep disturbance in persistent allergic rhinitis measured using actigraphy. Ann Allergy Asthma Immunol. 2009; 103(3): 190–194. [DOI] [PubMed] [Google Scholar]
- 35. Loewen A, Siemens A, Hanly P. Sleep disruption in patients with sleep apnea and end-stage renal disease. J Clin Sleep Med. 2009;5(4):324–329. http://www.ncbi.nlm.nih.gov/pubmed/19968009 Accessed October 8, 2016. [PMC free article] [PubMed] [Google Scholar]
- 36. Lauderdale DS, Chen JH, Kurina LM, Waite LJ, Thisted RA. Sleep duration and health among older adults: associations vary by how sleep is measured. J Epidemiol Community Health. 2016; 70(4): 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen JH, Waite L, Kurina LM, Thisted RA, McClintock M, Lauderdale DS. Insomnia symptoms and actigraph-estimated sleep characteristics in a nationally representative sample of older adults. J Gerontol A Biol Sci Med Sci. 2015; 70(2): 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006; 164(1): 5–16. [DOI] [PubMed] [Google Scholar]
- 39. Kurina LM, Thisted RA, Chen J-H, et al. Actigraphic sleep characteristics among older Americans. Sleep Heal. 2015;1(4):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shiovitz-Ezra S, Leitsch S, Graber J, Karraker A. Quality of life and psychological health indicators in the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 2009;64Suppl 1: i30–i37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roenneberg T, Kuehnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007; 11(6): 429–438. [DOI] [PubMed] [Google Scholar]
- 42. Zavada A, Gordijn MCM, Beersma DGM, Daan S, Roenneberg T. Comparison of the Munich Chronotype Questionnaire with the Horne-Ostberg’s Morningness-Eveningness Score. Chronobiol Int. 2005;22(2):267–278. http://www.ncbi.nlm.nih.gov/pubmed/16021843 Accessed January 26, 2017. [DOI] [PubMed] [Google Scholar]
- 43. Harrell FEJ. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001. [Google Scholar]
- 44. Skrondal A, Rabe-Hesketh S. Generalized Latent Variable Modeling. New York, NY: Chapman & Hall/CRC; 2004. [Google Scholar]
- 45. Weisberg S. Applied Linear Regression. Vol 528; 2005. [Google Scholar]
- 46. Binder DA. On the Variances of Asymptotically Normal Estimators from Complex Surveys. Int Stat Rev / Rev Int Stat. 1983;51(3):279. [Google Scholar]
- 47. StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp; 2015. [Google Scholar]
- 48. Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009; 94(12): 4801–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vgontzas AN, Bixler EO, Lin H-M, et al. Chronic Insomnia Is Associated with Nyctohemeral Activation of the Hypothalamic-Pituitary-Adrenal Axis: Clinical Implications. J Clin Endocrinol Metab. 2011. [DOI] [PubMed] [Google Scholar]
- 50. Dahlgren A, Kecklund G, Akerstedt T. Different levels of work-related stress and the effects on sleep, fatigue and cortisol. Scand J Work Environ Health. 2005;31(4):277–285. http://www.ncbi.nlm.nih.gov/pubmed/16161710 Accessed January 26, 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.