Abstract
STUDY QUESTION
Does the use of a vascular contrast agent facilitate earlier detection of maternal flow to the placental intervillous space (IVS) in the first trimester of pregnancy?
SUMMARY ANSWER
Microvascular filling of the IVS was demonstrated by contrast-enhanced ultrasound from 6 weeks of gestation onwards, earlier than previously believed.
WHAT IS KNOWN ALREADY
During placental establishment and remodeling of maternal spiral arteries, endovascular trophoblast cells invade and accumulate in the lumen of these vessels to form ‘trophoblast plugs’. Prior evidence from morphological and Doppler ultrasound studies has been conflicting as to whether the spiral arteries are completely plugged, preventing maternal blood flow to the IVS until late in the first trimester.
STUDY DESIGN, SIZE, DURATION
Uteroplacental flow was examined across the first trimester in human subjects given an intravenous infusion of lipid-shelled octofluoropropane microbubbles with ultrasound measurement of destruction and replenishment kinetics. We also performed a comprehensive histopathological correlation using two separately archived uteroplacental tissue collections to evaluate the degree of spiral artery plugging and evaluate remodeling of the upstream myometrial radial and arcurate arteries.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Pregnant women (n = 34) were recruited in the first trimester (range: 6+3 to 13+6 weeks gestation) for contrast-enhanced ultrasound studies with destruction-replenishment analysis of signal intensity for assessment of microvascular flux rate. Histological samples from archived in situ (Boyd Collection, n = 11) and fresh first, second, and third trimester decidual and post-hysterectomy uterine specimens (n = 16) were evaluated by immunohistochemistry (using markers of epithelial, endothelial and T-cells, as well as cell adhesion and proliferation) and ultrastructural analysis.
MAIN RESULTS AND THE ROLE OF CHANCE
Contrast agent entry into the IVS was visualized as early as 6+3 weeks of gestation with some variability in microvascular flux rate noted in the 6–7+6 week samples. Spiral artery plug canalization was observed from 7 weeks with progressive disintegration thereafter. Of note, microvascular flux rate did not progressively increase until 13 weeks, which suggests that resistance to maternal flow in the early placenta may be mediated more proximally by myometrial radial arteries that begin remodeling at the end of the first trimester.
LIMITATIONS REASONS FOR CAUTION
Gestational age was determined by crown-rump length measurements obtained by transvaginal ultrasound on the day of contrast-enhanced imaging studies, which may explain the variability in the earliest gestational age samples due to the margin of error in this type of measurement.
WIDER IMPLICATIONS OF THE FINDINGS
Our comprehensive in situ histological analysis, in combination with the use of an in vivo imaging modality that has the sensitivity to permit visualization of microvascular filling, has allowed us to reveal new evidence in support of increasing blood flow to the IVS from 6 weeks of gestation. Histologic review suggested the mechanism may be blood flow through capillary-sized channels that form through the loosely cohesive ‘plugs’ by 7 weeks gestation. However, spiral artery remodeling on its own did not appear to explain why there is significantly more blood flow at 13 weeks gestation. Histologic studies suggest it may be related to radial artery remodeling, which begins at the end of the first trimester.
STUDY FUNDING/COMPETING INTEREST(S)
This project was supported by the Oregon Health and Science University Knight Cardiovascular Institute, Center for Developmental Health and the Struble Foundation. There are no competing interests.
Keywords: Spiral artery, intervillous blood flow, contrast-enhanced ultrasound, placental perfusion, in vivo imaging, trophoblast plugs
Introduction
Many factors contribute to pregnancy success but arguably the most critical one is the establishment of the uteroplacental vasculature. Without the appropriate interplay between the maternal and fetal circulations, inadequate maternal-fetal exchange will inevitably result in compromised fetal growth and development. Remodeling of the maternal spiral arteries from tightly coiled vessels in the non-pregnant state, to wide-bore, low resistance conduits in pregnancy is fundamental to the establishment of the uteroplacental circulation. This complex conversion involves multiple processes that require the invasion of placental extravillous trophoblasts (EVTs) into the lumen of decidual spiral arteries (for review, (Pijnenborg et al., 2006; Whitley and Cartwright, 2010)). The EVTs accumulate in the lumen and form what has been referred to as ‘trophoblast plugs’. These collections of EVTs, or ‘plugs’, are postulated to obstruct maternal arterial blood flow into the intervillous space (IVS) before the end of the first trimester; yet, there is conflicting support for and against this hypothesis (Coppens et al., 1996; Valentin et al., 1996; Meekins et al., 1997; Burton et al., 1999, 2002; Carbillon et al., 2001; Jauniaux et al., 2001). Moreover, little is known about the breakdown of these ‘plugs’, how this process is initiated and regulated, and the relationship between disintegrating plugs and blood flow.
The primary challenge in addressing these questions is in situ access to the spiral arteries in relation to the placenta. Curettage specimens from miscarriage and elective pregnancy terminations may provide partial snap-shots, but they are limited by confounders such as tissue damage associated with collection and sample processing. In addition, current in vivo assessment of placental perfusion in early pregnancy is hindered by limitations in assessing perfusion through low flow and small diameter vessels like mid-first trimester decidual spiral arteries. In this study, we sought to overcome these obstacles using two approaches; firstly, using a vascular contrast agent to visualize vascular filling of the IVS by ultrasound in early pregnancy in a cohort of women with non-continuing pregnancies, and secondly, by histological examination of two separately archived uteroplacental tissue resources.
Contrast-enhanced ultrasound (CEUS) relies on the acoustic detection of gas-filled, lipid-encapsulated microbubbles to visualize and quantify microvascular perfusion (Kaufmann and Lindner, 2007; Kaufmann et al., 2007). The use of a contrast agent facilitates assessment of perfusion in small capillary networks that are difficult to assess solely with the use of Doppler ultrasound. Microbubbles have similar rheology to red blood cells (RBCs) and do not interfere with hemodynamics (Lindner et al., 2002). Work in nonhuman primate models has demonstrated the feasibility of using contrast agents to enhance detection of blood flow during implantation and early pregnancy (Simpson et al., 1997; Keator et al., 2011). Similarly, we recently implemented CEUS to visualize spiral arteries and vascular filling in the human placenta at 11–13 weeks of gestation (Roberts et al., 2016). Utilizing this technology, we are able to calculate the microvascular flux rate constant, which is a measure of in-flow velocity. Although this is not an absolute measure of ‘flow’ (volume/time), we will refer to the presence of contrast agent in the placenta as placental blood flow.
To compare uteroplacental arterial remodeling changes with changes in uteroplacental blood flow, we utilized the in situ placental hysterectomy collection at Cambridge University, UK (Boyd Collection). This previously published tissue resource is a collection of hysterectomies during pregnancy performed for reasons of uterine bleeding, trauma, pregnancy termination and fetal demise (Burton et al., 1999). Maternal age and parity are not available, but gestational age is estimated by embryonic and fetal crown-rump length (CRL). The advantage of the Boyd Collection is that the placenta and uterus have been sectioned into large blocks and embedded in paraffin, providing complete serial sections through the entire uteroplacental interface. Employment of the Boyd Collection, in combination with archived hysterectomy and decidual biopsies from our own institution, allowed for a comprehensive assessment of vascular remodeling, EVT plug immunophenotype and ultrastructural analysis of the disintegrating plugs in the first trimester.
Since, it has been previously suggested that the spiral artery plugs are only loosely cohesive and may even develop vascular channels communicating with the IVS (Burton et al., 1999), the primary objective of this study was to utilize CEUS to examine maternal blood flow to the human placenta in the first trimester, and to correlate these data with an independent histological review of spiral artery ‘plugs’ in early pregnancy.
Materials and Methods
Contrast-enhanced ultrasound
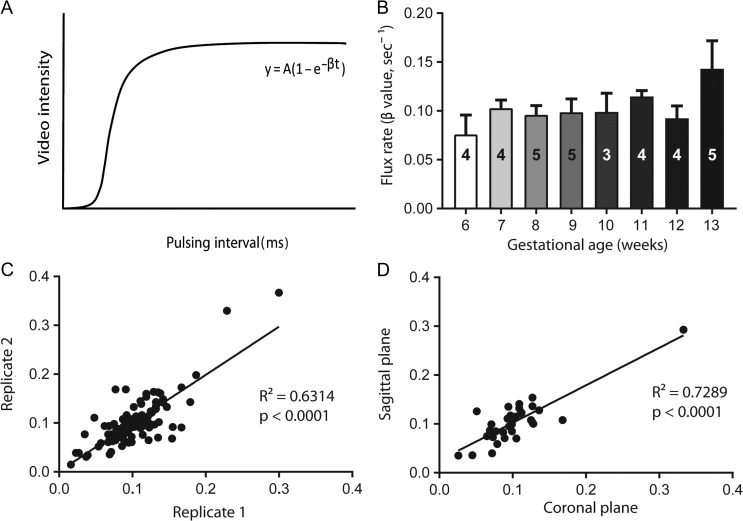
Women scheduled for elective termination of pregnancy (n = 34) underwent ultrasound studies at 6+3–13+6 weeks’ gestation. Patient demographics are given in Table I. Gestational age was determined by CRL on transvaginal ultrasound performed day of the procedure. CEUS was performed using a Sequoia system (Siemens Medical Systems, Mountain View, CA, USA) equipped with a 4C1-S transducer. Lipid-shelled octofluoropropane microbubble reagent (Definity®, Lantheus Medical Imaging, Billerica, MA, USA) was intravenously infused (rate: 60 ml/h) for visualization of uteroplacental perfusion, as previously described (Roberts et al., 2016). Microbubble re-entry in the IVS was recorded in three replicates for each study. Replenishment kinetic curves were generated and flux rate (β) calculated as the rate of refilling of the vascular space until signal saturation (Fig. 1A). Vascular filling of the entire placenta was visualized and captured in a single field of view within two visual planes: coronal and sagittal. The coronal plane was achieved by identifying bilateral uterine artery sources immediately after branching from the internal iliac artery. The sagittal plane was determined using the vesicocervical junction as a landmark with visualization of the internal iliac artery branching into the uterine artery.
Table I.
Contrast-Enhanced Ultrasound study participant demographics.
| Baseline Characteristics | Gestational age (weeks) | |||||||
|---|---|---|---|---|---|---|---|---|
| 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| n | 4 | 4 | 5 | 5 | 3 | 4 | 4 | 5 |
| Maternal age (y) | 28 | 23 | 27.6 | 22 | 22.7 | 27 | 21.7 | 27 |
| Nulliparous (%) | 25 | 25 | 20 | 60 | 33.3 | 25 | 50 | 20 |
| Race/Ethnicity (%) | ||||||||
| Caucasian | 100 | 100 | 80 | 100 | 33.3 | 75 | 75 | 60 |
| African American | - | - | 20 | - | 33.3 | - | - | - |
| Hispanic | - | - | - | - | - | 25 | 25 | 40 |
| Other | - | - | - | - | 33.3 | - | - | - |
| Body Mass Index (kg/m2) | 22 | 26.3 | 29.6 | 26.8 | 30.3 | 24.3 | 29.5 | 32.4 |
| Smoking (%) | ||||||||
| None | 50 | - | - | 20 | 66.7 | 50 | 75 | 40 |
| Current | 25 | 75 | 80 | 60 | 33.3 | 50 | - | - |
| Former | 25 | 25 | 20 | 20 | - | - | 25 | 60 |
Figure 1.
Contrast-enhanced ultrasound flux rate and variance. (A) Example replenishment kinetic curve demonstrating calculation of flux rate (β) from the slope of the curve (y). (B) Flux rate (β value in sec-1) plotted over 6–13 weeks of human gestation (mean + SEM). The number of participants is represented by the number overlay in each bar. The variance between measures of flux rate within (C) individual data acquisition replicates and (D) in two different anatomical orientations.
In situ placental histopathology: the Boyd collection
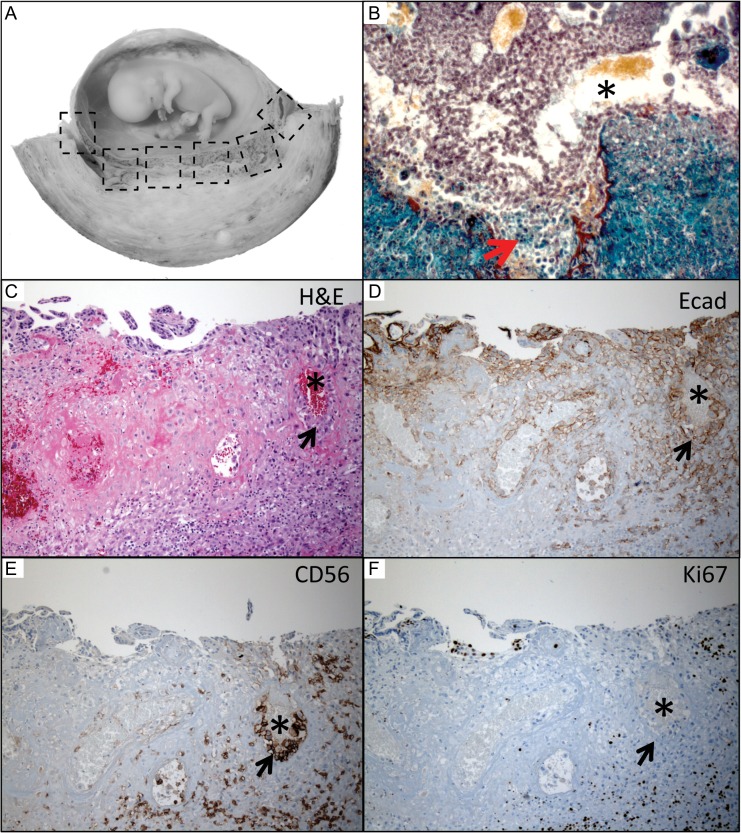
The majority of the in situ placental specimens of the Boyd Collection (Fig. 2 A, Table II) were fixed by immersion of the intact uterus in formalin. Excised blocks were secondarily fixed in formalin or Bouin's (e.g. case H916). One specimen (case H653) was perfused by the uterine artery with india ink, which may have affected spiral artery plug morphology and/or the presence or absence of maternal RBCs in the IVS. The width of decidua basalis viewed ranged from 1.5 to 7.0 cm (gestational ages 6–13 weeks based on CRL). These paraffin blocks had been previously serially sectioned, numbered and stained with hematoxylin and eosin, or Masson's trichrome.
Figure 2.
Characterization of channels within the spiral artery plugs. (A) En bloc sections from the Boyd Collection (gross example is from 10 weeks’ gestation) were available for serial section analysis revealing well-formed channels (*) through loosely cohesive endovascular trophoblast cells (B), which trichrome staining suggested may be related to cell death at the maternal arterial blood interface (arrow). (C) Elective termination specimens were employed for immunophenotyping (H&E: hematoxylin and eosin). (D) EVT cells expressed less E-cadherin (cell adhesion marker) than the strong staining seen in villous cytotrophoblast cells (top left). (E) The endovascular plugs had their own specific CD56 (cell adhesion marker: also stains uterine natural killer cells) staining pattern, which was absent in all other types of trophoblast. (F) Although the overlying anchoring villi were proliferating, the cells making up the spiral artery plugs were Ki-67 (proliferation marker) negative. Figure 2B was photographed using a 20× objective (~200×); C–F were photographed using a 10× objective (~100× magnification).
Table II.
Placental in Situ Pathology Specimens: Hysterectomies and Elective Termination Decidua Basalis in Paraffin.
| Specimens | Gestational age (CRL) | Number of arteries examined | Comments |
|---|---|---|---|
| Boyd collection | |||
| H710 | 6 weeks (4.0 mm) | 3 traced through serial sections | Loose plugs, no channels in 3/3 arteries |
| H750 | 7 weeks (10.0 mm) | 5 traced through serial sections | Loose plugs, 3/5 arteries with channels |
| H937 | 8 weeks (15.0 mm) | 4 traced through serial sections | Plugs breaking down, widening channels |
| H673 | 8 weeks (15.0 mm) | 7 traced through serial sections | Best example of channels opening plugs |
| H916 | 9 weeks (20.0 mm) | 4 traced through serial sections | Proximal spiral arteries now remodeling |
| H1029 | 9 weeks (20.0 mm) | 4 traced through serial sections | One of the distal radial arteries more dilated |
| H630 | 10 weeks (30.0 mm) | 5 traced through serial sections | EVTs seen around distal radial arteries |
| H653 | 11 weeks (46.0 mm) | 3 traced through serial sections | (India ink injected); distal arcuate dilated |
| H870 | 12 weeks (55.0 mm) | 3 traced through serial sections | Partial ‘plugs’ still seen with clear channels |
| H691 | 12.5 weeks (60.0 mm) | 5 traced through serial sections | Partial ‘plugs’ still seen with clear channels |
| H1094 | 13 weeks (73.0 mm) | 4 traced through serial sections | Partial ‘plugs’ still seen, but only in 2/4 |
| OHSU collection | |||
| Case 1 | 6.5 weeks (6.0 mm) | 2 cross-sections basalis surface | Loose plugs, no channels seen (EM study) |
| Case 2 | 7 weeks (11.0 mm) | 6 cross-sections basalis surface | 5/6 loose plugs with channels (EM study) |
| Case 3 | 7 weeks (10.0 mm) | 4 cross-sections basalis surface | 2/4 loose plugs with channels (IHC study) |
| Case 4 | 7.5 weeks (13.0 mm) | 2 cross-sections basalis surface | 2/2 loose plugs with channels (IHC study) |
| Case 5 | 8 weeks (14.0 mm) | 3 cross-sections basalis surface | 3/3 loose plugs with channels (EM study) |
| Case 6 | 8 weeks (16.0 mm) | 8 cross-sections basalis surface | 5/8 loose plugs with channels (IHC study) |
| Case 7 | 15 weeks (85.0 mm) | 6 cross-sections in hysterectomy | Partial plugs; proximal radial arteries dilated |
| Case 8 | 32 weeks (260.0 mm) | 3 cross-sections in hysterectomy | No plugs; arcuate arteries completely dilated |
| Cases 9–12 | Postmenopausal G0 | 4–7 cross-sections in hysterectomy | Arcuate arteries small and unremodeled |
| Cases 13–16 | Postmenopausal G2-5 | 2–6 cross-sections in hysterectomy | Radials and arcuates dilated and remodeled |
Boyd Collection is housed within the Center for Trophoblast Research, Cambridge, UK; it is a collection of serial sections through paraffin blocks taken from the entire uteroplacental interface from a series of hysterectomies performed in the 1950s. Archived decidua basalis paraffin blocks from primigravida elective terminations and four hysterectomy specimens were obtained from Oregon Health & Science University (OHSU).
CRL, crown-rump length; EVT, extravillous trophoblasts; EM, electron microscopy; IHC, immunohistochemistry.
All histological assessments were performed by one pathologist (T.K.M.). By reviewing the entire sequence of serial sections through the placental bed, spiral arteries were traced from their openings distally through the decidua basalis proximally and then into their upstream radial and arcuate arteries. Artery segments (spiral, radial, arcuate) were scored for the presence of placental trophoblast invasion, obstruction of the vascular lumen (plug), degree of plugging (complete, channels, partial, absence of plugs), and the relative dilation of the lumen (± >2-fold increase in diameter compared with unremodeled vessels in a nulligravida hysterectomy specimens).
Spiral artery plug ultrastructural and immunohistochemical analysis
Three nulligravida post-hysterectomy and five additional hysterectomies (two primigravida with placentas in situ and three multiparous postmenopausal cases) were obtained from the archives of the Department of Pathology, Oregon Health & Science University (OHSU, Table II). The OHSU archives also provided six first trimester decidua basalis specimens from primigravida women (age: 22–33 years) having an elective termination at 6–8 weeks gestation (confirmed by CRL). Histologic sections of these OHSU specimens were similarly scored, although decidua basalis from terminations only provided cross-sections of spiral arteries.
Decidua basalis specimens from elective terminations (at OHSU) were used to identify completely plugged and cannulated plugged spiral artery cross-sections. Serial sections were obtained for immunohistochemical studies; these spiral artery targets were then mapped onto the accompanying paraffin block and cored using a 14-gauge needle. The cores were deparaffinized, fixed in 2.5% glutaraldehyde and processed by the Electron Microscopy facility (OHSU). Complete plugs were analyzed for the presence or absence of tight junctions (endothelial cells served as positive controls), desmosomes, apoptosis (nuclear membrane integrity) and necrosis (cytoplasmic vacuolization). Serial sections were stained for pancytokeratin (epithelial marker), E-cadherin (cell adhesion marker), Ki-67 (proliferation marker), CD31 (endothelial marker), CD3 (T-cell marker) and CD56 (cell adhesion marker [also stains uterine natural killer cells]). Slides were scored for the presence or absence of staining compared with internal tissue controls within each decidual basalis histologic section. Patterns were assessed for reproducibility within and between multiple sections per case and between cases.
Statistical analysis
CEUS parameters were compared across gestation using an ANOVA with a Dunnett's multiple comparison post hoc test, and within data acquisition replicates by linear regression (PRISM software, version 7.01; GraphPad Software, Inc. La Jolla, CA, USA). A value of P < 0.05 was considered significant.
Ethical approval
The CEUS protocol was approved by the OHSU Institutional Review Board (IRB#10744) with written informed consent obtained from all participants.
Results
Placental perfusion in the first trimester
Using CEUS we were able to assess maternal blood flow into the IVS in samples from 6+3–13+6 weeks of gestation. Figure 1B shows the flux rate, a measure of vascular impedance, across this gestational age range. These data demonstrate maternal perfusion through the spiral arteries as early as 6 weeks and more clearly from 8 weeks onwards (Supplementary movie). Within the 6+3–7+6 week age range, we observed wide variability in flux rates from 0.041 to 0.125 s-1. Surprisingly, we do not demonstrate a progressive increase in microvascular flux rate with increasing gestational age across 6–13 weeks (Fig. 1B). However, when comparing the change in microvascular flux rate relative to 6 weeks, there is a significant increase at 13 weeks (1 versus 1.88 P < 0.05, one way ANOVA with Dunnett's post hoc test). We analyzed the variability within data acquisition replicates in one field of view (Fig. 1C) and between whole placenta perfusion data acquired from coronal and sagittal orientations (Fig. 1D). Replicates were highly correlated (P < 0.0001).
Channels in spiral artery trophoblastic plugs
Our objective was to evaluate the Boyd Collection (6–13 weeks gestation, Table II) within the context provided by CEUS data suggesting intervillous flow beginning as early as 6 weeks of gestation. Similar to a thrombus being cannulated by endothelial cells, we observed well-demarcated channels forming within the spiral artery trophoblastic plugs (Fig. 2B). The single 6-week specimen had spiral arteries apparently plugged by loosely cohesive EVTs, but maternal RBCs were also seen intermixed with these ‘plugs’. Vascular channels through the EVT plugs could be traced to the IVS by 7–8 weeks and these channels became more apparent with larger luminal diameters as gestational age increased. Additionally, increasing numbers of maternal RBCs were apparent in the IVS after 7 weeks, consistent with blood flow. Although maternal RBCs could have leaked into the IVS secondary to trauma (reason for hysterectomy), preparation artifacts, or possible retrograde flow through venous connections during handling, the presence of well-formed channels within the plugs suggests they are physiologic. After 8 weeks most of the spiral arteries examined showed only partial EVT obstruction (Fig. 2). These histological findings suggest that the collection of EVTs, termed ‘plugs’, do not completely obstruct flow to the IVS.
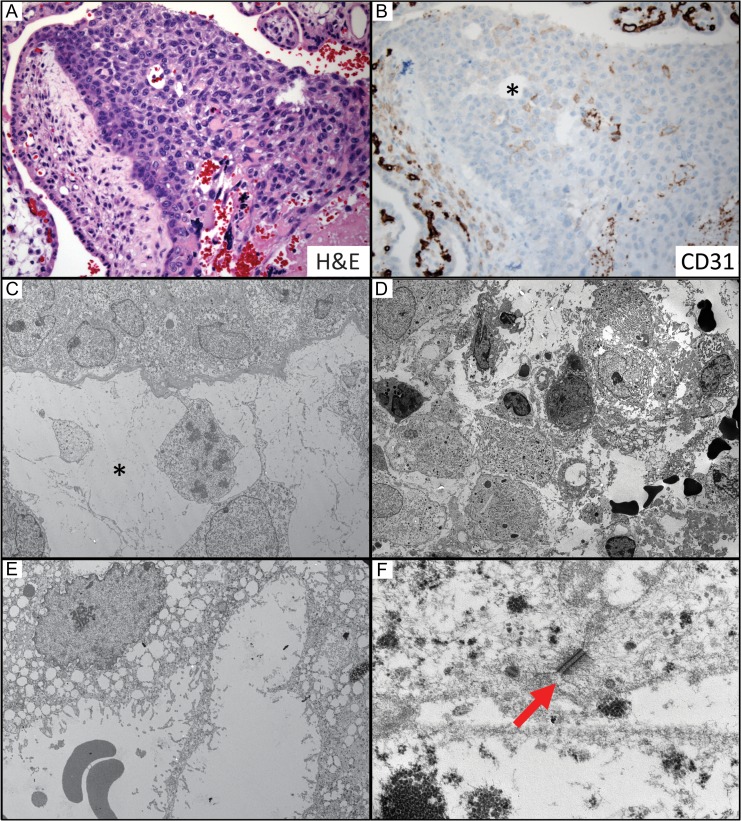
To further characterize the EVT plug channels we immunostained histologic sections and performed ultrastructural analysis. Unlike the cytotrophoblast cells rimming chorionic villi, the EVTs within spiral artery plugs lose E-cadherin expression and gain NCAM (neural cell adhesion molecule, CD56) (Fig. 2E). Interestingly, CD56 is also a marker of uterine natural killer cells, which are thought to contribute to spiral artery remodeling. Importantly, the EVTs in the ‘plugs’ lack mitotic activity and are negative for Ki-67 immunostaining (Fig. 2F). This finding contrasts with the trophoblasts in the anchoring villi, which have numerous conspicuous mitotic figures. Instead, EVT plugging cells appear to be undergoing necrosis as gestation progresses in a distal to proximal fashion (highlighted by the red to blue color change in trichrome stained sections, Fig. 2B). Apoptag staining of DNA strand breaks was negative suggesting that these cells are not undergoing apoptosis (data not shown).
To further investigate the cell adhesion characteristics of the EVTs in the plugs, we performed ultrastructural studies of decidua basalis samples at 6–8 weeks. These studies revealed that EVT plugs are loosely held together by desmosomes, not tight junctions (Fig. 3). Many of the trophoblasts in these plugs also showed evidence of necrosis with cytoplasmic vacuolization, but no nuclear membrane degradation (further data against apoptosis). Taken together, our histological observations of the progressive disintegration of EVT plugs and the formation of vascular channels into the IVS provide a correlation between tissue architecture and our CEUS data suggesting flow into the IVS in the first trimester. However, we do not observe a progressive increase in microvascular flux rate coincident with the progressive disintegration of the spiral artery plugs, which provided the rationale for examining proximal regulation of maternal blood flow to the IVS as a potential regulator of perfusion to the early placenta.
Figure 3.
Analysis of vascular channels through spiral artery plugs. (A) By 8 weeks gestation the channels were well-formed and clearly communicated with the intervillous space. (B) They were lined by trophoblast cells negative for CD31 (endothelial marker, *). (C–F) Electron microscopy showed loosely cohesive cells connected by desmosomes (red arrow) that were undergoing necrotic degeneration forming fibrin-lined channels. A and B were photographed using a 20× objective (~200× magnification). C and D 3000× (note RBCs); E 6000× (note RBCs); F 60 000×.
Pregnancy-induced radial and arcuate artery remodeling
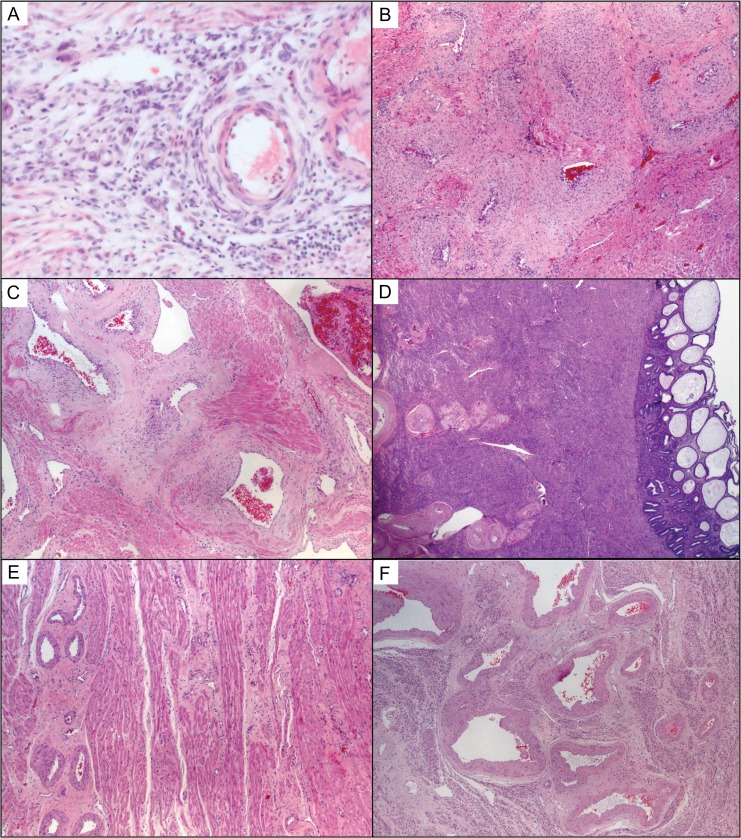
Previous reports of the Boyd Collection did not address the radial and arcuate arteries in the myometrium upstream from the decidual spiral arteries (Burton et al., 1999). Here, we scored these vessels for the presence or absence of luminal dilation compared with nulligravida hysterectomy controls and earlier gestational age specimens (Fig. 4). Our observations suggest that the distal radial arteries intersecting with the decidua basalis may begin dilating around 9 weeks (case H1029) with placental cells tracking along the loose perivascular adventitia by 15 weeks (Fig. 5). The placental cells did not invade the radial artery muscular walls, which were instead infused with CD3-positive T-cells. By 33 weeks the radial arteries are even more dilated than the second trimester cases in the Boyd Collection (Fig. 4C). This suggests a potential temporal sequence in radial vascular remodeling. Moreover, it appears the remodeling changes persist after the completion of pregnancy, because radial remodeling changes are still present in postmenopausal uterine specimens (Fig. 4D) and absent in the nulligravida cases. Similarly, the arcuate arteries upstream from the radial arteries remodel during pregnancy (Fig. 4E compared with 4F). Although, we see clear placental invasion of the radial artery perivascular adventitia in the 15-week case, the arcuate arteries are negative for perivascular trophoblasts in the 33-week specimen. A mechanism other than medial invasion and smooth muscle destruction may regulate radial and arcuate artery pregnancy-induced remodeling.
Figure 4.
Pregnancy-induced remodeling of myometrial radial and arcuate arteries. (A) The radial arteries appeared to begin remodeling at the end of the first trimester associated with perivascular lymphocytes and few EVT cells that track along the adventitia, but they did not invade the media. (B) By 15 weeks the upstream proximal radial arteries showed signs of remodeling that was clearly evident in the 33-week hysterectomy specimen (C) and left permanent fibrin changes seen in multigravida postmenopausal specimens (D). Compared with nulligravida uteri (E), the arcuate arteries also dilated and attenuated (F); this occurred in the absence of trophoblast. All sections stained with H&E and photographs taken using a 5× objective (~50× magnification).
Figure 5.
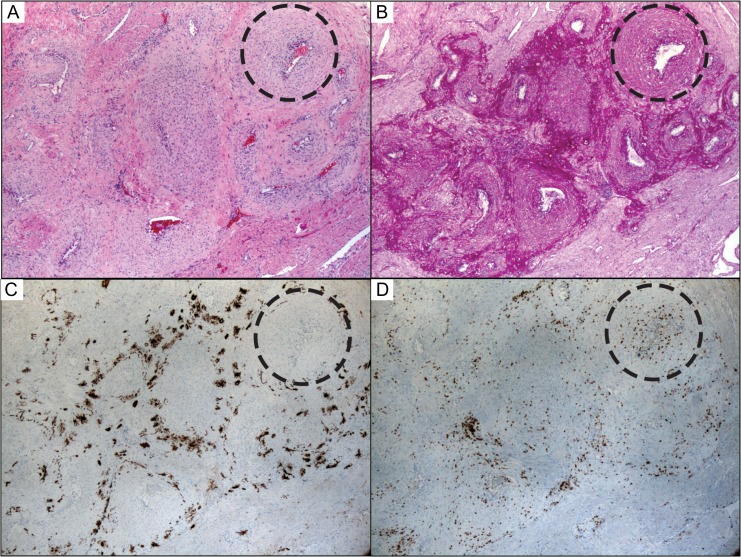
Radial artery remodeling involves perivascular trophoblasts and accumulation of medial CD3 positive and CD3 negative lymphocytes. (A) Remodeling radial artery at 15 weeks gestation by H&E stain showed medial muscular and extracellular matrix changes appreciated best in complete cross-sections (dashed circle). (B) Periodic acid–Schiff stain highlighted pervivascular adentitia that was invaded by cytokeratin positive EVT cells (C) that do not appear to invade the muscular media. (D) Instead, there are numerous lymphocytes, including CD3-positive T-cells in the walls of these remodeling arteries. Photographs taken using a 5× objective (~50× magnification).
Discussion
Our comprehensive in situ histological analysis, in combination with the use of an in vivo imaging modality that has the sensitivity, and higher resolution, to facilitate visualization of microvascular filling of the IVS, has allowed us to reveal new evidence of first trimester flow earlier in pregnancy than previously reported. Our novel CEUS data unequivocally demonstrate maternal blood flow in the IVS at 6–7 weeks of gestation, and are supported by morphological data that demonstrate the progressive disintegration of trophoblast plugs from 7 weeks onwards.
Maternal blood flow to the IVS has been addressed in several prior imaging studies mainly utilizing Doppler ultrasound with some evidence of blood flow as early as 5–7 weeks (Merce et al., 1996; Kurjak and Kupesic, 1998; Makikallio et al., 2004b), others suggesting absence of flow prior to 8 (Valentin et al., 1996; Makikallio et al., 1999, 2004a; Carbillon et al., 2005) or 10 weeks (Jauniaux et al., 2005) and general agreement that continuous flow is established by 12 weeks of gestation (Hustin et al., 1988; Jauniaux et al., 1991, 1992; Coppens et al., 1996). Yet uncertainty remains, and while flow has been demonstrated early, at best it is conceded that this is limited to slow, non-continuous flow prior to the end of 8 weeks of gestation (Burton et al., 1999). Additionally, it has been suggested that early flow is indicative of pregnancy failure, as correlations have been made that support this notion (Jaffe, 2001; Jauniaux et al., 2003; Merce et al., 2009). However, the sensitivity and resolution of the ultrasound system must be considered when interpreting these conflicting data sets. Pregnancies that are destined for spontaneous demise may indeed have higher flow but it is possible that the increased flow may be within the limits of detection of current Doppler capabilities, but does not confirm that early flow is not present in successful pregnancies, which may have lower, undetectable flow rates. Specifically, previous Doppler studies emphasizing no perfusion to the early placenta may have been impaired by limitations and error generated when attempting to quantify low velocity perfusion in small vessels. Using a contrast agent enhanced our ability to track vascular filling of the placenta. Importantly, in our study we were able to carefully observe microbubble replenishment post destruction and there was no evidence to suggest impeded spiral artery refilling, or obstructed flow to the IVS. Flow appears to be continuous and evenly distributed as opposed to slowly meandering around obstructive plugs. Using the CEUS analysis parameters, it is possible to calculate an estimate of flow within a vessel (Keator et al., 2011), however; we have limited our data presentation to microvascular flux rate as the placenta has a complex vascular network, and absolute blood volume and flow calculations are derived using equations and assumptions that are dependent on a standard capillary network. However, the entry of a contrast agent with similar rheology to RBCs, as detected by replenishment kinetic curves, is in itself strong evidence of the presence of flow at earlier gestational ages than previously assumed.
Despite inconsistencies in previously reported Doppler ultrasound studies attempting to determine if blood flow to the early human placenta was present, previous analysis of the Boyd Collection suggested the presence of channels in the spiral artery plugs as early as 7 weeks (Burton et al., 1999). Of interest, by the eighth week of pregnancy these channels were formed within the spiral artery plugs and overlying anchoring villi that communicated with the IVS and were measured to be 10–20 μm in diameter and by 9–10 weeks there were well defined channels (~100 μm in diameter) into the IVS, clearly suggesting that blood flow to the early placenta could be possible. The unequivocal demonstration of contrast agent in the IVS at 6–7 weeks of gestation identified by CEUS motivated our re-examination of the Boyd Collection. Our review of this Collection independently supported the observation of vascular channels. In an effort to characterize the temporal sequence of the formation of these channels, we extended these observations with immunohistochemistry and ultrastructural analysis.
Our histologic investigation of in situ preserved specimens from the Boyd Collection demonstrated that the trophoblasts in the loosely cohesive clusters of EVTs, so-called ‘plugs’, are not mitotically active, they are loosely held together by desmosomes, and they provide at least capillary-sized intercellular channels that would permit blood flow by 7 weeks. In contrast to the trophoblasts in the anchoring columns which proliferate and express E-cadherin, the EVTs do not proliferate and change cell surface adhesion markers from E-cadherin to CD56 (Kam et al., 1999). If the identified channels at 6–7 weeks provide capillary-sized pathways for intervillous blood flow, they may explain our microbubble reappearance kinetic data. These channels are reproducibly evident by 7–8 weeks gestation and we are confident they represent an in vivo differentiation rather than an artifact, because the Boyd Collection provides placentas fixed in situ. This approach avoids potential disruptions that may arise in elective termination curettage specimens.
Significantly, we do not observe a progressive increase in microvascular flux rate into the IVS with increasing gestational age, as would be predicted based on our histological assessment of the progressive disintegration of spiral artery plugs. Therefore, we suspect that the significant increase in flux rate at 13 weeks is more likely related to radial artery luminal diameter increases at the end of the first trimester, rather than loss of spiral artery plugs. This idea is supported by our histological analysis of a small cohort from the Boyd Collection, and we suggest that pregnancy-induced remodeling of the upstream vessels in the myometrium may be an important and under-appreciated component.
Spiral artery remodeling has been the most studied pregnancy-induced change in the uterine vascular network probably because of the accessibility of the tissue and the assumption that the spiral artery lumen must be the narrowest in the uterine vascular network. The rate of flow in a blood vessel is proportional to the fourth power of the radius of the vessel. If the spiral arteries are the narrowest lumen in the vascular tree, then dilation and opening of these vessels would be the key to regulating flow rate. In the Boyd Collection, it is clear that spiral arteries are more dilated and attenuated than the radial arteries with the exception of the trophoblastic plugs. The impedance in the conduit progressively disintegrates from 7 to 12 weeks. If flow was entirely dependent on the diameter of the channels within these plugs, we would expect a linear relationship between flow and gestational age. This was not the case. Therefore, we suspect the radial artery luminal diameter may be key to regulating uteroplacental blood flow in the first and early second trimester, although it is accepted that these observations are based on immersion, rather than perfusion-fixed material.
The uterine vascular network is complex and the spiral arteries are only a short segment in the distal-most aspect of this network. The uterine artery leads to the arcuate arteries that track in parallel with the serosal surface. The arcuates then penetrate the myometrium via perpendicular radial arteries, which feed the endometrial/decidual spiral arteries. Spiral arteries are angiogenic and grow and are shed each month with the menstrual cycle. During pregnancy the decidual spiral arteries are the first to dilate in a distal to proximal fashion from the IVS towards the radial arteries. The Boyd Collection illustrates this process clearly and shows dilated spiral arteries intersecting with more narrow unremodeled radial arteries. Others have described a progressive transformation of the uterine vascular network over time (Harris and Ramsey, 1966; Pijnenborg et al., 1980), and our CEUS data suggest to us the upstream radial artery remodeling may be the key to understanding early second trimester changes in uteroplacental flow resistance. In fact, new data from mice suggest radial artery diameter, not spiral artery diameter, may be the key resistance regulatory point for uteroplacental blood flow (Rennie et al., 2016), consistent with this hypothesis. Moreover, our findings suggest that like mice, radial/arcuate remodeling by mechanisms other than direct placental invasion of the vascular wall may be significant contributors to blood flow regulation in early human pregnancy. These findings should spur investigations into proximal vascular remodeling mechanisms and the relation with common obstetric complications such as pre-eclampsia (Ong et al., 2005).
Myometrial vascular remodeling may help explain why complications like pre-eclampsia are more common in a woman's first pregnancy. It is important to emphasize that only the decidual lining is lost after pregnancy and with it, the decidual spiral arteries. The myometrial radial and arcuate arteries remain. The precise timeline of complete radial and arcuate remodeling during pregnancy is yet to be established, but there are certainly changes that continue throughout pregnancy (Harris and Ramsey, 1966; Burchell, 1967) and changes that seem to remain embedded in the muscular walls of these myometrial blood vessels post-pregnancy. In our analysis, the OHSU post-hysterectomy cases provided four nulligravida and four multigravida postmenopausal uterine specimens for review: there were clear differences between radial and arcuate arteries from women who had been pregnant compared with nulligravidas.
Future studies need to investigate the mechanisms regulating myometrial artery remodeling. Similar to decidual spiral artery remodeling in cases of ectopic pregnancies (Craven et al., 1998), our histologic review of the radial arteries and the arcuate arteries suggests to us that the maternal inflammatory T-cell response may play an important role in this process. Although, the second trimester sample size is small, we did not observe placental trophoblast invasion of the vascular media in any of the radial or arcuate artery cross-sections. These hypotheses warrant further study by immunohistochemical analysis of placental in situ hysterectomy specimens and high resolution blood flow imaging analyzes.
Our CEUS cohort included four participants in the 6+3 to 6+6 week gestational age range with low flux rates measured in two women and higher flux in the other two. This variability may indicate that this is a critical time period in the establishment of maternal flow, although this interpretation is speculative at this time and requires further investigation. It is also important to note that gestational dating was determined by fetal biometry and therefore the margin of error in such measurements could be a contributing factor to the observed differences at this gestational age range. A possible future approach would be to implement super resolution ultrasound imaging in combination with microbubble use (Errico et al., 2015). This technology is still under development for placental imaging, but has the potential to provide invaluable flow data both for understanding normal physiology, and for the improved clinical identification and management of pregnancies at-risk for vascular compromise. Specifically, advances in imaging technology may aid our understanding of early fetal development and the pathophysiology of common obstetric problems, which in turn will inform the search for biomarker discovery.
In summary, here we demonstrate maternal blood flow to the placental IVS at 6 weeks of gestation. Although, we cannot comment on the resistance to flow prior to 6 weeks, our data support the onset of maternal flow into the IVS earlier than previously suspected. Our data are correlated with a comprehensive morphological and phenotypic review of spiral artery plugs and the uterine vascular network. The so-called ‘plugs’ are only loosely cohesive at 6 weeks and begin forming clear capillary-sized channels into the IVS by 7 weeks. We suggest there may be a two-step process that involves progressive remodeling of EVT cell clusters, or ‘plugs’, with reduced resistance to intervillous flow, and that this is protected hemodynamically by persistence of radial artery resistance until the end of the first trimester when the radial arteries start to remodel and develop more dilated lumens. We propose that the upstream radial and arcuate arteries may be significant regulators of uteroplacental blood flow, especially by the end of the first trimester.
Supplementary data
Supplementary data is available at Human Reproduction online.
Supplementary Material
Visualization of uteroplacental vascular filling in a human subject at gestational age 8 weeks and 4 days.
Acknowledgements
Thank you to the patients and staff of Planned Parenthood Columbia Willamette for their participation. Thank you to K. Kester, R. Kayton and L. Vecchirelli for their technical assistance with the histology and ultrastructure studies.
Authors’ roles
V.H.J.R. assisted with acquisition, and performed all analysis of the CEUS data, and wrote the manuscript. T.K.M. conceived of and designed the study, performed all histological review, and co-wrote the manuscript. P.B. facilitated and assisted with the CEUS studies, and reviewed the manuscript. M.M. assisted with data acquisition, histological sample processing and analysis, and reviewed the manuscript. G.J.B. facilitated the histological studies and edited the manuscript. J.O.L. acquired the CEUS data and reviewed the manuscript. A.E.F. conceived of and designed the study, acquired the CEUS data and edited the manuscript.
Funding
This project was supported by the Oregon Health and Science University Knight Cardiovascular Institute, Center for Developmental Health and the Struble Foundation.
Conflict of interest
None declared.
References
- Burchell RC. Arterial blood flow into the human intervillous space. Am J Obstet Gynecol 1967;3:303–311. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol 1999;3:718–724. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab 2002;6:2954–2959. [DOI] [PubMed] [Google Scholar]
- Carbillon L, Challier JC, Alouini S, Uzan M, Uzan S. Uteroplacental circulation development: Doppler assessment and clinical importance. Placenta 2001;10:795–799. [DOI] [PubMed] [Google Scholar]
- Carbillon L, Ziol M, Challier JC, Perrot N, Uzan M, Prevot S, Uzan S. Doppler and immunohistochemical evaluation of decidual spiral arteries in early pregnancy. Gynecol Obstet Invest 2005;1:24–28. [DOI] [PubMed] [Google Scholar]
- Coppens M, Loquet P, Kollen M, De Neubourg F, Buytaert P. Longitudinal evaluation of uteroplacental and umbilical blood flow changes in normal early pregnancy. Ultrasound Obstet Gynecol 1996;2:114–121. [DOI] [PubMed] [Google Scholar]
- Craven CM, Morgan T, Ward K. Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta 1998;4:241–252. [DOI] [PubMed] [Google Scholar]
- Errico C, Pierre J, Pezet S, Desailly Y, Lenkei Z, Couture O, Tanter M. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 2015;7579:499–502. [DOI] [PubMed] [Google Scholar]
- Harris J, Ramsey E. The morphology of human uteroplacental vasculature. Contrib Embryol, 1966;38:43–58. [Google Scholar]
- Hustin J, Schaaps J, Lambotte R. Anatomical studies of the uteroplacental vascularization in the first trimester of pregnancy In: Kaufmann P, Miller RK (eds). Trophoblast Research. Placental Vascularization and Blood Flow. New York: Springer, 1988, 49–60. [Google Scholar]
- Jaffe R. Development of early uteroplacental circulation. Early Pregnancy 2001;1:34–35. [PubMed] [Google Scholar]
- Jauniaux E, Greenwold N, Hempstock J, Burton GJ. Comparison of ultrasonographic and Doppler mapping of the intervillous circulation in normal and abnormal early pregnancies. Fertil Steril 2003;1:100–106. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Johns J, Burton GJ. The role of ultrasound imaging in diagnosing and investigating early pregnancy failure. Ultrasound Obstet Gynecol 2005;6:613–624. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Jurkovic D, Campbell S. In vivo investigations of the anatomy and the physiology of early human placental circulations. Ultrasound Obstet Gynecol 1991;6:435–445. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Jurkovic D, Campbell S, Hustin J. Doppler ultrasonographic features of the developing placental circulation: correlation with anatomic findings. Am J Obstet Gynecol 1992;2:585–587. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Watson A, Burton G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am J Obstet Gynecol 2001;5:998–1003. [DOI] [PubMed] [Google Scholar]
- Kam EP, Gardner L, Loke YW, King A. The role of trophoblast in the physiological change in decidual spiral arteries. Hum Reprod 1999;8:2131–2138. [DOI] [PubMed] [Google Scholar]
- Kaufmann BA, Lindner JR. Molecular imaging with targeted contrast ultrasound. Curr Opin Biotechnol 2007;1:11–16. [DOI] [PubMed] [Google Scholar]
- Kaufmann BA, Wei K, Lindner JR. Contrast echocardiography. Curr Probl Cardiol 2007;2:51–96. [DOI] [PubMed] [Google Scholar]
- Keator CS, Lindner JR, Belcik JT, Bishop CV, Slayden OD. Contrast-enhanced ultrasound reveals real-time spatial changes in vascular perfusion during early implantation in the macaque uterus. Fertil Steril 2011;4:1316–1321 e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjak A, Kupesic S. Parallel Doppler assessment of yolk sac and intervillous circulation in normal pregnancy and missed abortion. Placenta 1998;8:619–623. [DOI] [PubMed] [Google Scholar]
- Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr 2002;5:396–403. [DOI] [PubMed] [Google Scholar]
- Makikallio K, Jouppila P, Tekay A. First trimester uterine, placental and yolk sac haemodynamics in pre-eclampsia and preterm labour. Hum Reprod 2004. a;3:729–733. [DOI] [PubMed] [Google Scholar]
- Makikallio K, Tekay A, Jouppila P. Yolk sac and umbilicoplacental hemodynamics during early human embryonic development. Ultrasound Obstet Gynecol 1999;3:175–179. [DOI] [PubMed] [Google Scholar]
- Makikallio K, Tekay A, Jouppila P. Uteroplacental hemodynamics during early human pregnancy: a longitudinal study. Gynecol Obstet Invest 2004. b;1:49–54. [DOI] [PubMed] [Google Scholar]
- Meekins JW, Luckas MJ, Pijnenborg R, McFadyen IR. Histological study of decidual spiral arteries and the presence of maternal erythrocytes in the intervillous space during the first trimester of normal human pregnancy. Placenta 1997;5–6:459–464. [DOI] [PubMed] [Google Scholar]
- Merce LT, Barco MJ, Alcazar JL, Sabatel R, Troyano J. Intervillous and uteroplacental circulation in normal early pregnancy and early pregnancy loss assessed by 3-dimensional power Doppler angiography. Am J Obstet Gynecol 2009;3:315 e1–e8. [DOI] [PubMed] [Google Scholar]
- Merce LT, Barco MJ, Bau S. Color Doppler sonographic assessment of placental circulation in the first trimester of normal pregnancy. J Ultrasound Med 1996;2:135–142. [DOI] [PubMed] [Google Scholar]
- Ong SS, Baker PN, Mayhew TM, Dunn WR. Remodeling of myometrial radial arteries in preeclampsia. Am J Obstet Gynecol 2005;2:572–579. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980;1:3–19. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 2006;9–10:939–958. [DOI] [PubMed] [Google Scholar]
- Rennie MY, Whiteley KJ, Adamson SL, Sled JG. Quantification of gestational changes in the uteroplacental vascular tree reveals vessel specific hemodynamic roles during pregnancy in mice. Biol Reprod 2016;2:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts VH, Lo JO, Salati JA, Lewandowski KS, Lindner JR, Morgan TK, Frias AE. Quantitative assessment of placental perfusion by contrast-enhanced ultrasound in macaques and human subjects. Am J Obstet Gynecol 2016;3:369 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson NA, Nimrod C, De Vermette R, Fournier J. Determination of intervillous flow in early pregnancy. Placenta 1997;4:287–293. [DOI] [PubMed] [Google Scholar]
- Valentin L, Sladkevicius P, Laurini R, Soderberg H, Marsal K. Uteroplacental and luteal circulation in normal first-trimester pregnancies: doppler ultrasonographic and morphologic study. Am J Obstet Gynecol 1996;2:768–775. [DOI] [PubMed] [Google Scholar]
- Whitley GS, Cartwright JE. Cellular and molecular regulation of spiral artery remodelling: lessons from the cardiovascular field. Placenta 2010;6:465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Visualization of uteroplacental vascular filling in a human subject at gestational age 8 weeks and 4 days.