Abstract
Background
Gut microbiota dysbiosis has been linked to obesity-associated chronic inflammation. Microbiota manipulation may therefore affect obesity-related comorbidities. Blueberries are rich in anthocyanins, which have anti-inflammatory properties and may alter the gut microbiota.
Objective
We hypothesized that blueberry supplementation would alter the gut microbiota, reduce systemic inflammation, and improve insulin resistance in high-fat (HF)-diet–fed rats.
Methods
Twenty-four male Wistar rats (260–270 g; n = 8/group) were fed low-fat (LF; 10% fat), HF (45% fat), or HF with 10% by weight blueberry powder (HF_BB) diets for 8 wk. LF rats were fed ad libitum, whereas HF and HF_BB rats were pair-fed with diets matched for fiber and sugar contents. Glucose tolerance, microbiota composition (16S ribosomal RNA sequencing), intestinal integrity [villus height, gene expression of mucin 2 (Muc2) and β-defensin 2 (Defb2)], and inflammation (gene expression of proinflammatory cytokines) were assessed.
Results
Blueberry altered microbiota composition with an increase in Gammaproteobacteria abundance (P < 0.001) compared with LF and HF rats. HF feeding led to an ∼15% decrease in ileal villus height compared with LF rats (P < 0.05), which was restored by blueberry supplementation. Ileal gene expression of Muc2 was ∼150% higher in HF_BB rats compared with HF rats (P < 0.05), with expression in the LF group not being different from that in either the HF or HF_BB groups. Tumor necrosis factor α (Tnfa) and interleukin 1β (Il1b) gene expression in visceral fat was increased by HF feeding when compared with the LF group (by 300% and 500%, respectively; P < 0.05) and normalized by blueberry supplementation. Finally, blueberry improved markers of insulin sensitivity. Hepatic insulin receptor substrate 1 (IRS1) phosphorylation at serine 307:IRS1 ratio was ∼35% higher in HF rats compared with LF rats (P < 0.05) and HF_BB rats.
Conclusion
In HF-diet–fed male rats, blueberry supplementation led to compositional changes in the gut microbiota associated with improvements in systemic inflammation and insulin signaling.
Keywords: blueberry, gut microbiota, intestinal epithelial barrier, inflammation, insulin signaling
Introduction
Obesity has been characterized as a low-grade systemic inflammatory state (1). An increase in visceral adiposity is associated with macrophage infiltration, secretion of proinflammatory cytokines, and decreased insulin sensitivity (1–3). Cytokines, such as TNF-α and IL-1β, have been found to impair insulin signaling by promoting insulin receptor substrate 1 (IRS1) phosphorylation at serine 307 (p-IRS1, Ser307), inhibiting insulin action (3, 4).
There is evidence that obesity-associated inflammation originates, at least partially, from the gastrointestinal tract (5). Previous studies have shown that gut epithelial barrier integrity is impaired in the distal gut in response to high-fat (HF)-diet feeding (6, 7), with altered expression of gut-protecting mucins and defensins (8). The distal gut is home to >1014 bacteria, and impairment in gut permeability in combination with diet-driven microbiota dysbiosis can lead to translocation of bacterial proinflammatory factors such as LPS into the circulation (9, 10). LPS activates the transcription factor NF-κB to promote synthesis of proinflammatory cytokines (11). Chronic infusion of LPS in rodents led to weight gain, adipose tissue inflammation, and insulin resistance (9, 12). In addition, the manipulation of the microbiota composition by using prebiotics or antibiotics restored gut epithelial function and improved metabolic functions, especially insulin sensitivity (13, 14).
There is growing interest in the role of berries in disease prevention. Blueberries are high in anthocyanins, in addition to other polyphenolic compounds (15), and have antioxidant and anti-inflammatory properties (16–18) that may affect disease development. Dietary supplementation with whole blueberry and blueberry polyphenolics reduced biomarkers of oxidative stress (16, 17) as well as inflammatory gene expression (18). Prior et al. (19–21) showed that purified blueberry anthocyanins reduced body weight (BW) and improved glucose tolerance in HF-diet–fed male C57BL/6J mice, whereas whole blueberry powder did not. In contrast, others found that whole blueberry supplementation improved obesity-related insulin sensitivity, even without changes in BW, in HF-diet–fed mice (18) and Zucker Fatty rats (22). Blueberry supplementation also improved insulin sensitivity in obese, insulin-resistant men and women (23).
Blueberries are a source of fermentable fibers (24). In addition, high concentrations of anthocyanins have been found in the distal intestine where they can interact with, and be metabolized by, the gut microbiota (25). Therefore, blueberry may improve obesity-related inflammation via alteration of the gut microbiota composition. Berry extracts have exhibited antimicrobial and antiadhesion properties against pathogenic bacteria (26, 27). In rodents, dietary supplementation with whole blueberry altered microbiota composition (27, 28) and consumption of a wild blueberry powder beverage in men resulted in increases in Bifidobacterium spp. (29).
Blueberry-driven changes in gut microbiota could lead to changes in intestinal SCFAs. The most abundant intestinal SCFAs are acetate, propionate, and butyrate (30). Acetate and propionate have been shown to activate G-protein-coupled receptors (GPRs), such as GPR43, and promote production of gastrointestinal peptides, including glucagon-like peptide 1 (GLP1), a known incretin (31).
Previous studies that investigated whole blueberry supplementation did not examine changes in the gut microbiota in conjunction with effects on inflammation and insulin resistance. We hypothesized that the consumption of blueberry in HF-diet–fed rats would alter gut microbiota composition and reduce intestinal permeability, inflammation, and insulin resistance. To test this hypothesis, we fed rats an HF diet supplemented with 10% blueberry powder and investigated changes in gut microbiota composition, inflammation and glucose homeostasis while controlling for food and dietary fiber intake.
Methods
Animals and diets
Twenty-four male Wistar rats (200–220 g) were procured from Envigo and single-housed in a temperature-controlled room with a 12-h light-dark cycle. Rats were separated into 3 groups (n = 8/group) and fed low-fat (LF; 10% kcal as fat), HF (45% kcal as fat), or HF with 10 g freeze-dried blueberry powder/100 g (HF_BB) diets for 8 wk (Supplemental Tables 1–5). The blueberry powder was provided by the US Highbush Blueberry Council and was a Tifblue/Rubel 50/50 blend with 38.39 mg phenolics/g and 21.34 mg anthocyanins/g. HF and HF_BB diets were matched for sugars and soluble and insoluble fibers and were isocaloric (Research Diets). All of the diets were formulated to meet micronutrient requirements. Selection of the 10% blueberry concentration was based on previously published studies (16, 32). BW and food intake were monitored daily. The LF group was fed ad libitum while food intake was managed to ensure similar intakes between HF and HF_BB rats by pair-feeding the HF group with the HF_BB group. After 8 wk of being fed their respective diets, the rats were feed-deprived for 6 h and killed by using carbon dioxide inhalation. Sacrifice order was evenly distributed between treatment groups over 2 d, and all tissues were removed within 2.5 h after the beginning of the light cycle. Before being killed, a 24-h urine sample was collected and frozen at –80°C to be analyzed for F2-isoprostanes. Blood was collected by cardiac puncture, rested on ice for 15 min, and centrifuged at 1000 × g for 10 min at 4°C for serum collection. The liver, ileum, cecum, colon, and visceral fat pads (mesenteric, retroperitoneal, and epididymal) were collected and weighed; and an adiposity index was determined. Serum and all of the tissues were snap-frozen and stored at –80°C. All animal care procedures were approved by the Institutional Animal Care and Use Committee of the University of Georgia.
Oral-glucose-tolerance test
After 7 wk, rats were feed-deprived for 5 h before oral gavage with a glucose solution (2 g/kg BW by using 20% glucose; Sigma-Aldrich). Glycemia was measured by using a glucometer (Freestyle) before (0 min) and after (15, 30, 60, 90, and 120 min) glucose administration. Blood samples (∼100 μL) were collected at each time point and centrifuged as described above to obtain serum for insulinemia analysis.
Microbiota DNA sequencing
DNA was extracted from cecal contents by using the ZR Fecal DNA MiniPrep per the manufacturer's protocol (Zymo Research). Briefly, fecal contents were lysed by bead beating, and DNA was isolated by using fast-spin columns. DNA was filtered to remove humic acids and polyphenols, and the eluted DNA was sent to the University of California, Davis, Genomic Sequencing Center for sequencing. High-throughput sequencing was performed with Illumina MiSeq paired-end 250 base-pair runs. Amplification was performed on the V4 region of the 16S ribosomal RNA genes via PCR with the use of the following primers: F515 (5′-GTGCCAGCMGCCGCGGTAA-3′) and R806 (5′-GGACTACHVGGGTWTCTAAT-3′). Sequences were subsequently aligned to reference genomes. Bacterial abundance was normalized by log transformation, and multivariate statistical analysis and clustering (principal components analysis) were performed by using the METAGENassist platform (33). Differences in taxa abundance were assessed by using a 1-factor ANOVA (Fisher's post hoc test; METAGENassist). Linear discriminant analysis effect size analysis was performed on log-transformed abundance by using the Galaxy online module to identify discriminant taxa among groups (34).
SCFA analysis
SCFAs were quantified in serum by the Mayo Clinic Metabolomics Core via GC-MS by using previously published methods (35).
Blood analyses
LPS-binding protein (LBP; Biometec) and insulin (Alpco) in serum were measured by ELISA per the manufacturers’ instructions.
Lipid peroxidation markers
Analysis of urinary F2-isoprostanes was performed in the Vanderbilt University Eicosanoid Core Laboratory with GC/negative ion chemical ionization MS, and data were expressed per urinary creatinine. Liver malondialdehyde was measured by using ELISA per the manufacturers’ instructions (Oxford Biomedical Research).
Intestinal morphology
Gastrointestinal tissues were cryosectioned (5 μm; Leica CM1900; Leica Biosystems). Sections were stained with Alcian blue and nuclear fast red (Sigma-Aldrich). Villus height (in micrometers) and the number of goblet cells (per crypt) were measured manually in well-oriented sections (5 measurements/ileal section) by using a light microscope (BX40; Olympus) equipped with a digital camera (DP25; Olympus) and analysis software (DP2-BSW; Olympus).
Liver histology
Livers were cryosectioned (4 μm; Leica CM1900). Sections were stained at the University of Georgia College of Veterinary Medicine's Pathology Laboratory by using Oil Red O with hematoxylin as a counter nuclear stain. Sections were viewed under a light microscope (Nikon Eclipse E400; Nikon) at 200 × magnification. The Oil Red O–positive pixels were determined by using Scion Image (Scion Corporation).
PCR (qPCR)
Gene expression of inflammatory markers was determined in liver, fat, and ileum tissues by using qPCR. Gene expression of gut epithelial function was determined in ileum tissues. mRNA was extracted from liver, ileum, and mesenteric fat tissues by using the RNeasy Mini Kit or the Lipid Tissue Mini Kit (Qiagen) per the manufacturer's instructions and assessed for quantity and purity by using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). cDNAs were synthesized by using the RevertAidFirst Strand cDNA Synthesis Kit (Thermo Fisher Scientific). qPCR was performed on a StepOnePlus real-time PCR system (Thermo Fisher Scientific) by using SYBR Green PCR master mix (Thermo Fisher Scientific) with primers purchased from Integrated DNA Technologies (Supplemental Table 6). Data were analyzed according to the 2–ΔΔCt method (36).
Western blot
The phosphorylation of IRS1 in liver and NF-κB p65 in mesenteric fat was determined by Western blot. Liver proteins were extracted by using lysis buffer (Invitrogen) and protease and phosphatase inhibitors (Roche Diagnostics). Nuclear fraction proteins from mesenteric fat were extracted by using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fischer Scientific). A total of 20 μg (liver) or 30 μg (fat) proteins were loaded in precast Bolt 10% Bis-Tris Mini Gel (Life Technologies) for separation before being transferred to a polyvinylidene difluoride membrane and probed with primary antibodies (1:1000; Cell Signaling Technology): GAPDH, IRS1, phospho-IRS1(Ser307), phospho-NF-κB p65, and total NF-κB p65. IgG and anti-biotin rabbit HRP secondary antibodies (Cell Signaling Technology) were then probed onto the membrane. LiCor WesternSure chemiluminescent substrate was used as a detection agent. Blots were quantified by using a C-DiGit Blot Scanner and Image Studio Software (LiCor).
Statistical analysis
Data are presented as means ± SEMs. Statistical analysis was performed by using Prism software (Prism 6.0; GraphPad Software). Two-factor repeated-measures ANOVA was used to analyze BW, energy intake, and oral-glucose-tolerance test. One-factor ANOVA was performed to analyze data from adiposity, qPCR, Western blot, and biochemical analyses. Differences between groups were analyzed by using Fisher's least-significant-difference test. Correlations between SCFA concentrations and microbiota abundance were determined by using the nonparametric Spearman correlation. Differences were considered significant if P < 0.05.
Results
BW and glucose tolerance
There was no difference in final BW between LF, HF, and HF_BB rats (Figure 1A). As previously reported (12), HF feeding induced a significant increase in energy intake for the first week compared with LF feeding; and in week 2, HF_BB rats continued to have significantly higher intakes than LF rats. This may have been driven by diet palatability. However, there was no difference in intake between groups throughout the rest of the study and no difference in total energy intake between the HF and LF rats (Supplemental Figure 1). Despite no differences in BW, the overall adiposity index was significantly higher in HF and HF_BB rats than in the LF group (P < 0.05; Figure 1B). Although there were no significant differences between groups for mesenteric and epididymal fat depots, retroperitoneal fat pad weight was significantly higher in HF-BB rats than in LF rats (P < 0.05).
FIGURE 1.
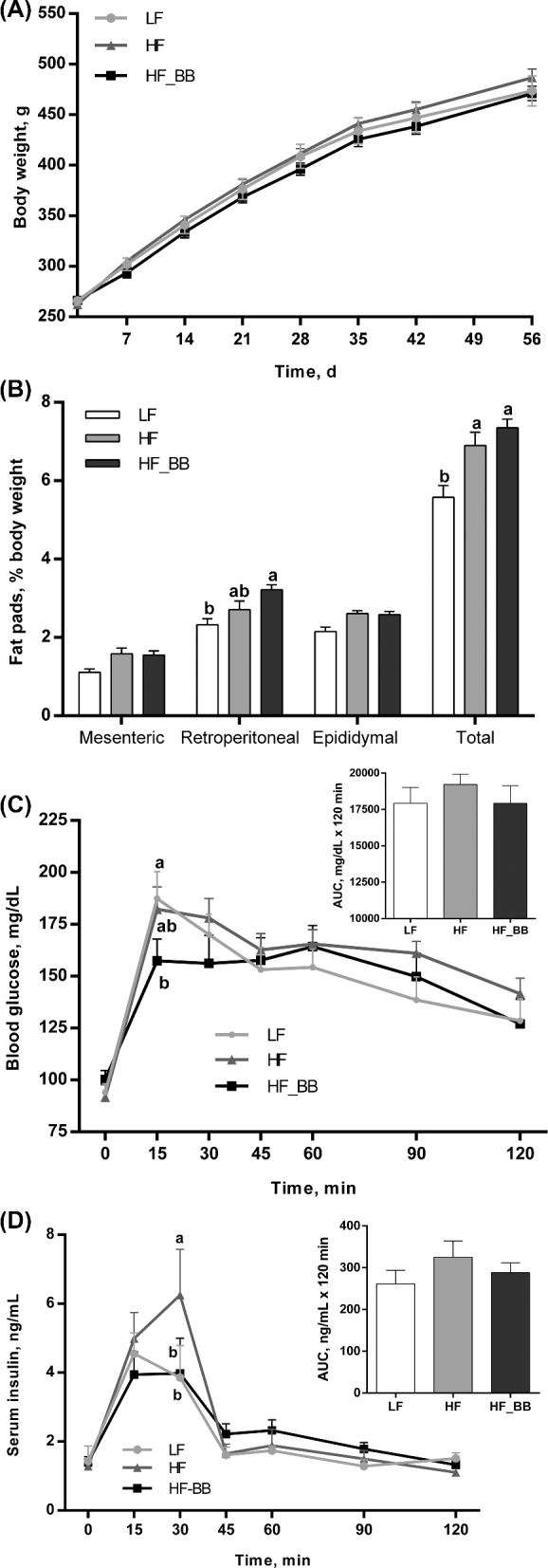
Body weight (A), adiposity index (B), blood glucose (C), and serum insulin (D) during an oral-glucose-tolerance test (2 mg/kg) in rats fed an LF, HF, or HF_BB diet for 8 wk. Values are means ± SEMs, n = 8/group. Labeled means at a time without a common letter differ, P < 0.05. HF, high fat; HF_BB, high fat with 10% blueberry; LF, low fat.
There was no difference in fasting (0 min) glycemia and insulinemia between groups (Figure 1C, D). After an oral-glucose-tolerance test, glycemia increased sharply in the 3 groups and peaked at 15 min postchallenge. Peak glycemia at 15 min for HF_BB rats was lower than in LF and HF rats, although comparison with the HF group failed to reach significance (P = 0.07). There were no significant differences in AUCs between groups. Insulin concentrations peaked 15 min post–oral-glucose challenge in the LF and HF_BB groups, but peaked at 30 min in HF rats and at that time was significantly higher than in the LF and HF_BB groups (P < 0.05). There was a 30% reduction in peak insulin in the HF_BB rats compared with the HF rats.
Microbiota composition and metabolites
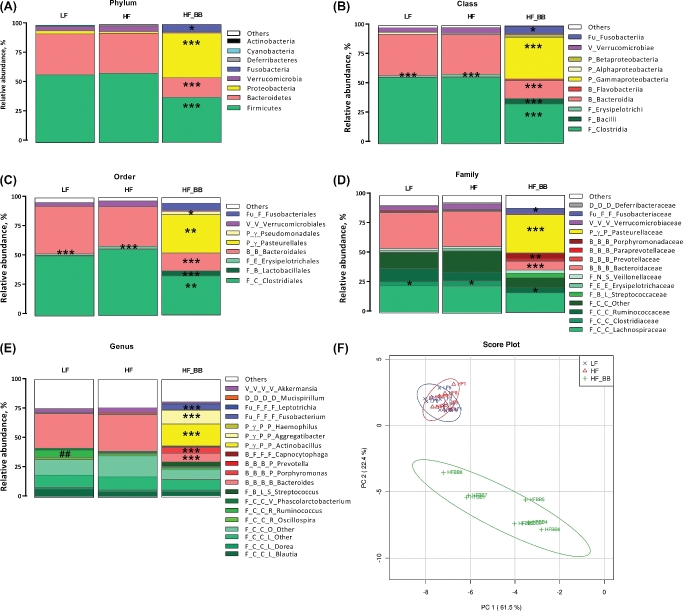
HF consumption alone did not have a major effect on microbiota composition. There were no differences in the abundance and ratio of the main phyla Firmicutes and Bacteriodetes between the HF and LF rats (Figure 2A). Blueberry supplementation had a much greater effect on microbiota composition. At the phylum level, blueberry supplementation led to significant decreases in both Firmicutes (P < 0.001) and Bacteriodetes (P < 0.001) abundance and significant increases in Proteobacteria (P < 0.001) and Fusobacteria (P < 0.05) abundance compared with HF and LF rats. Proteobacteria represented 37.99% of the identified bacteria in the HF_BB group compared with 2.17% in the LF rats and 1.56% in the HF rats.
FIGURE 2.
Microbial composition of rats fed an LF, HF, or HF_BB diet for 8 wk. The bacterial relative abundances at the phylum (A), class (B), order (C), family (D), and genus (E) levels and principal components analysis on all taxonomic levels (F) are shown. n = 8. *,**,***Differences between the HF_BB group and the LF and HF groups: *P < 0.05, **P < 0.01, ***P < 0.001. ##Differences between the LF and HF groups, P < 0.01. Bacteria in any shade of green belong to the Firmicutes phylum, shades of red indicate Bacteriodetes, yellow indicates Proteobacteria, purple indicates Verrucomicrobia, and blue indicates Fusobacteria. The phylogenic tree (phylum, class, order, and family in order) is indicated by the letters preceding the taxa. Phylum: F, Firmicutes; B, Bacteriodetes; Fu, Fusobacteria; D, Deferribacteres; V, Verrucomicrobia; P, Proteobacteria. Class: C, Clostridia; B, Bacilli; N, Negativictes; E, Erysipelotrichia; B, Bacteriodetes; F, Flavobacteria; γ, Gammaproteobacteria. Order: C, Clostridiales; L, Lactobacillales; S, Selenomonadales; E, Erysipelotrichiales; B, Bacteriales; F, Flavobacteriales; F, Fusobacteriales; D, Deferribacteriales; V, Verrucomicrobiales; P, Pasteurellales. Family: C, Clostridiaceae; R, Ruminococcaceae; S, Streptococcaceae; V, Veillonellaceae; P, Prevetollaceae; F, Flavobacteriaceae; V, Verrucomicrobiaceae; P, Pasteurellaceae. HF, high fat; HF_BB, high fat with 10% blueberry; LF, low fat; PC, principal component.
Abundance analysis at all taxonomic levels showed that microbiota composition in LF and HF rats was very similar, apart from a significant decrease in Ruminococcus (genus, P < 0.01) in HF rats when compared with the LF group (P < 0.01) (Figure 2E). The blueberry effect on Proteobacteria was driven by an increase in Gammaproteobacteria (class, P < 0.001), especially the Pasteurellales order (P < 0.01), including the genera Actinobacillus (P < 0.001) and Aggregatibacter (P < 0.001). The blueberry supplementation–induced increase in Fusobacteria abundance was driven by an elevation in Fusobacteriaceae (family; P < 0.05). Despite an overall decrease in Firmicutes, blueberry supplementation led to increased abundance of Bacilli (class), especially Lactobacillales (order; P < 0.001). Similarly, despite an overall decrease in Bacteriodetes, HF_BB rats showed a significant increase in Porphyromonadaceae (family; P < 0.01) abundance when compared with both LF and HF rats. Principal components analysis on all taxonomic levels showed that LF and HF rats had overall a very similar microbiota profile, whereas blueberry supplementation resulted in a radically different profile (Figure 2F).
Blueberry supplementation was associated with significant changes in serum SCFAs. Acetate was significantly elevated in the HF_BB group compared with the LF and HF groups (P < 0.05; Figure 3A). HF_BB rats had significantly higher concentrations of propionate than the LF group (P < 0.01) but were not different from the HF group. Finally, butyrate concentrations were significantly lower in HF_BB rats compared with the LF group (P < 0.05) but were not different from the HF group. There were no significant differences in serum acetate, propionate, or butyrate between the LF and HF rats. Correlation analysis showed significant positive relations between serum acetate and Proteobacteria (r = 0.42, P < 0.05), Gammaproteobacteria (r = 0.5, P < 0.05), Pasteurellales (r = 0.46, P < 0.05), Actinobacillus (r = 0.59, P < 0.01), and Aggregatibacter (r = 0.46, P < 0.05) abundance. Serum acetate was negatively correlated with Bacteroidetes (r = −0.61, P < 0.01) and positively correlated with Bacilli (r = 0.46, P < 0.05) and Lactobacillales (r = 0.55, P < 0.01) abundance. Butyrate concentrations tended to be negatively correlated to Fusobacterium (r = −0.38, P = 0.08) abundance.
FIGURE 3.

Serum SCFAs (A) and gene expression of Gpr43 and Glp1 in the ileum (B) of rats fed an LF, HF, or HF_BB diet for 8 wk. Values are means ± SEMs, n = 8. Labeled means without a common letter differ, P < 0.05. Glp1, glucagon-like peptide 1; Gpr43, G-protein-coupled receptor 43; HF, high fat; HF_BB, high fat with 10% blueberry; LF, low fat.
Blueberry supplementation led to a significant 3-fold increase in SCFA-target receptor, Gpr43 gene expression compared with the LF control (P < 0.01; Figure 3B). Gpr43 expression in HF_BB rats was also higher than in the HF group, but this difference did not reach significance (P = 0.1). HF rats had a significant decrease in Glp1 gene expression compared with LF and HF_BB rats (LF compared with HF and HF compared with HF_BB; P < 0.05).
Gastrointestinal barrier integrity and inflammation
HF feeding significantly reduced villus length compared with the LF control group (P < 0.0001; Figure 4A). Blueberry supplementation restored gastrointestinal integrity; ileal villus length in the HF_BB rats was similar to that in the LF rats and significantly higher than in the HF rats (P < 0.0001).
FIGURE 4.
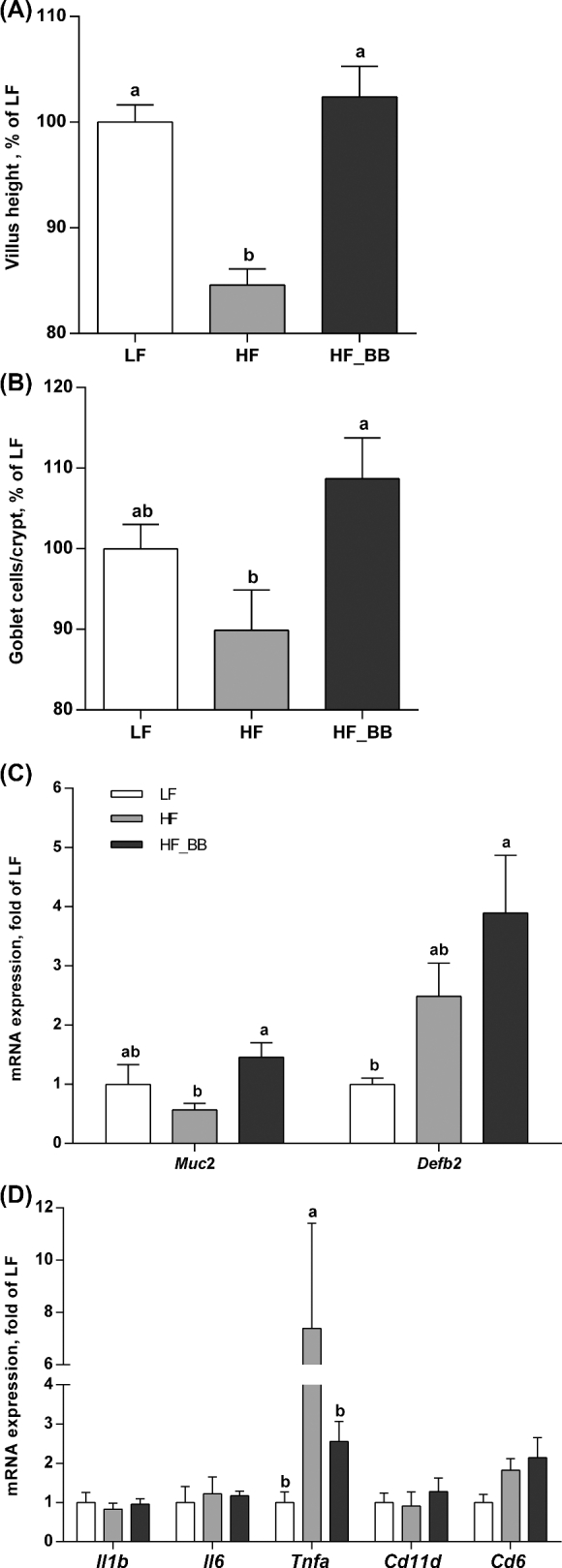
Villus length (A), goblet cells/crypt (B), Defb2 and Muc2 gene expression (C), and gene expression of inflammatory markers (D) in the ileum of rats fed an LF, HF, or HF_BB diet for 8 wk. Values are means ± SEMs, n = 8. Labeled means without a common letter differ, P < 0.05. Cd11d, cluster of differentiation 11d; Cd68, cluster of differentiation 68; Defb2, β-defensin 2; HF, high fat; HF_BB, high fat with 10% blueberry; Il1b, interleukin 1β; Il6, interleukin 6; LF, low fat; Muc2, mucin 2; Tnfa, tumor necrosis factor α.
Goblet cell number per crypt was significantly higher in the HF_BB rats compared with the HF rats (P < 0.05; Figure 4B). Goblet cell number in the LF control group did not differ from either the HF or HF_BB rats. Similarly, gene expression of the mucus protein, Muc2, in the ileum was significantly higher (2.5-fold) in the HF_BB rats than in the HF rats (P < 0.05). Muc2 expression in the LF group did not differ from that in either the HF or HF_BB rats (Figure 4C). HF_BB rats exhibited a significant increase in the antibacterial peptide Defb2 gene expression in the ileum compared with the LF rats (P < 0.05). The level of expression in the HF rats was not different from that in the LF or HF_BB rats.
In the ileum, HF feeding was associated with a significant increase in tumor necrosis factor α (Tnfa) gene expression (HF compared with LF; P < 0.001), which was normalized by blueberry supplementation (Figure 4D). There were no differences in other inflammatory genes assessed, including Il1b and Il6.
LBP and mesenteric fat inflammation
LBP was used as a proxy to assess circulating LPS concentrations. HF_BB rats showed a significant reduction in circulating LBP when compared with the HF rats (P < 0.05; Figure 5A). LBP serum concentrations in LF rats were not different from those in either HF or HF_BB rats. In line with these results, we observed a lower ratio of nuclear phospho- to total NF-κB p65 in the mesenteric fat tissue of the HF_BB group than in the other groups (Figure 5B). The difference was not significant (P = 0.07) between groups, but rats in the HF_BB group all clustered within the ratio of <0.1 (0.01–0.08), whereas the LF and HF groups showed inconsistent patterns of distribution (0.04–0.26).
FIGURE 5.

Circulating LPS (A), NF-κB p65 phosphorylation (B), gene expression of inflammatory markers (C), and gene expression of Ppara and Ppard (D) in adipose tissue of rats fed an LF, HF, or HF_BB diet for 8 wk. Values are means ± SEMs; n = 8, except for (B), n = 4–6. Labeled means without a common letter differ, P < 0.05. Cd11d, cluster of differentiation 11d; Cd68, cluster of differentiation 68; HF, high fat; HF_BB, high fat with 10% blueberry; Il1b, interleukin 1β; Il6, interleukin 6; LBP, LPS-binding protein; LF, low fat; Ppara, peroxisome proliferator-activated receptor α; Ppard, peroxisome proliferator-activated receptor δ; Tnfa, tumor necrosis factor α.
HF feeding significantly upregulated gene expressions of Il1b (HF compared with LF; P < 0.001) and Tnfa (HF compared with LF; P < 0.05) in mesenteric fat tissue, which were significantly downregulated to LF control levels by blueberry supplementation (Figure 5C). In addition, cluster of differentiation 11d (Cd11d) expression (a marker of macrophage infiltration) was significantly lower in blueberry-fed rats compared with the LF and HF groups (P < 0.05). Others have reported that blueberry feeding can alter expression of PPAR subtypes and affect lipid metabolism (22). Blueberry supplementation increased Ppara gene expression in mesenteric fat compared with HF rats (HF_BB compared with HF; P < 0.01; Figure 5D), although Ppara gene expression in HF and HF_BB rats was not significantly different than that in LF rats. Ppard expression significantly decreased in HF rats compared with LF rats (P < 0.05), and this was normalized by blueberry treatment (LF compared with HF_BB; P = 0.08; HF compared with HF_BB; P < 0.0001). We did not find any differences in Pparg gene expression (data not shown).
Hepatic measurements
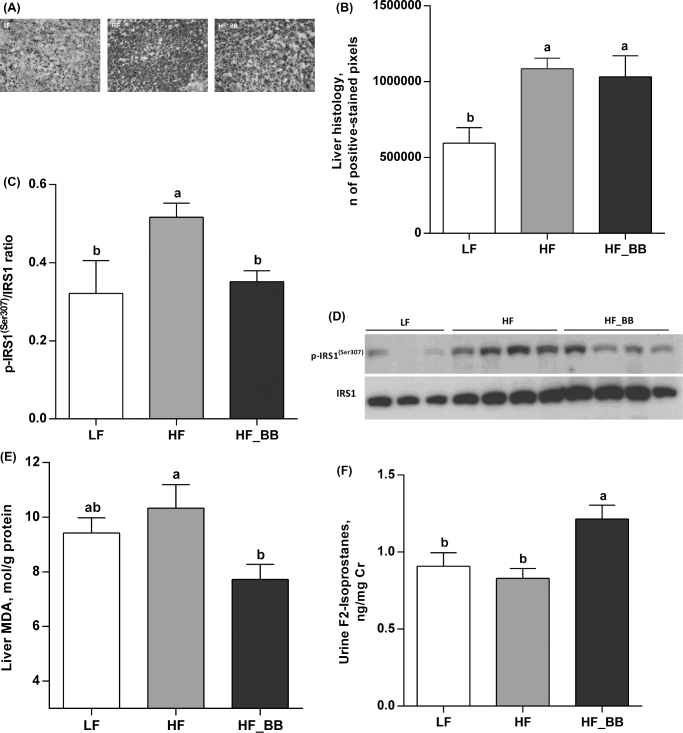
Compared with LF rats, HF feeding led to a significant increase in liver fat droplets (LF compared with HF; P < 0.01; LF compared with HF_BB; P < 0.05; Figure 6A, B), with no significant difference between HF and HF_BB groups. However, the hepatic p-IRS1 (Ser307) to IRS1 ratio was significantly increased in HF rats compared with LF rats and decreased to control concentrations by blueberry treatment (P < 0.05; Figure 6C, D). Hepatic malondialdehyde concentration, a marker of oxidative stress, was significantly reduced in HF_BB rats in comparison with the HF group (P < 0.05; Figure 6E), although neither the HF nor HF_BB groups had higher malondialdehyde than that in the LF group. F2-isoprostanes, a marker of systemic oxidative stress, were elevated in the urine of HF_BB rats relative to LF and HF rats (P < 0.05; Figure 6F).
FIGURE 6.
Histology (A, B), IRS1 phosphorylation (C, D), and MDA (E) in the liver and urinary F2-isoprostanes (F) of rats fed an LF, HF, or HF_BB diet for 8 wk. Values are means ± SEMs, n = 8; except for (A, B), n = 6–8. Labeled means without a common letter differ, P < 0.05. HF, high fat; HF_BB, high fat with 10% blueberry; IRS1, insulin receptor substrate 1; LF, low fat; MDA, malondialdehyde; p-IRS1(Ser307), insulin receptor substrate1 phosphorylation at serine 307.
Discussion
In this study we investigated the potential effects of blueberry on HF-diet–associated metabolic changes. Our hypothesis was that blueberry supplementation would trigger compositional changes in the gut microbiota associated with improved gut epithelial function, decreased systemic inflammation, and improved insulin signaling.
Blueberry supplementation resulted in a unique microbiota profile characterized by a high dominance of Gammaproteobacteria. These changes were associated with increases in villus height, goblet cell proliferation, and gene expression of Muc2 and Defb2 in the ileum, suggesting improvement in gut barrier integrity. Moreover, blueberry treatment suppressed local and systemic inflammation indexes and ameliorated hepatic oxidative stress. Finally, blueberry supplementation improved insulin sensitivity, which may be due to a decrease in hepatic p-IRS1 (Ser307) concentration, a marker of impaired insulin signaling (37), and the upregulation of ileal Glp1 gene expression.
There were no differences in BW and energy intake between HF and LF rats, whereas others found that 8 wk of HF feeding were sufficient to induce hyperphagia in rodents (5, 9). Blueberry supplementation may have reduced energy intake, and the pair-feeding paradigm prevented HF-diet–induced hyperphagia. Furthermore, all of the diets were matched for fiber content, and a recent study in mice (38) suggested that HF-diet–induced hyperphagia was driven by a lack of fiber in the diet. Thus, it is also possible that our diet composition was responsible for the lack of BW difference between the LF and HF groups. We still observed an increase in adiposity in both HF and HF_BB rats compared with the LF group. Adiposity in rats has previously been found to be proportional to dietary fat, regardless of BW (39). Although visceral fat was not reduced, gene expressions of Ppara and Ppard were significantly increased in HF_BB rats compared with HF rats, suggesting higher FA oxidation in the HF_BB group (40).
HF feeding alone had very little effect on microbiota composition; HF and LF rats’ gut microbiota profile was very similar. Noticeable exceptions included an HF-diet–associated decrease in Ruminococcus, which is a genus of the Ruminococcaceae family. Reduced abundance of Ruminococcaceae has previously been reported with HF feeding (41). We did not observe changes previously characterized in obese animals, such as an increase in Firmicutes and a decrease in Bacteroidetes abundance (42). This result may have been due to the similar fiber contents of the LF and HF diets, because fibers modulate gut microbiota composition (43).
Independently of dietary fiber content, the HF_BB diet induced a shift in the gut microbiota composition characterized by a significant decrease in Firmicutes and increases in Fusobacteria and Proteobacteria abundance. Despite an overall decrease in Firmicutes, blueberry supplementation led to increased abundance of Bacilli (class), especially Lactobacillales. Interestingly, blueberry extract has been shown to favor the growth of Lactobacillus spp. (44), suggesting that this effect may be anthocyanin-driven. The increase in Proteobacteria was driven by a dominance of the Gammaproteobacteria class, especially the Pasteurellales order, including the genus Actinobacillus and Aggregatibacter. Proteobacteria has been characterized as the least stable among the major phyla (45). Studies have shown that the relative abundance of Proteobacteria in the human gut transiently increases ≤45% without clinical signs (46), highlighting Proteobacteria’s sensitivity to environmental factors. Despite being traditionally thought to be proinflammatory (47), increases in Proteobacteria, especially Gammaproteobacteria, have been reported in association with metabolic improvements, notably after Roux-en-Y gastric bypass in humans and animals (48, 49). In these studies, similarly to our results, the abundance of Aggregatibacter was significantly increased.
Blueberry-driven changes in the microbiota may have improved gut health, resulting in reduced translocation of bacterial products such as LPS across the epithelial barrier (50). Epithelial barrier integrity is compromised with inflammation (5) and HF feeding (11). An HF diet notably led to increased circulating LPS, which induces the transcription of proinflammatory cytokines via NF-κB activation (11). In this study, we found significantly lower concentrations of serum LBP (a marker of circulating LPS) (51) in the HF_BB group compared with the HF group and reduced NF-κB activation in adipose tissue. Accordingly, Tnfa and IL1b gene expressions were downregulated in mesenteric fat of the HF_BB rats compared with that in the HF rats (12). These results confirmed that blueberry had an anti-inflammatory effect. DeFuria et al. (18) similarly reported that blueberry reduced HF diet–induced increases in Tnfα gene expression in visceral fat of HF-diet–fed mice.
Polyphenols previously have been shown to strengthen the intestinal epithelial barrier by upregulating the gene expression of MUC2 (52), the primary glycoprotein of the gastrointestinal mucus layer (53), and stimulating production of antimicrobial peptides, such as DEFβ2 (54). We found that blueberry supplementation had a positive effect on goblet cell count in HF rats and increased Muc2 and Defb2 gene expression in the ileum. DEFβ2 is upregulated by inflammation or bacterial stimuli (55). Thus, the observed increase in Proteobacteria in the HF_BB group may have acted as a triggering factor. The elevated Defb2 gene expression in HF rats may have resulted from gastrointestinal inflammation.
Blueberry may have improved epithelial barrier function through an increase in bacterial fermentation products, namely SCFAs (56). SCFAs have been shown to stimulate the proliferation and differentiation of enterocytes, ultimately contributing to increases in villus height and goblet cell proliferation (57, 58). Serum concentrations of acetate, propionate, and butyrate are a good proxy for bacterial fermentation (59). We found that blueberry supplementation led to increases in circulating acetate and propionate, while reducing butyrate. The amount and diversity of gut microbiota play a role in SCFA production (59). For example, the cecal concentration of butyrate has been previously correlated with the abundance of several Firmicutes taxa (60), which were low in the HF_BB group. In this study, there was a positive correlation between serum acetate and Proteobacteria taxa, including Gammaproteobacteria (class), Pasteurellales (order), Actinobacillus (genus), and Aggregatibacter (genus), which were primary contributors to the unique microbial composition of HF_BB rats.
Glucose homeostasis was only modestly impaired in HF rats, which could be related to the HF diet's fiber content (37). However, as previously reported (18, 22), blueberry supplementation improved insulin sensitivity in HF-fed rodents. HF_BB rats exhibited lower insulinemia than HF rats, showing that HF_BB rats required less insulin to clear glucose. Enhanced insulin sensitivity may have been due to a reduction in hepatic p-IRS1 (Ser307) concentration, a marker of cytokine-driven insulin resistance (37). HF feeding increased IRS1 Ser307 phosphorylation in the liver, which was normalized by blueberry treatment. Furthermore, HF diets can promote the production of reactive oxygen species (61) and oxidative stress can alter IRS phosphorylation (62, 63). Similarly to previous research with anthocyanins (64), hepatic malondialdehyde, a marker of oxidative stress, was reduced by blueberry supplementation and decreased oxidative stress may have contributed to the normalized IRS1 Ser307 phosphorylation in the HF_BB group.
Another possible mechanism for blueberry-induced changes in insulin sensitivity is through changes in ileal GLP1 expression. GLP1 improves both insulin secretion and sensitivity (65) and has previously been found to be downregulated by HF feeding (66). SCFAs, especially acetate and propionate, have been shown to stimulate GLP1 release via a GPR43-dependent pathway (31). In this study, ileal Gpr43 gene expression was higher in HF_BB rats than in HF rats and, although Glp1 gene expression was significantly decreased by HF feeding, it was restored by blueberry supplementation.
We also quantified urinary F2-isoprostanes as a biomarker of systemic oxidative stress (67). F2-isoprostanes have previously been shown to decrease with the consumption of high-anthocyanin foods (67), but were unaltered with blueberry supplementation in a previous study from our laboratory (19). In contrast to the liver malondialdehyde results, urinary F2-isoprostanes were slightly increased in the HF_BB group compared with both HF and LF groups, suggesting an increase in systemic oxidative stress. This may be related to specific changes in the gut microbiota, although further research would be necessary to confirm this.
There are limitations to this study that deserve consideration. First, we used a rodent model to test our hypothesis and the results cannot be directly extrapolated to humans due to differences in gut microbiota and physiology. Also, we showed the protective effect of blueberry on gut barrier integrity by measuring the concentration of serum LBP as a proxy to the LPS concentration in the circulation. A direct assessment of intestinal tight junction permeability would better confirm the role of blueberry in preserving the intestinal epithelial barrier. Last, although we showed that metabolic improvements with blueberry supplementation were found in association with compositional changes in the gut microbiota, the use of germ-free models would be needed to conclusively show that the gut microbiota is responsible for changes in inflammation and insulin sensitivity.
In conclusion, we show for the first time, to our knowledge, that blueberry-induced reductions in inflammation and insulin resistance in HF-diet–fed rats were found in conjunction with compositional changes in the gut microbiota and improved gut integrity. These changes may have prevented LPS translocation, resulting in reduced systemic inflammation and improved hepatic insulin sensitivity in HF-diet–fed rats. Thus, our study provides further support that blueberry may reduce obesity-related inflammation and insulin resistance.
Supplementary Material
Acknowledgments
We thank Kathie Wickwire for technical assistance. The authors’ responsibilities were as follows—SL, JGF, and CBdLS: designed the research; SL, KIK, RK, and ZIG: conducted the research; SL and KLK: analyzed the data; SL: wrote the manuscript; JGF and CBdLS: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by Agriculture and Food Research Initiative Competitive Grant 2014-67017-21757 from the US Department of AgricultureNational Institute of Food and Agriculture. We acknowledge the University of California, Davis, Host Microbe Systems Biology Core Facility for microbiota sample preparation and sequencing, the Mayo Clinic Metabolomics Core and its supporting grants (U24DK100469 and UL1TR000135) for SCFA analysis, the Vanderbilt University.
Supplemental Tables 1–6 and Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used:
- BW
body weight
- Defb2
β-defensin 2
- GLP1
glucagon-like peptide 1
- GPR
G-protein-coupled receptor
- HF
high fat
- HF_BB
high fat with 10% blueberry
- IRS1
insulin receptor substrate 1
- LBP
LPS-binding protein
- LF
low fat
- Muc2
mucin 2
- p-IRS1 (Ser307)
insulin receptor substrate 1 phosphorylation at serine 307
- Ppar
peroxisome proliferator-activated receptor
- Tnfa
tumor necrosis factor α.
References
- 1. Sam S, Haffner S, Davidson MH, D'Agostino RB Sr., Feinstein S, Kondos G, Perez A, Mazzone T. Relation of abdominal fat depots to systemic markers of inflammation in type 2 diabetes. Diabetes Care 2009;32:932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, Coussieu C, Basdevant A, Bar Hen A, Bedossa P et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 2006;55:1554–61. [DOI] [PubMed] [Google Scholar]
- 3. Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 2007;148:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 2002;277:1531–7. [DOI] [PubMed] [Google Scholar]
- 5. de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 2010;299:G440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, Jobin C, Lund PK. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One 2010;5:e12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamilton MK, Boudry G, Lemay DG, Raybould HE. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am J Physiol Gastrointest Liver Physiol 2015;308:G840–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tomas J, Mulet C, Saffarian A, Cavin JB, Ducroc R, Regnault B, Kun Tan C, Duszka K, Burcelin R, Wahli W et al. High-fat diet modifies the PPAR-gamma pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc Natl Acad Sci USA 2016;113:E5934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–72. [DOI] [PubMed] [Google Scholar]
- 10. Moreira AP, Texeira TFS, Ferreira AB, Peluzio MCG, Alfenas RCG. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr 2012;108:801–9. [DOI] [PubMed] [Google Scholar]
- 11. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 2009;1:a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de La Serre CB, de Lartigue G, Raybould HE. Chronic exposure to low dose bacterial lipopolysaccharide inhibits leptin signaling in vagal afferent neurons. Physiol Behav 2015;139:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 2006;55:1484–90. [DOI] [PubMed] [Google Scholar]
- 14. Carvalho BM, Guadagnini D, Tsukumo DM, Schenka AA, Latuf-Filho P, Vassallo J, Dias JC, Kubota LT, Carvalheira JB, Saad MJ. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia 2012;55:2823–34. [DOI] [PubMed] [Google Scholar]
- 15. Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem 2006;54:4069–75. [DOI] [PubMed] [Google Scholar]
- 16. Dulebohn RV, Yi W, Srivastava A, Akoh CC, Krewer G, Fischer JG. Effects of blueberry (Vaccinium ashei) on DNA damage, lipid peroxidation, and phase II enzyme activities in rats. J Agric Food Chem 2008;56:11700–6. [DOI] [PubMed] [Google Scholar]
- 17. Riso P, Klimis-Zacas D, Del Bo C, Martini D, Campolo J, Vendrame S, Moller P, Loft S, De Maria R, Porrini M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur J Nutr 2013;52:949–61. [DOI] [PubMed] [Google Scholar]
- 18. DeFuria J, Bennett G, Strissel KJ, Perfield JW II, Milbury PE, Greenberg AS, Obin MS. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J Nutr 2009;139:1510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prior RL, Wu X, Gu L, Hager TJ, Hager A, Howard LR. Whole berries versus berry anthocyanins: interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J Agric Food Chem 2008;56:647–53. [DOI] [PubMed] [Google Scholar]
- 20. Prior RL, Wu X, Gu L, Hager T, Hager A, Wilkes S, Howard L. Purified berry anthocyanins but not whole berries normalize lipid parameters in mice fed an obesogenic high fat diet. Mol Nutr Food Res 2009;53:1406–18. [DOI] [PubMed] [Google Scholar]
- 21. Prior RL, Wilkes SE, Rogers TR, Khanal RC, Wu X, Howard LR. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J Agric Food Chem 2010;58:3970–6. [DOI] [PubMed] [Google Scholar]
- 22. Seymour EM, Tanone II, Urcuyo-Llanes DE, Lewis SK, Kirakosyan A, Kondoleon MG, Kaufman PB, Bolling SF. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J Med Food 2011;14:1511–8. [DOI] [PubMed] [Google Scholar]
- 23. Stull AJ, Cash KC, Johnson WD, Champagne CM, Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr 2010;140:1764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin Z, Fischer J, Wicker L. Intermolecular binding of blueberry pectin-rich fractions and anthocyanin. Food Chem 2016;194:986–93. [DOI] [PubMed] [Google Scholar]
- 25. McGhie TK, Stevenson DE. Bioavailability and bioabsorption of anthocyanins. In: Wallace TC, Giusti MM, eds. Anthocyanins in health and disease. Boca Raton (FL): CRC Press; 2014. p. 91–113. [Google Scholar]
- 26. Puupponen-Pimia R, Nohynek L, Alakomi HL, Oksman-Caldentey KM. The action of berry phenolics against human intestinal pathogens. Biofactors 2005;23:243–51. [DOI] [PubMed] [Google Scholar]
- 27. Lacombe A, Li RW, Klimis-Zacas D, Kristo AS, Tadepalli S, Krauss E, Young R, Wu VC. Lowbush wild blueberries have the potential to modify gut microbiota and xenobiotic metabolism in the rat colon. PLoS One 2013;8:e67497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paturi G, Mandimika T, Butts CA, Zhu S, Roy NC, McNabb WC, Ansell J. Influence of dietary blueberry and broccoli on cecal microbiota activity and colon morphology in mdr1a(-/-) mice, a model of inflammatory bowel diseases. Nutrition 2012;28:324–30. [DOI] [PubMed] [Google Scholar]
- 29. Vendrame S, Guglielmetti S, Riso P, Arioli S, Klimis-Zacas D, Porrini M. Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J Agric Food Chem 2011;59:12815–20. [DOI] [PubMed] [Google Scholar]
- 30. Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987;28:1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012;61:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xie C, Kang J, Chen JR, Nagarajan S, Badger TM, Wu X. Phenolic acids are in vivo atheroprotective compounds appearing in the serum of rats after blueberry consumption. J Agric Food Chem 2011;59:10381–7. [DOI] [PubMed] [Google Scholar]
- 33. Arndt D, Xia J, Liu Y, Zhou Y, Guo AC, Cruz JA, Sinelnikov I, Budwill K, Nesbo CL, Wishart DS. METAGENassist: a comprehensive web server for comparative metagenomics. Nucleic Acids Res 2012;40:W88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol 2011;12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moreau NM, Goupry SM, Antignac JP, Monteau FJ, Le Bizec BJ, Champ MM, Martin LJ, Dumon HJ. Simultaneous measurement of plasma concentrations and 13C-enrichment of short-chain fatty acids, lactic acid and ketone bodies by gas chromatography coupled to mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2003;784:395–403. [DOI] [PubMed] [Google Scholar]
- 36. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 37. Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest 2001;107:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chassaing B, Miles-Brown J, Pellizzon M, Ulman E, Ricci M, Zhang L, Patterson AD, Vijay-Kumar M, Gewirtz AT. Lack of soluble fiber drives diet-induced adiposity in mice. Am J Physiol Gastrointest Liver Physiol 2015;309:G528–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boozer CN, Schoenbach G, Atkinson RL. Dietary fat and adiposity: a dose-response relationship in adult male rats fed isocalorically. Am J Physiol 1995;268:E546–50. [DOI] [PubMed] [Google Scholar]
- 40. Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta 2011;1812:1007–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Daniel H, Gholami AM, Berry D, Desmarchelier C, Hahne H, Loh G, Mondot S, Lepage P, Rothballer M, Walker A et al. High-fat diet alters gut microbiota physiology in mice. ISME J 2014;8:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 2005;102:11070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fak F, Jakobsdottir G, Kulcinskaja E, Marungruang N, Matziouridou C, Nilsson U, Stalbrand H, Nyman M. The physico-chemical properties of dietary fibre determine metabolic responses, short-chain Fatty Acid profiles and gut microbiota composition in rats fed low- and high-fat diets. PLoS One 2015;10:e0127252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hidalgo M, Oruna-Concha MJ, Kolida S, Walton GE, Kallithraka S, Spencer JP, de Pascual-Teresa S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J Agric Food Chem 2012;60:3882–90. [DOI] [PubMed] [Google Scholar]
- 45. Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL et al. The long-term stability of the human gut microbiota. Science 2013;341:1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Caporaso JG, Lauber CL, Costello EK, Berg-Lyons D, Gonzalez A, Stombaugh J, Knights D, Gajer P, Ravel J, Fierer N et al. Moving pictures of the human microbiome. Genome Biol 2011;12:R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 2012;13:R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shao Y, Ding R, Xu B, Hua R, Shen Q, He K, Yao Q. Alterations of gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in Sprague-Dawley rats. Obes Surg 2017;27:295–302. [DOI] [PubMed] [Google Scholar]
- 49. Graessler J, Qin Y, Zhong H, Zhang J, Licinio J, Wong ML, Xu A, Chavakis T, Bornstein AB, Ehrhart-Bornstein M et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J 2013;13:514–22. [DOI] [PubMed] [Google Scholar]
- 50. Osman N, Adawi D, Ahrné S, Jeppsson B, Molin G. Probiotics and blueberry attenuate the severity of dextran sulfate sodium (DSS)-induced colitis. Dig Dis Sci 2008;53:2464–73. [DOI] [PubMed] [Google Scholar]
- 51. Moreno-Navarrete JM, Ortega F, Serino M, Luche E, Waget A, Pardo G, Salvador J, Ricart W, Fruhbeck G, Burcelin R et al. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes (Lond) 2012;36:1442–9. [DOI] [PubMed] [Google Scholar]
- 52. Paturi G, Butts CA, Bentley-Hewitt KL, Ansell J. Influence of green and gold kiwifruit on indices of large bowel function in healthy rats. J Food Sci 2014;79:H1611–20. [DOI] [PubMed] [Google Scholar]
- 53. Johansson ME, Ambort D, Pelaseyed T, Schutte A, Gustafsson JK, Ermund A, Subramani DB, Holmen-Larsson JM, Thomsson KA, Bergstrom JH et al. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci 2011;68:3635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ling K. Polyphenol-mediated protective effects against bacterial translocation across intestinal epithelial cells and their mechanisms (Doctoral dissertation). University of Hong Kong, Pokfulam, Hong Kong SAR; 2015. Retrieved from http://dx.doi.org/10.5353/th_b5699899. [Google Scholar]
- 55. Wehkamp J, Harder J, Wehkamp K, Wehkamp-von Meissner B, Schlee M, Enders C, Sonnenborn U, Nuding S, Bengmark S, Fellermann K et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun 2004;72:5750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Henningsson AM, Bjorck IM, Nyman EM. Combinations of indigestible carbohydrates affect short-chain fatty acid formation in the hindgut of rats. J Nutr 2002;132:3098–104. [DOI] [PubMed] [Google Scholar]
- 57. Frankel WL, Zhang W, Singh A, Klurfeld DM, Don S, Sakata T, Modlin I, Rombeau JL. Mediation of the trophic effects of short-chain fatty acids on the rat jejunum and colon. Gastroenterology 1994;106:375–80. [DOI] [PubMed] [Google Scholar]
- 58. Park JH, Kotani T, Konno T, Setiawan J, Kitamura Y, Imada S, Usui Y, Hatano N, Shinohara M, Saito Y et al. Promotion of intestinal epithelial cell turnover by commensal bacteria: role of short-chain fatty acids. PLoS One 2016;11:e0156334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 2006;40:235–43. [DOI] [PubMed] [Google Scholar]
- 60. Zhong Y, Nyman M, Fak F. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol Nutr Food Res 2015;59:2066–76. [DOI] [PubMed] [Google Scholar]
- 61. Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokoyama M, Honda M, Miyamoto K et al. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism 2008;57:1071–7. [DOI] [PubMed] [Google Scholar]
- 62. Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 2011;50:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vinayagamoorthi R, Bobby Z, Sridhar MG. Antioxidants preserve redox balance and inhibit c-Jun-N-terminal kinase pathway while improving insulin signaling in fat-fed rats: evidence for the role of oxidative stress on IRS-1 serine phosphorylation and insulin resistance. J Endocrinol 2008;197:287–96. [DOI] [PubMed] [Google Scholar]
- 64. Wu T, Yin J, Zhang G, Long H, Zheng X. Mulberry and cherry anthocyanin consumption prevents oxidative stress and inflammation in diet-induced obese mice. Mol Nutr Food Res 2016;60:687–94. [DOI] [PubMed] [Google Scholar]
- 65. Vella A, Rizza RA. Extrapancreatic effects of GIP and GLP-1. Horm Metab Res 2004;36:830–6. [DOI] [PubMed] [Google Scholar]
- 66. Duca FA, Swartz TD, Sakar Y, Covasa M. Decreased intestinal nutrient response in diet-induced obese rats: role of gut peptides and nutrient receptors. Int J Obes (Lond) 2013;37:375–81. [DOI] [PubMed] [Google Scholar]
- 67. Milne GL, Dai Q, Roberts LJ II. The isoprostanes—25 years later. Biochim Biophys Acta 2015;1851:433–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.